Abstract
In neural crest cell development, the expression of the cell adhesion proteins cadherin-7 and cadherin-11 commences after delamination of the neural crest cells from the neuroepithelium. The canonical Wnt signaling pathway is known to drive this delamination step and is a candidate for inducing expression of these cadherins at this time. This project was initiated to investigate the role of canonical Wnt signaling in the expression of cadherin-7 and cadherin-11 by treating neural crest cells with Wnt3a ligand. Expression of cadherin-11 was first confirmed in the neural crest cells for the chicken embryo. The changes in the expression level of cadherin-7 and -11 following the treatment with Wnt3a were studied using real-time RT-PCR and immunostaining. Statistically significant upregulation in the mRNA expression of cadherin-7 and cadherin-11 and in the amount of cadherin-7 and cadherin-11 protein found in cell-cell interfaces between neural crest cells was observed in response to Wnt, demonstrating that cadherin-7 and cadherin-11 expressed by the migrating neural crest cells can be regulated by the canonical Wnt pathway.
Key words: neural crest, Wnt, cadherin-7, cadherin-11
Introduction
The neural crest originates from a population of cells at the dorsal surface of the neural tube. Premigratory neural crest cells undergo an epithelial-to-mesenchyme transition (EMT), leave the neural tube as mesenchymal cells to migrate along ventral or dorso-lateral pathways, and undergo differentiation into a wide variety of cell types (reviewed in refs. 1–3). At the trunk level, the ventrally migrating neural crest cells form the sensory, autonomic and enteric neurons, the glia of the peripheral nervous system and neurosecretory cells of the adrenal medulla, while the dorsolaterally migrating cells differentiate into melanocytes. The course of neural crest cell development engages dramatic changes in cell adhesion and motility, including changes in cell-to-cell and integrin-mediated adhesion to the substratum.
The classical cadherin proteins belong to a superfamily of transmembrane receptors that mediate cell-cell adhesion via the calcium-dependent homophilic binding of the extracellular domains.4,5 The Type I classical cadherins tend to participate in strong cell-cell adhesion and the Type II classical cadherins are associated with weaker cell-cell adhesion, the promotion of migratory behavior, and expression by cells with a mesenchymal cell phenotype. Several Type II or mesenchymal cadherins are expressed in neural crest cells in a spatially and temporally-regulated manner. The dynamic expression pattern of these proteins suggests that specific cadherins could play an important role in transition to a mesenchymal phenotype and later steps of neural crest development.6,7 In the chicken embryo, N-cadherin is strongly expressed throughout the neuroepithelium and the Type II family member cadherin-6B is expressed by dorsally-located neural crest progenitors. The neural crest cells emerging from the neural tube express other Type II cadherins, such as cadherin-7.8,9 In addition, cadherin-11 is known to be expressed in neural crest cells of rodent10–12 and Xenopus embryos13,14 while cadherin-19 mRNA has been reported in the neural crest cell-derived Schwann cell precursors of rat embryos.15
This dynamic expression of cadherins during neural crest development is important for the proper behavior of the neural crest cell through delamination, migration and localization. The downregulation of cadherin-6B by snail2 and the BMP4-dependent cleavage of N-cadherin protein are required for delamination of the neural crest cells from the neuroepithelium.16,17 Moreover, a knockdown of cadherin-6B in the presumptive neural crest region is sufficient for precocious departure of these cells from the neural tube.16 During active migration, most neural crest cells continue to maintain cell-cell contacts18,19 and Xu et al.20 showed evidence that direction and speed of cardiac neural crest cell motility in mouse embryos is influenced by N-cadherin and the gap junction protein, Cx43. In addition, there are indications that subpopulations of migrating neural crest cells express different cadherins and it is tempting to speculate that the type of cadherin expressed can not only influence migratory behavior (such as cadherin-7,21) but also mediate specific association of neural crest cells with their targets (e.g., between neural crest-derived glial cells and neurons) as well as coalescence into dorsal root and sympathetic ganglia at their final destination. Indeed, while full-length N-cadherin is inhibitory for the initial delamination, its function is later required for the coalescence of the neural crest-derived sympathetic ganglia.22
The expression of cadherins involved at specific phases of neural crest development is likely to be regulated by the signaling pathways responsible for coordinating the changes in cell behavior during these stages. Wnts play a pivotal role at several steps in neural crest cell development (reviewed in ref. 23). Burstyn-Cohen and colleagues24 demonstrated that the canonical branch of the Wnt pathway is required for the departure of neural crest cells from the neural epithelium to initiate migration and de Melker25 reported the nuclear localization of β-catenin, an indication of active Wnt signaling, at the initial delamination. The expression pattern of two Wnt family members, Wnt1 and Wnt3a, identifies them as likely candidates for inducing expression of molecules, such as cadherins, needed for delamination and initial migration of the neural crest.24,26,27 We tested whether treatment with Wnt3a ligand regulates the expression of two cadherins present in the migrating neural crest, cadherin-7 and cadherin-11.
In this study, we show that cadherin-11 is expressed by neural crest cells in the chicken embryo on both the transcript and protein level. We further demonstrate that the expression of cadherins in migrating neural crest cells is responsive to activation of the Wnt signaling pathway. Treatment with Wnt3a-conditioned medium upregulates the expression of cadherin-7 and cadherin-11 in neural crest cell cultures and increases the cadherin protein detected at cell-cell interfaces of neural crest cells. The neural crest field has made recent progress in identifying factors involved in neural crest induction and cell fate decisions. However, much remains to be known about the mechanisms underlying the changes in cell behavior during neural crest migration and the specific role(s) that cadherin proteins may play in these processes.
Results
Cadherin-11 expression in the embryo.
Cadherin-11 is reported to be expressed by migrating neural crest cells in rodent and Xenopus embryos.10–14 Because it has not been confirmed in chicken embryo neural crest cells, the expression of cadherin-11 was evaluated at the protein level by immunostaining of the embryo and neural tube explant culture, and at the transcript level by real-time RT-PCR in neural tube explant culture.
The expression of cadherin-11 protein in the migrating neural crest of a stage 17 (st 17) embryo and neural crest cell-derived tissues of a st 21 embryo was detected by immunostaining for cadherin-11 (Fig. 1). The anti-cadherin-11 antibody recognizes a cytoplasmic epitope (amino acids 651–796) of human cadherin-11,28 that is conserved with chicken cadherin-11. By western blot, this antibody detected a single band from a st 19 embryo lysate at approximately 110 kDa (Fig. 1H). To determine if cadherin-11 is expressed in the trunk level migrating neural crest, a st 17 embryo labeled with anti-cadherin-11 antibody was co-labeled with the HNK-1 antibody, a neural crest cell marker, to identify actively migrating neural crest cells in this region (Fig. 1A–D).29 In embryo sections at the hindlimb level, cadherin-11 is detected in a population of the HNK-1 positive neural crest cells (white arrows Fig. 1B–D) migrating through cadherin-11 positive mesenchyme (asterisks in Fig. 1B). At one day later in development (st 21), when the neural crest-derived cells are coalescing to form the dorsal root ganglia (DRG), the cadherin-11 and HNK-1 immunostaining becomes mostly complementary (Fig. 1E–G). The surrounding mesenchyme remains cadherin-11-positive while there are some cells faintly expressing cadherin-11 in the core of the DRG and a lateral stripe of cadherin-11 protein expression in the neuroepithelium (bracket, Fig. 1F).
Figure 1.

Cadherin-11 expression in the chicken embryo. (A–D) At the hindlimb level of a st 17 embryo, cadherin-11 (A–C, Cy3, red) is expressed in the mesenchyme (white asterisks in B) and in migrating neural crest cells (white arrows indicating examples in B–D), identified by co-labeling with the hNK-1 antibody (A, B and D, Cy2, green); (E–G) At st 21, HNK-1 and cadherin-11 signal are largely complementary in the dorsal root ganglia (DRG). Cadherin-11 is faintly expressed in the neural crest-derived DRG and remains strong in surrounding mesenchyme. Cadherin-11 is also expressed in the neural epithelium in a lateral stripe (bracket in F). Scale bar: (A) 50 mm and (E–G), 20 mm. (H) Western blot for cadherin-11 protein from a st 19 embryo lysate detects a single band at ∼110 kDa. Ladder: 250, 150, 100, 75, 50, 37, 25, 20, 15 kDa. DM, dermamyotome; L, ladder; NC, neural crest; NO, notochord; NT, neural tube.
Cadherin-11 is expressed in neural crest cells in neural tube explant culture.
Because cadherin-11 is expressed in multiple embryonic tissues including regions that the neural crest cells migrate through, neural tube explant culture was used to separate the neural crest cells from other embryonic tissues for the analysis using both real-time RT-PCR assay and immunostaining for cadherin-11. Neural tubes were dissected and placed into explant culture and the neural crest cells were allowed to migrate away from the neural tube for a 24 h period. After 24 h the neural tubes were removed from the culture dish and total RNA was isolated from neural tubes and neural crest cells separately. The RNA was subjected to real-time RT-PCR analysis for cadherin-11 and normalized to a control, GAPDH. The expression of cadherin-11 transcript in both the isolated neuroepithelium and the neural crest cells (Fig. 2A) confirmed the expression of cadherin-11 protein in the neuroepithelium and migrating neural crest detected by immunostaining in the embryo. In neural explant cultures immunostained for cadherin-11 and HNK-1, cadherin-11 expression is apparent in the neural crest cells after 24 h of culture (Fig. 2B).
Figure 2.
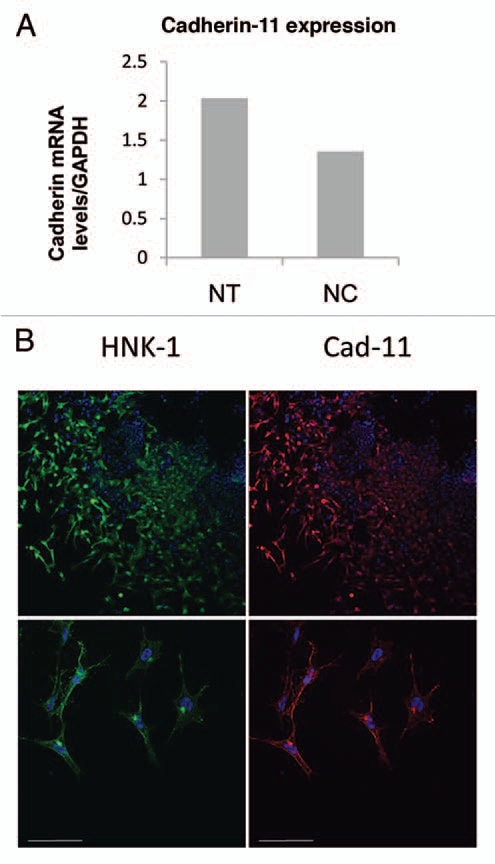
Analysis of cadherin-11 expression in neural crest cell culture by real-time RT-PCR and immunostaining. (A) Cadherin-11 transcript is detected by real-time RT-PCR in both the neuroepithelium (NT) and the neural crest (NC) cells in 24 h neural tube explant cultures; expression is normalized to GAPDH expression. (B) Immunostaining for HNK-1 (Cy2) and cadherin-11 (Cy3) in neural explant culture (both neural tube and neural crest cells) shows cadherin-11 expression in neural crest cells. Scale bar 50 µm.
Wnt positively regulates cadherin-7 and cadherin-11 expression in neural crest cells.
Real-time RT-PCR was used to evaluate the response of two cadherins, cadherin-7 and cadherin-11, expressed in the neural crest cells to Wnt3a ligand in explant culture. The canonical Wnt signaling pathway plays a key role in orchestrating the delamination step of neural crest from the neuroepithelium and, as part of this process, may also induce the transcription of cadherins expressed in the migrating neural crest cells at this time. To test this hypothesis, we treated neural tube explant cultures with Wnt3aconditioned medium to activate the canonical Wnt pathway. As a control, we included analysis of Sox10 expression as Sox10 is expressed in migrating neural crest cells30–32 and because it is reported to be responsive to Wnt signaling in the neural crest.33–36 As both cadherins are also expressed in the developing spinal cord, we removed the neural tube from the cultures and isolated RNA from the neural crest cells alone after allowing the neural crest cells to migrate away from the neural tube over a 24 h time period in the presence of Wnt3a-conditioned medium or control-conditioned medium. The total RNA from the neural crest cells was isolated, subjected to real-time RT-PCR analysis for cadherin-7, cadherin-11 and Sox10, and normalized to GAPDH (Fig. 3). As expected, Sox10 was expressed in the control neural crest cells and upregulated by 3.9-fold in response to Wnt (Fig. 3). Both cadherin-7 and cadherin-11 expression levels were upregulated in the neural crest cells in response to Wnt. Figure 3 shows that cadherin-7 expression was increased by 2.1 fold in the neural crest cells exposed to Wnt3a-conditioned medium compared to control-conditioned medium. Cadherin-11 expression was upregulated by 2.7 fold in the neural crest cells in response to Wnt when compared to control. The fold differences in the expression of cadherin-7, cadherin-11 and Sox10 in response to Wnt compared to control are statistically significant (p < 0.05; Mann-Whitney test). The Wnt regulation of cadherin-7, cadherin-11 and Sox10 was confirmed by three independent culture experiments. No-treatment controls (without control-conditioned medium) expressed similar levels of cadherin-7 and cadherin-11 compared to the control-conditioned medium expression levels (data not shown).
Figure 3.
Cadherin-7 and cadherin-11 expression is upregulated by Wnt3a in neural crest cells. Cadherin-7, cadherin-11 and Sox10 expression in neural crest cells after 24 h of culture with Wnt-conditioned medium (dark bars) or control-conditioned medium (light bars) first normalized to GAPDH and then normalized to the control. Wnt significantly elevated the expression of cadherin-7 (2.1-fold), cadherin-11 (2.7-fold) and Sox10 (3.9-fold) in the neural crest cells. Values are expressed as SEM of three independent experiments, p < 0.05 (Mann-Whitney test, p values less than 0.05 considered significant). CCM—control-conditioned medium; WCM—Wnt3a-conditioned medium.
Wnt increases the cadherin-7 and cadherin-11 protein at cell-cell interfaces of the neural crest.
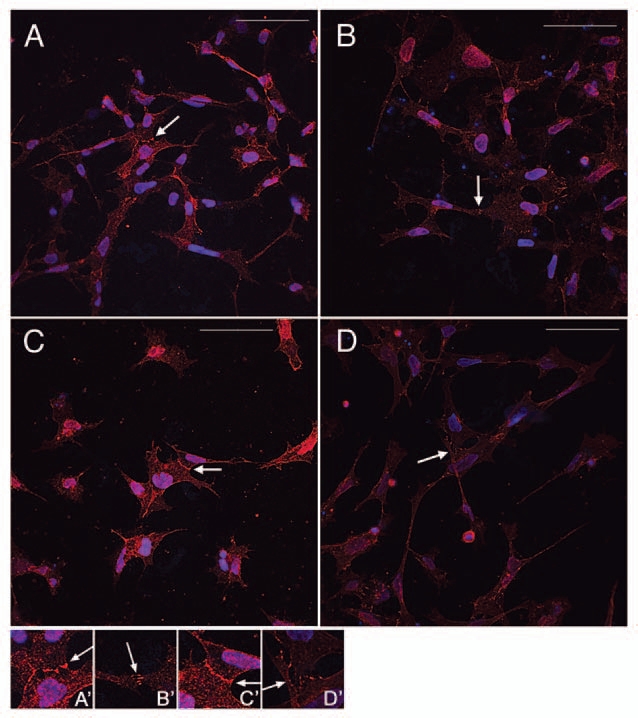
Cadherin function is regulated at many levels following activation of gene expression.5 To determine if the increase in cadherin-7 and cadherin-11 expression by activation of Wnt signaling resulted in changes of cadherin protein localized to inter-cellular junctions, we evaluated cadherin protein localized to the cell-cell interfaces in cultured neural crest cells. In order to assess the effect of exposure to Wnt on the cadherin-7 and cadherin-11 protein in a quantitative manner, the intensity of cadherin antibody staining at the cell-cell interfaces was measured in explant culture. The cultures were set up the same as for the real-time RT-PCR analysis and treated with the Wnt- or control-conditioned medium for 24 h followed by immunostaining for either cadherin-7 or cadherin-11. Using a constant PMT level for the laser set using explant cultures treated with control-conditioned medium, images were captured for each condition for both cadherin-7 and cadherin-11 immunostaining. In Figure 4, it is apparent that the cadherin immunostaining at cell-cell borders is higher in Wnt-treated cultures than for control cultures for both cadherin-7 (white arrows in Fig. 4A, Wnt; B, control) and cadherin-11 (Fig. 4C, Wnt; D, control). To quantify this, the intensities of cadherin-7 and cadherin-11 signal at cell-cell interfaces of neural crest cells were measured using tools associated with the Olympus Fluoview 1000 software. By quantification of this signal, Table 1 shows the difference between control and Wnt activation for cadherin-7 and cadherin-11 for three separate sets of analysis. The immunostaining intensity for both cadherins is significantly higher in Wnt-treated neural crest cells than for controls (see Table 1 for p values for each set), suggesting a Wnt-dependent effect on the amount of cadherin protein localized to intercellular junctions in neural crest cells.
Figure 4.

Cadherin-7 and cadherin-11 protein is upregulated by Wnt3a at cell-cell interfaces in neural crest cells. Neural crest cells cultured with Wnt-conditioned medium (A and C) or control-conditioned medium (B and D) were immunostained for cadherin-7 (A and B) or cadherin-11 (C and D) (Cy3) and counterstained with DAPI. In the presence of Wnt, the intensity of cadherin-7 (A) and cadherin-11 (C) at neural crest cell interfaces (denoted by white arrows) is higher than for neural crest cell cultured in the presence of control-conditioned medium (cadherin-7, B; cadherin-11, D). A higher magnification of a cell interface from each panel is shown in (A′–D′). These images are from those analyzed for quantification of the intensity of cadherin immunostaining at cell-cell borders (Table 1) as described in Materials and Methods.
Table 1.
Quantitation of cadherin immunostaining at cell-cell interfaces in response to exogenous Wnt3a
| Wnt | Control | |||||
| Intensity | ±SEM | CCIa | Intensity | ±SEM | CCIa | p valueb |
| Cadherin-7 | ||||||
| 3853 | 91.25 | 41 (37) | 3641 | 101.52 | 24 (24) | p < 0.05 |
| 4018 | 14.12 | 62 (114) | 3047 | 74.07 | 84 (116) | p < 0.001 |
| 3884 | 58.24 | 12 (37) | 3376 | 95.26 | 46 (69) | p < 0.001 |
| Cadherin-11 | ||||||
| 1657 | 57.93 | 61 (86) | 1356 | 55.03 | 45 (79) | p < 0.001 |
| 3870 | 42.45 | 54 (97) | 3461 | 46.04 | 93 (130) | p < 0.001 |
| 3665 | 60.07 | 36 (45) | 3447 | 87.65 | 29 (44) | p < 0.05 |
Three separate sets (cultures) were analyzed for intensity of cadherin-7 or cadherin-11 immunostaining at cell-cell interfaces in neural crest cell culture. Wnt3a increases the intensity of cadherin-7 and cadherin-11 immunostaining at cell-cell interfaces in cultured neural crest cells.
The total number of cell-cell interfaces (CCI) used for each of these observations. Numbers in parenthesis represent the total number of intensity values of all the peaks at all the interfaces.
p values <0.05 were taken to be significant, unpaired t-test for unequal variances was done to determine the p values.
Discussion
From induction to differentiation, the phases of neural crest cell development require dynamic alterations in how the neural crest cells interact with other cells and with their environment, involving changes in the molecules that mediate these interactions. The cell-cell adhesion proteins, cadherins, are one class of molecules regulated in a distinct temporal and spatial manner during this process. This study showed that, in addition to cadherin-7, cadherin-11 is also expressed in the migrating neural crest cells of chicken and that both cadherin-11 and cadherin-7 are positively regulated in neural crest cells by activation of the Wnt signaling pathway.
In the chicken embryo, analysis of immunostaining for cadherin-11 in a st 17 embryo indicated that cadherin-11 is expressed in early migrating neural crest cells as the neural crest cells migrate ventrally through cadherin-11-expressing mesenchyme. In later stages, the HNK-1 and cadherin-11 immunostaining is largely non-overlapping although there was a faint signal in a population of cells in the core of the DRG at st 21. This is unlike the pattern of other cadherin family members such as Pcdh1 which clearly co-localizes with HNK-1 to the periphery of the DRG in a st 22 embryo and cadherin-7 expression in the DRG at st 22.9,37 One possible explanation for the lack/very low levels of cadherin-11 expression in the neural crest-derived DRG is that as the neural crest cells coalesce to form the DRG, the decrease of cadherin-11 expression by cells that have stopped migrating may assist with segregation of the cells forming the DRG from the surrounding cadherin-11-positive mesenchyme. Overall, the expression pattern of cadherin-11 in the chicken embryo is comparable with characterization from other organisms. The presence of cadherin-11 protein in migrating neural crest cells as they traverse the sclerotome may serve to provide some level of cell-cell interaction without high levels of expression that may impede migration. There are other possible explanations, as cadherin-11 is cleaved to produce biologically-active fragments and is reported to promote cell migratory behavior in a non-cell adhesion dependent manner.38,39
The canonical Wnt signaling pathway regulating neural crest delamination induces a β-catenin-dependent transcriptional program, including targets such as cyclin D1.24 We asked if another target of the canonical Wnt signaling could be two cadherins whose expression is activated in the migrating neural crest cells. In support of this possibility, cadherin-11 is reported to be upregulated by Wnt8 in Xenopus animal cap assays40 and N-cadherin expression is upregulated by exposure toWnt7A in chicken primary limb mesenchymal cell cultures.41 In this study, treatment with Wnt3a ligand increased the expression levels of both cadherin-7 and cadherin-11 in neural crest cell cultures. The mechanism of activation by Wnt signaling could be direct (via β-catenin-TCF/LEF) or indirect through downstream target genes of the canonical Wnt pathway. For example, both FoxD3 and SoxE transcription factors are reported to ectopically activate the expression of cadherin-7 in the neural crest/neural epithelium30,32,42 and both FoxD343 and Sox1033–36 expression are known to be upregulated by Wnt. In chicken endocardial cushion cells, cadherin-11 is reported to be upregulated by Twist1,44 also a transcriptional target of Wnt.45 However, while these reports indicate a possible regulation of cadherins by Wnt, there have not been any reports describing regulation of cadherin-11 expression in the neural crest cells or the direct transcriptional regulatory control of either cadherin-11 or cadherin-7 in any cell type. To determine the direct transcriptional regulation of cadherin expression, genomic analysis of the cadherin-7 and cadherin-11 loci will be required.
As changes in transcription may not necessarily lead to an increase in cadherin protein levels or in functional adherens junctions, this led us to quantify the immunostaining for cadherin-7 and cadherin-11 at neural crest cell-cell interfaces following Wnt treatment. Not only did Wnt treatment increase the RNA levels for cadherin-7 and cadherin-11, Wnt also increased the amount of detectable cadherin protein localized to cell-cell interfaces in the neural crest cells. This may be a result of increased gene expression but there are other valid mechanisms for this observation. There could be increased trafficking of cadherin proteins, increased incorporation into adherens junctions, or even decreased turnover of the cadherin proteins. Irrespective of the mechanism, as a result of Wnt signaling, cadherin-7 and cadherin-11 expression is upregulated and there is more cadherin protein localized to cell-cell interfaces.
Materials and Methods
Fertile white Leghorn chicken eggs obtained from Sunnyside Farms, Inc. (Beaver Dam, WI, USA) were incubated at 38°C using a humidified force draft egg incubator (G.Q.F. Manufacturing) until reaching appropriate stages for experimental manipulation. Embryos were staged according to the criteria of Hamburger and Hamilton.46
Neural tube explant culture.
Neural tubes were dissected from stage 11–14 chicken embryos at +3 to +7 somite level, enzymatically digested with Pancreatin (USB) and all extraneous tissues were removed including notochord and somites. The isolated neural tubes were cultured in Ham's modified F-12 medium (Gibco) with 10% FBS (Hyclone) and 1% penicillin/streptomycin (Gibco) at 38°C and 5% CO2 in 35 mm tissue culture dishes (Corning) for RNA isolation or in 8-well chamber slides (Nunc) for immunostaining. Control-conditioned medium (CCM) and Wnt3a-conditioned medium (WCM) were collected from cultured L-cells (ATCC) and L-Wnt3a expressing cells (ATCC). The ability of the WCM to specifically activate the canonical Wnt pathway was verified as described in Galli et al.47 by evaluating stabilization of β-catenin protein in L-cells in response to Wnt. Due to the lack of cadherin expression in L cells, β-catenin protein is not bound to cadherins and is present at very low levels in the absence of Wnt signaling. Western blot analysis for β-catenin protein was done using protein lysates from L-cell cultures treated with CCM or WCM (mouse anti-β-catenin monoclonal IgG, BD Transduction Laboratories) (data not shown). For experiments involving Wnt pathway activation in explant cultures, either WCM or CCM was added to the dish or well for a final concentration of 50% of culture media volume, a concentration that was confirmed by western blotting for ability to stabilize β-catenin.
Real-time reverse transcriptase polymerase chain reaction (real-time RT-PCR).
Total RNA was isolated from the neural tube explants at designated time points using the RNAqueous kit (Ambion) and quantified using the RNA chip assay (Agilent Bioanalyzer). 100 nM of gene-specific probes (Applied Biosystems) were used for the real-time RT-PCR reaction for GAPDH-reference gene, cadherin-7 and cadherin-11, and for Sox10, TaqMan Gene Expression Assay (Applied Biosystems, cat. no. 4351372) was used. 10 ng of RNA template (as determined by optimization) was used for all real-time RT-PCR reactions. With the exception of Sox10, the sequences of the primers and probes were designed using the Primer Express software (Applied Biosystems): GAPDH: F 5′ GAA GCT TAC TGG AAT GGC TTT CC 3,′ R 5′ GGC AGG TCA GGT CAA CAA CAG 3′, TaqMan-Probe 5′ TGC CAA CCC CCA ATG 3′, Cadherin-7: F 5′CCA TGG TGG TGT CTG AAG CA 3′, R 5′ TGG GCA GCA ACA GTT CCA A 3′, TaqMan-Probe 5′ AAA AGT CGG CAC TAT C 3′, Cadherin-11: F 5′ AAT CAG GGA ACA TCC ATG CAA 3′, R 5′ CCG TGA GAG TGT ACT GAG CTC TCT 3′, TaqMan-Probe 5′ AAG ACA CTG GAC CGA GAG 3′. The TaqMan Real-time RT-PCR assay (Applied Biosystems 7000) was performed at 56°C (annealing temperature) for 40 cycles in a 96 well plate; two replicates of each RNA sample were analyzed. The results were analyzed by the relative quantification assay using the Q-base software (http://medgen.ugent.be/qbase/). Statistical analysis was done using the Mann-Whitney test and the values were expressed as standard error of the mean (SEM) for three independent experiments for a significance of p < 0.05.
Immunohistochemistry.
Whole embryo immunostaining. Embryos were fixed in 4% paraformaldehyde (PFA) for 2 h on ice, blocked with 5% heat-inactivated goat serum (Invitrogen) in Tris buffered saline with 0.2% Triton X-100 (Sigma) for 4 h and incubated in the cadherin-11 primary antibody overnight at 4°C, washed, followed by incubation with Cy3-labeled goat anti-mouse IgG secondary antibody overnight at 4°C. The embryos were photographed (Leica MZ16F microscope and QICAM camera) followed by paraffin embedding, sectioning at 10 µm, rehydration and immunostaining with HNK-1, using Cy2 goat-anti mouse IgM for the secondary.
Immunostaining on explant culture. Immunostaining on explant culture was done on neural tubes dissected from stage 11–14 embryos and explanted into culture as described above. The explants were cultured in eight-well slides with 3–4 neural tubes in each well. For each eight-well slide with explant culture, one well was treated with WCM and other with CCM. After 24 h these explants were fixed in 4% PFA on ice for 15 min and immunostained for cadherin-7 or cadherin-11 and counterstained with DAPI (0.5 µg/ml). Embryo sections and explant cultures were documented using an Olympus FluoView 1000 Laser Scanning Confocal Microscope. Primary antibodies used: mouse anti-cadherin-7 monoclonal IgG (1:100) (CCD7-1, Developmental Studies Hybridoma Bank), mouse anti-cadherin-11 monoclonal IgG (1:200), (Invitrogen), HNK-1 mouse monoclonal IgM (1:20) (ATCC), and secondary antibodies used: Cy3-labeled goat anti-mouse IgG (Jackson ImmunoResearch) and Cy2-labeled goat anti-mouse IgM (Jackson ImmunoResearch).
Intensity measurement for cadherin-7 and cadherin-11.
The analysis for quantifying the cadherin-7 and cadherin-11 protein was performed on images taken from three sets of experiments with neural crest explant cultures treated with WCM or CCM followed by immunostaining for cadherin-7 and cadherin-11. The Olympus Fluoview 1000 confocal imaging system was used to obtain images for the explant immunostaining. A constant PMT voltage for the laser was set using explants treat with CCM and the same PMT value was maintained for the WCM-treated explants during imaging. All images were taken from a single focal plane and image sizes kept constant at 1,024 × 1,024. The intensity measurement tool of Olympus Fluoview 1000 software was used for measuring the intensity of fluorescence for cadherin-7 and cadherin-11 immunostained proteins. A line was drawn through the immunostained cell-cell interfaces of the migrating neural crest cells and the intensity measurements were exported as excel files. As images were of a single focal plane with the focus on a given cell-cell interface, the peak values of each interface, which represents the area in focus for the immunostained localized protein, were taken for analysis. These peak values were then extracted and averaged to get the final mean for both Wnt3a- and control-conditioned medium treated explants. An unpaired t-test for unequal variances was performed to identify the statistical significance of the data.
Western blot analysis.
St 19 embryos were homogenized in lysis buffer (15 mM Tris, 1 mM EDTA, 1% Triton-X100 with 1X Protease Inhibitor Cocktail (Sigma)) followed by sonication. The embryo lysates were mixed with the 2X Laemmli sample buffer (Bio-Rad) and 5% BME (β-mercaptoethanol), boiled at 92°C for 3 min and loaded on to 10% gels of 1 mm thickness for which electrophoresis was carried out for 90 min at 100 V. Proteins were transferred onto PVDF (Bio-Rad) membranes at 300 mA for 90 min under cooling conditions. The membranes were blocked using Aqua block (EastCoast Bio) for 1 h at RT, incubated with the cadherin-11 primary antibody (mouse anti-human cadherin-11, 2 µg/mL, Invitrogen), washed with 1X TBST and incubated with the IRDye 680 nm-labeled secondary antibody, (1:5,000, LI-COR) for detection with the Odyssey infrared imaging system (LI-COR). Ladder: Precision Plus Protein Standards (Bio-Rad).
Acknowledgements
We thank Dr. Fran Day for assistance with the confocal microscopy, Dr. Evelyn Schlenker with the statistical analysis. The CCD7-1 mAb developed by S. Nakagawa and M. Takeichi was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. We gratefully acknowledge funding support by NIH Center of Biomedical Research Excellence (COBRE) Grant P20 RR015567 and a USD Research Catalyst Grant. The Genomics Core used for the real-time RT-PCR analysis is supported by NIH Grant Number 2 P20 RR016479 from the INBRE Program of the National Center for Research Resources.
Abbreviations
- DM
dermamyotome
- DRG
dorsal root ganglia
- EMT
epithelial to mesenchymal transition
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- ISH
in situ hybridization
- NC
neural crest
- NO
notochord
- NT
neural tube
- RT-PCR
reverse transcriptase polymerase chain reaction
- st
stage
- Pcdh1
protocadherin1
- PFA
paraformaldehyde
- WCM
Wnt-conditioned medium
- CCM
control-conditioned medium
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/12138
References
- 1.Kalcheim C. Mechanisms of early neural crest development: from cell specification to migration. Int Rev Cytol. 2000;200:143–196. doi: 10.1016/s0074-7696(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 2.Dupin E, Creuzet S, Le Douarin NM. The Contribution of the Neural Crest to the Vertebrate Body. In: Saint-Jeannet J-P, editor. Neural Crest Induction and Differentiation. Vol. 589. New York: Springer Science + Business Media; 2006. pp. 96–119. [DOI] [PubMed] [Google Scholar]
- 3.Duband JL. Neural Crest Cell Delamination and Migration: Integrating Regulations of Cell Interactions, Locomotion, Survival and fate. In: Saint-Jeannet J-P, editor. Neural Crest Induction and Differentiation. Vol. 589. New York: Springer Science + Business Media; 2006. pp. 45–77. [DOI] [PubMed] [Google Scholar]
- 4.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol. 2005;6:622–634. doi: 10.1038/nrm1699. [DOI] [PubMed] [Google Scholar]
- 5.Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. doi: 10.1101/gad.1486806. [DOI] [PubMed] [Google Scholar]
- 6.Pla P, Moore R, Morali OG, Grille S, Martinozzi S, Delmas V. Cadherins in neural crest cell development and transformation. J Cell Physiol. 2001;189:121–132. doi: 10.1002/jcp.10008. [DOI] [PubMed] [Google Scholar]
- 7.Taneyhill LA. To adhere, or not to adhere: The role of Cadherins in neural crest development. Cell Adh Migr. 2008;2:1–8. doi: 10.4161/cam.2.4.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- 9.Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann I, Balling R. Cloning and expression analysis of a novel mesodermally expressed cadherin. Dev Biol. 1995;169:337–346. doi: 10.1006/dbio.1995.1148. [DOI] [PubMed] [Google Scholar]
- 11.Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, et al. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite and limb bud of early mouse embryos. Dev Biol. 1995;169:347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- 12.Simonneau L, Kitagawa M, Suzuki S, Thiery JP. Cadherin-11 expression marks the mesenchymal phenotype: towards new functions of cadherins? Cell Adh Commun. 1995;3:115–130. doi: 10.3109/15419069509081281. [DOI] [PubMed] [Google Scholar]
- 13.Hadeball B, Brochers A, Wedlich D. Xenopus cadherin-11 (Xcadherin-11) expression requires the Wg/Wnt signal. Mech Dev. 1998;72:101–113. doi: 10.1016/s0925-4773(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 14.Vallin J, Girault J, Thiery J, Broders F. Xenopus cadherin-11 is expressed in different populations of migrating neural crest cells. Mech Dev. 1998;75:171–174. doi: 10.1016/s0925-4773(98)00099-9. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi M, Osumi N. Identification of a novel Type-II classical cadherin: rat cadherin19 is expressed in the cranial ganglia and Schwann cell precursors during development. Dev Dyn. 2005;232:200–208. doi: 10.1002/dvdy.20209. [DOI] [PubMed] [Google Scholar]
- 16.Coles E, Taneyhill L, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol. 2007;312:533–544. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development. 2007;134:491–501. doi: 10.1242/dev.02742. [DOI] [PubMed] [Google Scholar]
- 18.Kulesa PM, Fraser SE. In ovo time-lapse analysis of chick hindbrain neural crest cell migration shows cell interactions during migration to the branchial arches. Development. 2000;127:1161–1172. doi: 10.1242/dev.127.6.1161. [DOI] [PubMed] [Google Scholar]
- 19.Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Li WE, Huang GY, Meyer R, Chen T, Lou Y, et al. N-cadherin and Cx43alpha1 gap junctions modulates mouse neural crest cell motility via distinct pathways. Cell Commun Adh. 2001;8:321–324. doi: 10.3109/15419060109080746. [DOI] [PubMed] [Google Scholar]
- 21.Dufour S, Beauvais-Jouneau A, Delouvee A, Thiery JP. Differential function of N-cadherin and cadherin-7 in the control of embryonic cell motility. J Cell Biol. 1999;146:501–516. doi: 10.1083/jcb.146.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasemeier-Kulesa °C, Bradley R, Pasquale EB, Lefcort F, Kulesa PM. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development. 2006;133:4839–4847. doi: 10.1242/dev.02662. [DOI] [PubMed] [Google Scholar]
- 23.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Molec Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 24.Burstyn-Cohen T, Stanleigh J, Sela-Donenfeld D, Kalcheim C. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/Noggin signaling via G1/S transition. Development. 2004;131:5327–5339. doi: 10.1242/dev.01424. [DOI] [PubMed] [Google Scholar]
- 25.de Melker AA, Desban N, Duband JL. Cellular localization and signaling activity of beta-catenin in migrating neural crest cells. Dev Dyn. 2004;230:708–726. doi: 10.1002/dvdy.20091. [DOI] [PubMed] [Google Scholar]
- 26.Hollyday M, McMahon J, McMohon A. Wnt expression patterns in chick embryo nervous system. Mech Dev. 1995;52:9–25. doi: 10.1016/0925-4773(95)00385-e. [DOI] [PubMed] [Google Scholar]
- 27.Baranski M, Berdougo E, Sandler J, Darnell D, Burrus L. The dynamic expression pattern of frzb-1 suggests multiple roles in chick development. Dev Biol. 2000;217:25–41. doi: 10.1006/dbio.1999.9516. [DOI] [PubMed] [Google Scholar]
- 28.Akins MR, Benson DL, Greer CA. Cadherin expression in the developing mouse olfactory system. J Comp Neurol. 2007;501:483–497. doi: 10.1002/cne.21270. [DOI] [PubMed] [Google Scholar]
- 29.Tucker GC, Aoyama H, Lipinski M, Tursz T, Thiery JP. Identical reactivity of monoclonal antibodies HNK-1 and NC-1: conservation in vertebrates on cells derived from the neural primordium and on some leukocytes. Cell Differ. 1985;14:223–230. doi: 10.1016/0045-6039(84)90049-6. [DOI] [PubMed] [Google Scholar]
- 30.Cheung M, Briscoe J. Neural crest development is regulated by the transcription factor Sox9. Development. 2003;130:5681–5693. doi: 10.1242/dev.00808. [DOI] [PubMed] [Google Scholar]
- 31.Cheung M, Chaboissier MC, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival and delamination. Dev Cell. 2008;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 32.McKeown SJ, Lee VM, Bronner-Fraser M, Newgreen DF, Farlie PG. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn. 2005;233:430–444. doi: 10.1002/dvdy.20341. [DOI] [PubMed] [Google Scholar]
- 33.Aoki Y, Saint-Germain N, Gyda M, Magner-Fink E, Lee YH, Credidio C, et al. Sox10 regulates the development of neural crest-derived melanocytes in Xenopus. Dev Biol. 2003;259:19–33. doi: 10.1016/s0012-1606(03)00161-1. [DOI] [PubMed] [Google Scholar]
- 34.Honoré SM, Aybar MJ, Mayor R. Sox10 is required for the early development of the prospective neural crest in Xenopus embryos. Dev Biol. 2003;260:79–96. doi: 10.1016/s0012-1606(03)00247-1. [DOI] [PubMed] [Google Scholar]
- 35.Lewis JL, Bonner J, Modrell M, Ragland JW, Moon RT, Dorsky RI, et al. Reiterated Wnt signaling during zebrafish neural crest development. Development. 2004;131:1299–1308. doi: 10.1242/dev.01007. [DOI] [PubMed] [Google Scholar]
- 36.Dutton JR, Antonellis A, Carney TJ, Rodrigues FS, Pavan WJ, Ward A, et al. An evolutionarily conserved intronic region controls the spatiotemporal expression of the transcription factor Sox10. BMC Dev Biol. 2008;8:105. doi: 10.1186/1471-213X-8-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bononi J, Cole A, Tewson P, Schumacher A, Bradley RC. hicken protocadherin-1 functions to localize neural crest cells to the dorsal root ganglia during PNS formation. Mech Dev. 2008;125:1033–1047. doi: 10.1016/j.mod.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kashef J, K“hler A, Kuriyama S, Alfandari D, Mayor R, Wedlich D. Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases. Genes Dev. 2009;23:1393–1398. doi: 10.1101/gad.519409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCusker C, Cousin H, Neuner R, Alfandari D. Extracellular cleavage of cadherin-11 by ADAM metalloproteases is essential for Xenopus cranial neural crest cell migration. Mol Biol Cell. 2009;20:78–89. doi: 10.1091/mbc.E08-05-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borchers A, David R, Wedlich D. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development. 2001;128:3049–3060. doi: 10.1242/dev.128.16.3049. [DOI] [PubMed] [Google Scholar]
- 41.Tufan AC, Tuan RS. Wnt regulation of limb mesenchymal chondrogenesis is accompanied by altered N-cadherin-related functions. FASEB J. 2001;15:1436–1438. doi: 10.1096/fj.00-0784fje. [DOI] [PubMed] [Google Scholar]
- 42.Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- 43.Taneyhill L, Bronner-Fraser M. Dynamic alterations in gene expression after Wnt-mediated induction of avian neural crest. Mol Biol Cell. 2005;16:5283–5293. doi: 10.1091/mbc.E05-03-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shelton EL, Yutzey KE. Twist1 function in endocardial cushion cell proliferation, migration and differentiation during heart valve development. Dev Biol. 2008;317:282–295. doi: 10.1016/j.ydbio.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howe LR, Watanabe O, Leonard J, Brown AM. Twist is upregulated in response to Wnt1 and inhibits mouse mammary cell differentiation. Cancer Res. 2003;63:1906–1913. [PubMed] [Google Scholar]
- 46.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Int J Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- 47.Galli LM, Barnes T, Cheng T, Acosta L, Burrus L. Differential inhibition of Wnt-3a by Sfrp-1, Sfrp-2 and Sfrp-3. Dev Dyn. 2006;235:681–960. doi: 10.1002/dvdy.20681. [DOI] [PMC free article] [PubMed] [Google Scholar]


