Abstract
The vitamin A derivative all-trans retinoic acid (ATRA) is considered as a potent chemotherapeutic drug for its capability of regulating cell growth and differentiation. We aimed to study the effect of ATRA on MMP-9 in MDA-MB-231, human breast cancer cells and the probable molecular mechanisms through which ATRA exerts its effect. Our experimental findings demonstrate that ATRA enters into the nucleus and regulates various signaling pathways viz. Integrin, FAK, ERK, PI-3K, NFκB and also EGFR and downregulates pro-MMP-9 activity as well as its expression. As a result MDA-MB-231 cell migration on fibronectin medium gets retarded in presence of ATRA. ATRA upregulates TIMP-1 expression. Our study may help to understand the role of ATRA as a regulator of MMP-9 and the possible signaling pathways which are involved in this ATRA mediated downregulation of MMP-9.
Key words: MMP-9, ATRA, integrin, EGFR, NFκB
Introduction
Retinoids, including vitamin A and its analogues, regulate the growth and differentiation of a wide variety of cells. In some developing countries, vitamin A deficiency has been associated with an increased incident of cancer.1 Retinoids suppress tumor formation in many cancers.2 Retinoids act by binding to intracellular retinoic acid receptors (RARs) which interact with specific DNA response element retinoic acid response element (RARE) to regulate the transcriptional activity of retinoid target genes. There are two classes of retinoic acid receptors; RARs and RXRs, each with three subtypes α, β and γ.3 Matrix metalloproteinases (MMPs) have been reported to be inhibited by vitamin A and its analogues including all-trans retinoic acid (ATRA).4 The ability of ATRA to modulate differentiation, apoptosis, proliferation and MMP expression and activity has been suggested to explain its efficacy against tumors.
MMPs are involved in the hydrolysis of extracellular matrix (ECM) and cell surface molecules.5 The MMPs can be divided into five superfamilies and about 30 subfamilies. One superfamily, ‘the metzincins’, is distinguished by a conserved structural topology, a consensus motiff containing three histidines that bind zinc at the catalytic site and a conserved “Met-turn” motiff that sits below the active site zinc.6 The metzincins can be further subdivided into four distinct families, the (ADAMs)/adamalysins, the astacins, the serrlysins, and the matrix metalloproteinases (MMPs, matrixins).7 MMPs constitute a family of zinc dependent calcium containing endopeptidases.8 Gelatinases include MMP-2 and MMP-9. Gelatinases have a gelatin binding fibronectin domain, composed of three fibronectin repeats, inserted between the active site domain and Zn2+ binding domain.9 Gelatinase B (MMP-9) contains an additional ser/thr/pro rich collagen type IV domain.10 Pro gelatinase B have predicted molecular weights of 76 and 79 kD, but apparent molecular weight of 92 and 105 kD due to N- and O-linked glycosylation as well as 16 additional residue in the collagen-like hinge domain.11 MMP-9 is secreated as an inactive precursor that is subsequently activated by the removal of 73 amino acids at the amino terminus of the metalloproteinase.12 TGFβ also activates MMP-9 in breast and prostate cancer cells.13 Induction of MMP-9 is also regulated through cell-cell contacts and cell-ECM interaction.14 Elevated expression of MMP-9 is associated with increased metastatic potential in many cancer types including breast cancer, prostate cancer, brain cancer and melanoma.15 Overproduction of MMP-9 in non-metastatic rat embryo cells conferred a metastatic phenotype of these cells.16
In this present communication, our results shows that all-trans retinoic acid downregulates pro-MMP-9 activity by regulating tissue inhibitor metalloproteinase (TIMP-1), NFκB ex-pression, involving FAK-ERK-PI-3K pathways in MDA-MB-231 cells. Our results also demonstrates that ATRA mediated downregulation of MMP-9 inhibits MDA-MB-231 cell migration in fibronectin.
Results
Effect of ATRA on cell viability.
Cell viability (Fig. 1) was not affected significantly upon 20 µM ATRA treatment to the MDA-MB-231 cells (treated) for 48 h. ATRA treated cells showed approximately untreated (control) cells. T-test showed (p = 0.24231) that the difference between the control and treated cells were statistically not significant, i.e., there were no significant changes in cell viability between control and treated cells.
Figure 1.
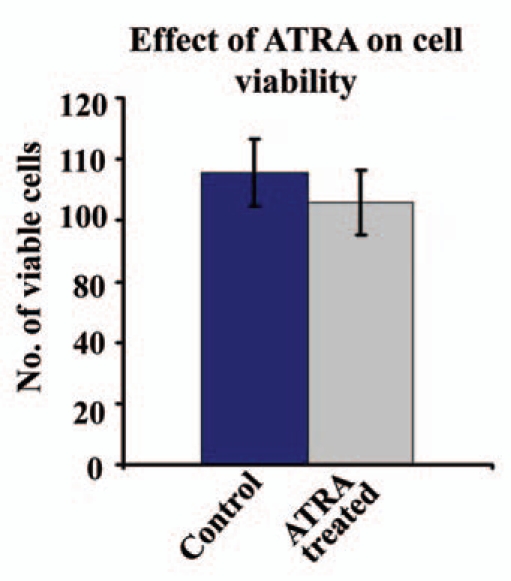
Control and 48 h 20 µM ATRA treated (Treated) MDA-MB-231 cells were checked for viability with trypan blue assay.
ATRA downregulates pro-MMP-9 activity.
Figure 2A showed appreciable reduction in pro-MMP-9 activity in the SFCM collected from MDA-MB-231 cells grown in presence of 20 µM ATRA for 48 h (lane 2) compared to the cells grown without ATRA (lane C). However, the inhibition of MMP-9 activity was not much pronounced when cells were treated with 20 µM ATRA for 24 h (lane 1). Lane M is the marker lane showing pro-MMP-9 and pro-MMP-2 activity.
Figure 2.
(A) MDA-MB-231 (300,000 cells/1.5 ml) cells were grown in absence (lane Control) and in presence of 20 µM ATRA for 24 h (lane 20 µM ATRA 24 h) and for 48 h (lane 20 µM ATRA 48 h). SFCMs collected in all cases were subjected to gelatine zymography. Lane M is the marker lane, showing pro-MMP-9 and pro-MMP-2 activity in the SFCM of HT-1080 cells. (B) Total RNA was extracted from control (lane −ATRA) and 48 h 20 µM ATRA treated (lane +ATRA) MDA-MB-231 cells. Two-step RT-PCR was performed with MMP-9 and TIMP-1 primers. PCR products were run on 2% agarose gel and bands were visualized under UV. (C) MDA-MB-231 (300,000 cells/1.5 ml) cells were grown in absence (−ATRA) and in presence (+ATRA) of 20 µM ATRA for 48 h. SFCM was collected and total protein was extracted. 50 µl of SFCM as well as 50 µg protein from both control and ATRA treated cells were subjected to assay with ELSA using anti-MMP-9 antibody.
One way ANOVA shows that there is significant difference between the groups (F2,6 = 495.40; p < 0.01). The corresponding Critical Difference (CD) at 1% level of significance (CD = 12.72) shows that the mean of control is significantly higher than that of 20 µM ATRA for 24 h and 48 h and there is also significant difference between 20 µM ATRA for 24 h and 48 h.
ATRA modulates MMP-9 and TIMP-1 expression.
MDA-MB-231 cells grown with (+ATRA) or without (−ATRA) 20 µM ATRA for 48 h showed suppression of MMP-9 expression both in SFCM and whole cell extract (Fig. 2C) in ATRA treated cells. mRNA expression (Fig. 2B) of MMP-9 was also downregulated upon 20 µM ATRA treatment. T-test showed that the difference in MMP-9 expression in SFCM (p = 0.00038) as well as in whole cell extract (p = 0.00244) between control and treated cells were statistically highly significant. However, TIMP-1 m-RNA expression (Fig. 2B) was found to be induced in presence of ATRA (+ATRA), compared to the control (−ATRA) cells.
ATRA downregulates EGFR and EGF-induced pro-MMP-9 activity.
Comparative zymogram (Fig. 3A) showed that pro-MMP-9 activity was upregulated upon 1,000 ng EGF treatment (lane 1) for 8 h, compared to the control (lane C) cells. However, pro-MMP-9 activity was inhibited appreciably in the SFCM collected from 20 µM ATRA (for 48 h) MDA-MB-231 cells (lane 2) prior to 8 h EGF treatment.
Figure 3.
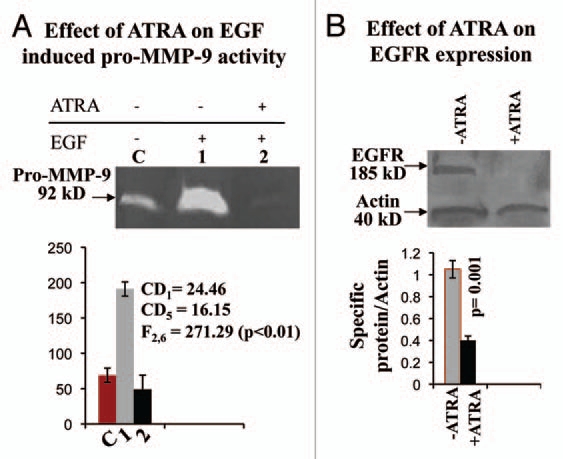
(A) SFCMs were collected from control and ATRA treated MDA-MB-231 cells, grown in presence and in absence of 1,000 ng/ml EGF for 8 h. Gelatin zymography was performed using sepharose 4B bead. Lane C denotes control MDA-MB-231 cells grown in absence of ATRA and EGF. Lane1 shows pro-MMP-9 activity of MDA-MB-231 cells grown in absence of ATRA, but in presence of EGF. Lane 2 represents pro-MMP-9 activity of ATRA treated MDA-MB-231 cells, grown in presence of EGF. (B) Total protein was extracted from both control (lane −ATRA) and ATRA treated (lane +ATRA) MDA-MB-231 cells. 100 µg of protein from each extract was subjected to western transfer on nitrocellulose membrane. Membrane was developed using anti-EGFR antibody, keeping actin as internal control.
One way ANOVA shows that there is significant difference between the groups (F2,6 = 495.40; p < 0.01). The corresponding CD at 1% level of significance = 24.46 and at 5% level of significance = 16.15. Thus the mean of EGF treated (lane 1) is significantly higher than control group and the mean of ATRA treated (lane 2) is significantly lower than that of EGF treated.
Western analysis (Fig. 3B) explained significant downregulation of EGFR expression upon 20 µM ATRA for 48 h (+ATRA), compared to the untreated (−ATRA) cells. Quantitative measurement showed that there is significant difference (p = 0.001) between control and ATRA treated EGFR expression.
ATRA downregulates integrin expression and the receptor ligand interactions.
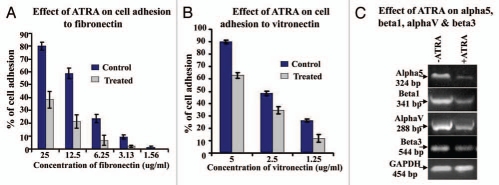
MDA-MB-231 cell adhesion to fibronectin (Fig. 4A) and vitronectin (Fig. 4B) was reduced appreciably upon 20 µM ATRA treatment (Treated) for 48 h, keeping the untreated (Control) cells as control. RT-PCR (Fig. 4C) described 20 µM ATRA for 48 h (+ATRA) downregulates m-RNA expression of α5, β1, αV and β3, comparing the control cells (−ATRA) grown without ATRA.
Figure 4.
(A) MDA-MB-231 cells grown in presence (Treated) and in absence (Control) of 20 µM ATRA were allowed to bind with different concentration of fibronectin (25 µg/ml, 12.5 µg/ml, 6.25 µg/ml, 3.125 µg/ml, 1.56 µg/ml) coated in 96 well plate. After 1.5 h incubation at 37°C wells were washed and cells were trypsinized. Numbers of bound cells were counted on a haemocytometer slide and % of adhesion was calculated. (B) MDA-MB-231 cells grown in presence (Treated) and in absence (Control) of 20 µM ATRA were allowed to bind with different concentration of vitronectin (5 µg/ml, 2.5 µg/ml, 1.25 µg/ml) coated in 96-well plate. After 1.5 h incubation at 37°C wells were washed and cells were trypsinized. Numbers of bound cells were counted on a haemocytometer slide and % of adhesion was calculated. (C) RT-PCR was performed in control (lane −ATRA) and ATRA treated (lane +ATRA) MDA-MB-231 cells with α5, β1, αv and β3 primer. GAPDH was used to confirm total RNA integrity and equal loading.
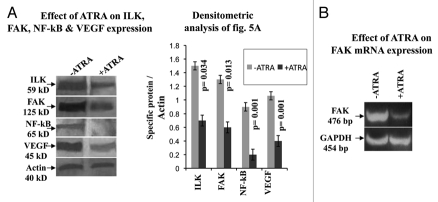
Effect of ATRA on ILK, FAK, NFκB and VEGF expression.
MDA-MB-231 cells grown in presence of 20 µM ATRA for 48 h (+ATRA) showed downregulation of ILK, FAK, NFκB and VEGF expression (Fig. 5) compared to the cells grown in absence of ATRA (−ATRA). Quantitative measurement showed that there is significant difference (p = 0.034(ILK); p = 0.013(FAK); p = 0.001(NFκB); p = 0.001(VEGF)) between control and ATRA treated ILK, FAK, NFκB and VEGF expression.
Figure 5.
(A) Western blots were performed in MDA-MB-231 cells grown in absence (lane −ATRA) and in presence (lane +ATRA) of 20 µM ATRA for 48 h as described in methods. Membranes were developed using anti-ILK, anti-FAK, anti-NFκB and anti-VEGF primary antibody, keeping actin as internal control. (B) RT-PCR of FAK was performed in MDA-MB-231 cells grown without (lane −ATRA) or with (lane +ATRA) 20 µM ATRA. PCR products were run on 2% agarose gel to visualize the bands.
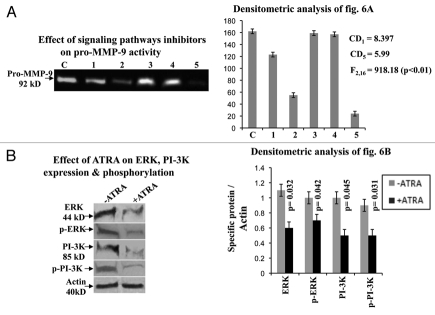
Effect of ATRA on different signaling molecules.
Comparative zymographic analysis (Fig. 6A) of pro-MMP-9 activity showed that the enzyme activity was inhibited appreciably in presence of PI-3K inhibitor (lane 2). Combined effect of ERK and PI-3K inhibitors (lane 5) also reduced the enzyme activity. However, pro-MMP-9 activity was not altered significantly in presence of ERK (lane 1), MEK (lane 3) and p38 (lane 4) inhibitors, compared to the control (lane C).
Figure 6.
(A) Gelatin zymography was performed using the SFCM, collected from MDA-MB-231 cells grown in absence (lane C) and in presence of ERK (lane 1), PI-3K (lane 2), MEK (lane 3), p38 (lane 4) and both ERK & PI-3K (lane 5) inhibitors. (B) Western blots were performed in control (lane −ATRA) and ATRA treated (lane +ATRA) MDA-MB-231 cells as before. Membranes were developed with anti-ERK, anti-p-ERK, anti-PI-3K and anti-p-PI-3K antibodies. Actin was used to confirm equal loading.
One way ANOVA shows that there is significant difference between the groups (F2,16 = 918.1765; p < 0.01). The corresponding CD at 1% level of significance = 8.397 and at 5% level of significance CD = 5.99. Thus the mean of control is significantly higher than that of lane 1, 2 and 5. However, there is no significant difference between control and lane 3, 4.
Immunoblot assay (Fig. 6B) showed that ATRA (+ATRA) suppressed expression and phosphorylation of ERK and PI-3K. Lane −ATRA demonstrated expression and phosphorylated status of ERK and PI-3K in control cells.
Quantitative measurement showed that there is significant difference (p = 0.032 (ERK); p = 0.042 (p-ERK); p = 0.045 (PI-3K); p = 0.031 (p-PI-3K)) between control and ATRA treated ERK, p-ERK, PI-3K and p-PI-3K expression.
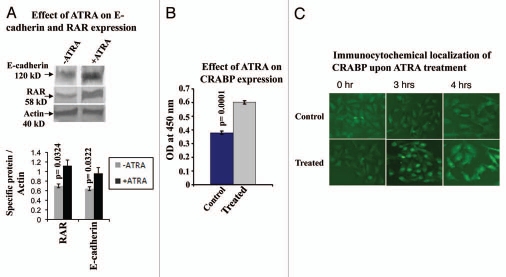
ATRA upregulates E-cadherin, RAR and CRABP expression.
Western blot analysis (Fig. 7A) of E-cadherin and RAR showed enhanced expression of the respective proteins in 20 µM ATRA treated (+ATRA) MDA-MB-231 cells, compared to the untreated control cells (−ATRA).
Figure 7.
(A) MDA-MB-231 cells were grown without (lane −ATRA) or with (lane +ATRA) ATRA for 48 h and total protein were extracted. Western blot was performed as before using anti-RAR and anti-e-cadherin antibodies. Actin was used as internal control. (B) ELISA of RAR and CRABP was performed as before in MDA-MB-231 cells, grown in presence (Treated) and in absence (Control) of ATRA for 48 h. (C) Immunocytochemical localization of CRABP was performed in MDA-MB-231 cells grown without (Control) or with (ATRA treated) 20 µM ATRA for 2, 3 and 4 h.
Quantitative measurement showed that there is significant difference (p = 0.0322(E-cadherin); p = 0.0324(RAR)) between control and ATRA treated E-cadherin, RAR expression.
Figure 7B showed that ATRA (Treated) also upregulates CRABP expression compared to the control cells (Control). T-test analysis showed (p = 0.00014) that the difference in the CRABP expression in control and treated cells were statistically highly significant.
Immunocytochemical localization of CRABP (Fig. 7C) showed that CRABP was initially present in the cytoplasm and was translocated into the nucleus within 3 h after 20 µM ATRA treatment (ATRA treated). Interestingly, after 4 h of treatment CRABP was again found to be exported in the cytoplasm. However, CRABP was found to be located in the cytoplasm in untreated (Control) cells even after 4 h.
ATRA inhibits migration of MDA-MB-231 cells in fibronectin medium.
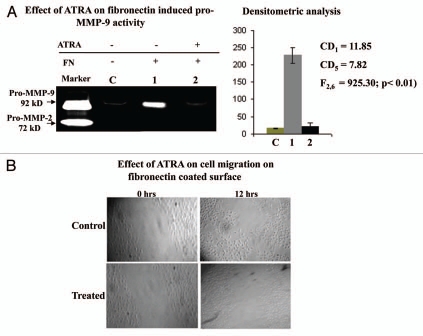
MDA-MB-231 cells grown without (lane 1) or with (lane 2) ATRA were allowed to grow in presence of 20 µg/ml fibronectin for 2 h. Zymographoic analysis (Fig. 8A) showed appreciable inhibition of fibronectin induced pro-MMP-9 activity in the SFCM of ATRA treated (lane 2) cells, compared to the control (lane 1). The MMP-9 activity was not much pronounced in absence of fibronectin (lane C), compared to fibronectin induced MMP-9 (lane 1).
Figure 8.
(A) MDA-MB-231 cells (300,000 cells/1.5 ml) grown in presence (lane 2) and in absence (lane 1) of 20 µM ATRA for 48 hrs. Both control and ATRA treated cells were then allowed to grow in presence of 20 µg/ml fibronectin for 2 h in SFCM. Lane C represents MDA-MB-231 cells grown in absence of fibronectin as well as ATRA. SFCMs were then subjected to gelatine zymography as before. Lane M is the marker lane, showing pro-MMP-9 and pro-MMP-2 activity in the SFCM, collected from HT-1080 cells. (B) Cell migration efficiency of Control and ATRA treated MDA-MB-231 cells were observed under inverted microscope by creating wounds in cell culture dishes. Cells were allowed to grow in presence of fibronectin ECM ligand to observe the efficacy of their migrations in SFCM.
One way ANOVA shows that there is significant difference between the groups (F2,6 = 925.30; p < 0.01). The corresponding CD at 1% level of significance = 11.85 and CD at 5% level of significance = 7.82. Therefore, the mean of FN treated (lane 1) MMP-9 is significantly higher than that of without FN MMP-9 (lane C) and also much higher than that of ATRA treated fn induced MMP-9 (lane 2).
When 20 µM ATRA treated (for 48 h) MDA-MB-231 cells (ATRA treated) were allowed to grow in presence of fibronectin over an wound (Fig. 8B), cell growth was observed to be restricted outside the wound and no or very less number of cells were observed to grow across the wound even after 12 h. However, cells which were not received any ATRA treatments (Control) were grown throughout the culture dish and after 12 h they were found to migrate across the wound.
Discussion
This study demonstrates downregulation of pro-MMP-9 activity in the serum free culture medium (SFCM) of MDA-MB-231 cells upon 20 µM ATRA treatment for 48 h. MMP-9 ex-pression in the both SFCM and whole cell extract was also reduced in ATRA treated MDA-MB-231 cells. Therefore, the reduced activity of pro-MMP-9 in the SFCM may be due to reduced expression of MMP-9 protein. Furthermore downregulation of MMP-9 m-RNA in ATRA treated cells describes that ATRA may inhibit pro-MMP-9 activity by down regulating MMP-9 gene ex-pression. To analyze the mechanism behind the ATRA mediated inhibition of gelatinolytic activity; TIMP-1 expression was studied and was found to be upregulated upon ATRA treatment. Activity of pro-MMP-9 in the extra cellular space is inhibited by TIMP-1.17 Upregulation of TIMP-1 expression therefore, may downregulate pro-MMP-9 activity. Cell viability was not found to be affected by ATRA treatment.
Several studies have shown that epidermal growth factor (EGF) stimulates the expression of MMP-9.18 Studies also showed that EGF increased the secretion of MMP-9.19 Epidermal growth factor receptor (EGFR) is a transmembrane protein with intrinsic protein tyrosin kinase (PTK) activity which is activated on EGF binding.20 EGFR activation involves homo- or hetero-dimerization of other EGFR family members, transphosphorylation of receptor, recruitment of various signaling proteins and activation of numbers of signaling pathways.21 1,000 ng EGF treatment for 8 h in the MDA-MB-231 cells induces pro-MMP-9 activity significantly. However, the activity was not induced appreciably in the ATRA treated MDA-MB-231 cells. The EGFR expression was found to be downregulated significantly after ATRA treatment. Therefore, ATRA mediated inhibition of pro-MMP-9 activity may result due to downregulation of EGFR in ATRA treated cells. Furthermore, integrins and EGFR can co-cluster at the cell surface and can co-activate common intracellular signaling cascades22 which may lead to gelatinolytic activity. ATRA inhibits the interaction between integrin receptors and fibronectin or vitronectin due to lower expression of α5b1 and αVβ3 in ATRA treated cells. A study by Rolli M, et al. a positive cooperation between MMP-9 and αVβ3 integrin receptors that contribute to migratory responses of metastatic breast cancer cells.23 Human MMP-9 contains an RGD sequence and this site may serve as recognition motif for α5β1 and αVβ3.24 In our experiment decreased expression of α5β1 and αVβ3 in ATRA treated cells possibly contribute to the reduced activity of pro-MMP-9.
Integrin proximal events are involved in the initiation of integrin-mediated signal transduction. A novel ankyrin repeat containing serine-threonine protein kinase (ILK) has been demonstrated to associate with the β1 and β3 subunit cytoplsmic domains24 and may be involved in regulating integrin-mediated signaling. Overexpression of ILK leads to alteration of cell adhesion to ECM and promotes anchorage-independent cell growth.25 Downregulation of ILK expression upon ATRA treatment to MDA-MB-231 cells possibly alter the cell adhesion to fibronectin and vitronectin and may alter the downstream signaling cascade. Furthermore, ILK over expression cells promote loss of expression or function of E-cadherin.26 E-cadherin, a calcium mediated membrane glycoprotein, mediates cell-cell adhesion interactions always in presence of Ca2+ ions.27 Dysfunction of E-cadherin has been correlated with malignancy or evidenced by tumor progression, loss of differentiation, invasion, metastasis and poor prognosis.28 Therefore, in our results downregulation of ILK expression by ATRA may reflect its effect upregulation of E-cadherin in ATRA treated MDA-MB-231 cells. ATRA mediated inhibition of cell-matrix association and stimulation of cell-cell contract may lead to ATRA mediated inhibition of cell migration on fibronectin.
FAK functions as an important integration point for the regulation of EGF and serum stimulated signal promoting human tumor cell motility and invasion respectively.29 The inhibition of FAK expression and function was found to result in decreased MMP-9 secretion.30 FAK can also associate with activated growth factor receptors through its FERM homology region and its activation can potentially affect integrin matrix-contact stability by regulation of MMP secretion.31 Studies showed that inhibition of FAK expression and function resulted in decrease MMP-9 secretion.32 ATRA treatment to the MDA-MB-231 cells resulted in downregulation of FAK expression could be a regulatory step in MMP-9 expression and activity.
The cross-talk between EGFR-integrin-FAK leads to activation of different signaling pathways such as ERK,33 PI-3K34 and regulate MMP-9 expression.35 ATRA by downregulating EGFR, α5-integrin and FAK may downregulate the expression and phosphorylation of ERK and PI-3K which in turn downregulate the expression of MMP-9 in the whole cell extract and in the SFCM. This lower expression of MMP-9 reflected in the gelatin zymography which showed reduced pro-MMP-9 activity in ATRA treated cells.
The MMP-9 promoter contains multiple transcription factor binding sites including AP1, SP1 and NFκB.36 The expression of NFκB and its binding to MMP-9 promoter was found to be reduced upon ATRA treatment. This ATRA mediated downregulation of NFκB expression is reflected on the ELISA and RT-PCR result which showed lower expression of MMP-9 in the whole cell extract.
MMP-9 is induced in premetastatic lung endothelial cells via VEGFR-1 receptor, which gets activated upon VEGF ligand binding.37 The block of MMP-9 induction via VEGFR-1 inhibition culd be useful for the prevention of tumor metastasis. In our study ATRA mediated inhibition of VEGF could inhibits the VEGF-VEGFR-1 interaction and the downstream signaling pathways which may affect pro-MMP-9 activity.
Downregulation of pro-MMP-9 by ATRA even in presence of fibronectin indicates lower efficiency of cell migration in fibronectin medium in presence of ATRA. Therefore, ATRA reduces cellular motility which may in turn downregulate cell invasion and may hamper the process of metastasis.38
The effect of ATRA is known to be mediated through two types of receptors: CRABPs and RARs. CRABPs serve to solubilize and transport their lipophilic ligand in the aqueous phase of cytosol. Ligand binding induces nuclear localization of CRABP-II and form a complex with RAR which mediates direct channeling of RA and facilitates the ligation of the receptor.38 Our results showed that CRABP is predominantly cytosolic in the absence of ATRA, but that it undergoes a dramatic nuclear localization upon binding of ATRA. Our experimental findings showed that CRABP interacts with RAR in a ligand dependent fashion and the whole complex enters into the nucleus within 3 h of ATRA treatment. The CRABP-RAR complex that mediates RA channeling is a short lived intermediate that rapidly dissociates following completion of transfer39 and our immunocytochemical study demonstrated nuclear localization of CRABP after 4 h of ATRA treatment. Furthermore, RAR and CRABP expression get stimulated upon ATRA treatment which may indicate the involvement of RAR and CRABP receptors in ATRA mediated signaling pathway.
Materials and Methods
Materials.
Minimal Essential Medium (MEM) and fetal bovine serum (FBS) were purchased from Invitrogen Corporation, USA. Fibronectin (440 kD) and protein G agarose was purchased from Roche, Germany. Vitronectin (75 kD) were purchased from BD Biosciences, USA. Gelatin Sepharose 4B beads was purchased from Amersham Biosciences, USA. All-trans Retinoic Acid (ATRA) was purchased from Sigma. Anti-FAK, anti-ILK, anti-MMP-9, anti-NFκB (p65), anti-ERK, anti-p-ERK, anti-PI-3K, anti-p-PI-3K, anti-EGFR, anti-VEGF, anti-RAR, anti-E-cadherin, anti-CRABP and anti-actin primary antibodies and alkaline phosphatase coupled, HRP-coupled and FITC-coupled secondary antibodies both monoclonal and polyclonal were purchased from Santa Cruz, USA. Super signal west pico chemiluminiscent substrate was purchased from Pierce, USA, Nitro blue tetrazolium/5-bromo-4-chloro-3-indoyl phosphate (NBT/BCIP, western blue stabilized substrate for alkaline phosphatase), ERK inhibitor (PD 98059), PI-3K inhibitor (LY294002), MEK inhibitor (U0126) and p38 inhibitor (SB203580) were purchased from Promega (Madison, WI, USA). Tetramethyl benzidine (TMB), GAPDH primers and 100 base pair DNA ladder were purchased from Bangalore Genei, India. RNAqueous 4 PCR (Total RNA isolation kit) and Retroscript (RT-PCR Kit) were purchased from Ambion, USA. MMP-9, FAK, TIMP-1, α5, β1, αV, β3 primers were purchased from Operon, USA.
Methods.
Cell culture. MDA-MB-231 human breast cancer cell line and HT1080 human fibrosarcoma cell line were obtained from National Centre for Cell Sciences (NCCS), Pune, India. MDA-MB-231 and HT1080 cells were grown and maintained in MEM containing 10% FBS in a CO2 incubator at 37°C.
Drug treatment. 7.5 mg ATRA was dissolved in 5 ml DMSO to prepare 5 mM stock solution. ATRA was added to the experimental dishes at concentrations of 10, 20, 30 µM. Control cells were treated with equal concentration of DMSO.
Cell viability assay. MDA-MB-231 cells (300,000/1.5 ml) were grown in absence (Control) and in presence of 20 µM ATRA for 48 h. Control and ATRA treated cells were collected by trypsinization. 10 µl of cell suspension (Control and ATRA treated) in PBS were taken and 10 µl Trypan blue was added into it. Cell suspensions were mixed well and kept for 3–5 min at room temperature. Numbers of stained and unstained cells were counted in a haemocytometer slide.
Zymography. MDA-MB-231 cells (300,000/1.5 ml) were initially grown in MEM supplemented with 10% FBS in petridishes, washed with serum free culture medium (SFCM) and treated with 20 µM concentrations of ATRA for 24 and 48 h in SFCM. Control cells were grown without ATRA but in presence of 1% DMSO (solvent for ATRA) for 48 h and serum free culture medium (SFCM) were collected. MDA-MB-231 cells were grown in absence and in presence of ERK, PI-3K, MEK, p38 inhibitors for 45 min and were then washed with fresh SFCM. SFCM were collected after 24 h. MDA-MB-231 cells, grown without or with 20 µM ATRA for 48 h were allowed to grow in presence of 1,000 ng EGF. SFCM were collected after 8 h. The MMPs in all the cases were separated from SFCM using Gelatin Sepharose 4B beads and shaking for 2 h at 4°C. The beads were washed ×3 with Tris-buffered saline with Tween-20 (TBS-T) and suspended in 50 µl of 1X sample buffer (0.075 gm Tris, 0.2 gm SDS in 10 ml water, pH 6.8). The suspension was incubated for 30 min. at 37°C and then centrifuged at 3,000 r.p.m. for 3 min. The supernatant was then subjected to zymography on 10% SDS-PAGE co-polymerized with 0.1% gelatin. Gel was washed in 2.5% Triton-X-100 for 30 min to remove SDS and was then incubated overnight in reaction buffer (50 mM Tris-HCl pH 7, 4.5 mM CaCl2, 0.2 M NaCl). After incubation, the gel was stained with 0.5% coomassie blue in 30% methanol and 10% glacial acetic acid. The bands were visualized by destaining the gel with water.
ELISA (enzyme linked immunosorbent assay). MDA-MB-231 cells (300,000/1.5 ml) were grown in absence and in presence of 20 µM ATRA for 48 h. The culture supernatants were collected by centrifugation at 3,000 r.p.m for 3 min and cells were extracted. The wells of microtitre plate were coated in triplicate with 50 µg of protein from control and ATRA treated cell extract and 50 µl culture SFCM from both control and experimental set and kept at 4°C overnight (plate was wrapped in wrap to prevent evaporation). Blank wells (with buffer in which samples are suspended) were also prepared. Next day wells were washed with blocking buffer (1% BSA in PBS) to block non-specific binding sites and incubated for 1 h at 37°C. Then the wells were washed three times with washing buffer (0.5% NP-40 and 0.5% BSA dissolved in PBS). Anti-MMP-9, anti-CRABP primary antibody solution (1:1,000 dilution) was added to respective wells and incubated at 37°C for 1 h. Wells were washed three times with washing buffer. Respective second antibody solution (1:1,000 dilution buffer) was added to wells and incubated at 37°C for 1 h. Wells were washed six times with washing buffer (3–5 min per wash). Substrate (TMB) was added to the wells (in dark) and kept as long as required (i.e., until color developed begins to become too intense). Then 1 M H2SO4 stop solution was added and reading was taken in ELISA reader at 450 nm.
RT-PCR. RNA was extracted from MDA-MB-231 cells grown in absence (Control) presence and of 20 µM ATRA for 48 h. The sequence of the primers used for PCR were: hMMP-9: 5′-GTA TTT GAT GGC ATC GCT CA-3′ (forward) and 5′-CAT TCC CTG CAA AGA ACA CA-3′ (reverse), hTIMP-1: 5′-hFAK: 5′-GCG CTG GCT GGA AAA AGA A-3′ (forward) and 5′-TCG GTG GGT GCT GGC TGG TAG G-3′ (reverse), hα5: 5′-CAT TTC CGA GTC TGG GCC AA-3′ (forward) and 5′-CAA AAC AGC CAG TAG CAA CAA-3′ (reverse), hβ1: 5′-TGT TCA GTG CAG AGC CTT CA-3′ (forward) and 5′-CCT CAT ACT TCG GAT TGA CC-3′ (reverse), hαV: 5′-GTT GGG AGA TTA GAC AGA GGA-3′ (forward) 5′-CAA AAC AGC CAG TAG CAA CAA-3 (reverse), 5′-hβ3. 5′-GGG GAC TGC CTG TGT GAC TC-3′ (forward) 5′-CTT TCC GGT CGT GGA TGG TG-3′ (reverse), GAPDH primers 5′-CGG AGT CAA CGG ATT TGG TCG TAT-3′ (forward) and 5′-AGC CTT CTC CAT GGT GGT GAA GAC-3′ (reverse) were used as control to normalize for mRNA integrity and equal loading. RT-PCR was carried out using two steps RT-PCR kit (Ambion, USA). Components were incubated at 42°C for 1 h and at 92°C for 10 min (to inactivate the reverse transcriptase). Conditions used for PCR consisted of 24 cycles for MMP-9 at 94°C for 30 sec, 63°C for 30 sec and 72°C for 60 sec, 25 cycles for FAK at 94°C for 30 sec, 60°C for 30 sec and 72°C for 1:30 min and 28 cycles for α5, β1, hαV and hβ3 at 94°C for 30 sec, 58°C for 30 sec and 72°C for 1:30 min with a final incubation at 72°C for 7 min in DNA thermal cycler. The predicted size of the PCR products were 198 base pairs (bp) for MMP-9, 476 bps for FAK, 324 for α5, 452 for β1, 288 for αV, 544 for β3 and 454 for GAPDH.
Immunoblot assay of NFκB, ERK, p-ERK, PI-3K, p-PI-3K, EGFR, VEGF, RAR and E-cadherin. MDA-MB-231 cells (300,000/1.5 ml) were grown in in absence (control) and in presence of 20 µM ATRA for 48 h. The respective cells were collected. Cell extraction was carried out using cell extraction buffer (37.5 mM Tris, 75 mM NaCl and 0.5% Triton-X-100) and the protein content of the extracts were estimated by Lowry's method. Equal amount of protein was taken and incubated with 1X sample buffer for 30 min followed by 5–8 min incubation with 0.1 volumes β-mercaptoethanol at 90°C. Samples were then subjected to electrophoresis on 7.5% SDS-PAGE. The proteins were transferred on to nitrocellulose membranes by western Blot at 300 mA for 3 h. The membranes were blocked with 1% BSA and subsequently washed three times with TBS-T. The immunoblots were reacted with anti NFκB, Anti-EGFR, anti-VEGF, anti-ERK, anti-p-ERK, anti-PI-3K, anti-p-PI-3K, anti-RAR, anti-E-cadherin primary antibodies respectively (1:1,000 dilution) for 1.5 h at 37°C followed by incubation with respective alkaline phosphatase coupled second antibodies. Bands were developed using NBT-BCIP as substrate. Actin was used to confirm equal loading.
Western blot of ILK and FAK. MDA-MB-231 cells (300,000/1.5 ml) were grown in in absence (control) and in presence of 20 µM ATRA for 48 h. The respective cells were collected. Cell extraction was carried out using cell extraction buffer (37.5 mM Tris, 75 mM NaCl and 0.5% Triton-X-100) and the protein content of the extracts were estimated by Lowry's method. Equal amount of protein was taken and incubated with 1X sample buffer for 30 min followed by 5–8 min incubation with 0.1 volumes β-mercaptoethanol at 90°C. Samples were then subjected to electrophoresis on 7.5% SDS-PAGE. The proteins were transferred to a nitrocellulose membrane. The membrane was blocked overnight at 4°C. The membrane was incubated with anti-ILK and anti- FAK primary antibodies (1:1,000) for 4.5 h at 37°C temperature. The membrane was washed 4 times, 15 min each, on a rocking platform at room temperature with TBST. Respective secondary antibodies conjugated with Horse Radish Peroxidase was diluted (1:40,000) with TBST containing 1% BSA at a ratio of 1:500 and was added to the membrane and was incubated at 37°C for 1.5 h. Washed with TBST 4 times, 15 min each. The membrane was transferred to a visualization solution (western blotting luminol reagent solution A and solution B) and soaked for 30 seconds. The membrane was removed, the excess fluid was drained off and placed inside a film cassette and a plastic wrap was folded back on to the membrane to form a tight enclosure. A photographic film cut accordingly was placed above the membrane, exposed for 1–3 min, developed and fixed. Finally, the film was washed with water and the examined for visualization of the target band.
Cell adhesion assay. The microtitre plate wells were coated with fibronectin (1.56 µg/ml, 3.125 µg/ml, 6.25 µg/ml, 12.5 µg and 25 µg/ml) or vitronectin (5 µg/ml, 2.5 µg/ml and 1.25 µg/ml). The ligands were allowed to bind for 1.5 h at 37°C. Wells were blocked with Buffer C (1% BSA, 1 mM CaCl2 and 1 mM MgCl2 in PBS) for 1 h at 37°C. Control and 20 µM ATRA treated (for 24 h and 48 h in complete medium) MDA-MB-231 cells were trypsinised from culture dishes, washed, suspended in Buffer C and added to microtitre plates (50,000 cells/well) and allowed to bind at 37°C for 1.5 h. The wells were washed ×3 with Buffer C. The bound cells were trypsinised, counted on haemocytometer and expressed as % of adhesion.
Immunocytochemistry of CRABP. MDA-MB-231 cells were allowed to grow overnight on coverslips in MEM containing 10% FBS. After washing with, the cells on the cover slips were treated with or without 20 µM ATRA for 2, 3 and 4 h in SFCM. The coverslips were then washed in PBS, fixed with 3.5% formaldehyde, permeabilised with 0.5% Triton-X100 and the nonspecific sites were blocked with 1% BSA. The coverslips were then treated with anti-CRABP primary antibody followed by FITC-coupled second antibody at 37°C in a humidified chamber. After washing 5 times in PBS, the cover slips were mounted on glass slides and observed under a fluorescence microscope.
Wound healing assay. MDA-MB-231 cells (300,000 cells/1.5 ml) grown in absence and in presence of 20 µM ATRA for 48 h. A wound was made in both treated and untreated cells with the fine tip of microtips. Cells were washed thoroughly with SFCM and were allowed to grow in presence of 20 µg/ml fibronectin and the migration of cells over the wound was observed under inverted microscope at 2 h intervals.
Statistical analysis. Statistical analyses were done with Student's t-test comparing between the control and treated groups. The analysis was done using Microsoft Excel (2003). In some cases statistical significance was done with ANOVA using SYSTAT 9.0.
Quantitative analysis of zymography and western blots were performed with Image J version 1.4.3.67.
Conclusion
Our experimental findings indicate that ATRA downregulate MMP-9 due to upregulation of TIMP-1, E-cadherin and downregulation of EGFR, NFκB, FAK and α5β1, αVβ3 integrin receptors in ATRA treated cells. ATRA mediated downregulation of MMP-9 may also inhibit cell migration on fibronectin. The effects of ATRA involve its cytoplasmic receptor CRABP to translocate the drug into nucleus where it binds to the nuclear receptor RAR.
Acknowledgments
We are thankful to Dr. Shyam Sundar Mandal, statistical officer, Chittaranjan National Cancer Institute, Kolkata (India) for the statistical calculations of the results. Grants, sponsors and Funding sources: Chittaranjan National Cancer Institute, Kolkata, India.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/11682 DOI: 10.4161/cam.4.3.11682
References
- 1.Silveira ER, Naves MM, Vannucchi H, Jordao Junior AA, Dagli ML, Moreno FS. Vitamin A and all-trans and 9-cis retinoic acids inhibit cell proliferation during the progression phase of hepatocarcinogenesis in Wistar rats. Nutr Cancer. 2001;39:244–251. doi: 10.1207/S15327914nc392_14. [DOI] [PubMed] [Google Scholar]
- 2.Orlandi M, Mantovani B, Ammar K, Avitabile E, Dal Monte P, Bartolini G. Retinoids and cancer: antitumoral effects of ATRA, 9-cis RA and the new retinoid IIF on the HL-60 leukemic cell line. Med Princ Pract. 2003;12:164–169. doi: 10.1159/000070753. [DOI] [PubMed] [Google Scholar]
- 3.Soprano DR, Qin P, Soprano KJ. Retinoic acid receptors and cancers. Ann Rev Nutr. 2004;24:201–221. doi: 10.1146/annurev.nutr.24.012003.132407. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Zang C, Fenner MH, Possinger K, Elstner E. PPARgamma ligands and ATRA inhibit the invasion of human breast cancer cells in vitro. Breast Cancer Res Treat. 2003;79:63–74. doi: 10.1023/a:1023366117157. [DOI] [PubMed] [Google Scholar]
- 5.Klein G, Vellenga E, Fraaije MW, Kamps WA, de Bont ES. The possible role of matrix metalloproteinase (MMP)-2 and MMP-9 in cancer, e.g., acute leukemia. Crit Rev Oncol Hematol. 2004;50:87–100. doi: 10.1016/j.critrevonc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 6.Gomis-Ruth FX. Structural aspects of the metzincin clan of metalloendopeptidases. Mol Biotechnol. 2003;24:157–202. doi: 10.1385/MB:24:2:157. [DOI] [PubMed] [Google Scholar]
- 7.Berthier C, Marti HP. Metzincins, including matrix metalloproteinases and meprin, in kidney transplantation. Swiss Med Wkly. 2006;136:789–794. doi: 10.4414/smw.2006.11408. [DOI] [PubMed] [Google Scholar]
- 8.Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 2003;4:216. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Opdenakker G, Van den Steen PE, Van Damme J. Gelatinase B: a tuner and amplifier of immune functions. Trends Immunol. 2001;22:571–579. doi: 10.1016/s1471-4906(01)02023-3. [DOI] [PubMed] [Google Scholar]
- 10.Van den Steen PE, Opdenakker G, Wormald MR, Dwek RA, Rudd PM. Matrix remodelling enzymes, the protease cascade and glycosylation. Biochim Biophys Act. 2001;1528:61–73. doi: 10.1016/s0304-4165(01)00190-8. [DOI] [PubMed] [Google Scholar]
- 11.Murphy G, Crabbe T. Gelatinases A and B. Methods Enzymol. 1995;248:470–484. doi: 10.1016/0076-6879(95)48030-7. [DOI] [PubMed] [Google Scholar]
- 12.Juarez J, Clayman G, Nakajima M, Tanabe KK, Saya H, Nicolson GL, Boyd D. Role and regulation of expression of 92-kDa type-IV collagenase (MMP-9) in 2 invasive squamous-cell-carcinoma cell lines of the oral cavity. Int J Cancer. 1993;55:10–18. doi: 10.1002/ijc.2910550104. [DOI] [PubMed] [Google Scholar]
- 13.Kim ES, Kim MS, Moon A. TGF-beta-induced upregulation of MMP-2 and MMP-9 depends on p38 MAPK, but not ERK signaling in MCF10A human breast epithelial cells. Int J Oncol. 2004;25:1375–1382. [PubMed] [Google Scholar]
- 14.Yan C, Tian F, Xiao F, Li K, Li C. Adhesion induces matrix metalloproteinase-9 gene expression in ovarian cancer cells. Zhonghua Zhong Liu Za Zhi. 2002;24:17–19. [PubMed] [Google Scholar]
- 15.Talvensaari-Mattila A, Turpeenniemi-Hujanen T. Preoperative serum MMP-9 immunoreactive protein is a prognostic indicator for relapse-free survival in breast carcinoma. Cancer Lett. 2005;217:237–242. doi: 10.1016/j.canlet.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 16.Bernhard EJ, Gruber SB, Muschel RJ. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proceedings of the Natl Acad Sci of USA. 1994;91:4293–4297. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankenberger M, Hauck RW, Frankenberger B, Haussinger K, Maier KL, Heyder J, Ziegler-Heitbrock HW. All trans-retinoic acid selectively downregulates matrix metalloproteinase-9 (MMP-9) and upregulates tissue inhibitor of metalloproteinase-1 (TIMP-1) in human bronchoalveolar lavage cells. Mol Med. 2001;7:263–270. [PMC free article] [PubMed] [Google Scholar]
- 18.Pellikainen JM, Ropponen KM, Kataja VV, Kellokoski JK, Eskelinen MJ, Kosma VM. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in breast cancer with a special reference to activator protein-2, HER2 and prognosis. Clin Cancer Res. 2004;10:7621–7628. doi: 10.1158/1078-0432.CCR-04-1061. [DOI] [PubMed] [Google Scholar]
- 19.Oc P, Modjtahedi H, Rhys-Evans P, Court WJ, Box GM, Eccles SA. Epidermal growth factor-like ligands differentially upregulate matrix metalloproteinase 9 in head and neck squamous carcinoma cells. Cancer Res. 2000;60:1121–1128. [PubMed] [Google Scholar]
- 20.Cao H, Lei ZM, Bian L, Rao CV. Functional nuclear epidermal growth factor receptors in human choriocarcinoma JEG-3 cells and normal human placenta. Endocrinology. 1995;136:3163–3172. doi: 10.1210/endo.136.7.7540549. [DOI] [PubMed] [Google Scholar]
- 21.Kuwada SK, Li X. Integrin alpha5/beta1 mediates fibronectin-dependent epithelial cell proliferation through epidermal growth factor receptor activation. Mol Biol Cell. 2000;11:2485–2496. doi: 10.1091/mbc.11.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growthfactor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- 23.Rolli M, Fransvea E, Pilch J, Saven A, Felding-Habermann B. Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc Nl Acad Sci USA. 2003;100:9482–9487. doi: 10.1073/pnas.1633689100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huhtala P, Humphries MJ, McCarthy JB, Tremble PM, Werb Z, Damsky CH. Cooperative signaling by alpha5beta1 and alpha4beta1 integrins regulates metalloproteinase gene expression in fibroblasts adhering to fibronectin. J Cell Biol. 1995;129:867–879. doi: 10.1083/jcb.129.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hannigan GE, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino MG, Radeva G, Filmus J, et al. Regulation of cell adhesion and anchorage-dependent growth by a new beta1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 26.Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J Biol Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- 27.Wu C, Keightley SY, Leung-Hagesteijn C, Radeva G, Coppolino M, Goicoechea S, et al. Integrin-linked protein kinase regulates fibronectin matrix assembly, E-cadherin expression and tumorigenicity. J Biol Chem. 1998;273:528–536. doi: 10.1074/jbc.273.1.528. [DOI] [PubMed] [Google Scholar]
- 28.Mittari E, Charalabopoulos A, Batistatou A, Charalabopoulos K. The role of E-cadherin/catenin complex in laryngeal cancer. Exp Oncol. 2005;27:257–261. [PubMed] [Google Scholar]
- 29.Chan AO. E-cadherin in gastric cancer. World J Gastroenterol. 2006;12:199–203. doi: 10.3748/wjg.v12.i2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Munshi HG, Wu YI, Ariztia EV, Stack MS. Calcium regulation of matrix metalloproteinase-mediated migration in oral squamous cell carcinoma cells. J Biol Chem. 2002;277:41480–4148. doi: 10.1074/jbc.M207695200. [DOI] [PubMed] [Google Scholar]
- 31.Rothhut B, Ghoneim C, Antonicelli F, Soula-Rothhut M. Epidermal growth factor stimulates matrix metalloproteinase-9 expression and invasion in human follicular thyroid carcinoma cells through Focal adhesion kinase. Biochimie. 2007;89:613–624. doi: 10.1016/j.biochi.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 32.Sein TT, Thant AA, Hiraiwa Y, Amin AR, Sohara Y, Liu Y, et al. A role for FAK in the Concanavalin A-dependent secretion of matrix metalloproteinase-2 and -9. Oncogene. 2000;19:5539–5542. doi: 10.1038/sj.onc.1203932. [DOI] [PubMed] [Google Scholar]
- 33.Segarra M, Vilardell C, Matsumoto K, Esparza J, Lozano E, Serra-Pages C, et al. Dual function of focal adhesion kinase in regulating integrin-induced MMP-2 and MMP-9 release by human T lymphoid cells. Faseb J. 2005;19:1875–1877. doi: 10.1096/fj.04-3574fje. [DOI] [PubMed] [Google Scholar]
- 34.Yao J, Xiong S, Klos K, Nguyen N, Grijalva R, Li P, Yu D. Multiple signaling pathways involved in activation of matrix metalloproteinase-9 (MMP-9) by heregulinbeta1 in human breast cancer cells. Oncogene. 2001;20:8066–8074. doi: 10.1038/sj.onc.1204944. [DOI] [PubMed] [Google Scholar]
- 35.Felx M, Guyot MC, Isler M, Turcotte RE, Doyon J, Khatib AM, et al. Endothelin-1 (ET-1) promotes MMP-2 and MMP-9 induction involving the transcription factor NFkappaB in human osteosarcoma. Clin Sci (Lond) 2006;110:645–654. doi: 10.1042/CS20050286. [DOI] [PubMed] [Google Scholar]
- 36.Hiratsuka S, Nakamura K, Iwai S, Murakami M, Itoh T, Kijima H, et al. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer cell. 2002;2:289–300. doi: 10.1016/s1535-6108(02)00153-8. [DOI] [PubMed] [Google Scholar]
- 37.Barkho BZ, Munoz AE, Li X, Li L, Cunningham LA, Zhao X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells. 2008;26:3139–149. doi: 10.1634/stemcells.2008-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dong D, Ruuska SE, Levinthal DJ, Noy N. Distinct roles for cellular retinoic acid-binding proteins I and II in regulating signaling by retinoic acid. J Biol Chem. 1999;274:23695–23698. doi: 10.1074/jbc.274.34.23695. [DOI] [PubMed] [Google Scholar]
- 39.Budhu AS, Noy N. Direct channeling of retinoic acid between cellular retinoic acid-binding protein II and retinoic acid receptor sensitizes mammary carcinoma cells to retinoic acid-induced growth arrest. Mol Cell Biol. 2002;22:2632–2341. doi: 10.1128/MCB.22.8.2632-2641.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]