Abstract
Multicellular organisms arise from the generation of different cell types and the organization of cells into tissues and organs. Cells of metazoa display two main phenotypes, the ancestral epithelial state and the recent mesenchymal derivative. Epithelial cells are usually stationary and reside in twodimensional sheets. By contrast mesenchymal cells are loosely packed and can move to new positions, thereby providing a vehicle for cell rearrangement, dispersal and novel cell-cell interactions. Transitions between epithelial and mesenchymal states drive key morphogenetic events in the early vertebrate embryo, including gastrulation, germ layer formation and somitogenesis. The cell behaviors and molecular mechanisms promoting transitions between these two states in the early mouse embryo are discussed in this review.
Key words: mouse embryo, EMT, MET, morphogenesis, gastrulation, somitogenesis, epiblast, mesoderm, endoderm, primitive streak, paraxial mesoderm
Introduction
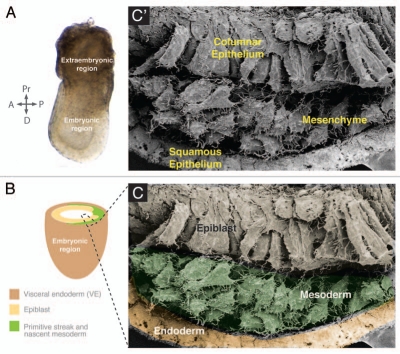
Metazoan cells display two major cellular phenotypes, epithelial or mesenchymal (Table 1 and Fig. 1C and C'). Epithelia consist of sheets of cells closely attached to each other by adherens junctions, tight junctions and gap junctions. Epithelial cells have apical-basal polarity with a localized distribution of cell-cell junctions, polarized organization of the actin cytoskeleton and an underlying basal lamina. The strong adhesiveness between cells provides integrity and mechanical rigidity to epithelia. In the embryo, epithelia serve as barriers to the external environment and between two different compartments. However, epithelia are not completely static entities. Cells are able to move horizontally within an epithelial layer by rearranging and remodeling their junctions and therefore allowing epithelial sheet morphogenesis as exemplified by germ-band extension in Drosophila.1–3 Epithelia are usually classified according to their morphology. Different epithelial types can be found in the embryo and adult. Squamous epithelia comprise flat, irregularly shaped cell layers, whereas cells of a columnar epithelium adopt a taller and columnar morphology. By contrast, mesenchymal cells exhibit neither a polarized distribution of membrane components nor apical-basal polarity. They are loosely attached to each other by focal contacts allowing for increased migratory capacity. Mesenchymal cells display two main modes of migration either individually or in chains displaying a front end-back end polarity.
Table 1.
Definitions
| Epithelial Cell | Cell with apical-basal polarity, intercellular adhesion complexes, polarized actin cytoskeleton and an underlying basal membrane |
| Squamous epithelium | Layer of flat epithelial cells with irregular boundaries |
| Columnar epithelium | Layer of tall epithelial cells with polygonal boundaries |
| Mesenchymal cell | Cell devoid of apical-basal polarity and adhesion complexes that exhibit elongate morphology, filopodia, front end-back end polarity and invasive motility |
| EMT | Epithelial to mesenchymal transition. Cells lose epithelial morphology and molecular identity and adopt mesenchymal properties |
| MET | Mesenchymal to epithelial transition. Cells downregulate mesenchymal markers and upregulate epithelial factors and they assume an epithelial morphology |
| Ingression | Epithelial cells undergo EMT and concomitantly leave an epithelial sheet of cells |
| Egression | Mesenchymal cells join a pre-established epithelial sheet and concomitantly undergo MET |
| Delamination | Cells leave an epithelium either via EMT or not |
| Relamination | Cells form/join an epithelium either via MET or not |
Figure 1.
Cell phenotypes in the early mouse embryo. (A) Gastrulating mouse embryo at embryonic day (E) 7.5. (C') Scanning electronic micrograph of a transverse section through a E7.5 mouse embryo showing the different cell phenotypes: columnar epithelium, mesenchyme and squamous epithelium. (B) Diagram of the embryonic part of a e7.5 mouse embryo. Dashed box outlines the three germ layers. (C) Scanning micrograph shown in (B and C') color-coded for the three germ layers.
Mesenchymal and epithelial phenotypes are reversible and cells can transition between them. Epithelial cells can transform into mesenchymal cells in a process known as epithelial to mesenchymal transition (EMT). EMT comprises a series of events whereby epithelial cells lose many of their characteristics and acquire mesenchymal features by altering their cellular morphology, adhesion properties and migratory capacity. After becoming specified to undergo EMT, epithelial cells start losing their apical-basal polarity and dismantle cell-cell junctions (reviewed in ref. 1). Loss of E-cadherin, a major component of adherens junctions and a hallmark of the epithelial phenotype, is a turning-point in the process.4 The basal membrane is disrupted and mesenchymal markers such as vimentin and N-cadherin are upregulated. Finally cells change their shape, extend protrusions and migrate. By contrast, mesenchymal cells can revert to the epithelial phenotype by undergoing a mesenchymal to epithelial transition (MET). In this process, cells epithelialize following the reverse order of steps previously mentioned. The order and extent of these sequences of events together with the molecular pathways that regulate them may vary at different sites within the embryo as well as being species-specific. Indeed many of the major signaling pathways operating during embryonic development including the Wnt, TGFb and RTK pathways contribute to different embryonic EMT and MET processes (reviewed in refs. 3 and 4).
The conversion from the epithelial cell to the mesenchymal cell phenotype is a key process in metazoan morphogenesis. Since epithelia are the primary tissue in the early embryo, EMT provides a mechanism for creating a new cell type. This differentiation and morphological switch from epithelial cells to motile mesenchymal cells facilitates cell movement, the generation of new tissue types and the reorganization of germ layers (Fig. 1C and C').5 Moreover, the morphogenetic function of EMT facilitates increased embryonic complexity by bringing together different tissues and enhancing further inductive patterning interactions.6
EMTs and METs occur in several ways, two key modes being ingression and egression, whereby cells leave or join pre-existing epithelia by EMT or MET, respectively (Fig. 2). However, not all EMTs consist of ingression as other modes can occur. One example is the somitic mesoderm relamintation in Hymenochirus, where epithelial cells undergo EMT, become motile, and migrate en masse to re-epithelialize after internalization.7 Nonetheless, all ingression events occur by EMT, for example the ingression of primary mesenchyme in sea urchins8 or the ingression of mesoderm through the subduction zones in the urodele.9 This is also the case for mouse gastrulation, which results in the formation of the three germ layers (discussed later). Also, not all MET is followed by egression, as in the case of mouse somitogenesis (discussed later). Although the process of egression is little explored,10 previous accounts of individual mesenchymal cells joining an epithelium during development have been reported.11,12 As we will discuss later, our recent observations lead us to propose that egression plays a fundamental role in mouse endoderm morphogenesis.
Figure 2.
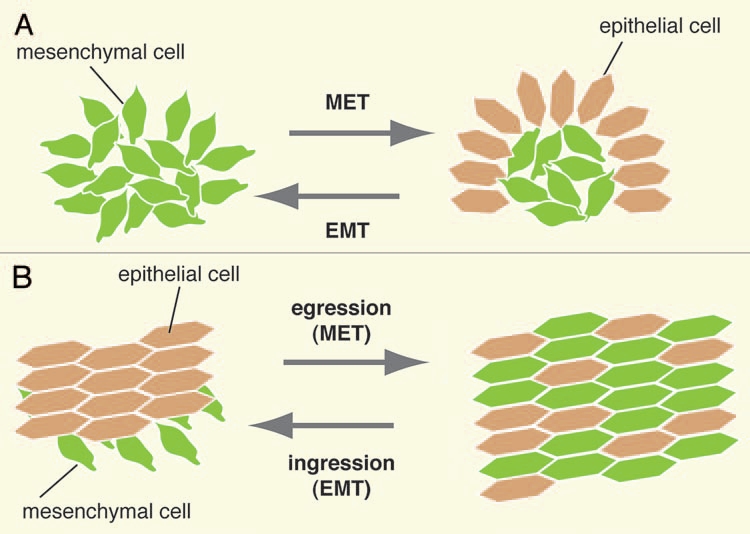
Diagrammatic representation of MET, EMT, egression and ingression. (A) Mesenchymal cells undergo MeT to epithelialize, as for example during somitogenesis. Conversely, epithelial cells undergo eMT to assume mesenchymal characteristics. (B) in an ingression, cells undergo EMT and leave an epithelium, while in an egression cells undergo MET and join a preexisting epithelium.
EMT and MET play pivotal roles during the cell movements and rearrangements occurring during development, wound healing and in cancer. In this review, we will focus on the interplay between epithelial and mesenchymal states that underlie key events during the morphogenesis of the early mouse embryo. In particular, we will discuss these processes in the 48 h period between embryonic day (E) 6.25 and E8.5, staged morphologically from the early streak (ES) to the 8–10 somite stage.13 Hence we will cover the morphogenetic events of gastrulation, germ layer formation and somitogenesis.
EMT During Mouse Gastrulation
The term gastrulation comes from the Greek “gaster” meaning stomach, and refers to the formation of the gut. However, the term gastrulation usually refers to the process by which the three embryonic germ layers ectoderm, mesoderm and endoderm are formed from an initial epithelial layer, the epiblast (also known as the embryonic ectoderm). Gastrulation comprises stereotypical mass cell movements that rearrange the embryo from a single cell layer to a multilayered structure. Once gastrulation is complete, the different cell populations are sorted and allocated, allowing organogenesis to begin. In mouse embryos, gastrulation occurs by ingression, whereby individual epithelial cells from the epiblast undergo an epithelial-to-mesenchymal transition, falling into the space between the epiblast and the juxtaposed extraembryonic endoderm (the visceral endoderm), and migrating away as two bilateral wings of mesoderm (Fig. 3A–C).
Figure 3.
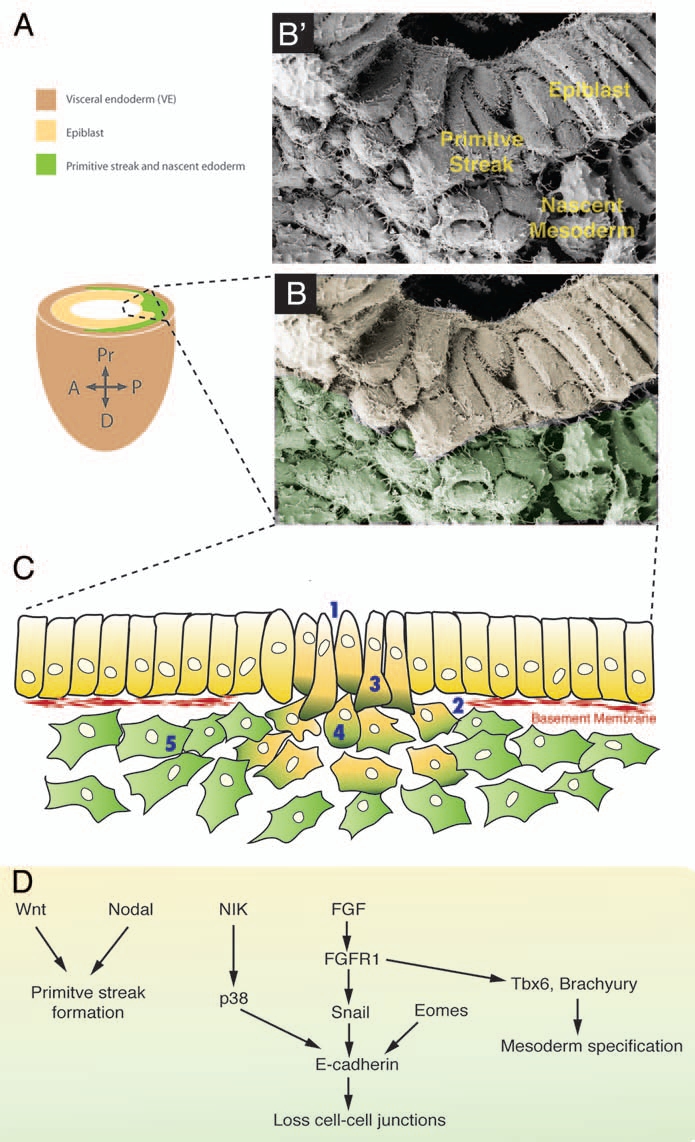
EMT at the mouse primitive streak. (B') Scanning electronic micrograph showing a transverse section through a E7.5 mouse primitive streak. (A) Scheme of the embryonic part of a E7.5 mouse embryo. Dashed box outlines the primitive streak. (B) Scanning micrograph of (A) color-coded for the different germ layers. (C) Cells undergo an eMT event at the primitive streak. (1) First intercellular spaces appear between cells and (2) basal lamina breaks down. (3) Cells acquire a bottle shape, (4) round up as they travel through the streak, and (5) finally acquire a stellate morphology and migrate away from the streak. (D) Signaling pathways that regulate the different EMT steps at the murine primitive streak.
Prior to the onset of gastrulation, the epiblast consists of a tall columnar pseudostratified epithelium with a basal lamina mainly composed of laminin and fibronectin.14,15 Epiblast cells posses tight junctions, adherens junctions, gap junctions and contact each other by microvilli and filopodia.15 Around E6.25 a morphologically distinct structure, the primitive streak, appears. The primitive streak breaks the bilateral symmetry and marks the posterior extremity of the embryo. In the mouse the primitive streak is the site of epiblast cell ingression during gastrulation (Fig. 3A–C). At the primitive streak epithelial disorganization becomes evident, intercellular spaces appear between neighboring cells and the basement membrane breaks down (Fig. 3C).16 Little is known about the mechanisms causing the disintegration of the basement membrane in the mouse primitive streak. However, studies in the rabbit show endocytotic pits with basal material in ingressing mesodermal cells suggesting that endocytosis may be involved,17 while in vitro studies in carcinoma cells have demonstrated the activation of different metalloproteases by Snail genes leading to basement membrane degradation.18 Recently, another pathway has been described in chick embryos involving Net1, an activator of RhoA. Loss of Net1 prior to EMT reduces basal RhoA levels causing basal microtubule destabilization and collapse of the epithelial cell-basal membrane junctions, thereby leading to the breakdown of the membrane.19
When cells at the primitive streak start ingressing, they elongate and acquire a bottle shape by narrowing their apical surface while maintaining their contacts to neighboring cells. Nuclei and mitochondria are mostly displaced apically while the cytoplasm bulges basally. Cells protrude filopodia basally towards the underlying endoderm.16 To maintain epithelial integrity, epiblast cells may vault over ingressing mesoderm cells as has been described in rabbits.17 Bottle shaped cells progressively lose their contact with the apical surface as finger-like projections of surrounding epiblast cells bridge over the ingressing cell and meet apically enclosing the ingressing cell. Therefore when an ingressing cell breaks down its adherens junctions to neighboring epiblast cells, epithelial continuity is maintained as cells remaining in the epithelium seal the gap by establishing new adherens junctions. Once cells detach from the epiblast layer, they round up as they traverse the primitive streak. These carefully orchestrated changes in cell shape are likely to be driven by cytoskeletal rearrangements. To this end, the gastrulation defects observed in the mouse mutant lulu, a null allele of the FERM protein Epb4.1.5, are associated with aberrant actin cytoskeletal organization whereby cells appear to be trapped in the primitive streak in an intermediate state of EMT.20 Cells undergo this transitional stage while traversing the primitive streak as they upregulate mesodermal markers including N-Cadherin and vimentin, while downregulating epithelial markers like E-Cadherin.21 Moreover, cytoskeletal rearrangements associated with higher-order cellular structures directly contribute to directional cell movement. In chick embryos, cells at the primitive streak appear organized in rosette-like structures and display polarized microtubule-arrays that may facilitate ingression of cells through the streak.22
Once cells have reached the mesoderm layer, the process of EMT is complete. Within the mesoderm cells are usually arranged in two or three layers, they acquire a stellate shape and project long filopodia as they migrate centrifugally from the primitive streak. Mesoderm cells migrate as a loosely packed cell sheet, but some cells near the area of the anterior primitive streak can be seen migrating as single cells or small groups of cells.15,16
Molecular Pathways that Regulate EMT at the Murine Primitive Streak
While some studies have focused on the description of the morphological events during mammalian gastrulation, there is growing interest in elucidating the genetic pathways that drive EMT at the primitive streak. Canonical Wnt signaling appears to be one of the main pathways required for primitive streak formation and mesoderm induction in the mouse. Prior to gastrulation, expression of Wnt3 demarcates the region of primitive streak formation. Wnt3 mutants fail to form a primitive streak23 as do β-catenin-deficient embryos, as well as Wnt receptor Lrp5/Lrp6 compound mutants.24,25 Conversely, stabilized β-catenin leads to premature EMT in the epiblast,26 while mutants lacking Axin2, a negative regulator of Wnt-signaling, show ectopic axes.27 In chick embryos, however, non-canonical Wnt signaling through Wnt5a/b and Wnt11b is involved in cell ingression during gastrulation.28
TGFβ signaling is also involved in the early steps of streak induction and gastrulation commitment. Nodal-deficient embryos fail to form and maintain a discrete primitive streak but do form some nascent mesoderm, although their spatial positioning is highly aberrant.29 On the other hand, the compound mutants of the Nodal-antagonists Cerberus-like/Lefty1 form ectopic primitive streaks.30 Moreover, Gdf1/Gdf3 compound mutants, two TGFβ family ligands, show affected mesoderm induction with variable expressivity.31 In the chick, the decision for a cell to ingress relies on FGF signaling. Tightly regulated expression of Churchill, an FGF-induced zinc-finger transcription activator, is involved in determining which epiblast cells will ingress and form mesoderm and which will remain in the epiblast thereby adopting a neural fate.32
In a subsequent step, once cells have started ingressing, FGF signaling is required to maintain mesoderm formation and EMT. In Fgf8 mutant embryos, epiblast cells undergo an EMT, but cells are unable to migrate away from the primitive streak.33 Loss of Fgf receptor 1 (Fgfr1) leads to arrest at gastrulation; EMT initiates but is not maintained.34 The failure to undergo EMT is likely due to the downregulation of the zinc-finger transcriptional repressor Snail1 at the primitive streak. Snail1 has been shown to repress E-cadherin expression by binding to E-box sequences within the E-cadherin promoter.2,35 Snail1 mutants form an aberrant mesodermal layer, where cells emerge from the primitive streak but continue to express E-cadherin and retain apical-basal polarity and an epithelial morphology.36 Loss of function studies in other model systems further support the key role of Snail in EMT during gastrulation.37,38 Whereas Snail1-deficient cells are able to migrate away from the streak and form axial and paraxial tissues albeit with abnormal morphology, Fgfr1-deficient epiblast cells accumulate at the primitive streak and mutant embryos show severe reductions in paraxial mesoderm formation. This would suggest that FGF signaling not only controls EMT at gastrulation but is also required for paraxial mesoderm cell fate specification by regulating the expression of the T-box transcription factors Tbx6 and T.34 Other transcription factors have been shown to regulate E-cadherin expression, and therefore control EMT. Conditional inactivation of the T-box factor Eomesodermin in the epiblast results in EMT arrest. In these mutants, even though Fgf8 and Snail are normally expressed, E-cadherin is only partially downregulated, suggesting a role for Eomesodermin in enhancing Snail-dependent E-cadherin downregulation, perhaps by activating Snail transcriptional partners or in epigenetic reprogramming.39 Downregulation of E-cadherin at the site of ingression is not only controlled at the transcriptional level, but also at the post-translational level. Disruption of p38 MAP kinase activation, due to loss of p38-interacting protein, leads to severe gastrulation defects.40 These proteins act downstream of the NCK-interacting kinase/Map4ke (NIK), loss of which also results in mesoderm cells accumulating at the primitive streak.41 This pathway controls E-cadherin expression by downregulating or destabilizing protein levels in an FGF-signaling independent way, ensuring precise control of E-cadherin during the EMT process.
As soon as cells have undergone EMT and reach the mesodermal layer, they migrate away from the primitive streak as two bilateral wings of mesoderm. In amniotes the different mesodermal cell lineages become specified and allocated according to the time and site of ingression at the primitive streak.42,43 Identity of the different mesodermal fates has been associated with the expression of defined transcription factors initiated at the site of gastrulation. Interestingly, mutant embryos lacking these factors not only show reduced or misshaped embryonic structures, but usually exhibit impaired mesoderm delamination and migration, suggesting a link between mesoderm movement and cell fate specification. The bHLH-containing MesP transcription factors are required for specifying anterior mesoderm. At the initiation of gastrulation, a population of newly ingressed mesodermal cells transiently expresses MesP1. In MesP1 mutants, MesP1-expressing cells pile up in the primitive streak and show reduced migratory activity resulting in abnormal cardiac morphogenesis.44 Moreover, MesP1/MesP2 double mutants exhibit a more severe phenotype with a greater accumulation of cells at the primitive streak and a failure to specify cranio-cardiac and paraxial mesoderm.45 T-box transcription factors are required for specifying posterior mesoderm with Tbx6 being essential for paraxial mesoderm specification.46 Tbx6 mutant embryos display an enlarged tail bud due to accumulation of cells at the streak, while in the posterior neural tubes form in place of somites.47
MET and the Morphogenesis of the Gut Endoderm
After having ingressed through the primitive streak, cells will either become mesoderm or endoderm. It is still an open question as to whether cells at this stage are bipotential, representing a mesendodermal population, or if they are already committed to one or the other fate before ingression. While fate mapping studies have indicated that some epiblast cells can contribute to different germ layer derivatives,48 it was recently demonstrated that the mesoderm marker Brachyury/T and the endoderm marker Foxa2 show mutually exclusive localization in the posterior pre-streak epiblast.49 Moreover, in a study addressing lineage segregation in the mouse embryo, genetic single-cell labeling analysis shows early segregation of endoderm from other lineages and is unable to define a mesendoderm-specific progenitor pool.50 In either case, cells fated to become definitive endoderm emerge from the anterior primitive streak with a mesenchymal morphology and therefore must undergo an MET in order to form the gut endoderm epithelium, which during gastrulation becomes established on the surface of the embryo. MET is therefore a key step in endoderm morphogenesis. Presently, there are two models put forward for the cell behaviors driving endoderm morphogenesis in the mouse embryo.
The Displacement Model
Fate mapping studies carried out in the mouse in the late 80s and early 90s as well as gene expression studies have led to the prevailing displacement model of mammalian endoderm morphogenesis (Fig. 4A).48,51–57 In the fate mapping experiments single visceral endoderm or epiblast cells were labeled, and their positions documented before and after in vitro culture. It was observed that axial (midline) visceral endoderm (VE) cells moved to extraembryonic regions of the conceptus, while epiblast cells at the anterior part of the primitive streak ended up at the embryo's surface overlying the epiblast and eventually in the gut tube. It was therefore suggested that a group of epiblast-derived cells emerging from the anterior primitive streak (APS) give rise to the definitive endoderm (DE) lineage. These cells exit the APS by moving to the surface at the distal tip of the embryo, inserting into the overlying VE and forming a congruent epithelium with it. As gastrulation proceeds and more cells reach the surface, the VE is displaced to proximal regions of the conceptus. There the VE exclusively gives rise to the yolk sac endoderm, while the DE layer, completely covering the embryonic portion of the conceptus, forms the gut endoderm, which in turn will give rise to the epithelial lining of the digestive and respiratory tracts and their associated organs including lungs, liver and pancreas.58
Figure 4.
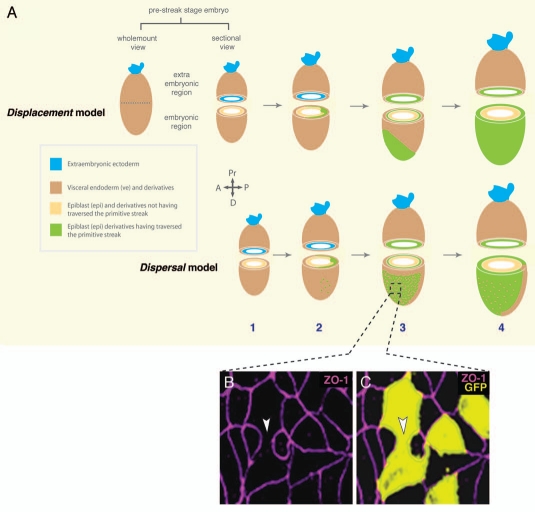
The two alternative models of endoderm morphogenesis in the mouse gastrula. (A) in the displacement model, the VE is dislodged to the extaembryonic region by the nascent DE as a coherent epithelium. In the dispersal model, the initially uniform VE epithelium (1) is interrupted by single egressing epiblast-derived cells at different sites (2). The VE-derived cells are further dispersed (3) until isolated as single cells in the gut epithelium (4). (B and C) Separating VE cells downregulate tight junction markers (bottom panels). GFp positive ve cells separating during the VE dispersal process downregulate the tight junction marker ZO-1 between their interfaces (white arrowhead), but keep tight junctions with surrounding DE cells intact. The two DE cells flanking the separating DE cells are possibly egressing and undergoing MET, thereby establishing tight junctions with the surrounding cells.
In this model, MET would occur when cells fated to become endoderm emerge on the surface of the embryo. EMT and then MET could occur in succession, since cells would ingress at the APS and then immediately re-epithelialize as they exited it. Further experiments using embryo painting and cell transplantation have lent support for this model.59,60 It has also been suggested that a subset of cells might directly delaminate from the epiblast to the surface epithelium, without passing through primitive streak. It has been proposed that these cells may not undergo EMT (and by extension MET) and simply translocate between the two epithelia,61 though detailed further analysis of such a mechanism has not been carried out.
The Dispersal Model
Genetic labeling and live imaging studies have led to an alternative model of endoderm morphogenesis (Fig. 4A).62 When embryos in which the entire VE was marked with a green fluorescent protein (GFP) reporter were live imaged, proximal displacement of the VE as a coherent sheet was not observed. Instead, these live imaging studies suggested that single epiblast-derived cells were inserting onto the embryo's surface providing widespread dispersal of the initially coherent VE layer. This mechanism would comprise widespread intercalation of embryonic epiblast-derived cells and extraembryonic VE. In this model cells destined to become DE would leave the primitive streak after ingression, and travel adjacent, or even within, the wings of mesoderm, between the inner epiblast and the outer VE. Prospective DE cells would traverse the circumference of the egg cylinder and sporadically incorporate into the overlying VE epithelium. Once these cells had emerged on the embryo's surface they were observed to divide, causing VE derivatives to become dispersed, first into small cohorts and by the end of gastrulation to single cells. Therefore, the dispersal model postulates that while the VE derivatives in extraembryonic regions are fated to form the epithelium of the yolk sac, VE-derived cells that remain overlying the epiblast may become incorporated into the embryonic gut tube along with the surrounding DE cells.62
In this model, epiblast-derived cells egress into the VE individually at multiple sites, and in doing so undergo MET. In this way widespread epiblast cell egression concomitantly mediates VE cell dispersal and dilution, and drives gut endoderm morphogenesis. To facilitate widespread egression, the initially compact VE epithelium might change its properties. For example the basement membrane between the visceral endoderm and mesodermal wings could present a barrier for cell egression and it may need to be broken down for cells to egress. Alternatively, the basement membrane may initially be scarce and only after germ layer formation is complete might be reinforced. Furthermore the rigidity of cell-cell junctions may need to be transiently weakened between neighboring VE cells allowing them to be pushed apart as epiblast-derived DE cells egress between them.
In support of such a model, analysis of the basement membrane underlying the VE has shown that it is less dense at the time when epiblast-derived cells are egressing as compared to subsequent stages after germ layer formation is complete.62 Also, the analysis of junction proteins reveals that VE-derived cells that are actively being separated during egression-mediated dispersal lack tight junctions between their common interfaces, but that tight junctions are present between latent VE cells and VE-derived cells and DE cells that have already joined the epithelium (Fig. 4B and C). By modulating cell-cell interactions the pre-established epithelium facilitates egressing cells that have already traversed the basal lamina to insert between its cells. This modulation of basement membrane and junctional proteins is likely to be crucial in allowing cells to emerge at the surface of the embryo.
Widespread egression could represent a commonly deployed mechanism for changing the composition of an epithelium. In the case of the gut endoderm it may facilitate the mixing of cells of two distinct origins, embryonic and extraembryonic. Since it is based on multiple sites of insertion, its efficiency and rapidity is likely to be higher than for the displacement of an epithelium based on a single site of intercalation, and in doing so might provide sufficient expansion in the surface area of the embryo to accommodate its rapid growth. It remains to be seen whether this type of morphogenetic mechanism is unique to gut endoderm morphogenesis, or if occurs in other instances during development, homeostasis, disease progression or regeneration.
When considering the disparities between the two proposed models of endoderm morphogenesis it may be useful to consider the technical limitations of the experiments carried out supporting each of the models. The fate mapping studies offer low resolution, following a single or few cells at the start and end points of the experiment. Also these studies have mostly have focused on axial (i.e., midline) VE cells, from which the model was extrapolated to the entire embryo. The analysis of VE markers that seemed to comply with the displacement model are in fact not informative of cell movements, since they depict cell states and not fates. Indeed it was shown that VE cells overlying the epiblast downregulate these markers upon gastrulation.62 On the other hand, the live imaging studies from which the dispersal model was derived, focus exclusively on lateral events and do not examine the midline. A reconciliation of the two apparently disparate models is indeed possible if in fact VE cells become displaced in the midline and dispersed laterally. This would mean that cells destined to become DE travel laterally along the wings of mesoderm and multifocally insert into the VE layer, and in separate but coordinated process VE cells along the midline become displaced proximally as the node forms and the notochord plate elongates. Future experiments will be required to determine whether a coordinate displacement-dispersal model accounts for the dynamic morphogenetic events taking place to shape the endoderm of mouse embryo.
Is Endoderm Specification a Simple Decision for Cells to Epithelialize?
Hypothetically, the cue to become endoderm could simply be the instruction of cells to epithelialize. As mesenchymal cells leave the primitive streak, key transcription factors in endoderm formation would become upregulated in a subset of cells, which insert into the embryo's surface layer to epithelialize. Embryos lacking either the HMG domain transcription factor Sox17 or the forkhead transcription factor FoxA2 exhibit defects in gut endoderm morphogenesis. In Sox17 mutant embryos only cells with VE-like character are present within the posterior gut tube.63 This might suggest that DE cells have failed to egress. Live imaging and lineage analysis will be required to determine whether these cells are indeed non-dispersed VE cells or DE cells that have egressed but adopted a different identity. Embryos lacking FoxA2 exhibit a gastrulation defect with a failure to form gut endoderm structures.64 In chimera experiments it was shown that epiblast-derived cells deficient for FoxA2 undergo all steps up until egression and even partially integrate into the VE, but fail to epithelialize and eventually leave the outer epithelium.49 More specifically, these cells do not acquire apical-basal polarity and fail to localize intercellular junction proteins. It is therefore possible that, at least during endoderm formation, FoxA2 is a driver of MET. Nonetheless, endoderm markers are expressed in FoxA2 mutant mice suggesting that cells can still be specified to become endoderm even without a FoxA2-driven epithelialization cue (our unpublished observations).
Further experiments will help determine whether other known key players in endoderm formation, such as Nodal-related TGFbeta ligands,65,66 the Mix-like family of homeodomain transcription factors,67,68 the Gata4/5/6 transcription factors,69,70 and the T-box transcription factor Eomes,71 are involved in the epithelialization step.
MET During Somite Epithelialization
The metameric pattern of vertebrate structures is formed through the sequential segmentation of the paraxial mesoderm into somites (Fig. 5B and C). Somitogenesis occurs in a rostro-caudal fashion when pairs of somites appear rhythmically along the body axis at a species-specific rate. This process is orchestrated by the coupling of a maturation gradient, called the wavefront, and an oscillating molecular clock, known as the segmentation clock, which drives the expression of a series of oscillating genes. Several genes have been shown to exhibit oscillating patterns of expression and they mainly comprise downstream targets of the Notch signaling pathway but also members of the FGF and Wnt pathways.72
Figure 5.
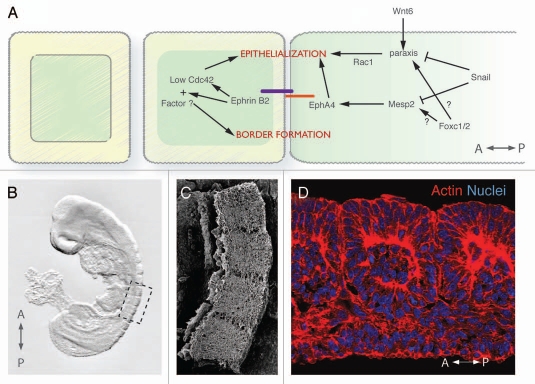
MET during somitogenesis. (A) Model showing the molecular pathways involved in intersomitic border formation and somite epithelialization in amniotes. Anterior to the left. (B) Somites (dashed box) appear in a rostro-caudal fashion at the dorsal side of a ten somite-stage mouse embryo. (C) Scanning electronic micrograph of 4 somitic blocks from a ten somite-stage mouse embryo. (D) Confocal image showing the mesenchymal core surrounded by epithelium in an epithelized somite. Phalloidin (red) labels the actin cytoskeleton and Hoechst (blue), the nuclei.
At the anterior end of the presomitic mesoderm (PSM), blocks of tissue segregate periodically forming an intersomitic boundary (gap) that separates the rostrally located forming somite from the caudal unsegmented mesoderm. Accompanying gap formation, a wave of epithelialization is initiated in the cells that are anteriorly facing the gap (the caudal part of the newly formed somite). Ventral-to-dorsal and caudal-to-rostral propagation of this wave of MET results in a spherical structure with a mesenchymal core surrounded by an outer epithelial layer (Fig. 5D).73,74 This newly epithelialized somite displays rostral and caudal compartment identities established before overt morphological segmentation. The concomitant processes of compartmentalization, epithelialization and gap formation, are all required for proper somite maturation and are regulated by distinct pathways.
Mesp2 is expressed in the PSM prior to somite formation and is essential for somite maturation.75 Mesp2 deficient embryos exhibit somite caudalization, as Mesp2 generates anteroposterior somite polarity through suppression of Dll1 in the presumptive anterior domain.75,76 An additional role for Mesp2 has been proposed in gap formation by restricting Lunatic fringe expression in the anterior PSM and thereby arresting Notch-dependent oscillations.77 Moreover, Mesp2 seems to be a key player during MET as Mesp2 deficient embryos fail to form epithelial somites. In chimeric embryos comprised of mutant and wt cells, Mesp1;Mesp2 double mutant cells do not contribute to epithelial somites arguing for a cell-autonomous requirement of these proteins.78 A further noncell autonomous role for these Mesp factors in the formation of a putative signaling center cannot be excluded since in chimeras comprised of wild type and Mesp2 deficient cells, wild type cells formed epithelial clusters instead of an integrated sheet.78 Additionally, ectopic expression of Mesp2 in somitic cells leads to aberrant epithelialization and gap formation.79
The mechanism by which Mesp2 initiates epithelialization has been elucidated in the chick embryo. cMeso1, the chick Mesp2 homologue, activates EphA4 expression in cells posteriorly facing the prospective boundary. EphA4 in turn interacts with EphrinB2 located at anteriorly juxtaposed cells, such that reverse signaling is sufficient to trigger gap formation and epithelialization by repressing Cdc42 activity via tyrosine phosphorylation. Low levels of Cdc42 were previously reported to be needed for somitic epithelialization, whereas cells would require high Cdc42 activity to maintain mesenchymal state.80 However, low Cdc42 levels are not sufficient to induce gap formation arguing for the presence of a still unknown factor that would collaborate with Cdc42 to create the intersomitic boundary. In a later step, EphA4 forward signaling will be needed for epithelialization in the posterior border cells.81
EphA4 has also been shown to be involved in boundary formation in zebrafish where EphA4 activation leads to cell polarization, apical distribution of β-catenin and acquisition of columnar morphology.82 In mouse, even though direct binding of Mesp2 to the EphA4 enhancer has been reported, EphA4 deficient mice show no somitic phenotype, possibly due to Mesp2 regulation of multiple Eph receptors with redundant activities.79,83
Another bHLH transcription factor, Paraxis, is also involved in somite morphogenesis. In Paraxis-null mice, somites appear segmented in loose mesodermal units with apparent boundaries but without terminal epithelialization.84 As in Mesp2 mutants, sclerotome and dermamyotome seem to be molecularly specified, suggesting that epithelialization is not required for the development of skeleton and muscles.75,84 Interestingly in Paraxis mutants PSM expression of Mesp2 and genes of the Notch pathway is unaffected, whereas genes expressed in the posterior domain of somites exhibit diffuse expression. Therefore a role for Paraxis in the maintenance of the rostro-caudal compartments after speci- fication in the PSM or as a necessary cofactor for Notch/Mesp2 antero-posterior specification has been suggested.85 However, the specific role of Paraxis in MET is still unclear. It might function to restrict the expression of genes directly involved in epithelialization, such as EphrinB2 which appears to be diffusely expressed throughout the whole somite in Paraxis mutants, or it may regulate the activity of downstream effectors such as the Rac1 GTPase, which has been shown to direct paraxis-promoted epithelialization.80,85 The GTPases Rac1 together with Cdc42 have been described as major regulators of cadherin-mediated cell-cell adhesion.86
The forkhead transcription factors Foxc1 and Foxc2 have been implicated in an earlier step of somite maturation. Compound Foxc1;Foxc2 homozygotes have no epithelial somites or segmented PSM. Paraxial mesoderm is specified, as mesodermal markers like Mox1 or pMesogenin1 appear to be normally expressed. However, markers of compartmentalization and border formation such as Paraxis, Mesp2 and Notch1 are downregulated. Mutant cells undergo cyclical oscillations in the PSM but they fail to mature once they have reached the anterior border. Thus Foxc1;Foxc2 might provide competence to respond to the putative wavefront maturation signal likely to be required to start the segmentation program.87
As during gastrulation, the Snail family of transcriptional repressors plays a central role in the maintenance of the mesenchymal state. Both mouse Snail1 and chick Snail2 transcripts display an oscillatory expression patterns in the PSM.88,89 Overexpression of chick Snail2 blocks segmentation, and results in Lnfg, cMeso1 and Paraxis downregulation and a failure in epithelialization. However, as in Mesp2 and Paraxis mutants, the segmentation block does not affect somite-derived structure formation. It has therefore been suggested that termination of FGF and Wnt expression at the determination front downregulates Snail and releases its blockage to the epithelialization and segmentation program.89
Interestingly not all factors promoting epithelialization act within the somites. In the chick, Wnt6, which is expressed in the ectoderm, has been shown to function as an epithelialization factor.90 To date no similar paracrine factor has been identified in the mouse.
Conclusion
Precisely coordinated transitions between epithelial and mesenchymal cell states are critical both for generating the complexity of different cell types and in the organization of these cells into the tissues and organs of the embryo. In this review we have discussed the stereotypical cell behaviors that drive gastrulation, germ layer formation and somitogenesis, three key sequential morphogenetic events taking place within the early mouse embryo. We have also highlighted our current understanding of the common and distinct molecular mechanisms involved in these transitions between the epithelial and mesenchymal cell states.
Acknowledgements
We thank Gloria Kwon, Floria Lupu and Sonja Nowotschin for discussions and comments on this review. Work in our laboratory is supported by the National Institutes of Health (RO1-HD052115 and RO1-DK084391) and NYSTEM. M.V. is supported by a Frank Lappin Horsfall award.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/10771 DOI: 10.4161/cam.4.3.10771
References
- 1.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:1–3. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 2.Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell. 2006;11:459–470. doi: 10.1016/j.devcel.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Pilot F, Lecuit T. Compartmentalized morphogenesis in epithelia: from cell to tissue shape. Dev Dyn. 2005;232:685–694. doi: 10.1002/dvdy.20334. [DOI] [PubMed] [Google Scholar]
- 4.Cano A, Pérez-Moreno M, Rodrigo I, Locascio A, Blanco M, del Barrio M, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadhrin expression. Nat Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 5.Hay E. Organization and fine structure of epithelium and mesenchyme in the developing chick embryo. Baltimore: Williams and Wilkins; 1968. [Google Scholar]
- 6.Tam P, Gad J, Kinder S, Tsang T, Behringer R. Morphogenetic tissue movement and the establishment of body plan during development from blastocyst to gastrula in the mouse. Bioessays. 2001;23:508–517. doi: 10.1002/bies.1070. [DOI] [PubMed] [Google Scholar]
- 7.Minsuk SB, Keller RE. Dorsal mesoderm has a dual origin and forms by a novel mechanism in Hymenochirus, a relative of Xenopus. Dev Biol. 1996;174:92–103. doi: 10.1006/dbio.1996.0054. [DOI] [PubMed] [Google Scholar]
- 8.Katow H, Solursh M. Ultrastructure of primary mesenchyme cell ingression in the sea urchin Lytechinus pictus. J Exp Zool. 1980;213:231–246. [Google Scholar]
- 9.Shook DR, Majer C, Keller R. Urodeles remove mesoderm from the superficial layer by subduction through a bilateral primitive streak. Dev Biol. 2002;248:220–239. doi: 10.1006/dbio.2002.0718. [DOI] [PubMed] [Google Scholar]
- 10.Schock F, Perrimon N. Molecular mechanisms of epithelial morphogenesis. Annu Rev Cell Dev Biol. 2002;18:463–493. doi: 10.1146/annurev.cellbio.18.022602.131838. [DOI] [PubMed] [Google Scholar]
- 11.Holtfreter J. A study of the mechanics of gastrulation. J Exp Zool. 1943;94:261–318. [Google Scholar]
- 12.Martins GG, Rifes P, Amandio R, Rodrigues G, Palmeirim I, Thorsteinsdottir S. Dynamic 3D cell rearrangements guided by a fibronectin matrix underlie somitogenesis. PLoS One. 2009;4:7429–7429. doi: 10.1371/journal.pone.0007429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Downs KM, Davies T. Staging of gastrulating mouse embryos by morphological landmarks in the dissecting microscope. Development. 1993;118:1255–1266. doi: 10.1242/dev.118.4.1255. [DOI] [PubMed] [Google Scholar]
- 14.Mitrani E. Primitive streak forming cells of the chick invaginate through a basement membrane. Roux's Arch Dev Biol. 1982;191:320–324. doi: 10.1007/BF00848491. [DOI] [PubMed] [Google Scholar]
- 15.Tam P, Williams E, Chan W. Gastrulation in the mouse embryo: ultrastructural and molecular aspects of germ layer morphogenesis. Microsc Res Tech. 1993;26:301–328. doi: 10.1002/jemt.1070260405. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto K, Nakatsuji N. Formation of the primitive streak and mesoderm cells in mouse embryos-detailed scanning electron microscopy study. Develop Growth & Differ. 1989;31:209–218. doi: 10.1111/j.1440-169X.1989.00209.x. [DOI] [PubMed] [Google Scholar]
- 17.Viebahn C, Mayer B, Miething A. Morphology of incipient mesoderm formation in the rabbit embryo: a light- and retrospective electron-microscopy study. Acta Anat (Basel) 1995;154:99–110. doi: 10.1159/000147756. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi A, Kitajima Y, Sumi K, Sato K, Hagiwara A, Koga Y, et al. Snail and SIP1 increase cancer invasion by upregulating MMP family in hepatocellular carcinoma cells. Br J Cancer. 2004;90:1265–1273. doi: 10.1038/sj.bjc.6601685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakaya Y, Sukowati E, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–775. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- 20.Lee J, Silva-Gagliardi N, Tepass U, McGrade C, Anderson K. The FERM protein Epb4.1l5 is required for organization of the neural plate and for the epithelial-mesenchymal transition at the primitive streak of the mouse embryo. Development. 2007;134:2007–2016. doi: 10.1242/dev.000885. [DOI] [PubMed] [Google Scholar]
- 21.Nakaya Y, Sukowati EW, Wu Y, Sheng G. RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol. 2008;10:765–775. doi: 10.1038/ncb1739. [DOI] [PubMed] [Google Scholar]
- 22.Wagstaff LJ, Bellett G, Mogensen MM, Munsterberg A. Multicellular rosette formation during cell ingression in the avian primitive streak. Dev Dyn. 2008;237:91–96. doi: 10.1002/dvdy.21390. [DOI] [PubMed] [Google Scholar]
- 23.Liu P, Wakamiya M, Shea M, Albrecht U, Behringer R, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- 24.Huelsken J, Vogel B, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. Requirement for betacatenin in anterior-posterior axis formation in mice. J Cell Biol. 2000;148:567–578. doi: 10.1083/jcb.148.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelly O, Pinson K, Skarnes W. The Wnt co-receptors Lrp5 and Lrp6 are essential for gastrulation in mice. Development. 2004;131:2803–2815. doi: 10.1242/dev.01137. [DOI] [PubMed] [Google Scholar]
- 26.Kemler R, Hierholzer A, Kanzler B, Kuppig S, Hansen K, Taketo M, et al. Stabilization of beta-catenin in the mouse zygote leads to premature epithelial-mesenchymal transition in the epiblast. Development. 2004;131:5817–5824. doi: 10.1242/dev.01458. [DOI] [PubMed] [Google Scholar]
- 27.Zeng L, Fagotto F, Zhang T, Hsu W, Vasicek T, Perry Wr, et al. The mouse Fused locus encodes Axin, an inhibitor of the Wnt signaling pathway that regulates embryonic axis formation. Cell. 1997;90:181–192. doi: 10.1016/s0092-8674(00)80324-4. [DOI] [PubMed] [Google Scholar]
- 28.Hardy K, Garriock R, Yatskieviych T, D'Agostino S, Antin P, Krieg P. Non-canonical Wnt signaling through Wnt5a/b and a novel Wnt11, Wnt11b, regulates cell migration during avian gastrulation. Dev Biol. 2008;320:391–401. doi: 10.1016/j.ydbio.2008.05.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conlon F, Lyons K, Takaesu N, Barth K, Kispert A, Herrmann B, et al. A primary requirement for nodal in the formation and maintenance of the primitive streak in the mouse. Development. 1994;120:1919–1928. doi: 10.1242/dev.120.7.1919. [DOI] [PubMed] [Google Scholar]
- 30.Perea-Gomez A, Vella F, Shawlot W, Oulad-Abdelghani M, Chazaud C, Meno C, et al. Nodal antagonists in the anterior visceral endoderm prevent the formation of multiple primitive streaks. Dev Cell. 2002;3:745–756. doi: 10.1016/s1534-5807(02)00321-0. [DOI] [PubMed] [Google Scholar]
- 31.Andersson O, Bertolino P, Ibáñez C. Distinct and cooperative roles of mammalian Vg1 homologs GDF1 and GDF3 during early embryonic development. Dev Biol. 2007;311:500–511. doi: 10.1016/j.ydbio.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 32.Sheng G, dos Reis M, Stern CD. Churchill, a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–613. doi: 10.1016/s0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]
- 33.Sun X, Meyers E, Lewandoski M, Martin G. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulation mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ciruna B, Rossant J. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev Cell. 2001;1:37–49. doi: 10.1016/s1534-5807(01)00017-x. [DOI] [PubMed] [Google Scholar]
- 35.Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. doi: 10.1038/35000034. [DOI] [PubMed] [Google Scholar]
- 36.Carver E, Jiang R, Lan Y, Oram K, Gridley T. The mouse snail gene encodes a key regulator of the epithelial-mesenchyml transition. Mol Cell Biol. 2001;21:8184–8188. doi: 10.1128/MCB.21.23.8184-8188.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blanco MJ, Barrallo-Gimeno A, Acloque H, Reyes AE, Tada M, Allende ML, et al. Snail1a and Snail1b cooperate in the anterior migration of the axial mesendoderm in the zebrafish embryo. Development. 2007;134:4073–4081. doi: 10.1242/dev.006858. [DOI] [PubMed] [Google Scholar]
- 38.Ip YT, Gridley T. Cell movements during gastrulation: snail dependent and independent pathways. Curr Opin Genet Dev. 2002;12:423–429. doi: 10.1016/s0959-437x(02)00320-9. [DOI] [PubMed] [Google Scholar]
- 39.Arnold S, Hofmann U, Bikoff E, Robertson E. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchymal transition and endoderm specification in the mouse. Development. 2008;135:501–511. doi: 10.1242/dev.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zohn I, Li Y, Skolnik E, Anderson K, Han J, Niswander L. p38 and p38-interacting protein are critical for downregulation of E-cadherin during mouse gastrulation. Cell. 2006;125:957–969. doi: 10.1016/j.cell.2006.03.048. [DOI] [PubMed] [Google Scholar]
- 41.Xue Y, Wang X, Li Z, Gotoh N, Chapman D, Skolnik E. Mesodermal patterning defect in mice lacking the Ste20 NCK interacting kinase (NIK) Development. 2001;128:1559–1572. doi: 10.1242/dev.128.9.1559. [DOI] [PubMed] [Google Scholar]
- 42.Iimura T, Pourquie O. Collinear activation of Hoxb genes during gastrulation is linked to mesoderm cell ingression. Nature. 2006;442:568–571. doi: 10.1038/nature04838. [DOI] [PubMed] [Google Scholar]
- 43.Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PP. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126:4691–4701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- 44.Saga Y, Miyagawa-Tomita S, Takagi A, Kitajima S, Miyazaki J, Inoue T. MesP1 is expressed in the heart precursor cells and required for the formation of a single heart tube. Development. 1999;126:3437–3447. doi: 10.1242/dev.126.15.3437. [DOI] [PubMed] [Google Scholar]
- 45.Kitajima S, Takagi A, Inoue T, Saga Y. MesP1 and Mesp2 are essential for the development of cardiac mesoderm. Development. 2000:127–127. doi: 10.1242/dev.127.15.3215. [DOI] [PubMed] [Google Scholar]
- 46.Chapman D, Cooper-Morgan A, Harrelson Z, Papaioannou V. Critical role for Tbx6 in mesoderm specification in the mouse embryo. Mech Dev. 2003;120:837–847. doi: 10.1016/s0925-4773(03)00066-2. [DOI] [PubMed] [Google Scholar]
- 47.Chapman D, Papaioannou V. Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature. 1998;391:695–697. doi: 10.1038/35624. [DOI] [PubMed] [Google Scholar]
- 48.Lawson KA, Meneses JJ, Pedersen RA. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 49.Burtscher I, Lickert H. Foxa2 regulates polarity and epithelialization in the endoderm germ layer of the mouse embryo. Development. 2009;136:1029–1038. doi: 10.1242/dev.028415. [DOI] [PubMed] [Google Scholar]
- 50.Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell. 2009;17:365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 51.Lawson KA, Meneses JJ, Pedersen RA. Cell fate and cell lineage in the endoderm of the presomite mouse embryo, studied with an intracellular tracer. Dev Biol. 1986;115:325–339. doi: 10.1016/0012-1606(86)90253-8. [DOI] [PubMed] [Google Scholar]
- 52.Lawson KA, Pedersen RA. Cell fate, morphogenetic movement and population kinetics of embryonic endoderm at the time of germ layer formation in the mouse. Development. 1987;101:627–652. doi: 10.1242/dev.101.3.627. [DOI] [PubMed] [Google Scholar]
- 53.Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, et al. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994;8:2466–2477. doi: 10.1101/gad.8.20.2466. [DOI] [PubMed] [Google Scholar]
- 54.Duncan SA, Manova K, Chen WS, Hoodless P, Weinstein DC, Bachvarova RF, et al. Expression of transcription factor HNF-4 in the extraembryonic endoderm, gut and nephrogenic tissue of the developing mouse embryo: HNF-4 is a marker for primary endoderm in the implanting blastocyst. Proc Natl Acad Sci USA. 1994;91:7598–7602. doi: 10.1073/pnas.91.16.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Law SW, Dugaiczyk A. Homology between the primary structure of alpha-fetoprotein, deduced from a complete cDNA sequence, and serum albumin. Nature. 1981;291:201–205. doi: 10.1038/291201a0. [DOI] [PubMed] [Google Scholar]
- 56.Taraviras S, Monaghan AP, Schutz G, Kelsey G. Characterization of the mouse HNF-4 gene and its expression during mouse embryogenesis. Mech Dev. 1994;48:67–79. doi: 10.1016/0925-4773(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 57.Yamamura K, Miki T, Suzuki N, Ebihara T, Kawai K, Kumahara Y, et al. Introduction of mouse C epsilon genes into Cos-7 cells and fertilized mouse eggs. J Biochem. 1985;97:333–339. doi: 10.1093/oxfordjournals.jbchem.a135058. [DOI] [PubMed] [Google Scholar]
- 58.Wells JM, Melton DA. Vertebrate endoderm development. Annu Rev Cell Dev Biol. 1999;15:393–410. doi: 10.1146/annurev.cellbio.15.1.393. [DOI] [PubMed] [Google Scholar]
- 59.Franklin V, Khoo PL, Bildsoe H, Wong N, Lewis S, Tam PP. Regionalisation of the endoderm progenitors and morphogenesis of the gut portals of the mouse embryo. Mech Dev. 2008;125:587–600. doi: 10.1016/j.mod.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Tam PP, Beddington RS. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development. 1987;99:109–126. doi: 10.1242/dev.99.1.109. [DOI] [PubMed] [Google Scholar]
- 61.Tam PP, Beddington RS. Establishment and organization of germ layers in the gastrulating mouse embryo. Ciba Found Symp. 1992;165:27–41. doi: 10.1002/9780470514221.ch3. [DOI] [PubMed] [Google Scholar]
- 62.Kwon GS, Viotti M, Hadjantonakis AK. The endoderm of the mouse embryo arises by dynamic widespread intercalation of embryonic and extraembryonic lineages. Dev Cell. 2008;15:509–520. doi: 10.1016/j.devcel.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, et al. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 64.Ang SL, Rossant J. HNF-3beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–574. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y, Festing M, Thompson JC, Hester M, Rankin S, El-Hodiri HM, et al. Smad2 and Smad3 coordinately regulate craniofacial and endodermal development. Dev Biol. 2004;270:411–426. doi: 10.1016/j.ydbio.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 66.Lowe LA, Yamada S, Kuehn MR. Genetic dissection of nodal function in patterning the mouse embryo. Development. 2001;128:1831–1843. doi: 10.1242/dev.128.10.1831. [DOI] [PubMed] [Google Scholar]
- 67.Hart AH, Hartley L, Sourris K, Stadler ES, Li R, Stanley EG, et al. Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development. 2002;129:3597–3608. doi: 10.1242/dev.129.15.3597. [DOI] [PubMed] [Google Scholar]
- 68.Germain S, Howell M, Esslemont GM, Hill CS. Homeodomain and winged-helix transcription factors recruit activated Smads to distinct promoter elements via a common Smad interaction motif. Genes De. 2000;14:435–451. [PMC free article] [PubMed] [Google Scholar]
- 69.Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, et al. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- 70.Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- 71.Arnold SJ, Hofmann UK, Bikoff EK, Robertson EJ. Pivotal roles for eomesodermin during axis formation, epithelium-to-mesenchyme transition and endoderm specification in the mouse. Development. 2008;135:501–511. doi: 10.1242/dev.014357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dequeant ML, Pourquie O. Segmental patterning of the vertebrate embryonic axis. Nat Rev Genet. 2008;9:370–382. doi: 10.1038/nrg2320. [DOI] [PubMed] [Google Scholar]
- 73.Duband J, Dufour S, Hatta K, Takeichi M, Edelman G, Thiery J. Adhesion molecules during somitogenesis in the avian embryo. J Cell Biol. 1987;104:1361–1361. doi: 10.1083/jcb.104.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sato Y, Takahashi Y. A novel signal induces a segmentation fissure by acting in a ventral-to-dorsal direction in the presomitic mesoderm. Dev Biol. 2005;282:183–191. doi: 10.1016/j.ydbio.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 75.Saga Y, Hata N, Koseki H, Taketo M. Mesp2: a novel mouse gene expressed in the presegmented mesoderm and essential for segmentation initiation. Genes Dev. 1997;11:1827–1839. doi: 10.1101/gad.11.14.1827. [DOI] [PubMed] [Google Scholar]
- 76.Morimoto M, Takahashi Y, Endo M, Saga Y. The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature. 2005;435:354–359. doi: 10.1038/nature03591. [DOI] [PubMed] [Google Scholar]
- 77.Sato Y, Yasuda K, Takahashi Y. Morphological boundary forms by a novel inductive event mediated by Lunatic fringe and Notch during somitic segmentation. Development. 2002;129:3633–3644. doi: 10.1242/dev.129.15.3633. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi Y, Hiraoka S, Kitajima S, Inoue T, Kanno J, Saga Y. Differential contributions of Mesp1 and Mesp2 to the epithelialization and rostro-caudal patterning of somites. Development. 2005;132:787–796. doi: 10.1242/dev.01597. [DOI] [PubMed] [Google Scholar]
- 79.Nakajima Y, Morimoto M, Takahashi Y, Koseki H, Saga Y. Identification of Epha4 enhancer required for segmental expression and the regulation by Mesp2. Development. 2006;133:2517–2525. doi: 10.1242/dev.02422. [DOI] [PubMed] [Google Scholar]
- 80.Nakaya Y, Kuroda S, Katagiri Y, Kaibuchi K, Takahashi Y. Mesenchymal-epithelial transition during somitic segmentation is regulated by differential roles of Cdc42 and Rac1. Dev Cell. 2004;7:425–438. doi: 10.1016/j.devcel.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 81.Watanabe T, Sato Y, Saito D, Tadokoro R, Takahashi Y. EphrinB2 coordinates the formation of a morphological boundary and cell epithelialization during somite formation. Proc Natl Acad Sci USA. 2009;106:7467–7472. doi: 10.1073/pnas.0902859106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Barrios A, Poole R, Durbin L, Brennan C, Holder N, Wilson S. Eph/Ephrin signalling regulates the mesechyma-to-epithelial transition of the paraxial mesoderm during somite morphogenesis. Curr Biol. 2003;13:1571–1582. doi: 10.1016/j.cub.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 83.Helmbacher F, Schneider-Maunoury S, Topilko P, Tiret L, Charnay P. Targeting of the EphA4 tyrosine kinase receptor affects dorsal/ventral pathfinding of limb motor axons. Development. 2000;127:3313–3324. doi: 10.1242/dev.127.15.3313. [DOI] [PubMed] [Google Scholar]
- 84.Burgess R, Rawls A, Brown D, Bradley A, Olson E. Requirement of the paraxis gene for somite formation and musculoskeletal patterning. Nature. 1996;384:570–573. doi: 10.1038/384570a0. [DOI] [PubMed] [Google Scholar]
- 85.Johnson J, Rhee J, Parsons S, Brown D, Olson E, Rawls A. The anterior/posterior polarity of somites is disrupted in paraxis-deficient mice. Dev Biol. 2001;229:176–187. doi: 10.1006/dbio.2000.9969. [DOI] [PubMed] [Google Scholar]
- 86.Fukata M, Kaibuchi K. Rho-family GTPases in cadherin-mediated cell-cell adhesion. Nat Rev Mol Cell Biol. 2001;2:887–897. doi: 10.1038/35103068. [DOI] [PubMed] [Google Scholar]
- 87.Kume T, Jiang H, Topczewska J, Hogan B. The murine winged helix transcription factor, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sefton M, Sánchez S, Nieto M. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development. 1998;125:3111–3121. doi: 10.1242/dev.125.16.3111. [DOI] [PubMed] [Google Scholar]
- 89.Dale J, Malapert P, Chal J, Vilhais-Neto G, Maroto M, Johnson T, et al. Oscillations of the snail genes in the presomitic mesoderm coordinate segmental patterning and morphogenesis in vertebrate somitogenesis. Dev Cell. 2006;10:355–366. doi: 10.1016/j.devcel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 90.Schmidt C, Stoeckelhuber M, McKinnell I, Putz R, Christ B, Patel K. Wnt6 regulates the epithelialisation process of the segmental plate mesoderm leading to somite formation. Dev Biol. 2004;271:198–209. doi: 10.1016/j.ydbio.2004.03.016. [DOI] [PubMed] [Google Scholar]