Abstract
Although epithelial to mesenchymal transitions (EMT) are often viewed as a unique event, they are characterized by a great diversity of cellular processes resulting in strikingly different outcomes. They may be complete or partial, massive or progressive, and lead to the complete disruption of the epithelium or leave it intact. Although the molecular and cellular mechanisms of EMT are being elucidated owing chiefly from studies on transformed epithelial cell lines cultured in vitro or from cancer cells, the basis of the diversity of EMT processes remains poorly understood. Clues can be collected from EMT occuring during embryonic development and which affect equally tissues of ectodermal, endodermal or mesodermal origins. Here, based on our current knowledge of the diversity of processes underlying EMT of neural crest cells in the vertebrate embryo, we propose that the time course and extent of EMT do not depend merely on the identity of the EMT transcriptional regulators and their cellular effectors but rather on the combination of molecular players recruited and on the possible coordination of EMT with other cellular processes.
Key words: epithelium-to-mesenchyme transition, embryonic development, neural crest, neurulation, cadherin switch, Snail transcription factors, Zeb transcription factors
Epithelial to Mesenchymal Transition: A Multifaceted Event Under the Control of a Few Regulatory Genes
Epithelial to mesenchymal transition (EMT) refers to the molecular and cellular program by which epithelial cells lose their characteristic organization as a mono- or multilayered sheet of compact and ordered cells to become a loose tissue of cells with variable shapes and often endowed with locomotory competence. The notion of EMT and the foundations of the underlying mechanisms have been primarily established in in vitro culture systems of epithelial cells treated with scattering factors, such as the MDCK kidney cells and the NBTII bladder carcinoma cells.1,2 However, although it was soon recognized as a potentially fundamental process for generating cellular and tissular plasticity, the concept of EMT gained considerable interest only after it was proposed to constitute a key step during tumor progression and that strong evidence for its physiological relevance during wound healing and morphogenesis has been accumulated.3–8
Cellular events during EMT.
It is now clearly established that EMT encompasses a complex series of cellular events extending well beyond the sole regulations of cell adhesion and cell shape.3,4,8–10 Indeed, epithelia are organized as continuous sheets of cuboidal or columnar cells that exhibit a highly polarized structure along their apico-basal axis and are connected one to another by a variety of intercellular junctions, thereby ensuring the mechanical coherence of the sheet and maintaining the integrity of the apical (luminal) and basal domains. Because of their junctions and intrinsic polarity, epithelial cells control the permeability between the apical and basal domains and constitute a selective barrier between adjacent tissues and organs or protect the whole organism from the external milieu. As a whole, the epithelial sheet constitutes a specific, defined microenvironment, in which each individual cell both receives appropriate signals necessary for its survival and must obey precise rules to undergo proliferation, movements and differentiation in concert with its neighbors, so that the structural and functional integrity of the tissue is preserved. In particular, the plane of division of epithelial cells is strictly defined and controled in relation with the structural changes of the tissue. When it is perpendicular to the apico-basal axis of the epithelial cell, cell division is symmetrical and allows the renewal, expansion or folding of the epithelium. In contrast, when it occurs parallel to the axis, cell division becomes asymmetrical and generates cellular diversity with the daughter cells acquiring distinct fates.11 If one of the daughter cell moves apically, it undergoes further differentiation and specialization as in multilayered epithelia; if it moves basally, it detaches from the epithelial sheet and is released in the extracellular matrix underneath. On the other hand, when an epithelial cell loses contact with its basement membrane or with its neighbors, either by accident due to failure in responding to a specific program or normally at the end of its life, it is systematically extruded from the sheet toward the luminal side and eliminated by a specific apoptotic program, called anoikis.12 In this respect, this process of cell extrusion must be clearly distinguished from EMT as both events end-up with remarkably opposite outcomes: while the former results from the loss of cell adhesion to the basement membrane and provokes cell expulsion and death in the apical side, the latter is associated with basement membrane disruption, fibrillar matrix invasion and active cell migration at the basal side.13
Due to the inherent complexity of the organization of epithelial sheets and to the great variety of mechanisms ensuring their integrity, cells undergoing EMT must accomplish a number of coordinated tasks in a defined spatio-temporal order. Moreover, they must fulfill many requirements in order to segregate completely from the other epithelial cells while protecting from the cell's checkpoints and safety programs.6 The primary event, but not necessarily the first one, is the disassembly of the cell-cell junctions and the concommitant loss of apico-basal polarity. Cells must also degrade the basement membrane material in order to penetrate the underlying fibrillar matrix as well as reorganize their adhesion sites to the matrix, from stable hemidesmosomes anchored to the cytokeratin network to more plastic focal adhesions connected with the actin bundles. Both events are believed to contribute to the acquisition of an active locomotory behavior. As the detaching cells move away from their native epithelium, they become rapidly deprived of their initial survival factors and must rapidly initiate new genetic programs that protect them from immediate apoptosis. Finally, in order to orient themselves toward their final destination, moving cells must acquire a battery of cell surface receptors to decipher their environmental clues and avoid inappropriate territories.
Diversity of EMT processes.
It should be stressed that this sequence of events may vary significantly from one cell type to another as a function of both the initial organization of the epithelium and the triggering signals eliciting EMT.3,5–7,9 Consequently, EMT may exhibit strikingly different phenotypes leading to remarkably different reorganizations of the tissues (Fig. 1). When in an epithelium, all cells undergo EMT simultaneously, the outcome is that the epithelial sheet is completely disrupted and entirely converted into a mesenchyme (Fig. 1A). Such complete EMT are usually achieved in vitro with cultured cell lines treated with scattering factors such as hepatocyte growth factor (HGF) or transforming growth factor-β (TGFβ), but can also be observed in vivo, e.g., in the vertebrate embryo, the sclerotome and the dermatome developing from the somite.5 In contrast, when the EMT program concerns only a few individuals within the epithelium or if it occurs stepwise over time, the epithelial sheet is maintained intact throughout the whole process which ends up with two separate, distinct tissues (Fig. 1B). This type of partial, progressive EMT is most common during embryogenesis, particularly during neural crest (NC) cell delamination at truncal levels (see below) and in the splanchnic mesoderm surrounding the gut. Finally, a third type of EMT can be described where groups of cells within an epithelial sheet are displaced en masse and all at once toward the basal side of the epithelium and become mesenchymal while the flanking epithelial cells fill the gap and heal over them (Fig. 1C). This process involves coordinated changes in cell shape and adhesion and generation of tractional forces in a defined portion of the epithelium by a mechanism involving apical constriction and functioning like a purse ring. Formation of the so-called bottle cells is typically associated with this type of en masse EMT. Examples of such EMT can be seen in particular among prospective mesodermal cells ingressing at gastrulation in invertebrates (in sea urchin or the ventral furrow in Drosophila) or in vertebrates (the blastopore in amphibians and the primitive streak in birds and mammals).6,7,14 As discussed below, delamination of cranial NC cells can to some extent also be assimilated to en masse EMT.
Figure 1.
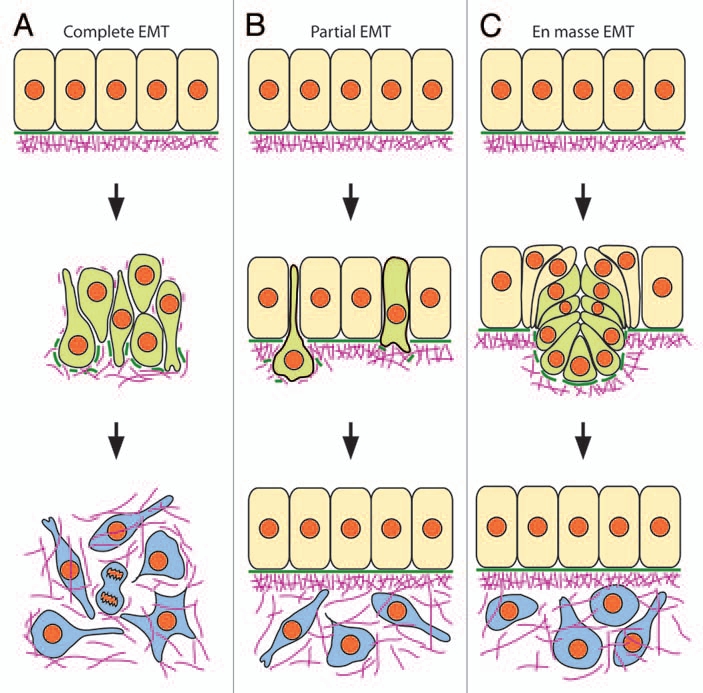
Different modes of EMT. (A) complete EMT. All epithelial cells undergo EMT coincidently, causing the complete dislocation of the epithelial structure and resulting in the formation of a single mesenchyme. (B) Partial EMT. A small number of epithelial cells undergo EMT individually and separately over time such that the epithelial structure is maintained intact during the whole process. (C) en masse EMT. Groups of cells in a defined portion of the epithelium undergo simultaneously coordinated movements of ingression so that they are displaced out of the epithelium which heals to fill the gap. Both partial and en masse EMT result in the formation of two distinct tissues: the epithelium which persists after EMT and the newly-formed mesenchyme. Comparison of the molecular and cellular events accompanying the different modes of EMT suggest that they do not differ in the molecular players that are involved but in the underlying regulatory processes. Epithelial cells are depicted in beige, cells undergoing EMT in green, and mesenchymal cells are in blue. Green solid bars: basement membrane; purple lines: fibrillar extracellular matrix.
Gene regulatory networks of EMT.
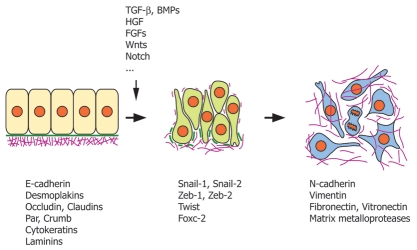
Molecular and cellular studies on cultured cell lines allowed to decipher the basic mechanisms of EMT and to identify the gene regulatory networks that control their occurrence (Fig. 2).3 First, EMT does not occur spontaneously in tissues, instead it is a very tightly-controled process that can be triggered by a plethora of inducers and signaling cascades. Notably, members of the TGFβ, Wnt, FGF and Notch families are regulators of EMT common to many cell types in both physiological and pathological situations. However, despite the great diversity of EMT inducers, all signaling pathways converge to a very limited set of EMT regulatory genes, which can be classified into three families of transcriptional regulators: the Snail, Zeb and Twist families.15–17 Moreover, although the complete list of the effectors of the EMT program is not yet entirely established and may vary with the cell types, the cell adhesion molecule E-cadherin emerges as the primary target of all signaling cascades causing EMT.17,18 Besides E-cadherin, additional targets, including components of the tight junctions (occludins, claudins) and apico-basal polarity factors (Crumb, Par), are also repressed by Snail, Zeb or Twist, resulting in the deterioration of all junctions, the loss of cell polarity and reorganization of the actin cytoskeleton. Snail, Zeb and Twist function essentially as transcriptional repressors, but numerous genes are activated either directly or indirectly in mesenchymal cells upon EMT, to allow cell migration and adaptation to a new environment. These include notably fibronectin, vimentin, matrix metalloproteases and N-cadherin, but their transcriptional regulators still await to be identified.16,18
Figure 2.
Molecular players involved in EMT. Studies on epithelial cell lines established in vitro and on cancer cells showed that transition between epithelial to mesenchymal cells is promoted by a great variety of growth factors and morphogens, e.g., TGFβ, BMPs, HGF/SF, FGF, wnt and Notch which all impinge via a few families of transcription factors (Snail, Zeb, Twist and Fox) on the expression of a variety of cellular components involved in the maintenance of the epithelial structure: cell adhesion molecules of the junctional complexes, such as E-cadherin, desmoplakins, occludins and claudins, cell polarity molecules, such as Par and Crumb, cytoskeletal components, such as cytokeratins, and basement membrane components, e.g., laminins. Conversely, mesenchymal cells express a new repertoire of adhesion, cytoskeletal and matrix components that enables them to populate their environment. Epithelial cells are depicted in beige, cells undergoing EMT in green, and mesenchymal cells are in blue. Green solid bars, basement membrane; purple lines, fibrillar extracellular matrix.
There is now strong evidence that this scenario applies to EMT that take place during tumor progression in carcinoma cells as well as during gastrulation in Drosophila and vertebrates.6,14 Most remarkably, these epithelia are all E-cadherin-expressing epithelia that are derived from the ectoderm and endoderm and are usually characterized by a great stability and a well-established apico-basal polarity. By nature, they constitute an impermeable barrier to the exterior of the organism and undergo EMT only under exceptional circumstances. In this respect, they differ strikingly from many epithelia encountered in neural and mesodermal tissues during embryonic development. These epithelia are often transient by nature, being considerably remodeled either partially or entirely into structures that sometimes no longer exhibit epithelial features. The somites, for example, undergo within a few hours several rounds of mesenchyme-to-epithelium transitions and EMT before they transform into the dermis, the skeletal muscles and the vertebrae. Likewise, the neural tube converts into a multilayered structure containing a great diversity of neuronal and glial cell types. Quite intriguingly, all embryonic epithelia highly susceptible to EMT express N-cadherin and not E-cadherin in adherens junctions.19–21 Given that the E- to N-cadherin transition is a hallmark of EMT in E-cadherinexpressing epithelia and that the Snail, Zeb and Twist transcription factors have been found to act as repressors for E-cadherin but not for N-cadherin,16,18 the question therefore remains as to which gene regulatory network operates in epithelial tissues in which N-cadherin is expressed in place of E-cadherin. Because it derives from the neural epithelium, a typical N-cadherin expressing epithelium, the NC population provides a remarkable paradigm to address this question and thus to examine the diversity in the molecular and cellular mechanisms underlying EMT.
The Neural Crest, a Cell Population Issuedby an EMT
NC cells are a population of cells unique to vertebrates, which is generated early during embryonic development, along the entire antero-posterior axis, at the boundary between the ectoderm (i.e., the prospective epidermis) and the primordium of the central nervous system, namely the neural plate. NC cells follow a distinct fate from the ectoderm and the neural plate, in the sense that they do not remain at their site of birth, but undergo extensive migration soon after they are being formed, to eventually populate a great diversity of areas in the embryo, often distant from their origin. NC cells have stem cell-like properties and are the source of many cell types ranging from neurons and glia of sensory, autonomic and enteric ganglia, to secretory cells of the medulla, melanocytes and, in the head, smooth muscle cells, bone and cartilage cells.22
At least in birds and amphibians, the natural history of the NC starts very precociously during gastrulation, long before individual NC cells can be detected undergoing migration. For example, in the chick, NC progenitors are located in a broad crescent-shape region in the anterior half of the blastoderm. Then, due to the complex movements of neurulation that involve convergence extension, cell intermixing as well as deep movements, they are gradually displaced toward the midline of the embryo, in a stereotype pattern along the anterio-posterior axis.23 During this process, nascent NC cells activate a specific transcriptional program, now refered to as a gene regulatory network,24 elicited by external cues released by the neighboring ectoderm, neural tube and paraxial mesoderm, and mediated by the BMP, Wnt and FGF signaling pathways.25,26 This program takes place in several consecutive steps throughout late gastrulation and neurulation and starts with the expression of a limited group of genes of the Pax, Msx and Zic families. The combined action of these genes contributes to define the region of competence of the NC at the interface between the ectoderm and the neural tube, hence their name of neural plate border specifiers, and allows the integration of new signaling inputs that will initiate the process of specification into bona fide NC cells. Specification of the NC can be defined as the process by which prospective NC cells acquire the competence to execute their subsequent developmental program, i.e., segregate from the neural epithelium and migrate throughout specific routes while being able to undergo cell fate decision into diverse lineages as well as maintaining the balance between proliferation, death and differentiation to control of the population size.24 It involves a large set of genes, the so-called NC specifiers, among which members of the Snail, Fox and SoxE families are predominant. These genes are also often refered to as NC markers, although it should be stressed that, individually, they may not be expressed throughout the entire duration of NC development nor be exclusively restricted to NC cells. In addition, in the absence of precise clonal analyses, it is not known yet whether in order to become a true NC cell, each individual NC precursor cell must express members of the different families of NC specifiers at once or only a combination of some of them. At completion of specification, nascent NC cells undergo an EMT, resulting in their complete segregation from the neural tube. This process is often refered to as NC cell delamination. Since the identity of the NC and their capacity to differentiate into numerous cell types rely primarily on their ability to separate irreversibly from the neural tube, the EMT process in itself constitutes the true signature of this cell population.
A variety of in vivo and in vitro cellular and molecular analyses in chick and Xenopus along with genetic studies in the mouse and zebrafish allowed to identify the basic cellular processes underlying EMT during NC cell delamination as well as some of the triggering signaling cascades. Since several in-depth reviews have been published regularly over the years on NC cell delamination,24,27–37 this review is aimed essentially at presenting aspects that have been poorly discussed, notably the description of NC cell delamination in the context of neurulation, and at re-interpreting data at the light of our current view on the EMT process in epithelial cell lines and tumors.
Neurulation and Deploymentof the EMT Program During NC Cell Delamination
Due to their initial position at the interface between the prospective ectoderm and neural plate, specification and positioning of NC progenitors at the dorsal aspect of the neural tube are intimately connected with the process of neurulation. Neurulation is commonly described as the series of morphogenetic movements that result in the transformation of the neural plate into an elongated, hollow neural tube sitting along the midline of the embryo and covered by the epidermis. However, due to a great diversity in the initial spatial organization of neural plate cells, the cellular mechanisms driving neurulation differ considerably from one species to another as well as with the axial level of the embryo (Fig. 3).
Figure 3.
Different strategies of neurulation in vertebrates. (A) Primary neurulation in the chick at the midbrain level. This process involves columnarization of a preexisting epithelium which then rolls, folds and bends dorsally into a tube. Note that during primary neurulation, the notochord generally preexists to the neurulation event. In amphibians, the whole neuraxis forms by primary neurulation whereas in amniotes, only the rostral neural tube in the head and upper trunk are concerned. (B) Secondary neurulation in the chick. It involves condensation of a mesenchymal blastema followed by epithelialization, first dorsally then progressively more ventrally. A lumen then appears by cavitation in the midline to form a neural tube. During secondary neurulation, the notochord forms coincident with the neurulation event. This type of neurulation occurs in the lumbosacral region of amniotes. (C) Neurulation in zebrafish. In this species, the neural tube does not form by rolling up and elevation of folds but by movements of convergence extension in a multilayered neural plate. This results in the formation first of a keel, then a rod. Cavitation also occurs in the midline but most likely involves different cellular events than during secondary neurulation. The neural tube is depicted in orange; the notochord in red, the ectoderm in light purple and the mesoderm in white.
In amphibians, such as Xenopus or Axolotl, as well as in the head and anterior trunk regions in amniotes, neurulation consists of the rolling up of a flat sheet of epithelial cells into an elongated, hollow tube (Fig. 3A).38–41 The neural tube forms by bending of the neural plate along the midline, resulting in a groove. Then, owing to forces exerted medially by the ectoderm, the lateral margins of the plate elevate into neural folds which later come in apposition in the midline and fuse. The closure of the neural tube and the healing of the overlying ectoderm mark the separation of the two tissues which then follow individual fates. Thus, neurulation proceeds from the neural plate epithelium which is initially in continuity with the ectoderm and shares with it many morphological, cellular and functional traits. Both tissues are composed of cuboidal cells expressing cytokeratins and associated to each other by tight junctions and E-cadherin-containing adherens junctions, and they constitute a physical barrier to the embryo's exterior.19,42,43 After neural tube closure, in contrast, neural epithelial cells display strikingly different morphological features, they do not present any more contact with the external milieu and they no longer function as a barrier.
In birds and mammals, the caudal part of the neural tube (future lumbar, sacral and tail levels) forms in a completely different manner (Fig. 3B).39,44 This process, called secondary neurulation by reference to the primary neurulation found at rostral levels, involves the tail bud, a blastema situated along the midline, under the overlying ectoderm which is completely sealed. Tail bud cells do not constitute an epithelium in contact with the embryo's exterior; instead they form a dense mesenchyme that does not differ much from the neighboring mesoderm. They show no obvious cell polarity, no basement membrane, and no tight junctions and there is no pre-existing lumen; in addition, they express N-cadherin and not E-cadherin. Neurulation proceeds essentially via coalescence of the tail bud cells into a solid cord. A lumen then forms in its midline by cavitation. A detailed analysis of cell movements during secondary neurulation is still missing, but it cannot be excluded that, like primary neurulation,38 it involves convergence extension and cell intercalation.
Finally, in the zebrafish, neurulation is characterized by the complete absence of both neural folds and a neural groove (Fig. 3C).40,45 The neural plate starts out as a multilayered tissue, composed of deep and superficial cells. Deep cells have a columnar, epithelial-like morphology and are anchored to a basement membrane whereas superficial cells are more cuboidal in shape. However, neither cell types are true epithelial cells as they do not exhibit elaborated junctional complexes. Neurulation involves coordinated movements of both superficial and deep cells, which as a result become intermingled and assemble into a monolayered epithelium protruding ventrally into the embryo, the so-called neural keel. As for secondary neurulation in amniotes, the lumen forms secondarily by cavitation involving mirror-symmetric cell division that positions cells to opposite sides of the neural tube.46
Most remarkably, although the mode of neurulation varies considerably at the morphological level, the outcome of the whole process is invariably the same in all species and at all axial levels: the formation of a tubular structure in which individual neural epithelial cells exhibit the same shape and possess similar cellular properties. They are elongated, radially-oriented, and constitute a pseudo-stratified epithelium in which cells seem overlaping although they extend from the basal lamina to the lumen of the neural tube. Such a peculiar arrangement of cells is due to the position of the nucleus which shuttles during the cell cycle from the apical side at mitosis to the basal side in the S phase, a process known as interkinetic nuclear migration. Cell divisions occur apically and, at stages prior to neuron birth, they are essentially symmetrical, resulting in two identical daughter cells which soon re-establish contact with the basement membrane.47 Albeit neural epithelial cells are fully polarized,48 they do not exhibit the whole range of cellular and molecular features of flat, cuboidal epithelia. In particular, they lack tight junctions and express vimentin intead of cytokeratins.42,43,49 In addition, they form adherens junctions containing N-cadherin instead of E-cadherin.20,21 This organization prevails in the lateral sides of the neural tube but is also detected in its dorsal and ventral sides, but less obviously as cells are less elongated due to the curvature of the epithelium.
Because neurulation evolves from radically-different spatial arrangements of cells to end up with a unique structure, it proceeds through completely different cellular and molecular processes. During primary neurulation, morphological transformation of neural epithelial cells is accompanied by the disappearance of tight junctions from the apical side of the cells.42,49 Concommitantly, vimentin replaces cytokeratins in the cytoskeleton.43 These events are reminiscent of those occuring during EMT of epithelial cell lines in culture, although the neural tube does not convert into a mesenchyme and retains epithelial traits while simultaneously exhibiting a number of features typical of mesenchymal cells. Secondary neurulation, in contrast, proceeds through an almost converse process, related to some extent to mesenchyme-to-epithelium transition, while in zebrafish, it involves mostly the transition between an immature epithelial-like multilayered tissue composed of poorly organized cells into a more stable pseudo-stratified epithelium with fully polarized cells.45
Thus, depending on their origin, the natural history of neural epithelial cells varies considerably, and it is therefore likely that such a diversity will be reflected by the cellular and molecular events accompanying NC cell EMT and/or by their kinetics. However, so far, this question remains elusive as it is only relatively recently that the fate map of NC cell progenitors has been established for the anterior regions of the embryo.23,50 We still do not known with precision how prospective NC cells are generated in more caudal regions, and particularly in regions of secondary neurulation. Likewise, how NC progenitors are driven to the dorsal aspect of the neural tube during formation of the neural keel in the zebrafish has not been described with precision. 51 Moreover, the problem is further complicated by the fact that the timing of NC cell delamination is not synchronized with the formation of the neural tube, regardless of the mode of neurulation. For example, in regions of primary neurulation, the time when NC cells commence migration occurs generally around the time of neural tube closure but the exact timing varies much with the axial level considered. At cranial levels, the first NC cells are generally seen to separate from the neural tube immediately after closure, prior to healing of the ectoderm. This is true for almost all the species examined so far among amphibians, birds and mammals,29 including humans.52 The mouse embryo constitutes an exception to this, as the timing of neural tube closure is considerably delayed compared to the development of the rest of the head. In this species, EMT begins while the neural tube is wide open. At levels posterior to the otic placode, i.e., at somitic levels, including in regions of secondary neurulation, NC cell migration starts well after neural tube closure and ectoderm healing, and this is true for all amphibian and amniote species studied so far.29,53
The consequence of the different time lags between NC cell delamination and neural tube closure in the head and trunk is that the relative spatial positioning of the NC, ectodermal and neural tube populations, their organizations and mutual interactions, as well as their states of differentiation are different. At cranial levels and particularly in the chick midbrain (Fig. 4A), NC progenitors are located precisely in the neural folds.54 At the end of invagination of the neural epithelium, they become bottle shaped. As soon as the folds become apposed one to another, NC cells in each side lose contact with the basal lamina, get more rounded, and start to mingle with cells from the other side. At the same time, dorsally, ectodermal cells from either sides separate from NC cells, move and heal over them, while ventrally, neural tube cells execute the same mirror movement. Consequently, the entire NC population becomes physically segregated from the ectoderm and the neural tube, and it immediately undergoes migration in between both tissues. Thus, NC cells pour out of the neural tube as dense, multilayered bulges in a short time scale of less than 12 h in birds. In summary, cranial NC cell delamination occurs all at once and massively in a discrete portion of the neural epithelium, immediately after its invagination, all features characteristic of en masse EMT.54–56
Figure 4.
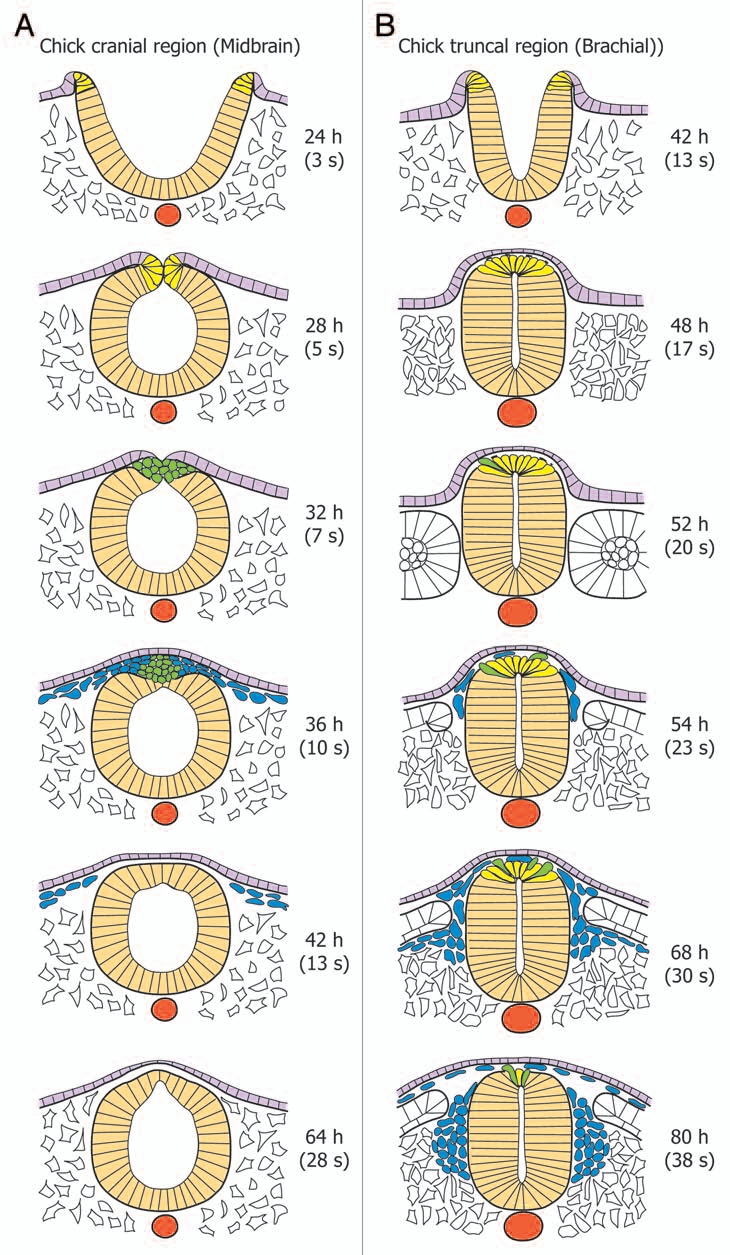
Kinetics of NC cell specification, delamination and migration during primary neurulation at cranial and truncal levels of the chick embryo. (A) In the midbrain, NC undergo massively EMT soon after neural tube closure, resulting in large streams of migrating cells. The total duration of delamination does not exeed 12 h. (B) in the anterior trunk, at the brachial level, NC cells undergo EMT about 8–12 h after neural tube closure. Delamination is progressive and concerns a limited number of cells at the same time. Thus, the duration of the delamination process lasts about 30–40 h. The neural tube is depicted in beige; the notochord in red, the ectoderm in light purple, the mesoderm in white. NC cells in the process of specification are in yellow, delamination in green, and migration in blue.
In contrast to the head, the onset of NC cell delamination in the trunk is clearly uncoupled from closure of the neural tube, indicating that, in this region, the determinism of the triggering of EMT does not reside in the fold fusion (Fig. 4B). However, this does not necessarily mean that any failure in neural tube closure may not impact on EMT itself.39,57,58 NC progenitors are integrated in the dorsal neural tube after closure. They do not yet constitute, as in the head, a separate cell population and they cannot be discriminated morphologically from the other neural epithelial cells. In striking contrast with the massive and rapid emergence of cranial NC cells, truncal NC cells emigrate individually from the dorsal neural tube, in a dripping fashion over a long period of time extending beyond 40 h.59–62 This very gradual segregation of NC cells in the trunk stems primarily from the fact that in this region, EMT is coupled to the cell cycle.55,63 In the avian embryo, NC cells synchronously emigrate from the neural tube in the S-phase, when the nucleus is closest to the basal side, and specific inhibition of the G1/S transition provokes severe reduction in delamination, without affecting specification.63 Moreover, recent studies have shown that NC cell progenitors are initially distributed in a broad region in the dorsal region of the neural tube and that they become gradually confined to a narrow band at the apex of the tube as delamination progresses.62 Thus, NC cell delamination in the trunk is a typical partial, progressive EMT that leaves the dorsal neural tube morphologically intact. Intriguingly, a very similar kinetics of NC cell delamination has been described for the zebrafish trunk or for regions of secondary neurulation in amniotes, even though they differ in their neurulation processes.53,64
In conclusion, while the cellular events accompanying NC cell formation and delamination are likely to be influenced by the mode of neurulation (i.e., primary vs. secondary), the timing when delamination occurs seems defined by rules independent of neurulation (i.e., cranial vs. truncal/somitic), thus raising the possibility that the regulatory events triggering EMT may be governed by processes independent of the mode of neurulation and conserved among vertebrates.
Molecular Players of EMT in NC Cells
EMT effectors during NC cell delamination.
The E- to N-cadherin switch, neurulation and NC cell delamination. A switch from E-cadherin to N-cadherin has been shown to be a critical event during EMT in epithelial cell lines and in tumors, and to promote tumor cell dispersion both in vitro and in vivo.18 Although the neural tube expresses primarily N-cadherin in adherens junctions, its transformation from a flat neural plate into a tube in regions of primary neurulation is accompanied by a switch from E-cadherin to N-cadherin.65 During secondary neurulation, in contrast, tail bud cells that contribute to the neural tube do not undergo this shift. Therefore, the question is whether this shift has any influence on NC cell delamination and to which extent it is required for triggering EMT. At the present time, relatively few is known about this question. Indeed, surprisingly enough, there is no detailed description of the time course of the disappearance of E-cadherin in the neural ectoderm and its replacement by N-cadherin in most species,65 except a recent report in the Xenopus embryo.66 Thus, when the E- to N-cadherin switch actually occurs and whether both cadherins show exclusive expression domains or may be transiently co-expressed in the same cells in the neural epithelium are simply not known!
A variety of experiments in different animal models have uncovered the importance of N-cadherin in early neural development, but none of them directly addressed in functional terms the problem of the switch between E- and N-cadherin. Genetic studies in the mouse showed that in the absence of N-cadherin, the neural tube can form but it is undulated, indicating that its final shaping is affected.67 In the zebrafish, cellular rearrangements during movements of convergence and intercalation are impaired in N-cadherin mutants, resulting in the absence of a hollow neural tube.45,68,69 Intriguingly, both in mouse and fish, the defects resulting from the absence of N-cadherin are relatively late, suggesting that other cadherins, possibly E-cadherin, can compensate transiently for its absence. Recent studies in the Xenopus embryo are, however, suggestive of a precocious implication of N-cadherin in neurulation.66 Depletion of N-cadherin causes inhibition of cell movements in the neural tube, resulting in defects in its closure followed by spina bifida. Interestingly, E-cadherin is also required for neurulation but it is specifically implicated in cell movements in the epidermis. Furthermore, E-cadherin and N-cadherin cannot substitute for each other for the promotion of cell movements, illustrating the functional specificity of cadherins during neurulation. This study, however, did not investigate the impact of the cadherin swaping in the process of NC cell delamination.
A few reports argue for a role of the E- to N-cadherin switch in promoting migration of NC cells. In particular, a mouse mutant has been described in which Zeb-2, a repressor of E-cadherin (see below), has been knocked-out.70 Homozygote Zeb-2-deficient embryos show maintenance of E-cadherin expression in the neural epithelium, associated with alteration in the expression of the neural marker Sox-2 and strong reduction of NC cell migration in the vagal region. These observations indicate that maintenance of E-cadherin expression in the neural tube affects its developmental program as well as NC cell delamination, at least at some axial levels. However, the phenotype of the embryos has not been characterized further. In particular, neither the presence of N-cadherin nor the expression of the EMT transcriptional regulators have been analyzed in NC cells. It should be stressed that although suggestive of the requirement of E-cadherin downregulation during NC delamination, these experiments do not demonstrate that the E- to N-cadherin switch constitutes by itself the triggering event of EMT in NC cells. Indeed, by the end of the whole process of neurulation and neuronal patterning, the neural epithelium does not turn into a mesenchyme, and, contrary to what has been observed in epithelial cell lines, not all neural epithelial cells undergoing E- to N-cadherin switch execute the EMT program. Rather, EMT occurs in a tiny proportion of neural epithelial cells situated in a discrete region of the neural tube. Additional factors and events have then to be invoqued to account for the very precise spatiotemporal control of NC cell delamination.
Cadherin switch and NC cell delamination. Despite the lack of any conclusive data on the role of the E- to N-cadherin switch during NC cell EMT, this process is characterized by a cadherin switch implicating different cadherins. However, the repertoire of cadherins implicated varies from one species to another, suggesting that it is not much the type of cadherins that matters in the progress of EMT but rather the switch between different types of cadherin. Moreover, this switch is quite intricate as in almost all species and at all axial levels, prospective NC cells express more than one cadherin prior to delamination.71 Indeed, beside N-cadherin which is common to all neural tube cells, NC cells specifically express an additional cadherin: cadherin-6 in mouse and zebrafish72,73 and cadherin-6B, a variant of cadherin-6, in birds;74 the Xenopus embryo is an exception to this, as so far cadherin-6 has not been reported in the premigratory NC cell population.75 The significance of the co-expression of several cadherins in prospective NC cells is not understood yet, but it may be related with the selective recognition of NC cells among the other neural epithelial cells at the time of delamination or with the necessity to maintain cell cohesion in a region of the neural tube subjected to intense remodeling and physical constraints. Moreover, it is noteworthy that cadherin-6B remains expressed in the roof plate after completion of NC delamination, suggesting that its function extends beyond the sole specification and delamination of the NC population.
The cadherin switch at the time of NC cell delamination has been particularly well documented in the chick.71 At all axial levels, once NC cells delaminate from the neural tube, they lose both N-cadherin and cadherin-6B expression. However, cranial and trunk NC cells differ in the timing of cadherin regulation. In the head, both transcripts and proteins are absent from the entire prospective NC cell population at the time of fold fusion, such that an N-cadherin-free zone clearly delineates cells undergoing EMT from their neighbors.20,21,76 Coincidently, N-cadherin expression is reinforced in neural epithelial cells at the vicinity of the NC, most likely to consolidate the closing neural tube and prevent its re-opening. In the trunk, N-cadherin transcripts are maintained in NC cell progenitors until their complete separation from the neural tube.77 However, N-cadherin proteins decrease in amount on the lateral surfaces of pre-migrating NC cells (but remain present in their luminal side), and disappear completely when cells are fully segregated from the dorsal neural tube.21,76–78 As to cadherin6B, its transcripts are diminished at the time of fold fusion, but only in a discrete region corresponding to the midbrain, while proteins disappear later from the cells' surface at the onset of migration.74,79,80 Elsewhere in the head as well as in the whole trunk, cadherin-6B messengers and proteins are detectable in NC progenitors until they are completely segregated from the neural tube.78–80 It should be noted that in the mouse, contrary to chick cadherin-6B, cadherin-6 is not repressed in NC cells after delamination.72 It persists in migrating cells until differentiation, where it becomes confined to the Schwann cell lineage.
After delamination, NC cells undergoing migration immediately turn on a new cadherin program. In the chick, at truncal levels, cadherin-7 becomes strongly upregulated over the entire cell body in NC cells that have entirely detached from the neural tube.78 Less is known about cadherin-7 expression at cranial levels, but in situ hybridization studies suggest that it is also upregulated at least in a subset of migrating NC cells.74 Surprisingly, although data are still very sketchy, cadherin-7 does not seem to be expressed in migrating NC cells in other vertebrate species. In the zebrafish and rat, cadherin-7 and, in Xenopus, F-cadherin, a close relative to chick cadherin-7, are expressed in discrete regions of the central nervous system in patterns often similar to that described in chick, but they do not show up in migrating NC cells.81–84 The situation in the mouse is rather confusing as several cadherin-7 genes have been reported with sometimes highly divergent sequences.85–87 In all these species, however, a cadherin switch does occur even though it does not implicate cadherin-7. In Xenopus and most likely in the mouse, cadherin-11, a cadherin often associated with mesenchymal cells, is expressed in migrating NC cells particularly in the head but also in the trunk.88–90 Whether cadherin-7 and cadherin-11 fulfill similar functions in migrating NC cells and can substitute for each other remains as yet undefined.
Recently, the mechanisms involved in the regulation of N-cadherin expression and function during NC delamination have been unravelled in the case of chick trunk NC cells.77 Due to the great stability and long half-life of the molecule, N-cadherin activity is shut off at different levels, first at the functional level to ensure rapid disruption of cell-cell contacts, then at the translational and transcriptional ones, possibly to prevent restoration of strong cohesion during migration. N-cadherin molecule is removed from the surface of NC cells by a two-tiered mechanism that relies on its sequential proteolytic cleavage. At first, a major part of the extracellular domain of N-cadherin is cleaved by ADAM-10, a metalloprotease expressed in the dorsal neural tube,91 leaving a membrane-anchored domain CTF1.92 This process results in the deterioration of the intercellular junctions and most likely constitutes a decisive event triggering EMT. Consistent with this, premigratory NC cells show no N-cadherin staining when an antibody to N-cadherin that recognizes the N-terminus of the molecules is used (i.e., the NCD2, GC4 and FA5 monoclonal antibodies20,76–78), while they are clearly positive with an antibody to an epitope close to the membrane (the ID723 antibody21). During the second step, the remainder of the N-cadherin molecule is further cleaved off from the membrane into an intracellular CTF2 peptide which shuttles to the nucleus where it acts as a transcriptional regulator.93 Interestingly, overexpression of the CTF2 domain in chick trunk neural tubes stimulates NC cell delamination,77 suggesting a dual function of the proteolytic degradation of N-cadherin on the surface of NC cells in enabling them to separate from each other and activating a migration-stimulatory cascade. Whether this model also applies to trunk NC cells in the other species and to cranial NC remains to be established. On the other hand, it is not known at present whether cadherin-6B also undergoes the same series of proteolytic cleavages. Interestingly, recent studies on cranial NC migration in the Xenopus embryo revealed that cadherin-11 is also subjected to proteolytic cleavage by ADAM-13, another member of the ADAM family of proteases, thereby suggesting that proteolytic cleavage of cadherin may play a fondamental role in the control of both NC cell delamination and migration.94,95 However, while cleavage of N-cadherin is part of a global down-regulation of this protein necessary for EMT and releases a cytoplasmic fragment that regulates gene expression, cleavage of cadherin-11 is essential for cranial NC cell migration, it occurs continuously during migration and leaves a membrane-anchored fragment that retains its interaction with β-catenin. Moreover, this fragment is able to recruit and activate GTPases, it localizes to cell protrusions and stimulates filopodia and lamellipodia formation.
Functional studies mainly in the chick model strongly argue for a role of cadherin downregulation in NC cell delamination. Overexpression of N-cadherin in the trunk premigratory NC or of cadherin-6B in the head results in a strong reduction of cell migration both in vivo and in vitro.77,79,80 However, both cadherins differ in their mode of action. NC cells with elevated levels of cadherin-6B form clusters attached to the neural tube or accumulated into its lumen, and they show alteration neither in expression of the NC specifiers nor in cell proliferation and survival (except for those extruded into the lumen). Unlike cadherin-6B, N-cadherin overexpression inhibits delamination by provoking a substantial reduction in NC cell proliferation and cyclin D1 transcription. Interestingly, N-cadherin mutants retaining both the cell- and β-catenin-binding domains but lacking the juxtamembrane domain necessary for cleavage have no effect on cell delamination. Moreover, high amounts of N-cadherin cause massive disruption of the neural tube morphology while cadherin-6B has no apparent deleterious effect on neural epithelial cells. These observations suggest that cadherin-6B would prevent cell delamination merely by a mechanical effect, whereas N-cadherin would instead operate through signaling. However, it should be stressed that the study on cadherin-6B was performed in the head while that on N-cadherin concerned the trunk, and the differences observed may then stem also from the fact that both regions differ in their dependence to cell cycle for delamination.
Additional knock-down experiments in the chick head suggest that at least in this region, the cadherin-6B switch is both necessary and sufficient for NC cell delamination.79 However, it should be stressed that no data are available yet for the trunk region. Moreover, a variety of other observations indicate that, in the case of N-cadherin, cadherin downregulation is not an absolute requirement for cell delamination and that NC cells may to some extent cope with, or even require, a basal expression of cadherins on their surface for migration. Firstly, trunk NC cells electroporated with moderate quantities of N-cadherin cDNA do migrate normally toward the ventral side of the embryo and form sympathetic ganglia.96 Conversely, excess of cadherin-7 or cadherin-11 which are normally expressed by migrating NC cells also perturb delamination and/or migration, indicating that any cadherin in excess, regardless of its type, is deleterious for migration.78,97 Secondly, trunk NC cells cultured in vitro have been found to express significant amounts of N-cadherin on their surface, but maintain reduced cell-cell contacts by a mechanism involving active endocytosis and degradation.98,99 Thirdly, cardiac NC cells from N-cadherin-deficient mice show a reduced rate of migration, resulting from decreased persistence of movement albeit increased individual cell velocity.100 In this respect, both in culture and in vivo, migrating NC cells need to maintain almost continuous contacts with their neighbors for persistence of movement and oriented migration.98,99,101,102 Thus, both excessive and insufficient cadherin-dependent cell-cell contacts affect NC cell migration, thereby illustrating the fact that they are requested at tightly controled levels during various steps of NC cell development for maintaining alternatively strong cell adhesion, cell sorting and cell signaling.
Cell-matrix adhesion, proteases and NC cell delamination. Beside the dynamic regulation of cadherin-mediated cell adhesion, EMT in epithelial cell models is accompanied by thorough reorganizations of cell-matrix adhesion, involving basement membrane degradation, synthesis of new extracellular matrix components, change in integrin repertoire and formation of labile and dynamic cell-matrix adhesion sites. Although such changes are believed to occur during NC cell delamination, still relatively few is known about the molecular changes that actually take place at onset of migration, their role in the time course of EMT and how they are coordinated.
At cranial levels in chick and frog, the basal lamina of the neural plate is initially in continuity with that of the non-neural ectoderm.28,29 During folding of the neural plate and the subsequent apposition of the epidermal ectoderm with the neural tube, it becomes disrupted at the hinge point between both tissues, coinciding with the area of prospective NC cells. Then, healing of the ectoderm, dorsally, and of the neural tube, ventrally, is accompanied by the progressive extension of a basal lamina deposited along each tissue, further isolating the NC population from their neighbors. Once the last NC cells have emigrated from the neural tube, the ectoderm and neural tube are continuously limited by basal laminae. Thus, in the head, the duration of NC cell delamination is marked by the rupture of the basal lamina until its complete restoration over the neural tube, thereby revealing a possible direct role of basal lamina break-down in initiation of migration.
At truncal levels, the basal lamina overlying the dorsal region of the neural tube is also either absent or frequently interrupted.28,29 However, unlike in the cranial region, because NC cell emigration is delayed with respect to neural tube closure, break-down of the basal lamina is not immediately followed by EMT. This led to the assumption that the lack of a continuous basal lamina under NC progenitors does not constitute a triggering signal for onset of delamination and may be only permissive for migration. A very recent report, however, suggests that the absence of basal lamina in the dorsal neural tube may directly contribute to the initiation of migration. Indeed, in the medaka embryo, integrin interaction with laminin has been found to regulate interkinetic nuclear migration in neural epithelial cells. Mutant embryos for laminin deposition show abnormal mitotic pattern in the neural tube, with a basal displacement of mitosis.103 Given that trunk NC cell delamination occurs at the S phase, it is tempting to propose that, in the dorsal part of the neural tube, the absence of an elaborated basal lamina may result in the positioning of the nuclei toward the basal side and, thus, favor delamination.
Break-down of the basal lamina along premigratory NC cells results from a conjunction of several processes. Although its influence has not been addressed directly, the process of neural tube closure and ectodermal healing generates important tensions and physical constraints, particularly at the hinge point between the ectoderm and neural tube. In this region, folding occurs at the basal side of the cells whereas anywhere else along the neural tube, it occurs at the apical side. Thus, the surface of the basal side of NC cells becomes extremely reduced, possibly causing cell detachment from the basal lamina and disruption of the epithelial structure. Among the other factors that cause dissolution of the basal lamina, proteases are the most prominent. Initial studies revealed that at least in vitro both trunk and cranial NC cells synthesize plasminogen activator during migration.104–106 In this respect, it is noteworthy that a sequence in the promoter of the plasminogen activator gene driving specific expression in the neural crest lineage has been characterized in human and used for tracing and targeting NC cells in transgenic mice.107 More recently, it has been found that trunk NC cells produce the matrix metalloprotease MMP-2 when they disperse out of the neural tube, and that inhibitors of MMPs prevent NC cell delamination but not their migration.108 Consistent with this, the transcriptional regulator Ets-1 known to induce expression of MMPs is upregulated in cranial NC cells in the chick and frog, precisely at the time of NC cell delamination, and overexpression of Ets-1 in the neural tube causes ectopic delamination of neural epithelial cells associated with degradation of the basal lamina.55,109 Other studies showed that a subset of NC cells in the hindbrain at the origin of the cardiac NC do not produce MMP-2 by themselves but use for migration MMP-2 released in their environment by mesodermal cells.110,111 As mentionned before, NC cells also synthesize ADAM proteases such as ADAM-10 in the chick91 and ADAM-13 in the frog.112 Furthermore, ADAM-13 has been shown to cleave fibronectin and proposed to facilitate cell migration by reorganizing locally the fibronectin network.113 Finally, previously unsuspected players may also contribute to the reorganization of the basal lamina at the time of NC cell delamination. This is in particular the case of the Wnt antagonist Frzb-1, which is strongly expressed in the dorsal neural tube at the time of NC cell delamination.114–116 Recent, interesting studies in the Xenopus embryo demonstrated that Frzb-1 promotes the dissolution of the basal lamina at the junction between the ectoderm and endoderm in the primary mouth primordium by decreasing levels of fibronectin and laminin transcripts.117 Consistent with this view, overstimulation of the Wnt signaling pathway in vitro by LiCl or exogenous Wnt-1 or in vivo after electroporation of Wnt-1 or of a constitutively active form of β-catenin has been found to cause a marked inhibition of trunk NC cell delamination in birds.118,119
Contrary to epithelial cell models, NC cell EMT is not accompanied by a switch from a preferential laminin adhesion to fibronectin adhesion. Chick trunk NC cells show equal adhesion to fibronectin and laminin (at least laminin-111 also known as laminin-1) prior to and after initiation of migration, and laminin was found to promote rapid cell migration as efficiently as fibronectin.120–122 Consistent with this, in vivo, trunk NC cells invading the somites migrate preferably along basal lamina of the myotome over the fibrillar matrix of the sclerotome, even though they can accommodate for migration to the absence of basal lamina after experimental removal of the dermamyotome.123 Moreover, migrating NC cells do not synthesize fibronectin except a subset of cranial cells,124 but they synthesize vitronectin.125 In the Xenopus, however, cranial NC cells were found to show a great preference for fibronectin over laminin for migration, although both molecules can support efficient adhesion.126
A number of changes have been observed in the integrin repertoire of NC cells at delamination. However, it should be stressed that we are far from a comprehensive view of the integrin pattern during NC development in vivo, although this has been thoroughly investigated in avian trunk NC cells in vitro.120,125,127 At cranial levels, the α5β1 integrin, a fibronectin receptor, is expressed in migrating NC cells in Xenopus and mediates adhesion and migration over fibronectin,126 while the α6β1 integrin, a laminin receptor often encountered in epithelia, is absent from migrating NC cells.128 However, whether both integrins are regulated in relation with induction of migration has not been specifically investigated. In the chick, both α4β1, a fibronectin receptor involved in migration of a large variety of cell types, and αvβ3, a promiscuous receptor for many extracellular matrix components, become conspicuous in cranial NC cells at onset of migration.129,130 The function of αvβ3 has not been defined yet, but perturbation studies in vitro showed that α4β1 is implicated in cell migration.129,131 Finally, in the mouse, α4β1 and possibly α5β1 (based on the analysis of knocked-out mice), but not αvβ3 are induced in migrating cranial NC.130,132,133 At the trunk level in birds, no major change have been detected during delamination in α1β1 integrin, the major laminin-binding integrin involved in NC cell migration.134 α4β1 has been detected in migrating trunk NC cells,129,131 but in contrast to cranial levels, its expression is relatively low and it is not clear yet whether it coincides with the initiation of migration. Thus, in Xenopus and chick, cranial NC cell delamination is accompanied by the de novo expression of a set of integrins that are known to play a role in cell migration. In contrast, no such switch has been found at truncal level, suggesting that it is merely the interpretation of the adhesive signal by the same integrins that is responsible for initiation of migration. For example, it has been shown recently that trunk NC cells at the time of delamination and early migration express the Nedd9, a Cas-family scaffolding protein within the integrin signaling pathway, which regulates cell migration by modulating formation of focal adhesion sites and actin dynamics.135
Rho GTPases and NC cell delamination. More than a decade ago, the GTPase RhoB has been identified in a screen for genes specifically expressed in the dorsal neural tube and has been shown to exhibit a very dynamic pattern in prospective NC cells prior to and during early migration.136 In addition, blockade of Rho activity using the C3 exotoxin inhibits NC cell delamination,136 but it should be stressed that, as the C3 exotoxin shows no selectivity for any particular Rho, this experiment did not allow to ascribe a role in EMT exclusively to RhoB. Overexpression of either a wild type or a dominant active form of RhoB in the neural tube causes severe distortion of the neural tube resulting from massive cell death.137 However, if RhoB is coexpressed with Sox9, a NC specifier, no cell death is observed, instead cells undergo massive EMT correlating with basement membrane breakdown. These results have been interpreted as cooperation between RhoB and Sox-9 playing separate and complementary roles, respectively by triggering EMT and by protecting cells from apoptosis and confering them with NC cell features.137 Consistent with this, RhoB has been shown to ly downstream BMP-4 and Snail-2 in the genetic cascade involved in NC cell development in the chick trunk.138,139 Other studies performed on cranial NC in the zebrafish embryo also ascribed an active role to Rho GTPases in NC cell EMT. Hindbrain NC cell delamination is impaired by treatment with ROCKi, an inhibitor of Rho kinase, which inhibits cell blebbing activity.51
However, this view on the requirement of Rho GTPases for triggering NC delamination has been challenged recently. Groysman et al.140 found that activation of endogenous Rho by lysophosphatidic acid inhibits NC cell delamination and, reciprocally, that loss-of-function of RhoA or RhoB or of overall Rho signaling by C3 transferase or with the Y27632 compound, an inhibitor of Rho Kinase, accelerate and enhance NC emigration. Consistent with these findings, the Y27632 compound has been demonstrated to change cell fates in the neural tube, by inducing Snail expression, to induce EMT and to stimulate migration by altering cytoskeletal organization and cell adhesion both in chick and frog.141,142 On the other hand, Kinoshita et al.143 showed that during neural tube formation, Rho GTPases accumulate at the apical side of chick neural epithelial cells under the control of the Wnt/PCP signaling pathway, and that inhibition and continuous activation of Rho both result in neural tube defects, suggesting that correct spatiotemporal regulation of Rho is essential for neural tube morphogenesis. Likewise, Nishimura and Takeichi144 found that Shroom-3, an actin-binding protein known to be a key player in epithelial apical constriction, binds Rho kinase, a target of Rho, and is required for cranial neural tube closure. Finally, in the Drosophila wing imaginal disc epithelium, the GTPase Rho-1, is involved in the transition between cuboidal to columnar cell shape under the control of Dpp, the homolog of BMPs.145 All together, these experiments suggest that rather than being involved in triggering NC cell delamination, Rho GTPases are necessary for maintenance of the epithelial integrity of the neural tube. Alternatively, the differences observed between RhoB function, notably in avian and fish embryos, may reflect differences in the process of neurulation, requiring recruitment of the same molecular player for opposite functions.
Finally, it is worth-mentioning other studies that have uncovered new players of the Rho family involved in NC development. In Xenopus, RhoV has been identified as an early NC marker whose activity is essential for NC cell induction.146 RhoV messengers accumulate shortly after gastrulation in the NC region, and its depletion impairs expression of the NC specifiers such as Sox9, Snail-2 or Twist but not Snail-1. Moreover, RhoV knockdown causes a dramatic loss of cranial NC derived structures, whereas it overexpression expands the NC territory at the expense of the neural tube. In the chick, two related members of the Rho family, RhoU and RhoV show complementary patterns in the ectoderm and prospective NC area at the time of their specification.147
EMT transcriptional regulators during NC cell delamination.
Of the three Snail, Zeb and Twist families of transcriptional regulators of EMT, the Snail family is undubiously the one which received much attention in the process of NC cell delamination.16,148 In this respect, it should be reminded that the first demonstration of the role of Snail in EMT has been achieved owing to the NC system.149 Much less is known, in contrast, about the implication of the other factors in EMT of NC cells although more data have been collected during the recent years.
The snail family of znc-finger transcription factors. Two members of the Snail family have been identified in NC cells, Snail-1 and Snail-2 (formerly known as Slug). In the chick, NC cells express Snail-2 and not Snail-1 whereas in the mouse and zebrafish, it is the reverse situation, cells express Snail-1 instead of Snail-2 and lastly, in the Xenopus, both factors coexist in NC cells both cranial and truncal.150–152 Snail genes are generally induced very precociously during specification of the NC, particularly in the head where they appear in the neural folds early during neurulation, long before neural tube closure. In the Xenopus head, Snail-1 appears at mid gastrula in an arc that surrounds the prospective neural plate whereas Snail-2 appears later in the folds.152,153 In the chick head, Snail-2 is first detected in the neural folds during neurulation almost coincident with Sox-9, a major NC specifier.154 High expression persists in prospective NC cells throughout the delamination process as well as in early migrating NC cells to decline gradually as they reach the ventral regions of the embryo. In the chick trunk, Snail-2 expression is less conspicuous than in the head, possibly reflecting the progressive, slow emigration of NC cells. In addition, it appears relatively late in NC progenitors, i.e., after neural tube closure, and it is repressed soon after delamination once cells start migrating.139 Thus, Snail expression in the neural tube is restricted to the NC cell population and marks the specification and delamination steps, suggesting that it plays a decisive role in both processes.
To date, it has not been possible to determine whether Snail genes play a role only in specification, in delamination or both. This is largely due to the fact that both events are intimately connected, as the purpose of the specification process is to establish the grounds for EMT and to provide the cell with the competence to undergo EMT and survive in the environment out of the neural tube. Therefore, defining the transition between specification and delamination is elusive and arbitrary and it is not very easy to define the exact timing when an individual cell is fully specified and undergoes EMT. Except for the few genes (such as Ets-1) that are expressed late during specification and mark the EMT step,55 the difficulty is to define precisely whether a gene is required specifically for specification of the NC lineage or for EMT.
Loss of function experiments in chick and Xenopus result in a strong deficit in cranial NC cell migration.149,152,155 Conversely, gain-of-function experiments reveal that Snail-1 in Xenopus and Snail-2 in chick are sufficient to induce expansion of the NC territory in the head and production of a greater number of migrating NC cells,138,152 therefore arguing for the requirement of Snail genes in NC cell specification and delamination. Moreover, these experiments have uncovered a functional hierarchy between Snail-1 and Snail-2 in the Xenopus,152 although both factors have also been found to be interchangeable to some extent in chick.138 While Snail-1 is able to induce the expression of numerous NC specifiers (such as Zic-5, Foxd-3, Twist and Snail-2) both in embryos and in animal cap assays, Snail-2 lies downstream of Snail-1 in the genetic cascade leading to NC formation and, alone, it is not able to induce NC markers in animal caps.152 Nonetheless, numerous observations suggest that Snail genes may be neither sufficient nor necessary for NC cell specification and delamination. Indeed, overexpression of Snail-1/2 in chick and frog causes expansion of the NC population only at cranial levels and in the area contiguous to the endogenous NC-forming region.138,152 Expression of NC cell markers and EMT are never observed in the trunk and in more lateral and ventral regions of the neural tube, indicating that Snail-expressing cells must either receive additional inputs or express other transcriptional regulators to achieve specification and execute the EMT program. In the mouse, the conditional knockout of Snail-1, either alone or in combination with Snail-2, does not provoke complete inhibition of NC cell delamination and migration in the head.156 A substantial number of cells expressing bona fide NC markers, including Sox-10, Crabp-1 and p75, are seen to emigrate from the neural tube both in vivo and in vitro. It has been concluded that both Snail genes are dispensable for NC cell formation and delamination, and that other NC specifiers and EMT regulators can compensate for their loss. It should be noted, however, that neither the presence of NC cells at the trunk level nor the capacity of the migrating cranial NC cells to survive and differentiate into their normal derivatives have been investigated in the mutant embryos. Moreover, it cannot be excluded that some NC cell subpopulations are selectively eliminated in the absence of Snail-1/2.
E-cadherin and tight junction components, such as occludin and claudins, are among the main transcriptional targets of Snail in epithelial cells in culture and in tumors. Although there is at present no clear picture on the spatio-temporal expression pattern of Snail in relation with those of E-cadherin, occludin and claudin in the chick for example, its restricted expression in prospective NC cells cannot account for their progressive loss from the entire neural tube. However, several recent studies indicate that Snail-2 can directly regulate target genes involved in cell adhesion in NC cells. In the mouse embryo, E-cadherin and Snail-1 messengers exhibit non-overlapping, complementary profiles at the boundary between the ectoderm and the neural tube in the head region, consistent with a role for Snail-1 in the regulation of E-cadherin.157 In Xenopus, the Ajuba family of LIM proteins have been identified as functional co-repressors of Snail. They interact with Snail in the nucleus and cooperate for repression of E-cadherin in MCF7 epithelial cells. Interestingly, these proteins are also components of adherens junctions and may function as mediators between the cell surface and the nucleus.158,159 Finally, cadherin-6B has been demonstrated as a direct target of Snail-2 in chick NC cells. Snail-2 can bind to E boxes in the cadherin-6B regulatory sequence and modulates NC cell delamination in a cadherin-6B-dependent fashion.80 However, Snail-2 is probably not the sole regulator of cadherin-6B expression in NC cells at least in the trunk, as both genes show extensive regions of coexpression and that cadherin-6B expression persists in the dorsal neural tube long after cessation of NC cell delamination.74,78 In addition, it remains to be determined why, in the mouse embryo, cadherin-6 is not repressed in migrating NC cells, albeit they exhibit Snail-1 expression.
Several lines of evidence indicate that Snail may be essential for the maintenance of NC cell survival at the onset of migration. Indeed, in invertebrates, the primary function of the Snail family of transcription factors is to protect cells from apoptotic cell death16 and, in MDCK cells, Snail-1 has been found to confer resistence to apoptosis induced by serum deprivation and to block cell cycle progression.160 Furthermore, Snail-2 electroporation in the chick head reduces the number of apoptotic NC cells.160 Consistent with this, Snail-2 has been found to cooperate with other transcriptional regulators to promote complete EMT of neural epithelial cells. For example, neural tube cells transfected with Ets-1 alone undergo partial EMT, but fail to invade the neighboring tissues; either they form clusters anchored to the neural tube at its basal side or they die if they are isolated. In contrast, when these cells coexpress Snail-2 and Ets-1, they express NC cell markers and become able to colonize the periphery of the neural tube.55 Likewise, forced expression of Sox-9 in the trunk neural tube in chick can cause significant delamination only when used in combination with Snail-2. Conversely, in mouse Sox-9 mutants, Snail-2 is dramatically downregulated in truncal NC cells which instead undergo massive cell death at the onset of migration.137
In conclusion, Snail is likely to be involved in distinct cellular events to regulate NC cell formation and delamination: control of induction and maintenance of specifier genes, regulation of cell-cell interactions and prevention of apoptosis. These roles require the cooperation with other transcription regulators that do not function in a simple linear cascade but rather in a complex network of mutual interactions. Clearly, in order to understand its roles during NC cell development, much is needed to establish the complete list of Snail partners and targets.
The Zeb family of transcription factors. The Zeb family of transcription factors contains two members, Zeb-1 also known as δEF-1 and Zeb-2 also known as SIP-1, encoded by two separate genes, Zfhx-1a and Zfhx-1b.15,17 These proteins are characterized by a central homeodomain flanked by two zinc-finger clusters that bind to bipartite E-boxes in the promoter region of their target genes. Zeb factors function primarily as transcriptional repressors, e.g., for E-cadherin, brachyury and several junctional proteins, but also as activators, e.g., for α4 integrin.17,161 Interestingly, while Zeb-1 can activate BMP signaling, Zeb-2 binds phosphorylated Smad-1 (hence its other name Smad1interacting protein) and has been proposed to act as a sensor of BMP signaling in cells. It also plays a role in the genetic cascade involved in neural induction in fish, frog and chick.162–164 Intriguingly, Zeb-2 has been demonstrated to perform the same roles as Snail-1/2 at least in cells cultured in vitro: it represses the same range of targets, it blocks cell cycle progression by repressing cyclin-D1 expression, and it protects cells from apoptotic cell death.165,166
The expression profiles of Zeb-1 and Zeb-2 in early development at the time of NC cell formation have been in part determined.163,167–169 Most remarkably, in all species, Zeb-2 shows expression overlapping that of Snail-1/2 in NC cells, particularly at the time of delamination and early migration. Furthermore, Zeb-1 and Zeb-2 are differentially expressed in tissues, in patterns indicative of functional specificities.167 In Xenopus, Zeb-1 is first detected in the paraxial mesoderm and its expression then expands to the neural tube. Zeb-2 in contrast is found in the whole neural tube at both truncal and cranial levels at early neurula stage, and, from late neurula stage, it becomes restricted to premigratory NC cells where expression is conspicuous. Unlike Zeb-2, Zeb-1 is absent from premigratory NC cells but both of them are coexpressed once cells are migratory at cranial levels. In the zebrafish, the two Zeb-2 orthologues are expressed in pre-migratory and migrating NC cells.163 Finally, in the chick and mouse, while Zeb-1 is apparently not related with NC delamination, being expressed only after migration,168,169 Zeb-2 is found in the whole neural tube early during neurulation and later it is strongly expressed by migrating NC cells both in the head and trunk170 (Dady and Duband JL, unpublished).
The function of Zeb proteins in NC development have been essentially defined by genetic analyses in the mouse and fish. Zeb1-deficient mice die perinatally and exhibit multiple skeletal defects, but no overt anomalies in the nervous system.171 In contrast, Zeb-2 mutants have numerous neural tube defects: the neural tube fails to close and there is no sharp boundary between the ectoderm and neural plate. In addition, E-cadherin expression persists in the neural tube while cranial NC cell migration is retarded and vagal NC cells at the origin of the enteric ganglia are virtually missing.70 Furthermore, specific knockout of Zeb-2 in NC cells using Wnt-Cre mice results in specific anomalies in craniofacial and heart development, as well as in strong deficits in the number of melanocytes and peripheral neurons.170 Although the conditional knockout of Zeb-2 in NC cells cannot be directly compared with the Snail1 mutant described by Murray and Gridley156 because of the nature of the transgenic mouse lines used, it exhibits a much more impressive list of deficiencies in NC derivatives, suggesting that Snail-1 is more dispensable than Zeb-2 for NC development. Consistent with the anomalies observed in mice, fish embryos treated with Zeb-2 morphants exhibit severe defects in post-otic cranial NC cell migration resulting in the absence of NC derivatives in the posterior pharyngeal arches and a loss of enteric neurons in the intestine.163
All together these experiments suggest that Snail and Zeb proteins may perform complementary roles during neural tube and NC development. Snail1/2 exhibiting a restricted pattern in prospective and early migrating NC cells would be essentially involved in their specification by controling expression of other specifier genes. Conversely, Zeb-2 being broadly expressed in the neural tube would participate in the transformation of the neural ectoderm into neural plate, possibly by repressing ectodermal genes including E-cadherin. Finally, during NC delamination and migration, both Snail and Zeb-2 genes would function in synergy to modulate the adhesive and proliferative properties of NC progenitors and protect them from apoptosis once out of the neural tube.
The E-cadherin transcriptional repressors of the bHLH family. So far, Twist-1 and E47 are the only reported E-cadherin repressors belonging to the bHLH family of transcription factors.15 While little is known about the expression profile and function of E47 during embryonic development in general,172 Twist received much greater attention, largely because mouse Twist-null mutants exhibit major craniofacial defects.173 Moreover, in cancer cells, Twist is overexpressed and has been shown to exhibit dual function: it promotes EMT through downregulation of E-cadherin and it interferes with the p53 tumor suppressor pathway, thereby allowing cells to escape from apoptotic failsafe programs and acquire invasive potential.174,175
Twist is strongly expressed in migrating NC cells at cranial levels but not in truncal NC cells at any stage of their development.176–178 In Xenopus for example, it has been one of the first cranial NC cell marker identified:176 it is induced in the neural folds by Snail-1 signals prior to delamination and is maintained in NC cells during migration to the branchial arches.153 Surprisingly enough, its function and targets in the genetic cascade accompanying NC development have not been determined in this species. Data on the role of Twist in NC cells come essentially from analyses of the mouse mutant. Twist-null embryos show numerous cranial defects, including failure of neural tube closure at forebrain and midbrain levels and severe anomalies in the branchial arches.173,177,179,180 In particular, NC cells populate inappropriate locations in the first brancial arch and display defective osteogenic and odontogenic differentiation. In addition, loss of Twist impacts on the patterning of cranial ganglia and nerves. Interestingly, defects in the neural tube and NC at the midbrain and anterior hindbrain levels result primarily from the abnormalities in the cranial mesenchyme.180 However, other defects have been reported in cardiac NC cells, resulting in anomalies in the cardiac outflow tract, and have been in contrast attributed to defects in NC cell delamination at the posterior hindbrain level.181
The mechanisms by which Twist controls NC cell delamination remain unknown. Interestingly, clues may come from recent studies that deciphered the mechanisms controling its expression in Drosophila. During gastrulation, germ band extension causes an endogenous compression of stomodeal cells at the anterior of the embryo. It has been found that this compression leads to nuclear translocation of Armadillo (the homolog of β-catenin in Drosophila) functioning as a mechanosensor which in turn increases Twist expression and induces midgut differentiation.182 Because NC progenitors are situated in a region of the neural tube which is subjected to intense compression, it is tempting to speculate that Twist would be induced in response to neural fold movements during neural tube closure and elicit a transcriptional cascade leading to NC delamination. Since Twist is not expressed in the trunk region, it would be interesting to determine whether this is in relation with differences in cell movements during neurulation and if so, whether applying local compression in the trunk neural tube might induce its expression.
Other transcription factors. Recently, members of the forkhead box family of transcriptional regulators emerged as important players in cancer.183 In particular, FoxC-2 expression has been found to correlate with the metastatic capacities of cancer cells and in cultured cell lines, it is induced by EMT-promoting genes, e.g., Snail-1 and Twist. In addition, forced expression of FoxC-2 causes epithelial cells to adopt a mesenchymal phenotype associated with expression of vimentin, N-cadherin and fibronectin, but unlike Snail-1, it does not repress expression of epithelial markers such as E-cadherin.184
So far, the presence of FoxC-2 has not been reported in NC cells in any species. Yet another member of the Fox family, Foxd3, has been shown to play a key role in NC cell development. In all species and at all axial levels, Foxd-3 expression matches with NC induction, delamination and migration.185–189 Its expression starts early during NC induction approximately coincident with that of Snail-1 in Xenopus or zebrafish or Snail-2 in chick, but in contrast to the latter, it remains expressed in most NC cells during migration, except for melanoblasts. Gain and loss of function experiments in chick and frog as well as genetic analyses in mouse and zebrafish have ascribed multiple functions to Foxd-3 in NC cells, possibly through recruitment of different partners at defined steps of development. First, it has been implicated in NC cell specification. Thus, in the Xenopus head, when ectopically overexpressed, Foxd-3 is a potent inducer of NC cell markers, including Snail-2 and Twist,188 while in the chick trunk, it induces Sox-10 but not Snail-2 at the expense of interneuron markers.186 In addition, in contrast to Snail, it is able to induce expression of NC genes in distant locations from the NC prospective area. Conversely, attenuation of Foxd-3 expression in the Xenopus embryo reduces formation of NC cell progenitors.188 Likewise, in the zebrafish, Foxd-3 mutants have normal numbers of premigratory NC cells, but these cells express reduced levels of Snail-1 and Sox-10, implicating Foxd-3 as an essential regulator of these transcription factors in the premigratory NC.189 Second, Foxd-3 controls NC cell delamination. In Xenopus, it controls expression of Ets-1, independently of Snail-2.188 Ectopic expression of Foxd-3 in the neural tube of the chick trunk results in massive EMT with decrease in N-cadherin expression, alteration in cell polarity, and upregulation fo cadherin-7.137,186 However, Foxd-3 effect is not immediate and is only observed after 48 h suggesting that it acts by an indirect mechanism. Consistent with a role in delamination, the onset of NC migration is delayed in zebrafish Foxd-3 mutants, and there is a reduction in the number of migratory trunk NC cells, particularly along the medial migration pathway.189 Third, Foxd-3 is also involved in NC cell survival during migration as evidenced by analyses of mouse and zebrafish mutants. Complete absence of Foxd-3 results in a catastrophic loss of NC-derived structures: craniofacial dysmorphogenesis, skeletal anomalies, pharyngeal defects and peripheral nervous system defects.189–191 Interestingly, the melanogenic NC lineage is virtually not affected in mutants,190,191 in line with experiments in chick showing that Foxd-3 represses the melanocytic lineage in the NC population.192 Thus, Foxd-3 has been proposed to selectively specify premigratory NC cells for a neuronal, glial or cartilage fate, by inducing the expression of lineage-associated transcription factors in these cells and regulating their subsequent delamination and migration.
Beside Forkhead box transcription factors, a number of other transcriptional regulators not previously implicated in EMT of cultured epithelial cells or of cancer cells have been identified in NC cells and proposed to play a role in cell delamination. This is in particular the case of c-Myb.193 In the chick trunk, c-Myb is expressed in prospective and migrating NC cells at higher levels than in the rest of the neural tube and attenuation of c-Myb expression causes partial inhibition of NC cell formation. In addition, overexpression of c-Myb in neural tube explants induces expression of Snail-2 and forces EMT of neural epithelial cells. These results then suggest that c-Myb might control NC cell delamination through Snail-2 activation, but it cannot be excluded that it may also regulate other targets. For example, recent studies have demonstrated that c-Myb in association with Ets-1 and Sox-9 to control Sox-10 expression in cranial NC.194 Likewise, in hematopoietic cells, c-Myb again in cooperation with Ets-1 has been shown to control α4 integrin expression.195,196
Members of the Id subfamily of Helix-Loop-Helix (HLH) proteins are negative regulators of bHLH transcription factors that have also been shown to regulate NC cell specification and possibly their EMT. In the chick, Id-2 is expressed specifically in cranial NC cells prior to delamination and is later repressed at onset of migration. No Id-2 is found in trunk NC cells. Intringingly, ectopic expression of Id-2 in the non-neural ectoderm converts cells to a NC fate with expression of the HNK-1 marker and loss of epithelial features.197 Other Id factors, Id-1 and Id-4, are sequentially expressed during cranial NC development in chick, but their function has not been defined.198,199 In the Xenopus head, only one member of the family is expressed in the NC. Id-3 is expressed precociously in cranial and truncal prospective NC, and its depletion causes loss of cranial skeletal structures derived from the NC. Unlike Id-2 in chick which is merely involved in NC cell specification, Id-3 plays an essential role in their proliferation and survival as well as in the maintenance of a precursor pool of NC cells.200,201
Coordination of transcriptional regulator activity during NC cell delamination. The above descriptive and functional studies indicate that NC cell delamination is achieved through cooperation of multiple transcriptional regulators in addition to members of the Snail, Zeb and bHLH families. Even though it is not yet possible to draw a coherent sketch of the transcriptional network controling NC cell EMT, data collected form perturbation experiments based on either loss of function or ectopic gain of function allowed to identify some of the epistatic relationships between these factors and NC specifiers in the prospective NC lineage. In Xenopus cranial NC, there is a highly complex crossregulation of the Snail-1, Snail-2, Foxd-3, Sox-9 and Sox-10 genes, but Snail-1 emerges as a key player able to induce expression of numerous other NC specifiers and EMT regulators. It can enhance its own expression and induce that of Snail-2, Twist, Foxd-3 and Ets-1.152 Like Snail-1, Foxd-3 is able to induce its own expression and that of Snail-2 (Snail-1 was unfortunately not tested), Twist, Ets-1, but unlike Snail-1, it also induces pan neural genes such as Sox-2 and N-CAM.188 In the chick cranial NC and as well in the mouse, Snail-2 is upregulated by Sox-9, one of the earliest NC specifiers.137,154 However, unlike in the Xenopus and in cancer cells, Foxd-3 and Snail-2 belong apparently to independent signaling cascades.186
Additional information regarding the interplay between transcription factors during NC cell development were gained from the analyses of the regulatory sequences of their genes. Thus, Xenopus Snail-2 and mouse Sox-9 promoters were found to contain binding sites for the Lef/Tcf transcription factors, indicating direct regulation of their expression by the canonical Wnt signaling pathway.202,203 In addition, both mouse Sox-9 and chick Sox-10 exhibit in their regulatory sequence enhancers that drive specifically precocious expression in cranial NC cells, thereby revealing the presence of unique gene regulatory networks in this particular NC population.194,202 Interestingly, these enhancers contain binding sites for Ets-1, which is restricted to delaminating NC cells in the head. Detailed dissection of the Sox-10 enhancer driving expression in the cranial NC reveals Sox-9, Ets-1 and c-Myb as coregulators of enhancer activity. Notably, ChiP, DNA-pull down, and gel-shift assays demonstrate their direct binding to the enhancer in vivo while mutation of their corresponding binding sites abolishes endogenous Sox-10 expression.194 Interestingly, beside this “spatial” enhancer responsible for Sox-10 expression in cranial NC cells, a “temporal” enhancer has been identified in the promoter that regulates expression of Sox-10 in vagal and truncal NC cells at later stages of development, i.e., during migration and differentiation.194
Finally, a very recent study has uncovered epigenetic regulation of key NC specifiers both in human and Xenopus.204 CHD-7, an ATP-dependent chromatin remodeler homologous to the Drosophila trithorax group protein Kismey was found to be essential for activation of NC specifiers and EMT genes such as Sox-9, Snail-2 and Twist, but not of the neural plate border specifier genes, Msx-1, Zic-1 and Pax-3. In Xenopus, knockdown of CHD-7 results in extensive defects in cranial NC cell derivatives, while in human, CHD-7 in association with several partners bind to the NC-specific enhancer of the Sox-9 gene and a conserved element upstream the Twist gene. Consistently, mutation in the gene encoding CHD-7 in human results in a complex congenital anomalies of the NC known as CHARGE syndrome. These observations therefore highlight the major transcriptional reprogramming event that accompany NC cell development and provide insight into the synergistic control of enhancer activity of the NC specifiers by chromatin remodelers, thereby orchestrating NC gene expression programs.
EMT inducers during NC cell delamination.
While a great variety of extrinsic factors can induce EMT in cultured epithelial or tumor cells,3 only a limiter number of morphogens have been found to control NC cell delamination. To date, most of the information available concerns the trunk NC in the chick but data obtained in chick and mouse head suggest that the same triggering signals are operative in both regions in different species. In addition, although studies on the Xenopus cranial NC focussed essentially on the specification step and did not specifically addressed the question of delamination (reviewed in ref. 205), current data suggest, however, that it is the same families of morphogens, BMPs and Wnts, that are involved in NC cell specification and delamination, further emphasizing the continuity between both events.206 The mechanisms by which members of the BMP family control onset of migration in time and space have been depicted in detail. Early during neurulation, BMP-4 is expressed by the superficial ectoderm in contact with the neural plate. After closure of the neural tube, BMP-4 expression shifts from the ectoderm to the dorsal region of the neural tube, approximately to the prospective NC area.207 Addition of BMP-4 to neural tube explants stimulates production of NC cells,139 in line with previous observations showing that members of the TGFβ can provoke premature trunk NC cell migration in vitro by regulating integrin activity.208 However, BMP-4 expression is not restricted to the time window when NC cells are released, and instead it is expressed uniformly throughout a long portion of the neural axis in a pattern consistent with a role in specification, delamination and migration. In contrast to BMP-4, its antagonist Noggin shows a dynamic expression in the dorsal neural tube along a caudo-rostral gradient that coincides precisely with onset of NC emigration. Addition of Noggin in vitro or in vivo to neural tubes at the time of NC cell migration prevents their delamination, thereby demonstrating that the delamination promoting activity of BMP-4 is regulated spatially and temporally by Noggin.139,209 Consistent with these observations, NC cells delaminate precociously and in greater amounts in mouse embryo lacking Noggin.210 Additional studies in the chick embryo suggest that Noggin expression is under the control of the paraxial mesoderm. Specifically, the dorsomedial region of the dissociating somite was proposed to be the source of an inhibitory factor of unknown nature that downregulates Noggin expression in the dorsal neural tube.209 However, it remains to be determined whether this process can be transposed to cranial NC where the mesoderm is not partitioned into somites. Studies in the mouse and chick, though, indicate that NC formation and migration in the head are also under the influence of BMP signaling under the control of Noggin. BMP-4 can induce expression of Snail-2 and Sox-9 in avian cranial neural plate explants followed by EMT.154 Likewise, NC cells are generated in excess in mouse Noggin mutants while in BMP-2 mutants, cranial NC development is markedly impaired.210,211
The BMP-4 signaling cascade controls NC cell delamination through regulation of both cell adhesion and proliferation. BMP-4 effectors involved in cell adhesion are multiple: it can induce in a temporal sequence expression of Snail-2, cadherin-6B, Rho-B and cadherin-7 and it stimulates cleavage of N-cadherin while activating integrins.77,139,208 Conversely, Noggin inhibition of BMP-4 maintains expression of N-cadherin and causes repression of Rho-B and cadherin-6B but surprisingly, not of Snail-2.77,139 Concerning cell proliferation, BMP-4 effect involves Wnt-1 signaling. Noggin overexpression inhibits the G1/S transition in the neural tube, while blocking the G1/S transition abrogates BMP-induced EMT. Moreover, Wnt-1 expression is stimulated by BMP-4 and interfering with Wnt signaling by blocking β-catenin or Lef inhibits G1/S transition, cell delamination and transcription of several BMP-dependent genes.212 Moreover, BMP-4-mediated N-cadherin proteolytic cleavage has been found to stimulate transcription of cyclin-D1, thereby raising the intriguing possibility of functional connections between regulation of cell adhesion and cell proliferation by BMP-4 signaling.77 These observations led Kalcheim et al. to propose that NC cell EMT involves a linear cascade of events elicited by BMP-4 under the control of Noggin.77,140 Downregulation of Noggin in the dorsal neural tube by somite-derived factors would allow BMP-4 to be active and to induce expression of Wnt-1, which in turn activates Cyclin-D1 expression through β-catenin and stimulates G1/S transition. Concommitantly, BMP-4 induces cleavage of N-cadherin protein by ADAM-10 and γ-secretase, allowing both deterioration of cell-cell junctions and stimulation of β-catenin and Cyclin-D1 expression.
This model therefore assigns key roles to BMP-4, N-cadherin and the G1/S transition at the center of the EMT process, but it does not account entirely for the great complexity of the NC delamination process. Indeed, G1/S transition is not sufficient to drive NC cell EMT as, at cranial levels, NC cells can delaminate independently of it and that at all axial levels, ventral neural tube cells do not undergo EMT although they also display interkinetic nuclear migration.55 Moreover, truncal NC cells can be forced to undergo EMT in a non-coordinated manner with the cell cycle when overexpressing Ets-1,55 suggesting that that EMT may to some extent be uncoupled from the cell cyle (reviewed in ref. 213). As discussed above, it is rather the conjunction between the presence of the nucleus at the basal side with the disruption of the basal lamina that favors EMT.103 On the other hand, it is well known that NC cell departure is not synchronized with somitogenesis in most of the neural axis.27 Thus, Noggin control by somites may be only circumstancial. Instead, its expression may be regulated by a factor released by the paraxial mesoderm, not correlated with somite maturation but with other as yet undefined events. In addition, Wnt-1 expression in the dorsal neural tube does not match well with onset of NC cell delamination at levels rostral to the 20th somite. In these regions, its expression precedes by far migration and persists in the neural tube long after cessation of migration114,214 (our unpublished data). Finally, NC cells have been shown to produce Wnt antagonists at the time of their emigration, thereby raising the intriguing possibility that they may not be responsive to Wnt signals at this step.114–116
Lineage Decisions and EMT
EMT potential in the ectoderm and neural tube.
NC cells are often regarded as the only cell population of the neurectoderm capable of undergoing EMT and endowed with migration properties. By opposition, the rest of the neural epithelium and the ectoderm are essentially considered as static because they show little or no tendency to undergo EMT in vivo. Thus, understanding whether and why neural tube and ectodermal cells are incapable of executing the EMT program should provide additional clues on NC cell delamination.
Although the ectoderm is a very flexible structure which frequently changes shape during early morphogenesis, EMT is not commonly observed in this tissue under normal conditions. This correlates with the absence in ectodermal cells of transcriptional regulators of EMT, such as Snail, Zeb-2 or Twist, or of the regulators specific to NC specification and delamination such as Foxd3, Ets-1, Rho-B, Id-2 and Sox-9/10. However, there are notable exceptions to this rule in the cranial neurogenic placodes, such as the trigeminal and epibranchial placodes. These placodes are local thickenings of the ectodermal epithelium, from which neuron precursors of the cranial sensory ganglia emerge and mingle with NC cell streams migrating into the branchial arches.215,216 Studies in the chick embryo have shown that although of a very similar origin, placodal neurons do not delaminate the same way as NC cells. In particular, the epithelium from which they derive is pluristratified: superficial cells remain proliferative and epithelial while cells in the deep layer become quiescent and leave the epithelium owing to a breach in the underlying basement membrane. In addition, these cells express both E- and N-cadherin prior to delamination and retain expression of N-cadherin after, but they never express Snail-2 or Rho-B217,218 (Daddy and Duband JL, unpublished). Finally, neurons originating from the placode do not migrate extensively, instead they associate with NC cells underneath. Thus, the molecular players controling the release of placodal cells are clearly distinct from those involved in NC cell EMT but remain to be determined.
The notion that, with the exclusion of the placodes, ectodermal cells do not normally undergo EMT has been challenged recently. Indeed, based on the presence of cells coexpressing the PDGF receptor and E-cadherin in the lateral ectoderm in mouse cranial regions, Weston et al.219 proposed that cells at the origin of the mesectoderm (also called ectomesenchyme) from which skeleton and connective tissues of the head are formed do not derive from the NC in the neural folds but from more lateral, non-neural ectoderm. Further studies in the mouse embryo using Cre recombinase-based fate mapping of ectodermal cells recently confirmed the ectodermal origin of cells released in the underlying mesenchyme.220 These results therefore indicate that the ectoderm near the neural epithelial retains at least for some time the ability to undergo EMT and to produce ectomesenchymal cells. However, it remains to be established whether this schema only applies to the mouse species, due to its peculiar organization of the neural tube and ectoderm, if all ectomesenchymal cells derive from this particular portion of the ectoderm, and which EMT genes are expressed to account for this cell delamination.
Although they differ strikingly from NC cells, neural epithelial cells exhibit a number of molecular features suggesting that they may at least to some extent be competent for executing the EMT program (see above). The fact that they do not normally undergo EMT in vivo may be explained by the presence of growth factors or morphogens produced by neighboring tissues that would restrict their potential. In this respect, Shh has been shown to inhibit integrin activity in both NC cells and neural epithelial cells, thereby limiting their migratory capacities.221,222 The EMT capacity of neural epithelial cells has been evaluated directly on neural tube fragments collected at different stages of neurulation and explanted in vitro under conditions free of the environmental influences and physical constraints present in the embryo.142 Although neural epithelial cells show the ability to spread on the dish and migrate as a sheet during the initial stages of neurulation, they fail to undergo EMT even after prolonged cultures. Only, fibroblastic cells expressing NC cell markers may appear over time in culture indicating that EMT can occur spontaneously in neural tube explants but only in predetermined NC cells. Thus, neural epithelial cells are not entirely static cells as they are transiently able to spread and locomote on two-dimensional substrata, but they differ from NC cells by their intrinsic inability to spontaneously undergo EMT.
A mean to further evaluate the EMT potential of neural and non-neural epithelial cells is to define whether they can be forced to undergo EMT and if this can be achieved independently of the acquisition of a NC identity. Experiments in chick and frog showed that neural epithelial cells can delaminate from the neural tube when they are converted into NC cells, e.g., after treatment with BMP-4 or transfection with NC specifiers,137,207,223 indicating that even in ventral regions, acquitition of a NC cell identity is sufficient to provide cells with the ability to execute the EMT program. In a few cases, however, neural epithelial cells have been found to undergo EMT independently of their phenotypic conversion into NC cells. For example, electroporation of Ets-1 or HIP-1, an antagonist of Shh signals, results in massive cell delamination in the neural tube without induction of Sox-10 or HNK-1 markers.55,222 Of interest, in these experiments, cells lose their epithelial structure, but they fail to migrate at distance from the neural tube and generally remain anchored to the neural epithelium or die, indicating that acquisition of NC cell traits is critical for complete delamination and efficient migration and survival.
In contrast to the neural epithelium, little is known about the influence of acquisition of a NC identity on the induction of overexpression and EMT in the ectoderm. The only documented example concerns Id-2 overexpression in the cranial chick ectoderm using recombinant retroviruses, which results in the massive conversion of ectodermal cells into fibroblastic, HNK-1-positive cells assimilated to NC cells.197 Thus, the ectoderm responds to NC-specific genes very similarly to neural epithelial cells but, to our knowledge, whether they can be forced to undergo EMT independently of a NC or a placodal identity has not yet been directly addressed.
Lessons from NC cell-free regions.
In all vertebrates, NC cells emerge from the neural epithelium along the entire neuraxis, except in a few discrete regions which contribute only to a limited number of NC cells or which are characterized by a complete absence of cells. These are regions corresponding to the telencephalon at the anterior neuropore, the rhombomeres 3 and 5 (r3, r5), and the caudal trunk. Comparison of the gene regulatory networks and/or of the cellular events that operate in these regions with those from other, “normal” axial levels provides complementary information on the mechanisms leading to NC cell segregation from the neural tube.
Several possible explanations may account for the absence of NC in discrete regions of the neural tube. First, inductive signals are present only in regions where NC cells are normally induced, but absent from the regions where they are reduced or absent. Second, the inductive signals are present throughout the neural axis, but they are locally inhibited by antagonistic signals released in the prospective NC environment. Third, NC cells are generated as in other regions but they do not undergo EMT or are selectively eliminated by apoptosis. The second mechanism was found recently to prevail in the anterior neuropore.224 Grafting experiments in the Xenopus embryo demonstrated that in this region, the neurectoderm is capable of giving rise to NC cells and that the prechordal mesoderm inhibits NC formation through release of the Wnt/β-catenin antagonist Dickkopf-1 (Dkk-1). Moreover, Dkk-1 loss of function in Xenopus and mouse is sufficient to induce formation of NC cells in the forebrain. Likewise, this can be mimicked by injection of Wnt-8 or β-catenin in the blastomere fated to give rise to the anterior neural folds. Interestingly, induction of extra-NC cells in the anterior regions does not occur at the expense of other neural and placodal tissues, and these NC cells possess migration and differentiation capabilities very similar to those produced more posteriorly at the midbrain level.
Absence of NC cells from r3 and r5 has been proposed to be accounted for by multiple independent processes acting at the delamination and migration steps.225–229 In particular, although these two rhombomeres produce NC cells which migrate anteriorly or posteriorly to join the streams derived from the adjacent r2, r4 and r6, a number of NC cells from these segments die by apopotosis prior to migration. This cell death program is elicited by BMP-4 produced in r3 and r5 under the control of the adjacent rhombomeres. However, all NC cells arising at the hindbrain are primed to respond to the pro-apoptotic effect of BMP-4, but at least at r4 and r6, they escape from cell death by expressing high levels of the BMP-4 antagonist Noggin.228,229 Moreover, beside being selectively eliminated by apoptosis, NC cells from r3 and r5 differ from the other cranial NC by their gene regulatory network (our unpublished results). Thus, while NC cells emerging at r4 and r6 express AP-2, cadherin-6B, cyclin-D1, Ets-1, Foxd3, Id-2, the α4 and β3 integrins, neuropilin-2, Rho-B, Snail-2 and Sox-10, those at r3 express only AP-2, cadherin-6B, Id-2 and Snail-2, and not the other genes. In addition, interesting differences were detected between NC cell populations originating from r3 and r5. NC cells from r5 express a panel of genes closer to NC at r4 than at r3, as all genes are expressed but cyclin-D1, neuropilin-2 and Rho-B. Notably, Foxd-3, Ets-1, integrins and Sox-10 are clearly detected at least transiently in those cells. This suggests that NC cells generated at r3 would undergo normal specification, but that not all genes involved in their EMT and migration are induced in these cells. Alternatively, these cells may be eliminated prematurely before expression of genes necessary for EMT and migration. NC cells from r5 in contrast would be specified and capable of undergoing EMT, but they may be eliminated after being released. This is consistent with the fact that emigration of NC cells from r3 was found to cease earlier than in other rhombomeres.226
Lastly, selective cell death has also been found to cause the absence of NC cells in the caudal-most region, but contrary to the hindbrain, it is not due to the presence of BMP-4 combined with Noggin absence.230 The most caudal region in both mammals and birds is characterized by the absence of motor nerves in the spinal cord and a lack of peripheral ganglia. In the chick embryo, this part of the neural tube corresponds to the region of somites 47–53 which are the last pairs of somites to be formed. Analysis of the expression pattern of NC specifiers in this region as well as markers of migrating NC cells reveal that although NC cells are specified and generated almost normally in this region, they migrate in low numbers and delay compared to more rostral NC cells. These cells do not provide neurons but only melanocytes and Schwann cells. The scarcity and delayed migration of caudal NC cells are primarily due to extensive cell apoptosis caused by the absence of BMP-4 and Wnt-1 signals in the dorsal neural tube. Indeed, instead of being repressed, Noggin persists in the neural tube together with BMP-4. In addition, Wnt-1 is absent, while Wnt-3a remains normally expressed. Forced expression of Noggin in more rostral levels of the trunk neural tube produces a significant reduction of NC cells due to massive apoptosis and a complete repression of Wnt-1, thereby mimicking to some extent the situation observed in the caudal region. Conversely, Noggin repression in the caudal neural tube restores both NC cells with neurogenic potential and Wnt-1 expression. This study therefore reveals that NC progenitors are normally generated in the caudal neural tube but those with neurogenic potential are selectively eliminated due to the lack of adequate BMP-4/Wnt-1 signals. NC cells with melanogenic potential are in contrast spared and migrate normally, indicating that they do not need BMP-4/Wnt-1 signals for delamination, migration and survival. Thus, these data suggest the intriguing possibility that the regulatory mechanisms involved in NC cell delamination are dependent on the nature of their derivatives. If so, this would mean that different types of delamination processes would have evolved for each type of NC derivatives.
Acquisition of stem cell properties and NC cell delamination.
Recent evidence suggests that breast cancer cells that undergo EMT acquire stem cell-like characteristics,231 probably allowing dispersing cells to cope with changes in their environment and become responsive to different stimuli. This process is also believed to generate multiple distinct cellular subpopulations within the tumor contributing to intratumoral heterogeneity.3,8 Interestingly, there is now growing evidence that embryonic stem cells normally undergo EMT during differentiation in three-dimensional matrices, and that acquisition of a mesenchymal phenotype may to some extent confer cells with pluripotency.3,8 The question of acquisition of stemness and EMT is therefore relevant to the NC as these cells are known for their ability to provide a large variety of cell types. There is now a general consensus that NC cells do not constitute an homogeneous population at the time when they segregate from the neural tube; rather it consists of coexisting pluripotent or bipotent cells and of fate-restricted cells precociously engaged into a single lineage.22,232 Evidence has accumulated that NC cell progenitors of sympathetic and sensory neurons, glia and melanocytes are segregated even before delamination. Moreover, NC progenitors for each cell lineage may be spatially segregated within the dorsal neural tube and depend on distinct factors for survival. For example, c-Kitpositive melanocyte precursors in the mouse embryo are both spatially and temporally segregated from p75-positive cells at the origin of peripheral neurons and glia. Melanocytes progenitors are situated in the dorsal midline of the neural tube and undergo migration later than neural progenitors which are situated more laterally in the neural tube and migrate precociously.233 In the chick trunk, as discussed above, BMP-4/Wnt-1 signals would be required for neuronal progenitor survival prior to migration but dispensable for the melanocyte progeny which instead relies preferentially on Wnt-3a.230 Likewise, Cre recombinase-fate mapping studies in the mouse trunk showed that GDF-7, a member of the BMP family produced in the dorsal neural tube, restricts a pool of late-emigrating NC cell precursors to the sensory lineage.234 Finally, very recent data from the Kalcheim's laboratory suggest that in the chick trunk, progenitors of the different NC derivatives are spatially segregated along the ventro-dorsal axis of the neural tube and emigrate in an ordered fashion so that the most ventral sympathetic neurons are generated first while melanocytes migrating dorsally are the last cells to leave the neural tube.62 These data therefore indicate that the concept of generation of stemness upon EMT may not be readily applicable to NC cells as their fate is preestablished prior to migration. Consistent with this, in chick, Sox-2, a marker of immature neural cells implicated in stemness is lost at the time of NC cell EMT and acts as a negative regulator of NC cell formation.235 However, a number of genes involved in the maintenance of pluripotency have been identified in NC at the time of specification and delamination. This is in particular the case of Foxd-3 which has been found to be required for the maintenance of pluripotent cells in the mouse embryo236 as well as of laminin 10/11 (also known as laminin 511) which has been found to promote mouse embryonic stem cell renewal.237 Indeed, the laminin a5 chain has been previously identified in a screen for genes upregulated in response to chick truncal NC induction.35 Interestingly, laminin a5 null mice display anomalies suggestive of a role in the maintenance of NC cells into a progenitor state.238 In particular, regroupment of NC cells into ganglia is delayed and migrating cells tend to disperse farther than in normal embryos. Thus, it cannot be excluded, that as for tumor cells, the EMT process in the NC may also contribute to the maintenance of a pool of pluripotent cells among an heterogeneous population of multiple progenitors.
Cellular Analysis of NC Cell Delamination in the Trunk Reveals the “Unpredictable Nature” of NC Cell EMT in the Chick Trunk
Most of the functional studies performed so far analyzed NC cell delamination essentially from a molecular or genetic point of view, but did not allow to draw a complete picture of the cellular events driving EMT. A major break-through has been recently achieved in the appreciation of these cellular mechanisms owing to tracking of individual NC cells with confocal time-lapse imaging.213 This study performed at the trunk level of chick embryos allowed to visualize directly the dynamics of NC cell EMT and solved several issues regarding the cellular mechanism of the EMT, such as the sequence of events, modes of detachment and the role of cell division in generating NC cells. A major conclusion is that NC cells can separate from the neural tube by a great diversity of cellular mechanisms, thereby revealing that their EMT program is governed by a complex network of non-linear, independent and loosely-connected mechanisms that can occur in multiple orders and combinations to allow NC cells to escape from the neural epithelium. In accord with the prevailing model that deterioration of junctional complexes is a key event of EMT, a majority of NC cells were found to delaminate following a classical sequence of detachment and retraction of the tail from the luminal side and translocation of the cell body out of the epithelium. However, there were notable exceptions to this sequence. For example, the cell tail is ruptured during retraction leaving cell fragments behind at the apical surface, revealing that downregulation of cell junctions is not a prerequisite and that cell traction may be sufficient for pulling the cell out of the epithelium. Also, separation occurs sometimes as the cell rounds up during mitosis, indicating that in the dorsal neural tube cell division may not necessarily occur at the apical side. This observation is fully consistent with the recent finding that absence of basement membrane results in the basal displacement of mitosis in neural epithelial cells.103 Moreover, the plane of cell division does not predict the occurrence of EMT and generation of NC cells. Finally, retraction of the cell tail is not necessarily accompanied by redistribution of epithelial apico-basal polarity markers. Whether this diversity of processes relates with the establishment of the different lineages issues from the NC remains however to be determined.
A number of striking features also emerge from this study, challenging several previous hypotheses. First, NC cells were found to emigrate from any region of the dorsal neural tube with the same probability, and not solely from a discrete region such as the dorsal midline. Second, only very few cells in the dorsal neural tube actually undergo EMT, explaining some of the characteristics of NC cell emigration in the trunk. Based on the proportion of labeled neural tube cells undergoing EMT in a very large number of samples, an estimate of 8% of the dorsal neural cells was found to emigrate. This is in striking contrast with the relatively homogeneous expression patterns of the NC specifiers in the dorsal neural tube, such as Snail-2, Foxd-3, Sox-9 as well as other genes involved in EMT, including cadherin-6B. This suggests that most cells expressing the whole range of NC genes do not undergo EMT and raises the intriguing question of their fate. Do they undergo apoptosis or do they switch to another differentiation program? In addition, delaminating NC cells were not different from other neural tube cells regarding their cellular behavior, thereby excluding any obvious forerunner
signs that will predict EMT. Additional, as yet undefined cellular and molecular events must account for the choice of undergoing EMT in the dorsal neural tube.
Concluding Remarks
Although, we are still far from a comprehensive view of the gene regulatory network controling NC cell delamination, several conclusions can be drawn from the comparison of molecular actors involved in EMT of epithelial cell lines and of NC cells. First, rather than a linear cascade, NC cell delamination must be considered as the result of a conjunction of a great variety of cellular events that ultimately control cell and matrix adhesion, cell proliferation, cell fate and cell survival. The intricacy and interweaving of these events within a discrete heterogenous cell population over a relatively short period of time make it extremely difficult to decipher the function of each molecular player during this process. Clearly, only functional analyses at the single cell level will allow solving this problem. Second, although it is tempting to propose a unifying model describing the molecular cascade leading to NC cell delamination, it becomes more and more obvious that, due to the heterogeneity of the NC population along the neural axis and the diversity of the neurulation processes in vertebrates, NC cell EMT involves distinct cellular and molecular mechanisms regulated by separate processes. This is particularly striking for the cranial and truncal NC cells. Cranial NC cell delamination is massive and predictable. It involves cooperation between multiple transcriptional regulators recruited all together and acting within a short time period during the intense tissular remodelings of neurulation (Figs. 4 and 5). In contrast, trunk NC cell delamination is progressive, unpredictable, limited to individual cells and delayed with respect to the movements of neurulation. It is connected with the cell cycle and recruits a more restricted panel of transcription factors in a limited number of cells (Figs. 4 and 5). Third, the comparison between EMT processes involved in NC cell dispersion and in tumor cell delamination indicates strong conservation of the molecular players, but suggest a great diversity in the spatio-temporal sequences of events. Notably, NC cell EMT involves fine tuning of the regulatory processes which limit both spatially and temporally its occurence in an exquisite manner. In addition, coordination of EMT process with other cellular events, such as cell proliferation and mechanical constraints may account for a such a diversity.
Figure 5.
Molecular players of EMT during NC cell delamination at cranial and truncal levels in the chick embryo. The massive delamination of NC cells at cranial levels is correlated with expression of a greater number of transcriptional regulators than at truncal levels, suggesting cooperation between transcription factors to allow coordination of EMT events in cells. + moderate expression; ++ strong expression; +/− expression in a limited number of cells (e.g., for cad7 at cranial levels) or gradually decreasing during migration (e.g., for RhoB in migrating cells). Expression of Sox-9 and Sox-10, two major genes involved in NC specification and migration, is indicated for reference.
Acknowledgements
The author wishes to thank Guojun Sheng for his kind invitation to write this review and for his patience and comprehension, to thank Alwyn Dady, Claire Fournier-Thibault and Liliana Osório for fruitful discussions and to express his sympathy to all his colleagues working in the field for their stimulating contributions.
Footnotes
Previously published online: www.landesbioscience.com/journals/celladhesion/article/12501
References
- 1.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:3–8. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 2.Boyer B, Tucker GC, Vallés AM, Franke WW, Thiery JP. Rearrangements of desmosomal and cytoskeletal proteins during the transition from epithelial to fibroblastoid organization in cultured rat bladder carcinoma cells. J Cell Biol. 1989;109:1495–1509. doi: 10.1083/jcb.109.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelialmesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 5.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baum B, Settleman J, Quinlan MP. Transitions between epithelial and mesenchymal states in development and disease. Semin Cell Dev Biol. 2008;19:294–308. doi: 10.1016/j.semcdb.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Shook D, Keller R. Mechanisms, mechanics and function of epithelial-mesenchymal transitions in early development. Mech Dev. 2003;120:1351–1383. doi: 10.1016/j.mod.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9:265–273. doi: 10.1038/nrc2620. [DOI] [PubMed] [Google Scholar]
- 9.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal tansitions. Nat Rev Mol Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 10.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Cur Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Knoblich JA. Asymmetric cell division during animal development. Nat Rev Mol Cell Biol. 2001;2:11–20. doi: 10.1038/35048085. [DOI] [PubMed] [Google Scholar]
- 12.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–562. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 13.Radtke F, Clevers H. Self-renewal and cancer of the gut: two sides of a coin. Science. 2005;307:1904–1909. doi: 10.1126/science.1104815. [DOI] [PubMed] [Google Scholar]
- 14.Nakaya Y, Sheng G. Epithelial to mesenchymal transition during gastrulation: an embryological view. Dev Growth Differ. 2008;50:755–766. doi: 10.1111/j.1440-169X.2008.01070.x. [DOI] [PubMed] [Google Scholar]
- 15.Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. doi: 10.1038/nrc2131. [DOI] [PubMed] [Google Scholar]
- 16.Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. doi: 10.1242/dev.01907. [DOI] [PubMed] [Google Scholar]
- 17.Vandewalle C, Van Roy F, Berx G. The role of the ZEB family of transcription factors in development and disease. Cell Mol Life Sci. 2009;66:773–787. doi: 10.1007/s00018-008-8465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 19.Thiery JP, Delouvée A, Gallin W, Cunningham BA, Edelman GM. Ontogenetic expression of cell adhesion molecules: L-CAM is found in epithelia derived from the three primary germ layers. Develop Biol. 1984;102:61–78. doi: 10.1016/0012-1606(84)90175-1. [DOI] [PubMed] [Google Scholar]
- 20.Hatta K, Takagi S, Fujisawa H, Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Develop Biol. 1987;120:215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- 21.Duband J-L, Volberg T, Sabanay I, Thiery JP, Geiger B. Spatial and temporal distribution of the adherensjunction-associated adhesion molecule A-CAM during avian embryogenesis. Development. 1988;103:325–344. doi: 10.1242/dev.103.2.325. [DOI] [PubMed] [Google Scholar]
- 22.Le Douarin NM, Kalcheim C. The Neural Crest. Second Edition. New York: Cambridge University Press; 1999. [Google Scholar]
- 23.Ezin AM, Fraser SE, Bronner-Fraser M. Fate map and morphogenesis of presumptive neural crest and dorsal neural tube. Dev Biol. 2009;330:221–236. doi: 10.1016/j.ydbio.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sauka-Spengler T, Bronner-Fraser M. A gene regulatory network orchestrates neural crest formation. Nat Rev Mol Cell Biol. 2008;9:557–568. doi: 10.1038/nrm2428. [DOI] [PubMed] [Google Scholar]
- 25.Steventon B, Carmona-Fontaine C, Mayor R. Genetic network during neural crest induction: from cell specification to cell survival. Semin Cell Dev Biol. 2005;16:647–654. doi: 10.1016/j.semcdb.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Monsoro-Burq AH, Wang E, Harland R. Msx1 and Pax3 cooperate to mediate FGF8 and WNT signals during Xenopus neural crest induction. Dev Cell. 2005;8:167–178. doi: 10.1016/j.devcel.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 27.Newgreen DF, Erickson CA. The migration of neural crest cells. Int Rev Cytol. 1986;103:89–145. doi: 10.1016/s0074-7696(08)60834-7. [DOI] [PubMed] [Google Scholar]
- 28.Erickson CA, Perris R. The role of cell-cell and cell-matrix interactions in the morphogenesis of the neural crest. Develop Biol. 1993;159:60–74. doi: 10.1006/dbio.1993.1221. [DOI] [PubMed] [Google Scholar]
- 29.Duband J-L, Monier F, Delannet M, Newgreen DF. Epithelium-mesenchyme transition during neural crest development. Acta Anat. 1995;154:63–78. doi: 10.1159/000147752. [DOI] [PubMed] [Google Scholar]
- 30.Duband JL. Neural crest delamination and migration: integrating regulations of cell interactions, locomotion, survival and fate. Adv Exp Med Biol. 2006;589:45–77. doi: 10.1007/978-0-387-46954-6_4. [DOI] [PubMed] [Google Scholar]
- 31.Nieto MA. The early steps of neural crest development. Mech Develop. 2001;105:27–35. doi: 10.1016/s0925-4773(01)00394-x. [DOI] [PubMed] [Google Scholar]
- 32.Kalcheim C. Mechanism of early neural crest development: From cell specification to migration. Int Rev Cytol. 2000;200:143–196. doi: 10.1016/s0074-7696(00)00004-8. [DOI] [PubMed] [Google Scholar]
- 33.Kalcheim C, Burstyn-Cohen T. Early stages of neural crest ontogeny: formation and regulation of cell delamination. Int J Dev Biol. 2005;49:105–116. doi: 10.1387/ijdb.041949ck. [DOI] [PubMed] [Google Scholar]
- 34.LaBonne C, Bronner-Fraser M. Molecular mechanisms of neural crest formation. Annu Rev Cell Dev Biol. 1999;15:81–112. doi: 10.1146/annurev.cellbio.15.1.81. [DOI] [PubMed] [Google Scholar]
- 35.Gammill LS, Bronner-Fraser M. Genomic analysis of neural crest induction. Development. 2002;129:5731–5741. doi: 10.1242/dev.00175. [DOI] [PubMed] [Google Scholar]
- 36.Morales AV, Barbas JA, Nieto MA. How to become neural crest: from segregation to delamination. Semin Cell Dev Biol. 2005;16:655–662. doi: 10.1016/j.semcdb.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Raible DW. Development of the neural crest: achieving specificity in regulatory pathways. Curr Opin Cell Biol. 2006;18:698–703. doi: 10.1016/j.ceb.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Davidson LA, Keller RE. Neural tube closure in Xenopus laevis involves medial migration, directed protrusive activity, cell intercalation and convergent extension. Development. 1999;126:4547–4556. doi: 10.1242/dev.126.20.4547. [DOI] [PubMed] [Google Scholar]
- 39.Colas J-F, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Develop Dyn. 2001;221:117–145. doi: 10.1002/dvdy.1144. [DOI] [PubMed] [Google Scholar]
- 40.Lowery LA, Sive H. Strategies of vertebrate neurulation and a re-evaluation of teleost neural tube formation. Mech Dev. 2004;121:1189–1197. doi: 10.1016/j.mod.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 41.Copp AJ. Neurulation in the cranial region—normal and abnormal. J Anat. 2005;207:623–635. doi: 10.1111/j.1469-7580.2005.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aaku-Saraste E, Hellwig A, Huttner WB. Loss of occludin and functional tight junctions, but not ZO-1, during neural tube closure-remodeling of the neuroepithelium prior to neurogenesis. Develop Biol. 1996;180:664–679. doi: 10.1006/dbio.1996.0336. [DOI] [PubMed] [Google Scholar]
- 43.Erickson CA, Tucker RP, Edwards BF. Changes in the distribution of intermediate-filament types in Japanese quail embryos during morphogenesis. Differentiation. 1987;34:88–97. doi: 10.1111/j.1432-0436.1987.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 44.Schoenwolf GC, Delongo J. Ultrastructure of secondary neurulation in the chick embryo. Am J Anat. 1980;158:43–63. doi: 10.1002/aja.1001580106. [DOI] [PubMed] [Google Scholar]
- 45.Hong E, Brewster R. N-cadherin is required for the polarized cell behaviors that drive neurulation in the zebrafish. Development. 2006;133:3895–3905. doi: 10.1242/dev.02560. [DOI] [PubMed] [Google Scholar]
- 46.Tawk M, Araya C, Lyons DA, Reugels AM, Girdler GC, Bayley PR, et al. A mirror-symmetric cell division that orchestrates neuroepithelial morphogenesis. Nature. 2007;446:797–800. doi: 10.1038/nature05722. [DOI] [PubMed] [Google Scholar]
- 47.Wilcock AC, Swedlow JR, Storey KG. Mitotic spindle orientation distinguishes stem cell and terminal modes of neuron production in the early spinal cord. Development. 2007;134:1943–1954. doi: 10.1242/dev.002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Afonso C, Henrique D. PAR3 acts as a molecular organizer to define the apical domain of chick neuroepithelial cells. J Cell Sci. 2006;119:4293–4304. doi: 10.1242/jcs.03170. [DOI] [PubMed] [Google Scholar]
- 49.Simard A, Di Pietro E, Ryan AK. Gene expression pattern of Claudin-1 during chick embryogenesis. Gene Expr Patterns. 2005;5:553–560. doi: 10.1016/j.modgep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Bastidas F, De Calisto J, Mayor R. Identification of neural crest competence territory: role of Wnt signaling. Dev Dyn. 2004;229:109–117. doi: 10.1002/dvdy.10486. [DOI] [PubMed] [Google Scholar]
- 51.Berndt JD, Clay MR, Langenberg T, Halloran MC. Rho-kinase and myosin II affect dynamic neural crest cell behaviors during epithelial to mesenchymal transition in vivo. Dev Biol. 2008;324:236–244. doi: 10.1016/j.ydbio.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas S, Thomas M, Wincker P, Babarit C, Xu P, Speer MC, et al. Human neural crest cells display molecular and phenotypic hallmarks of stem cells. Hum Mol Genet. 2008;17:3411–3425. doi: 10.1093/hmg/ddn235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Osorio L, Teillet MA, Palmeirim I, Catala M. Neural crest ontogeny during secondary neurulation: a gene expression pattern study in the chick embryo. Int J Dev Biol. 2009;53:641–648. doi: 10.1387/ijdb.072517lo. [DOI] [PubMed] [Google Scholar]
- 54.Tosney KW. The segregation and early migration of cranial neural crest cells in the avian embryo. Develop Biol. 1982;89:13–24. doi: 10.1016/0012-1606(82)90289-5. [DOI] [PubMed] [Google Scholar]
- 55.Théveneau E, Duband J-L, Altabef M. Ets-1 confers cranial features on neural crest delamination. PLoS ONE. 2007;2:1142. doi: 10.1371/journal.pone.0001142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duband J-L, Thiery JP. Distribution of fibronectin in the early phase of avian cephalic neural crest cell migration. Develop Biol. 1982;93:308–323. doi: 10.1016/0012-1606(82)90120-8. [DOI] [PubMed] [Google Scholar]
- 57.Copp A, Cogram P, Fleming A, Gerrelli D, Henderson D, Hynes A, et al. Neurulation and neural tube closure defects. Methods Mol Biol. 2000;136:135–160. doi: 10.1385/1-59259-065-9:135. [DOI] [PubMed] [Google Scholar]
- 58.Newgreen DF, Kerr RS, Minichiello J, Warren N. Changes in cell adhesion and extracellular matrix molecules in spontaneous spinal neural tube defects in avian embryos. Teratology. 1997;55:195–207. doi: 10.1002/(SICI)1096-9926(199703)55:3<195::AID-TERA4>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 59.Thiery JP, Duband J-L, Delouvée A. Pathways and mechanism of avian trunk neural crest cell migration and localization. Develop Biol. 1982;93:324–343. doi: 10.1016/0012-1606(82)90121-x. [DOI] [PubMed] [Google Scholar]
- 60.Tosney KW. The early migration of neural crest cells in the trunk region of the avian embryo: An electron microscopic study. Develop Biol. 1978;62:317–333. doi: 10.1016/0012-1606(78)90219-1. [DOI] [PubMed] [Google Scholar]
- 61.Sadaghiani B, Thiébaud CH. Neural crest development in the Xenopus laevis embryo, studied by interspecific transplantation and scanning electron microscopy. Develop Biol. 1987;124:91–110. doi: 10.1016/0012-1606(87)90463-5. [DOI] [PubMed] [Google Scholar]
- 62.Krispin S, Nitzan E, Kassem Y, Kalcheim C. Evidence for a dynamic spatiotemporal fate map and early fate restrictions of premigratory avian neural crest. Development. 2010;137:585–595. doi: 10.1242/dev.041509. [DOI] [PubMed] [Google Scholar]
- 63.Burstyn-Cohen T, Kalcheim C. Association between the cell cycle and neural crest delamination through specific regulation of G1/S transition. Develop Cell. 2002;3:383–395. doi: 10.1016/s1534-5807(02)00221-6. [DOI] [PubMed] [Google Scholar]
- 64.Raible DW, Wood A, Hodson W, Henion PD, Weston JA, Eisen JS. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Dev Dyn. 1996;195:29–42. doi: 10.1002/aja.1001950104. [DOI] [PubMed] [Google Scholar]
- 65.Takeichi M. The cadherins: Cell-cell adhesion molecules controlling animal morphogenesis. Development. 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- 66.Nandadasa S, Tao Q, Menon NR, Heasman J, Wylie C. N- and E-cadherins in Xenopus are specifically required in the neural and non-neural ectoderm, respectively, for F-actin assembly and morphogenetic movements. Development. 2009;136:1327–1338. doi: 10.1242/dev.031203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Develop Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 68.Lele Z, Folchert A, Concha M, Rauch GJ, Geisler R, Rosa F, et al. parachute/n-cadherin is required for morphogenesis and maintained integrity of the zebrafish neural tube. Development. 2002;129:3281–3294. doi: 10.1242/dev.129.14.3281. [DOI] [PubMed] [Google Scholar]
- 69.Harrington MJ, Hong E, Fasanmi O, Brewster R. Cadherin-mediated adhesion regulates posterior body formation. BMC Dev Biol. 2007;7:130. doi: 10.1186/1471-213X-7-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Van de Putte T, Maruhashi M, Francis A, Nelles L, Kondoh H, Huylebroeck D, et al. Mice lacking Zfhx1b, the gene that codes for Smad-interacting protein-1, reveal a role for multiple neural crest cell defects in the ethiology of Hirschsprung disease-mental retardation syndrome. Am J Hum Genet. 2003;72:465–470. doi: 10.1086/346092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Taneyhill LA. To adhere or not to adhere: the role of Cadherins in neural crest development. Cell Adh Migr. 2008;2:223–230. doi: 10.4161/cam.2.4.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Inoue T, Chisaka O, Matsunami H, Takeichi M. Cadherin-6 expression transiently delineates specific rhombomeres, other neural tube subdivisions, and neural crest subpopulations in mouse embryos. Dev Biol. 1997;183:183–194. doi: 10.1006/dbio.1996.8501. [DOI] [PubMed] [Google Scholar]
- 73.Liu Q, Liu B, Wilson AL, Rostedt J. Cadherin-6 message expression in the nervous system of developing zebrafish. Dev Dyn. 2006;235:272–278. doi: 10.1002/dvdy.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakagawa S, Takeichi M. Neural crest cell-cell adhesion controlled by sequential and subpopulation-specific expression of novel cadherins. Development. 1995;121:1321–1332. doi: 10.1242/dev.121.5.1321. [DOI] [PubMed] [Google Scholar]
- 75.David R, Wedlich D. Xenopus cadherin-6 is expressed in the central and peripheral nervous system and in neurogenic placodes. Mech Dev. 2000;97:187–190. doi: 10.1016/s0925-4773(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 76.Akitaya T, Bronner-Fraser M. Expression of cell adhesion molecules during initiation of and cessation of neural crest cell migration. Dev Dynamics. 1992;194:12–20. doi: 10.1002/aja.1001940103. [DOI] [PubMed] [Google Scholar]
- 77.Shoval I, Ludwig A, Kalcheim C. Antagonistic roles of full-length N-cadherin and its soluble BMP cleavage product in neural crest delamination. Development. 2007;134:491–501. doi: 10.1242/dev.02742. [DOI] [PubMed] [Google Scholar]
- 78.Nakagawa S, Takeichi M. Neural crest emigration from the neural tube depends on regulated cadherin expression. Development. 1998;125:2963–2971. doi: 10.1242/dev.125.15.2963. [DOI] [PubMed] [Google Scholar]
- 79.Coles EG, Taneyhill LA, Bronner-Fraser M. A critical role for Cadherin6B in regulating avian neural crest emigration. Dev Biol. 2007;312:533–544. doi: 10.1016/j.ydbio.2007.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Taneyhill LA, Coles EG, Bronner-Fraser M. Snail2 directly represses cadherin6B during epithelial-to-mesenchymal transitions of the neural crest. Development. 2007;134:1481–1490. doi: 10.1242/dev.02834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Espeseth A, Marnellos G, Kintner C. The role of F-cadherin in localizing cells during neural tube formation in Xenopus embryos. Development. 1998;125:301–312. doi: 10.1242/dev.125.2.301. [DOI] [PubMed] [Google Scholar]
- 82.Liu Q, Marrs JA, Londraville RL, Wilson AL. Cadherin-7 function in zebrafish development. Cell Tissue Res. 2008;334:37–45. doi: 10.1007/s00441-008-0664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu B, Joel Duff R, Londraville RL, Marrs JA, Liu Q. Cloning and expression analysis of cadherin7 in the central nervous system of the embryonic zebrafish. Gene Expr Patterns. 2007;7:15–22. doi: 10.1016/j.modgep.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takahashi M, Osumi N. Expression study of cadherin7 and cadherin20 in the embryonic and adult rat central nervous system. BMC Dev Biol. 2008;8:87. doi: 10.1186/1471-213X-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Faulkner-Jones BE, Godinho LN, Reese BE, Pasquini GF, Ruefli A, Tan SS. Cloning and expression of mouse Cadherin-7, a type-II cadherin isolated from the developing eye. Mol Cell Neurosci. 1999;14:1–16. doi: 10.1006/mcne.1999.0764. [DOI] [PubMed] [Google Scholar]
- 86.Moore R, Larue L. Cell surface molecules and truncal neural crest ontogeny: a perspective. Birth Defects Res C Embryo Today. 2004;72:140–150. doi: 10.1002/bdrc.20014. [DOI] [PubMed] [Google Scholar]
- 87.Moore R, Champeval D, Denat L, Tan SS, Faure F, Julien-Grille S, et al. Involvement of cadherins 7 and 20 in mouse embryogenesis and melanocyte transformation. Oncogene. 2004;23:6726–6735. doi: 10.1038/sj.onc.1207675. [DOI] [PubMed] [Google Scholar]
- 88.Hadeball B, Borchers A, Wedlich D. Xenopus cadherin-11 (Xcadherin-11) expression requires the Wg/Wnt signal. Mech Dev. 1998;72:101–113. doi: 10.1016/s0925-4773(98)00022-7. [DOI] [PubMed] [Google Scholar]
- 89.Vallin J, Girault J-M, Thiery JP, Broders F. Xenopus cadherin-11 is expressed in different populations of migrating neural crest cells. Mech Dev. 1998;75:171–174. doi: 10.1016/s0925-4773(98)00099-9. [DOI] [PubMed] [Google Scholar]
- 90.Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, et al. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Develop Biol. 1995;169:347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- 91.Hall RJ, Erickson CA. ADAM 10: an active metalloprotease expressed during avian epithelial morphogenesis. Dev Biol. 2003;256:146–159. doi: 10.1016/s0012-1606(02)00133-1. [DOI] [PubMed] [Google Scholar]
- 92.Reiss K, Maretzky T, Ludwig A, Tousseyn T, de Strooper B, Hartmann D, et al. ADAM10 cleavage of N-cadherin and regulation of cell-cell adhesion and beta-catenin nuclear signalling. EMBO J. 2005;24:742–752. doi: 10.1038/sj.emboj.7600548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marambaud P, Wen PH, Dutt A, Shioi J, Takashima A, Siman R, et al. A CBP binding transcriptional repressor produced by the PS1/epsilon-cleavage of N-cadherin is inhibited by PS1 FAD mutations. Cell. 2003;114:635–645. doi: 10.1016/j.cell.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 94.Kashef J, Kohler A, Kuriyama S, Alfandari D, Mayor R, Wedlich D. Cadherin-11 regulates protrusive activity in Xenopus cranial neural crest cells upstream of Trio and the small GTPases. Genes Dev. 2009;23:1393–1398. doi: 10.1101/gad.519409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCusker C, Cousin H, Neuner R, Alfandari D. Extracellular cleavage of cadherin-11 by ADAM metalloproteases is essential for Xenopus cranial neural crest cell migration. Mol Biol Cell. 2009;20:78–89. doi: 10.1091/mbc.E08-05-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kasemeier-Kulesa JC, Bradley R, Pasquale EB, Lefcort F, Kulesa PM. Eph/ephrins and N-cadherin coordinate to control the pattern of sympathetic ganglia. Development. 2006;133:4839–4847. doi: 10.1242/dev.02662. [DOI] [PubMed] [Google Scholar]
- 97.Borchers A, David R, Wedlich D. Xenopus cadherin-11 restrains cranial neural crest migration and influences neural crest specification. Development. 2001;128:3049–3060. doi: 10.1242/dev.128.16.3049. [DOI] [PubMed] [Google Scholar]
- 98.Monier-Gavelle F, Duband J-L. Control of N-cadherinmediated intercellular adhesion in migrating neural crest cells in vitro. J Cell Sci. 1995;108:3839–3853. doi: 10.1242/jcs.108.12.3839. [DOI] [PubMed] [Google Scholar]
- 99.Monier-Gavelle F, Duband J-L. Cross-talk between adhesion molecules: Control of N-cadherin activity by intracellular signals elicited by β1 and β3 integrins in migrating neural crest cells. J Cell Biol. 1997;137:1663–1681. doi: 10.1083/jcb.137.7.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xu X, Li WEI, Huang GY, Meyer R, Chen T, Luo Y, et al. Modulation of mouse neural crest motility by N-cadherin and connexin 43 gap junction. J Cell Biol. 2001;154:217–229. doi: 10.1083/jcb.200105047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Thomas LA, Yamada KM. Contact stimulation of cell migration. J Cell Sci. 1992;103:1211–1214. doi: 10.1242/jcs.103.4.1211. [DOI] [PubMed] [Google Scholar]
- 102.Teddy JM, Kulesa PM. In vivo evidence for short- and long-range cell communication in cranial neural crest cells. Development. 2004;131:6141–6151. doi: 10.1242/dev.01534. [DOI] [PubMed] [Google Scholar]
- 103.Tsuda S, Kitagawa T, Takashima S, Asakawa S, Shimizu N, Mitani H, et al. FAK-mediated extracellular signals are essential for interkinetic nuclear migration and planar divisions in the neuroepithelium. J Cell Sci. 2010;123:484–496. doi: 10.1242/jcs.057851. [DOI] [PubMed] [Google Scholar]
- 104.Valinsky JE, Le Douarin NM. Production of plasminogen activator by migrating cephalic neural crest cells. EMBO J. 1985;4:1403–1406. doi: 10.1002/j.1460-2075.1985.tb03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Erickson CA, Isseroff RR. Plasminogen activator activity is associated with neural crest cell motility in tissue culture. J Exp Zool. 1989;251:123–133. doi: 10.1002/jez.1402510203. [DOI] [PubMed] [Google Scholar]
- 106.Agrawal M, Brauer PR. Urokinase-type plasminogen activator regulates cranial neural crest cell migration in vitro. Dev Dyn. 1996;207:281–290. doi: 10.1002/(SICI)1097-0177(199611)207:3<281::AID-AJA5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 107.Pietri T, Eder O, Blanche M, Thiery JP, Dufour S. The human tissue plasminogen activator-Cre mouse: a new tool for targeting specifically neural crest cells and their derivatives in vivo. Develop Biol. 2003;259:176–187. doi: 10.1016/s0012-1606(03)00175-1. [DOI] [PubMed] [Google Scholar]
- 108.Duong TD, Erickson CA. MMP-2 plays an essential role in producing epithelial-mesenchymal transformations in the avian embryo. Dev Dyn. 2004;229:42–53. doi: 10.1002/dvdy.10465. [DOI] [PubMed] [Google Scholar]
- 109.Tahtakran SA, Selleck MA. Ets-1 expression is associated with cranial neural crest migration and vasculogenesis in the chick embryo. Gene Expr Patterns. 2003;3:455–458. doi: 10.1016/s1567-133x(03)00065-6. [DOI] [PubMed] [Google Scholar]
- 110.Cai DH, Vollberg TM, Sr, Hahn-Dantona E, Quigley JP, Brauer PR. MMP-2 expression during early avian cardiac and neural crest morphogenesis. Anat Rec. 2000;259:168–179. doi: 10.1002/(SICI)1097-0185(20000601)259:2<168::AID-AR7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 111.Cai DH, Brauer PR. Synthetic matrix metalloproteinase inhibitor decreases early cardiac neural crest migration in chicken embryos. Dev Dyn. 2002;224:441–449. doi: 10.1002/dvdy.10129. [DOI] [PubMed] [Google Scholar]
- 112.Alfandari D, Wolfsberg TG, White JM, DeSimone DW. ADAM13: A novel ADAM expressed in somitic mesoderm and neural crest cells during Xenopus laevis development. Develop Biol. 1997;182:314–330. doi: 10.1006/dbio.1996.8458. [DOI] [PubMed] [Google Scholar]
- 113.Alfandari D, Cousin H, Gaultier A, Smith K, White JM, Darribere T, et al. Xenopus ADAM 13 is a metalloprotease required for cranial neural crest-cell migration. Curr Biol. 2001;11:918–930. doi: 10.1016/s0960-9822(01)00263-9. [DOI] [PubMed] [Google Scholar]
- 114.Baranski M, Berdougo E, Sandler JS, Darnell DK, Burrus LW. The dynamic expression pattern of frzb-1 suggests multiple roles in chick development. Develop Biol. 2000;217:25–41. doi: 10.1006/dbio.1999.9516. [DOI] [PubMed] [Google Scholar]
- 115.Ladher RK, Church VL, Allen S, Robson L, Abdelfattah A, Brown NA, et al. Cloning and expression of the Wnt antagonists Sfrp-2 and Frzb during chick development. Develop Biol. 2000;218:183–198. doi: 10.1006/dbio.1999.9586. [DOI] [PubMed] [Google Scholar]
- 116.Jin E-J, Erickson CA, Takada S, Burrus LW. Wnt and BMP signaling govern lineage segregation of melanocytes in the avian embryo. Develop Biol. 2001;233:22–37. doi: 10.1006/dbio.2001.0222. [DOI] [PubMed] [Google Scholar]
- 117.Dickinson AJ, Sive HL. The Wnt antagonists Frzb1 and Crescent locally regulate basement membrane dissolution in the developing primary mouth. Development. 2009;136:1071–1081. doi: 10.1242/dev.032912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmidt C, McGonnell IM, Allen S, Otto A, Patel K. Wnt6 controls amniote neural crest induction through the non-canonical signaling pathway. Dev Dyn. 2007;236:2502–2511. doi: 10.1002/dvdy.21260. [DOI] [PubMed] [Google Scholar]
- 119.de Melker AA, Desban N, Duband JL. Cellular localization and signaling activity of β-catenin in migrating neural crest cells. Develop Dyn. 2004;230:708–726. doi: 10.1002/dvdy.20091. [DOI] [PubMed] [Google Scholar]
- 120.Desban N, Duband J-L. Avian neural crest cell migration on laminin: Interaction of the a1b1 integrin with distinct laminin-1 domains mediates different adhesive responses. J Cell Sci. 1997;110:2729–2744. doi: 10.1242/jcs.110.21.2729. [DOI] [PubMed] [Google Scholar]
- 121.Desban N, Lissitzky J-C, Rousselle P, Duband J-L. alpha1 beta1-integrin engagement to distinct laminin-1 domains orchestrates spreading, migration and survival of neural crest cells through independent signaling pathways. J Cell Sci. 2006;119:3206–3218. doi: 10.1242/jcs.03057. [DOI] [PubMed] [Google Scholar]
- 122.Newgreen DF. Adhesion to extracellular materials by neural crest cells at the stage of initial migration. Cell Tiss Res. 1982;227:297–317. doi: 10.1007/BF00210888. [DOI] [PubMed] [Google Scholar]
- 123.Tosney KW, Dehnbostel DB, Erickson CA. Neural crest cells prefer the myotome's basal lamina over the sclerotome as a substratum. Develop Biol. 1994;163:389–406. doi: 10.1006/dbio.1994.1157. [DOI] [PubMed] [Google Scholar]
- 124.Newgreen DF, Thiery JP. Fibronectin in early avian embryos: Synthesis and distribution along the migration pathways of neural crest cells. Cell Tiss Res. 1980;211:269–291. doi: 10.1007/BF00236449. [DOI] [PubMed] [Google Scholar]
- 125.Delannet M, Martin F, Bossy B, Cheresh DA, Reichardt LF, Duband J-L. Specific roles of the aVb1, αvβ3 and aVb5 integrins in avian neural crest cell adhesion and migration on vitronectin. Development. 1994;120:2687–2702. doi: 10.1242/dev.120.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alfandari D, Cousin H, Gaultier A, Hoffstrom BG, DeSimone DW. Integrin alpha5beta1 supports the migration of Xenopus cranial neural crest on fibronectin. Develop Biol. 2003;260:449–464. doi: 10.1016/s0012-1606(03)00277-x. [DOI] [PubMed] [Google Scholar]
- 127.Testaz S, Delannet M, Duband J-L. Adhesion and migration of avian neural crest cells on fibronectin require the cooperating activites of multiple integrins of the β1 and β3 families. J Cell Sci. 1999;112:4715–4728. doi: 10.1242/jcs.112.24.4715. [DOI] [PubMed] [Google Scholar]
- 128.Lallier TE, Whittaker CA, DeSimone DW. Integrin alpha6 expression is required for early nervous system development in Xenopus laevis. Development. 1996;122:2539–2554. doi: 10.1242/dev.122.8.2539. [DOI] [PubMed] [Google Scholar]
- 129.Kil SH, Krull CE, Cann G, Clegg D, Bronner-Fraser M. The α4 subunit of integrin is important for neural crest cell migration. Develop Biol. 1998;202:29–42. doi: 10.1006/dbio.1998.8985. [DOI] [PubMed] [Google Scholar]
- 130.Pietri T, Thiery JP, Dufour S. Differential expression of beta3 integrin gene in chick and mouse cranial neural crest cells. Dev Dyn. 2003;227:309–313. doi: 10.1002/dvdy.10299. [DOI] [PubMed] [Google Scholar]
- 131.Testaz S, Duband J-L. Central role of the α4β1 integrin in the coordination of avian truncal neural crest adhesion, migration and survival. Develop Dyn. 2001;222:127–140. doi: 10.1002/dvdy.1181. [DOI] [PubMed] [Google Scholar]
- 132.Pinco K, Liu S, Yang JT. a4 integrin is expressed in a subset of cranial neural crest cells and in epicardial progenitor cells during early mouse development. Mech Develop. 2001;100:99–103. doi: 10.1016/s0925-4773(00)00503-7. [DOI] [PubMed] [Google Scholar]
- 133.Goh KL, Yang JT, Hynes RO. Mesodermal defects and cranial neural crest apoptosis in α5 integrin-null embryos. Development. 1997;124:4309–4319. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- 134.Duband J-L, Belkin AM, Syfrig J, Thiery JP, Koteliansky VE. Expression of a1 integrin, a laminin-collagen receptor, during myogenesis and neurogenesis in the avian embryo. Development. 1992;116:585–600. doi: 10.1242/dev.116.3.585. [DOI] [PubMed] [Google Scholar]
- 135.Aquino JB, Lallemend F, Marmigere F, Adameyko II, Golemis EA, Ernfors P. The retinoic acid inducible Cas-family signaling protein Nedd9 regulates neural crest cell migration by modulating adhesion and actin dynamics. Neuroscience. 2009;162:1106–1119. doi: 10.1016/j.neuroscience.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Liu J-P, Jessel TM. A role for rhoB in the delamination of neural crest cells from the dorsal neural tube. Development. 1998;125:5055–5067. doi: 10.1242/dev.125.24.5055. [DOI] [PubMed] [Google Scholar]
- 137.Cheung M, Chaboissier M-C, Mynett A, Hirst E, Schedl A, Briscoe J. The transcriptional control of trunk neural crest induction, survival and delamination. Dev Cell. 2005;8:179–192. doi: 10.1016/j.devcel.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 138.del Barrio MG, Nieto MA. Overexpression of Snail family members highlights their ability to promote chick neural crest formation. Development. 2002;129:1583–1593. doi: 10.1242/dev.129.7.1583. [DOI] [PubMed] [Google Scholar]
- 139.Sela-Donenfeld D, Kalcheim C. Regulation of the onset of neural crest migration by coordinated activity of BMP4 and Noggin in the dorsal neural tube. Development. 1999;126:4749–4762. doi: 10.1242/dev.126.21.4749. [DOI] [PubMed] [Google Scholar]
- 140.Groysman M, Shoval I, Kalcheim C. A negative modulatory role for rho and rho-associated kinase signaling in delamination of neural crest cells. Neural Dev. 2008;3:27. doi: 10.1186/1749-8104-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Broders Bondon F, Chesneau A, Romero-Oliva F, Mazabraud A, Mayor R, Thiery JP. Regulation of XSnail2 expression by Rho GTPases. Dev Dyn. 2007;236:2555–2566. doi: 10.1002/dvdy.21273. [DOI] [PubMed] [Google Scholar]
- 142.Duband J-L, Blavet C, Jarov A, Fournier-Thibault C. Spatio-temporal control of neural epithelial cell migration and epithelium-to-mesenchyme transition during neural tube development. Dev Growth Diff. 2009;51:25–44. doi: 10.1111/j.1440-169X.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 143.Kinoshita N, Sasai N, Misaki K, Yonemura S. Apical accumulation of Rho in the neural plate is important for neural plate cell shape change and neural tube formation. Mol Biol Cell. 2008;19:2289–2299. doi: 10.1091/mbc.E07-12-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nishimura T, Takeichi M. Shroom3-mediated recruitment of Rho kinases to the apical cell junctions regulates epithelial and neuroepithelial planar remodeling. Development. 2008;135:1493–1502. doi: 10.1242/dev.019646. [DOI] [PubMed] [Google Scholar]
- 145.Widmann TJ, Dahmann C. Dpp signaling promotes the cuboidal-to-columnar shape transition of Drosophila wing disc epithelia by regulating Rho1. J Cell Sci. 2009;122:1362–1373. doi: 10.1242/jcs.044271. [DOI] [PubMed] [Google Scholar]
- 146.Guémar L, de Santa Barbara P, Vignal E, Maurel B, Fort P, Faure S. The small GTPase RhoV is an essential regulator of neural crest induction in Xenopus. Dev Biol. 2007;310:113–128. doi: 10.1016/j.ydbio.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 147.Notarnicola C, Le Guen L, Fort P, Faure S, de Santa Barbara P. Dynamic expression patterns of RhoV/Chp and RhoU/Wrch during chicken embryonic development. Dev Dyn. 2008;237:1165–1171. doi: 10.1002/dvdy.21507. [DOI] [PubMed] [Google Scholar]
- 148.Nieto M. The Snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–166. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 149.Nieto MA, Sargent MG, Wilkinson DG, Cooke J. Control of cell behavior during vertebrate development by Slug, a zinc finger gene. Science. 1994;264:835–839. doi: 10.1126/science.7513443. [DOI] [PubMed] [Google Scholar]
- 150.Sefton M, Sanchez S, Nieto MA. Conserved and divergent roles for members of the Snail family of transcription factors in the chick and mouse embryo. Development. 1998;125:3111–3121. doi: 10.1242/dev.125.16.3111. [DOI] [PubMed] [Google Scholar]
- 151.Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail-1 gene and its expression in wild-type, spadetail and no-tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- 152.Aybar MJ, Nieto MA, Mayor R. Snail precedes slug in the genetic cascade required for the specification and migration of the Xenopus neural crest. Development. 2003;130:483–494. doi: 10.1242/dev.00238. [DOI] [PubMed] [Google Scholar]
- 153.Linker C, Bronner-Fraser M, Mayor R. Relationship between gene expression domains of Xsnail, Xslug and Xtwist and cell movement in the prospective neural crest of Xenopus. Develop Biol. 2000;224:215–225. doi: 10.1006/dbio.2000.9723. [DOI] [PubMed] [Google Scholar]
- 154.Sakai D, Suzuki T, Osumi N, Wakamatsu Y. Cooperative action of Sox9, Snail2 and PKA signaling in early neural crest development. Development. 2006;133:1323–1333. doi: 10.1242/dev.02297. [DOI] [PubMed] [Google Scholar]
- 155.LaBonne C, Bronner-Fraser M. Snail-related transcriptional repressors are required in Xenopus for both the induction of the neural crest and its subsequent migration. Develop Biol. 2000;221:195–205. doi: 10.1006/dbio.2000.9609. [DOI] [PubMed] [Google Scholar]
- 156.Murray SA, Gridley T. Snail family genes are required for left-right asymmetry determination, but not neural crest formation, in mice. Proc Natl Acad Sci USA. 2006;103:10300–10304. doi: 10.1073/pnas.0602234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcriptional factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biol. 2000;2:76–83. doi: 10.1038/35000025. [DOI] [PubMed] [Google Scholar]
- 158.Langer EM, Feng Y, Zhaoyuan H, Rauscher FJ, 3rd, Kroll KL, Longmore GD. Ajuba LIM proteins are snail/slug corepressors required for neural crest development in Xenopus. Dev Cell. 2008;14:424–436. doi: 10.1016/j.devcel.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ayyanathan K, Peng H, Hou Z, Fredericks WJ, Goyal RK, Langer EM, et al. The Ajuba LIM domain protein is a corepressor for SNAG domain mediated repression and participates in nucleocytoplasmic Shuttling. Cancer Res. 2007;67:9097–9106. doi: 10.1158/0008-5472.CAN-07-2987. [DOI] [PubMed] [Google Scholar]
- 160.Vega S, Morales AV, Ocana OH, Valdes F, Fabregat I, Nieto MA. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004;18:1131–1143. doi: 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, et al. SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res. 2005;33:6566–6578. doi: 10.1093/nar/gki965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sheng G, dos Reis M, Stern CD. Churchill a zinc finger transcriptional activator, regulates the transition between gastrulation and neurulation. Cell. 2003;115:603–613. doi: 10.1016/s0092-8674(03)00927-9. [DOI] [PubMed] [Google Scholar]
- 163.Delalande JM, Guyote ME, Smith CM, Shepherd IT. Zebrafish sip1a and sip1b are essential for normal axial and neural patterning. Dev Dyn. 2008;237:1060–1069. doi: 10.1002/dvdy.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nitta KR, Takahashi S, Haramoto Y, Fukuda M, Tanegashima K, Onuma Y, et al. The N-terminus zinc finger domain of Xenopus SIP1 is important for neural induction, but not for suppression of Xbra expression. Int J Dev Biol. 2007;51:321–325. doi: 10.1387/ijdb.062252kn. [DOI] [PubMed] [Google Scholar]
- 165.Mejlvang J, Kriajevska M, Vandewalle C, Chernova T, Sayan AE, Berx G, et al. Direct repression of cyclin D1 by SIP1 attenuates cell cycle progression in cells undergoing an epithelial mesenchymal transition. Mol Biol Cell. 2007;18:4615–4624. doi: 10.1091/mbc.E07-05-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Sayan AE, Griffiths TR, Pal R, Browne GJ, Ruddick A, Yagci T, et al. SIP1 protein protects cells from DNA damage-induced apoptosis and has independent prognostic value in bladder cancer. Proc Natl Acad Sci USA. 2009;106:14884–14889. doi: 10.1073/pnas.0902042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.van Grunsven LA, Taelman V, Michiels C, Opdecamp K, Huylebroeck D, Bellefroid EJ. deltaEF1 and SIP1 are differentially expressed and have overlapping activities during Xenopus embryogenesis. Dev Dyn. 2006;235:1491–1500. doi: 10.1002/dvdy.20727. [DOI] [PubMed] [Google Scholar]
- 168.Takagi T, Moribe H, Kondoh H, Higashi Y. DeltaEF1, a zinc finger and homeodomain transcription factor, is required for skeleton patterning in multiple lineages. Development. 1998;125:21–31. doi: 10.1242/dev.125.1.21. [DOI] [PubMed] [Google Scholar]
- 169.Funahashi J, Sekido R, Murai K, Kamachi Y, Kondoh H. Delta-crystallin enhancer binding protein delta EF1 is a zinc finger-homeodomain protein implicated in postgastrulation embryogenesis. Development. 1993;119:433–446. doi: 10.1242/dev.119.2.433. [DOI] [PubMed] [Google Scholar]
- 170.Van de Putte T, Francis A, Nelles L, van Grunsven LA, Huylebroeck D. Neural crest-specific removal of Zfhx1b in mouse leads to a wide range of neurocristopathies reminiscent of Mowat-Wilson syndrome. Hum Mol Genet. 2007;16:1423–1436. doi: 10.1093/hmg/ddm093. [DOI] [PubMed] [Google Scholar]
- 171.Higashi Y, Moribe H, Takagi T, Sekido R, Kawakami K, Kikutani H, et al. Impairment of T cell development in deltaEF1 mutant mice. J Exp Med. 1997;185:1467–1479. doi: 10.1084/jem.185.8.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sobrado VR, Moreno-Bueno G, Cubillo E, Holt LJ, Nieto MA, Portillo F, et al. The class I bHLH factors E2-2A and E2-2B regulate EMT. J Cell Sci. 2009;122:1014–1024. doi: 10.1242/jcs.028241. [DOI] [PubMed] [Google Scholar]
- 173.Chen ZF, Behringer RR. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 174.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, et al. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 175.Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, et al. Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell. 2008;14:79–89. doi: 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 176.Hopwood ND, Pluck A, Gurdon JB. A Xenopus mRNA related to Drosophila twist is expressed in response to induction in the mesoderm and the neural crest. Cell. 1989;59:893–903. doi: 10.1016/0092-8674(89)90612-0. [DOI] [PubMed] [Google Scholar]
- 177.Soo K, O'Rourke MP, Khoo PL, Steiner KA, Wong N, Behringer RR, et al. Twist function is required for the morphogenesis of the cephalic neural tube and the differentiation of the cranial neural crest cells in the mouse embryo. Dev Biol. 2002;247:251–270. doi: 10.1006/dbio.2002.0699. [DOI] [PubMed] [Google Scholar]
- 178.Germanguz I, Lev D, Waisman T, Kim CH, Gitelman I. Four twist genes in zebrafish, four expression patterns. Dev Dyn. 2007;236:2615–2626. doi: 10.1002/dvdy.21267. [DOI] [PubMed] [Google Scholar]
- 179.O'Rourke MP, Tam PP. Twist functions in mouse development. Int J Dev Biol. 2002;46:401–413. [PubMed] [Google Scholar]
- 180.Ota MS, Loebel DA, O'Rourke MP, Wong N, Tsoi B, Tam PP. Twist is required for patterning the cranial nerves and maintaining the viability of mesodermal cells. Dev Dyn. 2004;230:216–228. doi: 10.1002/dvdy.20047. [DOI] [PubMed] [Google Scholar]
- 181.Vincentz JW, Barnes RM, Rodgers R, Firulli BA, Conway SJ, Firulli AB. An absence of Twist1 results in aberrant cardiac neural crest morphogenesis. Dev Biol. 2008;320:131–139. doi: 10.1016/j.ydbio.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Desprat N, Supatto W, Pouille PA, Beaurepaire E, Farge E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev Cell. 2008;15:470–477. doi: 10.1016/j.devcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 183.Myatt SS, Lam EW. The emerging roles of forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 2007;7:847–859. doi: 10.1038/nrc2223. [DOI] [PubMed] [Google Scholar]
- 184.Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, et al. Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers. Proc Natl Acad Sci USA. 2007;104:10069–10074. doi: 10.1073/pnas.0703900104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Labosky PA, Kaestner KH. The winged helix transcription factor Hfh2 is expressed in neural crest and spinal cord during mouse development. Mech Dev. 1998;76:185–190. doi: 10.1016/s0925-4773(98)00105-1. [DOI] [PubMed] [Google Scholar]
- 186.Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- 187.Kos R, Reedy MV, Jonhson RL, Erickson CA. The winged-helix transcription factor FoxD3 is important for establishing the neural crest lineage and repressing melanogenesis in avian embryos. Development. 2001;128:1467–1479. doi: 10.1242/dev.128.8.1467. [DOI] [PubMed] [Google Scholar]
- 188.Sasai N, Mizuseki K, Sasai Y. Requirement of FoxD3class signaling for neural crest determination in Xenopus. Development. 2001;128:2525–2536. doi: 10.1242/dev.128.13.2525. [DOI] [PubMed] [Google Scholar]
- 189.Stewart RA, Arduini BL, Berghmans S, George RE, Kanki JP, Henion PD, et al. Zebrafish foxd3 is selectively required for neural crest specification, migration and survival. Dev Biol. 2006;292:174–188. doi: 10.1016/j.ydbio.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 190.Teng L, Mundell NA, Frist AY, Wang Q, Labosky PA. Requirement for Foxd3 in the maintenance of neural crest progenitors. Development. 2008;135:1615–1624. doi: 10.1242/dev.012179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lister JA, Cooper C, Nguyen K, Modrell M, Grant K, Raible DW. Zebrafish Foxd3 is required for development of a subset of neural crest derivatives. Dev Biol. 2006;290:92–104. doi: 10.1016/j.ydbio.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 192.Thomas AJ, Erickson CA. FOXD3 regulates the lineage switch between neural crest-derived glial cells and pigment cells by repressing MITF through a non-canonical mechanism. Development. 2009;136:1849–1858. doi: 10.1242/dev.031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Karafiat V, Dvorakova M, Krejci E, Kralova J, Pajer P, Snajdr P, et al. Transcription factor c-Myb is involved in the regulation of the epithelial-mesenchymal transition in the avian neural crest. Cell Mol Life Sci. 2005;62:2516–2525. doi: 10.1007/s00018-005-5297-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Betancur P, Bronner-Fraser M, Sauka-Spengler T. Genomic code for Sox10 activation reveals a key regulatory enhancer for cranial neural crest. Proc Natl Acad Sci USA. 2010;107:3570–3575. doi: 10.1073/pnas.0906596107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rosen GD, Barks JL, Iademarco MF, Fisher RJ, Dean DC. An intricate arrangement of binding sites for the Ets family of transcription factors regulates activity of the α4 integrin gene promoter. J Biol Chem. 1994;269:15652–15660. [PubMed] [Google Scholar]
- 196.Rosen GD, Birkenmeier TM, Dean DC. Characterization of the α4 integrin gene promoter. Proc Natl Acad Sci USA. 1991;88:4094–4098. doi: 10.1073/pnas.88.10.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Martinsen BJ, Bronner-Fraser M. Neural crest specification regulated by the helix-loop-helix repressor Id2. Science. 1998;281:988–991. doi: 10.1126/science.281.5379.988. [DOI] [PubMed] [Google Scholar]
- 198.Kee Y, Bronner-Fraser M. Id4 expression and its relationship to other Id genes during avian embryonic development. Mech Dev. 2001;109:341–345. doi: 10.1016/s0925-4773(01)00576-7. [DOI] [PubMed] [Google Scholar]
- 199.Kee Y, Bronner-Fraser M. Temporally and spatially restricted expression of the helix-loop-helix transcriptional regulator Id1 during avian embryogenesis. Mech Dev. 2001;109:331–335. doi: 10.1016/s0925-4773(01)00574-3. [DOI] [PubMed] [Google Scholar]
- 200.Kee Y, Bronner-Fraser M. To proliferate or to die: role of Id3 in cell cycle progression and survival of neural crest progenitors. Genes Dev. 2005;19:744–755. doi: 10.1101/gad.1257405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Light W, Vernon AE, Lasorella A, Iavarone A, LaBonne C. Xenopus Id3 is required downstream of Myc for the formation of multipotent neural crest progenitor cells. Development. 2005;132:1831–1841. doi: 10.1242/dev.01734. [DOI] [PubMed] [Google Scholar]
- 202.Bagheri-Fam S, Barrionuevo F, Dohrmann U, Gunther T, Schule R, Kemler R, et al. Long-range upstream and downstream enhancers control distinct subsets of the complex spatiotemporal Sox9 expression pattern. Dev Biol. 2006;291:382–397. doi: 10.1016/j.ydbio.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 203.Vallin J, Thuret R, Giacomello E, Faraldo MM, Thiery JP, Broders F. Cloning and characterization of three Xenopus slug promoters reveal direct regulation by Lef/beta-catenin signaling. J Biol Chem. 2001;276:30350–30358. doi: 10.1074/jbc.M103167200. [DOI] [PubMed] [Google Scholar]
- 204.Bajpai R, Chen DA, Rada-Iglesias A, Zhang J, Xiong Y, Helms J, et al. CHD7 cooperates with PBAF to control multipotent neural crest formation. Nature. 2010;463:958–962. doi: 10.1038/nature08733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Steventon B, Araya C, Linker C, Kuriyama S, Mayor R. Differential requirements of BMP and Wnt signalling during gastrulation and neurulation define two steps in neural crest induction. Development. 2009;136:771–779. doi: 10.1242/dev.029017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Raible DW, Ragland JW. Reiterated Wnt and BMP signals in neural crest development. Semin Cell Dev Biol. 2005;16:673–682. doi: 10.1016/j.semcdb.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 207.Liem KF, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 208.Delannet M, Duband J-L. Transforming growth factor-b control of cell-substratum adhesion during avian neural crest cell emigration in vitro. Development. 1992;116:275–287. doi: 10.1242/dev.116.1.275. [DOI] [PubMed] [Google Scholar]
- 209.Sela-Donenfeld D, Kalcheim C. Inhibition of Noggin expression in the dorsal neural tube by somitogenesis: a mechanism for coordinating the timing of neural crest emigration. Development. 2000;127:4845–4854. doi: 10.1242/dev.127.22.4845. [DOI] [PubMed] [Google Scholar]
- 210.Anderson RM, Stottmann RW, Choi M, Klingensmith J. Endogenous bone morphogenetic protein antagonists regulate mammalian neural crest generation and survival. Dev Dyn. 2006;235:2507–2520. doi: 10.1002/dvdy.20891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kanzler B, Foreman RK, Labosky PA, Mallo M. BMP signaling is essential for development of skeletogenic and neurogenic cranial neural crest. Development. 2000;127:1095–1104. doi: 10.1242/dev.127.5.1095. [DOI] [PubMed] [Google Scholar]
- 212.Burstyn-Cohen T, Stanleigh J, Sela-Donenfeld D, Kalcheim C. Canonical Wnt activity regulates trunk neural crest delamination linking BMP/noggin signaling with G1/S transition. Development. 2004;131:5327–5339. doi: 10.1242/dev.01424. [DOI] [PubMed] [Google Scholar]
- 213.Ahlstrom JD, Erickson CA. The neural crest epithelialmesenchymal transition in 4D: a ‘tail’ of multiple non-obligatory cellular mechanisms. Development. 2009;136:1801–1812. doi: 10.1242/dev.034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Cauthen CA, Berdougo E, Sandler J, Burrus LW. Comparative analysis of the expression patterns of Wnts and Frizzleds during early myogenesis in chick embryos. Mech Develop. 2001;104:133–138. doi: 10.1016/s0925-4773(01)00369-0. [DOI] [PubMed] [Google Scholar]
- 215.Ayer-Leliívre CS, Le Douarin NM. The early development of cranial sensory ganglia and the potentialities of their component cells studied in quail-chick chimeras. Develop Biol. 1982;94:291–310. doi: 10.1016/0012-1606(82)90349-9. [DOI] [PubMed] [Google Scholar]
- 216.Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- 217.Graham A, Blentic A, Duque S, Begbie J. Delamination of cells from neurogenic placodes does not involve an epithelial-to-mesenchymal transition. Development. 2007;134:4141–4145. doi: 10.1242/dev.02886. [DOI] [PubMed] [Google Scholar]
- 218.Shiau CE, Bronner-Fraser M. N-cadherin acts in concert with Slit1-Robo2 signaling in regulating aggregation of placode-derived cranial sensory neurons. Development. 2009;136:4155–4164. doi: 10.1242/dev.034355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Weston JA, Yoshida H, Robinson V, Nishikawa S, Fraser ST. Neural crest and the origin of ectomesenchyme: neural fold heterogeneity suggests an alternative hypothesis. Dev Dyn. 2004;229:118–130. doi: 10.1002/dvdy.10478. [DOI] [PubMed] [Google Scholar]
- 220.Breau MA, Pietri T, Stemmler MP, Thiery JP, Weston JA. A nonneural epithelial domain of embryonic cranial neural folds gives rise to ectomesenchyme. Proc Natl Acad Sci USA. 2008;105:7750–7755. doi: 10.1073/pnas.0711344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Testaz S, Jarov A, Williams KP, LE L, Koteliansky VE, Fournier-thibault C, et al. Sonic hedgehog restricts adhesion and migration of neural crest cells independently of the Patched-Smoothened-Gli signaling pathway. Proc Natl Acad Sci USA. 2001;98:12521–12526. doi: 10.1073/pnas.221108698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fournier-Thibault C, Blavet C, Jarov A, Bajanca F, Thorsteinsdottir S, Duband JL. Sonic hedgehog regulates integrin activity, cadherin contacts, and cell polarity to orchestrate neural tube morphogenesis. J Neurosci. 2009;29:12506–12520. doi: 10.1523/JNEUROSCI.2003-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.McKeown SJ, Lee VM, Bronner-Fraser M, Newgreen DF, Farlie PG. Sox10 overexpression induces neural crest-like cells from all dorsoventral levels of the neural tube but inhibits differentiation. Dev Dyn. 2005;233:430–444. doi: 10.1002/dvdy.20341. [DOI] [PubMed] [Google Scholar]
- 224.Carmona-Fontaine C, Acuna G, Ellwanger K, Niehrs C, Mayor R. Neural crests are actively precluded from the anterior neural fold by a novel inhibitory mechanism dependent on Dickkopf1 secreted by the prechordal mesoderm. Dev Biol. 2007;309:208–221. doi: 10.1016/j.ydbio.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 225.Nieto MA, Sechrist J, Wilkinson DG, Bronner-Fraser M. Relationship between spatially-restricted Krox-20 gene expression in branchial neural crest and segmentation in the chick hindbrain. EMBO J. 1995;14:1697–710. doi: 10.1002/j.1460-2075.1995.tb07159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Sechrist J, Scherson T, Bronner-Fraser M. Rhombomere rotation reveals that multiple mechanisms contribute to the segmental pattern of hindbrain neural crest migration. Development. 1994;120:1777–1790. doi: 10.1242/dev.120.7.1777. [DOI] [PubMed] [Google Scholar]
- 227.Trainor PA, Sobieszczuk D, Wilkinson D, Krumlauf R. Signalling between the hindbrain and paraxial tissues dictates neural crest migration pathways. Development. 2002;129:433–442. doi: 10.1242/dev.129.2.433. [DOI] [PubMed] [Google Scholar]
- 228.Graham A, Francis-West P, Brickell P, Lumsden A. The signalling molecule BMP4 mediates apoptosis in the rhombencephalic neural crest. Nature. 1994;372:684–686. doi: 10.1038/372684a0. [DOI] [PubMed] [Google Scholar]
- 229.Smith A, Robinson V, Patel K, Wilkinson DG. The EphA4 and EphB1 receptor tyrosine kinases and ephrin-B2 ligand regulate targeted migration of branchial neural crest cells. Curr Biol. 1997;7:561–570. doi: 10.1016/s0960-9822(06)00255-7. [DOI] [PubMed] [Google Scholar]
- 230.Osorio L, Teillet MA, Catala M. Role of noggin as an upstream signal in the lack of neuronal derivatives found in the avian caudal-most neural crest. Development. 2009;136:1717–1726. doi: 10.1242/dev.028373. [DOI] [PubMed] [Google Scholar]
- 231.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–715. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Le Douarin NM, Creuzet S, Couly G, Dupin E. Neural crest cell plasticity and its limits. Development. 2004;131:4637–4650. doi: 10.1242/dev.01350. [DOI] [PubMed] [Google Scholar]
- 233.Wilson YM, Richards KL, Ford-Perriss ML, Panthier JJ, Murphy M. Neural crest cell lineage segregation in the mouse neural tube. Development. 2004;131:6153–6162. doi: 10.1242/dev.01533. [DOI] [PubMed] [Google Scholar]
- 234.Lo L, Dormand EL, Anderson DJ. Late-emigrating neural crest cells in the roof plate are restricted to a sensory fate by GDF7. Proc Natl Acad Sci USA. 2005;102:7192–7197. doi: 10.1073/pnas.0502581102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Wakamatsu Y, Endo Y, Osumi N, Weston JA. Multiple roles of Sox2, an HMG-box transcription factor in avian neural crest development. Dev Dyn. 2004;229:74–86. doi: 10.1002/dvdy.10498. [DOI] [PubMed] [Google Scholar]
- 236.Hanna LA, Foreman RK, Tarasenko IA, Kessler DS, Labosky PA. Requirement for Foxd3 in maintaining pluripotent cells of the early mouse embryo. Gen Dev. 2002;16:2650–2661. doi: 10.1101/gad.1020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Domogatskaya A, Rodin S, Boutaud A, Tryggvason K. Laminin-511 but not -332, -111 or -411 enables mouse embryonic stem cell self-renewal in vitro. Stem Cells. 2008;26:2800–2809. doi: 10.1634/stemcells.2007-0389. [DOI] [PubMed] [Google Scholar]
- 238.Coles EG, Gammill LS, Miner JH, Bronner-Fraser M. Abnormalities in neural crest cell migration in laminin a5 mutant mice. Develop Biol. 2006;289:218–228. doi: 10.1016/j.ydbio.2005.10.031. [DOI] [PubMed] [Google Scholar]