Abstract
This study investigated whether Terminalia catappa L. hydrophilic extract (TCLW) prevents photoaging in human dermal fibroblasts after exposure to UVB radiation. TCLW exhibited DPPH free radical scavenging activity and protected erythrocytes against AAPH-induced hemolysis. In the gelatin digestion assay, the rates of collagenase inhibition by TCL methanol extract, TCLW, and its hydrolysates were greater than 100% at the concentration of 1 mg/mL. We found that serial dilutions of TCLW (10–500 μg/mL) inhibited collagenase activity in a dose-dependent manner (82.3% to 101.0%). However, TCLW did not significantly inhibit elastase activity. In addition, TCLW inhibited MMP-1 and MMP-9 protein expression at a concentration of 25 μg/mL and inhibited MMP-3 protein expression at a concentration of 50 μg/mL. TCLW also promoted the protein expression of type I procollagen. We also found that TCLW attenuated the expression of MMP-1, -3, and -9 by inhibiting the phosphorylation of ERK, JNK, and p38. These findings suggest that TCLW increases the production of type I procollagen by inhibiting the activity of MMP-1, -3 and -9, and, therefore, has potential use in anti-aging cosmetics.
1. Introduction
The leaves of Terminalia catappa L. (Combretaceae) are commonly used as folk medicine in Southeast Asia to treat dermatitis and hepatitis [1]. Extracts of the leaves and bark of the plant have been reported to have chemopreventive [2, 3], antioxidant, and superoxide radical scavenger effects [4], anti-HIV reverse transcriptase activity [5], and hepatoprotective [4] and anti-inflammatory effects [6]. Terminalia catappa extract has also been shown to decrease the protein expression of MMP-2 and MMP-9 in lung cancer cells [3]. The phytochemicals in this plant include tannins (punicalagin, punicalin, terflavins A and B, chebulagic acid), flavanoids (isovitexin, vitexin, isoorientin, rutin) and triterpenoids (ursolic acid and asiatic acid) [7–9]. The hepatoprotective activity of Terminalia catappa extract has been shown to be due primarily to the superoxide anion and hydroxyl radical scavenging activity of ursolic acid and asiatic acid [10].
Wrinkles, laxity, and hyperpigmentation characterize aging [11]. Skin aging can be divided into two basic processes, intrinsic, and extrinsic aging. Extrinsic aging is generally referred to as photoaging due to chronic exposure to short wavelength UV light (UVB) and is characterized by severe wrinkling and pigmentary changes, such as solar lentigo and mottled pigmentation on exposed areas such as the face, neck, and forearm. The most abundant structural protein in skin connective tissue is type I collagen, which is synthesized primarily by fibroblasts and is responsible for conferring strength and resiliency [12]. Ultraviolet- (UV-) induced skin damage principally manifests as degradation of extracellular matrix (ECM) proteins, including type I collagen, elastin, proteoglycans, and fibronectin [13, 14]. It has been shown that UV irradiation leads to the formation of reactive oxygen species (ROS) that activate the mitogen-activated protein (MAP) kinase pathway, which subsequently induces the expression and activation of matrix metalloproteinases (MMPs) in human skin in vivo [15, 16]. MMPs are known to be upexpressed in human fibroblasts within hours after exposure to UV irradiation and are, therefore, considered key factors in the photoaging process. Therefore, agents with the ability to elevate ECM protein levels or inhibit the major collagen-degrading enzymes like MMPs would prove to be useful in the development of effective anti-aging agents.
This study investigated the effects of Terminalia catappa extract and its hydrolysates on protein expression of MMPs, elastase, and type I procollagen in human dermal fibroblasts after exposure to UVB and investigated the mechanism by which the extract protects against photodamage.
2. Material and Methods
2.1. Chemicals
Leaves of Terminalia catappa L. were harvested in Taichung County, Taiwan. Human foreskin fibroblasts were obtained from the Bioresource Collection and Research Center (Hsinchu, Taiwan). Bradford Reagent was supplied by Bio-Rad Laboratories (CA, USA). Elastase substrate IV and porcine elastase were purchased from Calbiochem (San Diego, CA, USA). Coomassie blue R-250, dibasic sodium phosphate, Igepal CA-630, tris and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from USB (Cleveland, OH, USA). Collagenase was purchased from Calbiochem, Merck (Darmstadt, Germany). Fetal bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA, and Dulbecco's Modified Eagle's Medium (DMEM) were purchased from Gibco, Invitrogen (Carlsbad, CA, USA). Fluorogenic Peptide Substrate I was purchased from R&D systems (Wiesbaden, Germany). Gelatin, agarose, hydrochloric acid, methanol, dimethyl sulfoxide (DMSO), propylene glycol (PG), doxycycline hyclate, calcium chloride (CaCl2), 1,1-diphenyl-2-picrylhydrazy (DPPH) and 2,2′-azobis (2-methylpropionamidine) dihydrochloride (AAPH), and DL-dithiothreitol were purchased from Sigma-Aldrich Chemicals (St. Louis, MO, USA).
2.2. Preparation of Terminalia catappa L. Extract and Its Hydrolysates
The dried leaves were ground and then extracted twice with a 30-fold volume of methanol for 1 h by ultrasonication. The supernatant was filtered and then evaporated to dryness in vaucum to obtain methanol extract of Terminalia catappa L. (TCLM). The hydrophilic extract of Terminalia catappa L. (TCLW) was prepared in the same manner as TCLM except that the solvent was replaced with water. Acid hydrolysis of TCLW was carried out at 80°C in the presence of 1.2 N HCl (2 mL) for 30 min and 2.4 N HCl (2 mL) for 60 min. After hydrolysis, the solution was partitioned with ethyl acetate (EA). The EA layer was evaporated to dryness in vaucum to obtain TCLW hydrolysate (TCLWH). The abbreviations used for and the hydrolytic conditions of TCLWH are as follows: TCLWH1, 0.6 N HCl for 0.5 h; TCLWH2, 1.2 N HCl for 0.5 h; TCLWH3, 0.6 N HCl for 1 h; TCLWH4, 1.2 N HCl for 1 h. The TCLW extract and TCLW hydrolysates were stored at −20°C before use.
2.3. Total Phenolic Content of TCLW
Total phenolic content of TCLW was determined by the Folin-Ciocalteu reaction. TCLW was mixed with 2-fold 10% Folin-Ciocalteu phenol reagent. The mixture was allowed to stand at room temperature for 5 min and then sodium carbonate (700 mM) was added to the mixture. The resulting blue complex was then measured at 760 nm. Gallic acid was used as a standard for the calibration curve. The phenolic compound contents were calibrated using the linear equation based on the calibration curve. The contents of phenolic compounds are expressed as μg gallic acid equivalent/mg TCL dry weight.
2.4. DPPH Radical Scavenging Activity of TCLW
Reaction mixtures containing 200 μM DPPH (100 μL) and serial dilutions of sample (concentration of sample ranging from 25 to 1000 μg/mL) were placed in a 96-well microplate at room temperature in the dark for 30 min. After incubation, the absorbance was read at 517 nm by an ELISA reader (Tecan, Grodig, Austria). Ascorbic acid was used as the positive control. Scavenging activity was determined by the following equation:
| (1) |
2.5. Preparation of Erythrocyte Suspensions and Hemolysis Assay
Whole blood was obtained from male SD rats via cardiopuncture and collected in an EDTA-containing tube. The erythrocytes were isolated by centrifugation at 3000 × g for 10 min, washed four times with PBS, and then resuspended to the desired hematocrit level using the same buffer. In order to induce free radical chain oxidation in the erythrocytes, aqueous peroxyl radicals were generated by thermal decomposition of AAPH in oxygen. An erythrocyte suspension at 5% hematocrit was incubated with PBS (control) or preincubated with TCLW (10–50 μg/mL) at 37°C for 30 min, followed by incubation with or without 25 mM AAPH in PBS at pH 7.4. This reaction mixture was shaken gently while being incubated for a fixed interval at 37°C. A 200-μL aliquot of the reaction mixture was removed and centrifuged at 3000 ×g for 2 min, with absorbance of the supernatant determined at 540 nm. Reference values were determined using the same volume of erythrocytes in a hypotonic buffer (5 mM phosphate buffer at pH 7.4; 100% hemolysis). The hemolysis percentage was calculated using the formula: [(A sample/A control)] × 100.
2.6. Gelatin Digestion Assay
Agarose solution (1%) was prepared in collagenase buffer (50 mM Tris-HCl, 10 mM CaCl2, 0.15 M NaCl, pH 7.8) with 0.15% porcine gelatin (Sigma Aldrich, Cat. G-2500) and allowed to solidify on plates. Various concentrations of TCLW and TCLWH (10 μL) dissolved in 10% DMSO were incubated with 10 μL of bacterial collagenase-1 (0.1 mg/mL) in 80 μL of collagenase buffer for 1 h at room temperature. Doxycycline hyclate was used as positive control. The samples (40 μL) were loaded onto paper disks placed on gelatin-agarose gel and incubated for 18 h at 37°C. The degree of gelatin digestion in agarose gel was visualized by Coomassie Blue staining after removal of the paper disks. Following destaining, the area of the light translucent zone over blue background was determined by a densitometric program (TINA) to estimate gelatinase activity.
2.7. MMP Activity Assays
Enzyme activity assays were performed in 50 mM tris buffer (pH 7.8), 0.15 M NaCl, and 10 mM CaCl2. Various concentrations of TCLW and TCLWH were tested for their ability to digest a synthetic fluorogenic substrate (a general MMP substrate). Each concentration of TCLW and TCLWH was incubated with 1 μM substrate at 37°C for 20 h. Fluorescence intensity was measured at 320 nm (excitation) and 450 nm (emission) with a fluorescence reader. The rate of collagenase inhibition was calculated by the following equation:
| (2) |
where A indicates the absorbance with enzyme but without sample, B indicates the absorbance without enzyme and sample, C indicates the absorbance with enzyme and sample, and D indicates the absorbance without enzyme but with sample.
2.8. Measurement of Elastase Activity
The elastase inhibition test on TCLW and TCLWH was investigated using elastase derived from porcine pancreas. Elastase (500 U) was dissolved in 5 mL of 10 mM tris buffer solution (pH 6.0) and 5 mg elastase substrate IV was dissolved in 5 mL of 100 mM tris buffer solution (pH 8.0). To measure elastase activity, 100 μL of 100 mM tris buffer solution (pH 8.0), 25 μL of elastase substrate IV solution, 50 μL of sample solution, and 25 μL of elastase solution were dispensed into each well of a 96-well plate and then preincubated for 20 min at room temperature. The elastase activity was quantified by measuring light absorbance at 405 nm using a microplate reader (Tecan, Grodig, Austria). Each assay was carried out in triplicate.
The inhibition rate of elastase was calculated by the following equation:
| (3) |
where A indicates the absorbance with enzyme but without sample, B indicates the absorbance without enzyme and sample, C indicates the absorbance with enzyme and sample, and D indicates the absorbance without enzyme but with sample.
2.9. Cell Culture
Human foreskin fibroblasts (Hs68) were obtained from neonatal foreskins and cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 U/mL streptomycin in a humidified atmosphere of 5% CO2 at 37°C. Hs68 cells were plated at 80%–90% confluence in all experiments.
2.10. MTT Assay for Cell Viability
The fibroblasts were plated at a density of 104 cells/well in a 96-well plate and then treated with various concentrations of extracts dissolved in DMSO (<0.1%) for 24 h. Mitochondrial dehydrogenase activity, which can be used as an index of cell viability, was assessed using the MTT assay as previously described [17]. Viability was quantified by measuring the absorbance at 570 nm using a microplate reader (Tecan, Grodig, Austria).
2.11. UV Irradiation
Cells were cultured until 80% confluent, washed twice with phosphate-buffered saline (PBS), and then exposed to UVB irradiation in PBS (302 nm, CL-1000M, UVP, USA). In our previous study, the dose of 80 mJ/cm2 UVB irradiation was determined to induce MMP without being cytotoxic (data not shown). Subsequently, cells were incubated for 24 h in 37°C in a humidified atmosphere of 5% CO2 in serum-free DMEM containing various concentrations of TCLW and TCLWH.
2.12. Western Blotting Analysis
Western blotting assay was performed using whole cell lysates prepared from Hs68 cells at a density of 5 × 105 cells. Cells were harvested and homogenized with lysis buffer containing 10 mM Na3VO4, 10 mg/mL leupeptin, 10 mg/mL PMSF and RIPA, and then the lysates were subjected to centrifugation at 12000 ×g for 10 min. All reactions were performed in triplicate. Protein concentration in the culture medium was measured using Bradford reagent (Bio-Rad, Hercules, CA, USA) with bovine serum albumin as the standard. Cell lysates containing equal amounts of total protein were separated by electrophoresis on SDS-polyacrylamide gel and then transferred to a PVDF membrane (Hybond ECL, Amersham Pharmacia Biotech Inc., Piscataway, NJ, USA). Nonspecific binding was blocked with nonfat milk in TBST ((10 mM Tris-HCl, pH 7.5, 150 mM NaCl) containing 0.05% Tween 20). The membrane was incubated with goat polyclonal antibodies against MMP-1 (1 : 500) and type I procollagen (1 : 500), and mouse polyclonal antibodies against MMP-3 (1 : 500), MMP-9 (1 : 500), ERK (1 : 500), JNK (1 : 500), p38 (1 : 500), p-ERK (1 : 500), p-JNK (1 : 500) and p-p38 (1 : 500) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Anti-immunoglobulin G-horseradish peroxidase (Santa Cruz Biotechnology Inc.) was used as the secondary antibody. Immunoreactive proteins were detected with the ECL Western blotting detection system (Fujifilm, LAS-4000, Japan). Signal strengths were quantified using a densitometric program (multi Gauge V2.2).
2.13. Statistical Analysis
Each experiment was performed in triplicate and all data are presented as mean ± SD. Significant differences between groups were analyzed by ANOVA followed by the Scheffe's test. A P value <.05 was considered significant.
3. Results
3.1. Extraction Yield and Total Phenolic Content of TCLW
The extraction yield of TCLW was 22.5% and that of TCLM was 13.5%. The total phenolic content, expressed as μg gallic acid equivalents (GAE) per mg of dry weight (TCL), was 102.0 ± 0.2 μg GAE/mg.
3.2. Scavenging of DPPH Radicals
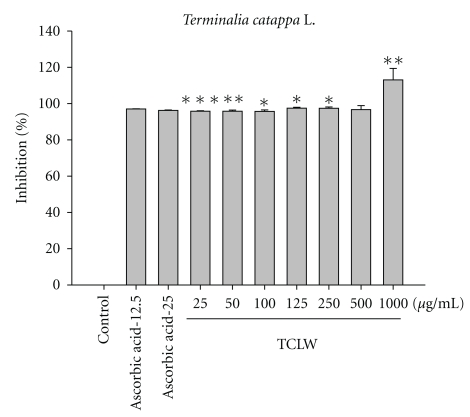
Figure 1 shows the free radical scavenging activity of TCLW (25–1000 μg/mL) and ascorbic acid (25 mg/mL); TCLW exhibited excellent DPPH radical scavenging activity. Our results indicated that DPPH radical scavenging activity of TCLW at 25 μg/mL (95.8 ± 0.3%) was similar to that of ascorbic acid at an equal concentration (96.2 ± 0.2%).
Figure 1.
DPPH radical scavenging activity of TCLW. The TCLW provided a strong inhibitory effect and in a dose-dependent manner (25–1000 μg/mL) on DPPH scavenging. (n = 4; *P < .05; **P < .01; ***P < .001).
3.3. Erythrocyte Hemolysis Assay
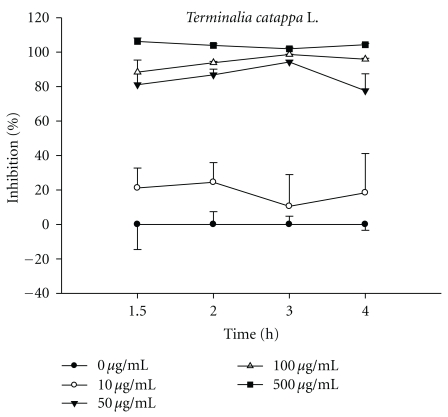
The influence of TCLW (10–500 μg/mL) on in vitro erythrocyte hemolysis was examined by incubating rat erythrocytes in the presence of 25 mM AAPH as an initiator of oxidation. TCLW (50–500 μg/mL) exhibited a strong dose-dependent inhibitory effect against erythrocyte hemolysis (Figure 2).
Figure 2.
The time course inhibition of TCLW on AAPH-induced lysis of rat erythrocyte. The TCLW provided a strong inhibitory effect and in a dose-dependent manner (50–500 μg/mL) against erythrocyte hemolysis.
3.4. TCLW and TCLWH Inhibited Bacterial Collagenase-1
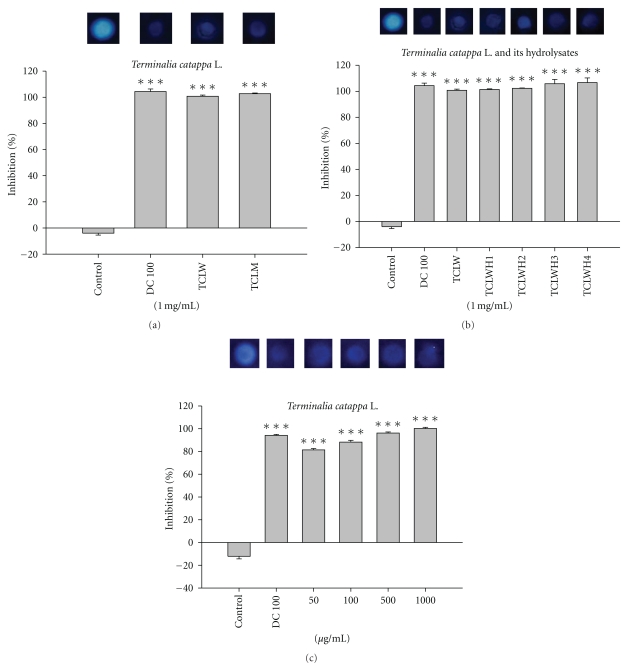
For visual investigation of the inhibitory effect of TCLM, TCLW, and TCLWH on MMPs, an indirect assay was developed using bacterial collagenase-1. Following incubation of bacterial collagenase-1 with various concentrations of TCLW and TCLWH, the inhibition of enzyme activity was compared with enzyme activity of the control. As Figure 3(a) shown, DMSO as the control group reaction with the reagent exhibited the highest gelatinolytic activity in the discrete zone; the doxycycline, a well-known MMP inhibitor, was the positive control resulting clear zone. The collagenase-1 inhibition of TCLW and TCLM was similar to doxycycline. As shown in Figure 3(b), the rates of collagenase-1 inhibition were 94.3 ± 0.8% for doxycycline (100 μg/mL), 100.7 ± 0.9% for TCLW (1000 μg/mL), 101.4 ± 0.5% for TCLWH1, 102.2 ± 0.4% for TCLWH2, 105.8 ± 3.4% for TCLWH3, and 106.7 ± 3.5% for TCLWH4. The rates for collagenase-1 inhibition for various concentrations (50–1000 μg/mL) of TCLW ranged from 81.9 ± 0.7% to 100.7 ± 0.7%. In addition, TCLW inhibited gelatinolytic activity in a dose-dependent manner (Figure 3(c)).
Figure 3.
The inhibition of Terminalia catappa L. water extract, methanol extract, and its hydrolysates on collagenase activity. (a) Terminalia catappa L. water extract (TCLW), and methanol extract (TCLM), (b) Terminalia catappa L. water extract (TCLW) and its hydrolysates 1 mg/mL, and (c) Terminalia catappa L. water extract (TCLW) 0.05–1 mg/mL (H1: hydrolyzed by 0.6 N HCl for 30 min; H2: 1.2 N HCl for 30 min; H3: hydrolyzed by 0.6 N HCl for 60 min; H4: 1.2 N HCl for 60 min) (positive control, DC 100: doxycycline 100 μg/mL; control: dd water) (n = 4; *P < .05; **P < .01; ***P < .001).
3.5. TCLW Inhibited Bacterial Collagenase-1
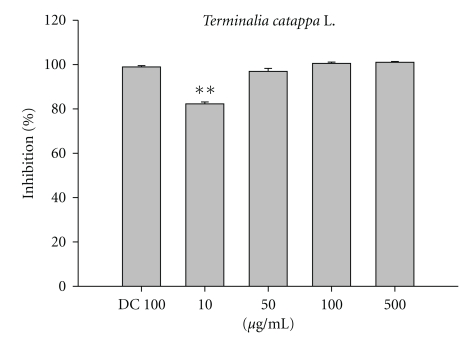
Fluorescence-conjugated gelatin was used to measure the inhibitory effect of TCLW on bacterial collagenase-1 protein expression. Fluorescence-conjugated substrate was incubated with bacterial collagenase-1 for 20 h in the presence of different concentrations of TCLW or doxycycline hyclate (positive control) at 37°C. TCLW exhibited a significant inhibitory effect on bacterial collagenase-1; the inhibition rate was >95% of the control at concentrations ≥50 μg/mL (Figure 4).
Figure 4.
The inhibition rate (%) of TCLW on bacterial collagenase activity using fluorometric assay. (n = 4; **P < .01).
3.6. The Effect of TCLW on Elastase Activity
This assay measures the synthesis and activity of elastase in cells exposed to TCLW. As shown in Figure 5, TCLW (10–500 μg/mL) did not have a significant effect on elastase activity.
Figure 5.
The inhibition rate of TCLW on porcine elastase. (n = 4).
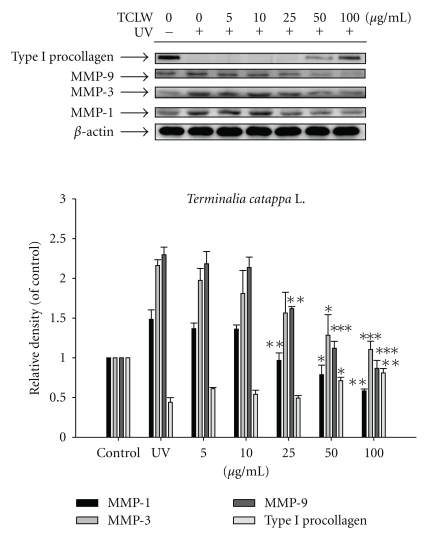
3.7. TCLW Inhibited the Protein Expression of MMPs
UVB irradiation of untreated Hs68 cells resulted in a 1.5-fold increase in MMP-1 expression, a 2.2-fold increase in MMP-3 expression, and a 2.3-fold increase in MMP-9 expression relative to control levels. TCLW treatment (25–100 μg/mL), however, suppressed the UVB-induced upregulation of MMPs (Figure 6). TCLW at concentrations of 25 μg/mL and higher led to a significant decrease in MMP-1 and -9 expression to basal level. TCLW treatment at concentrations of 50 μg/mL and higher significantly inhibited MMP-3 expression (Figure 6).
Figure 6.
Effect of TCLW on the UVB-induced expression of MMP-1, 3, 9, and type I procollagen in human fibroblasts. (n = 4; *P < .05; **P < .01; ***P < .001).
3.8. TCLW Upregulates Type I Procollagen Expression
Fibroblasts were treated with TCLW (5–100 μg/mL) for 24 h after exposure to UVB (80 mJ/cm2). TCLW treatment (≥50 μg/mL) led to a significant increase in the expression of type I procollagen (Figure 6).
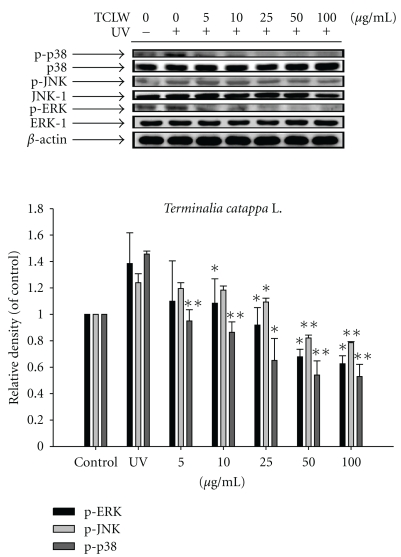
3.9. Effect of TCLW on MAP Kinase Expression
UVB irradiation of untreated Hs68 cells resulted in a 1.4-fold increase in p-ERK expression, a 1.2-fold increase in p-JNK expression, and a 1.5-fold increase in phosphorylated p38 relative to control levels. TCLW treatment (5–100 μg/mL), however, significantly inhibited the UVB-induced overexpression of those MAP kinases. P-ERK was suppressed to the basal level at 10 μg/mL TCLW, p-JNK was suppressed to the basal level at 25 μg/mL TCLW, and phosphorylated p38 was suppressed to the basal level at 5 μg/mL TCLW (Figure 7).
Figure 7.
Effect of TCLW on the UVB-induced expression of MAP kinases in human fibroblasts. (n = 4; *P < .05; **P < .01).
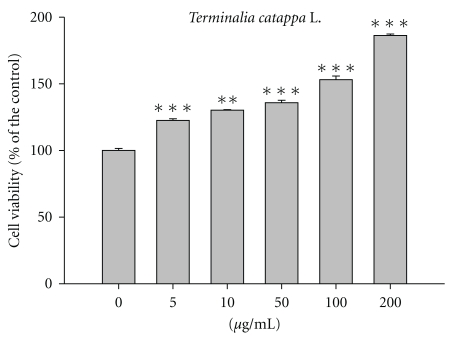
3.10. Effect of TCLW on Cell Viability
Hs68 cells were treated with various concentrations of TCLW (5–200 μg/mL) and cell viability was measured. As shown in Figure 8, TCLW did not exhibit cytotoxic effects on the proliferation of cells (cell viability >90% of control). In addition, TCLW promoted cell proliferation in a dose-dependent manner.
Figure 8.
Cell viability of TCLW on human fibroblasts. (n = 4; **P < .01; ***P < .001).
4. Discussion
Aging is a complex phenomenon that is modulated by multiple factors, including genetics, life style, and exposure to sunlight and pollutants. UV radiation produces ROS, which activate MAPkinase pathway to induce the expression and activation of MMPs that degrade extracellular matrix proteins including type I collagen, elastin, and glycosaminoglycans in skin [18, 19].
It has been shown that Moringa oleifera extract, which has a total phenolic content of 52.5 μg GAE/mg, has potent DPPH scavenging activity [20]. In addition, Fraxinus chinensis extract scavenges UVA-induced DPPH free radicals and inhibits MMP-1 mRNA and protein expression in human skin fibroblasts [21]. Sorbus commixta Hedl, a compound with a high total phenolic content, exhibits free radical scavenging effect and decreases MMP-1 mRNA expression [22]. Terminalia catappa L. leaves are rich in polyphenol antioxidants. In addition, studies have shown that the antioxidant activities of polyphenols reduce the risk of skin diseases [23]. We speculate that TCLW can protect against oxidative stress-induced photodamage because of its high total phenolic content (102 μg GAE/mg), DPPH free radical scavenging activity, as well as its ability to prevent AAPH-induced hemolysis and activate MMP.
UV stimulates collagenase expression, which causes matrix protein degradation and, subsequently, skin photoaging [24, 25]. Collagenase-1 (MMP-1) degrades collagen, gelatin, and proteoglycan link protein, and collagenase-3 (MMP-3) is involved in several MMP activation cascades including activation of MMP-1 [26]. Agents that inhibit collagenase activity would be ideal candidates for the prevention or treatment of photoaging. Botanical extracts have been reported to suppress collagenase activity. For example, Viscum coloratum inhibits collagenase release [27] and Morinda citrifolia inhibits elastase and tyrosinase activities [28]. In addition, xanthorrhizol, a sesquiterpenoid isolated from ethyl acetate extract of Curcuma xanthorrhiza, inhibits UVB-induced MMP-1 expression and increases the level of type I procollagen in human fibroblasts [29]. Erythrodiol-3-acetate from Spiraea japonica stem extract has been shown to upregulate type I procollagen expression in fibroblasts after exposure to UVB irradiation [30]. Furthermore, Magnolia obovata extract (150 μg/mL) and Polypodium leucotomos (50 μg/mL) have been reported to attenuate MMP-1 expression [31, 32]. We found that TCLW (25 μg/mL) inhibited MMP-1, -3 and -9 expression, a finding consistent with that reported by Yang et al. [33].
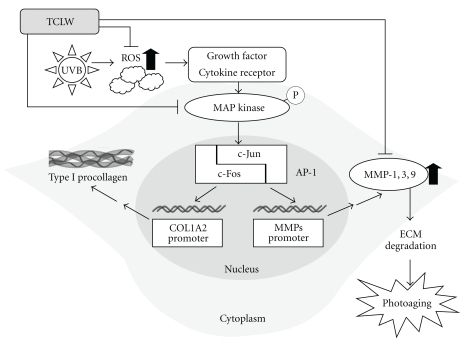
MAP kinases are upstream regulators of MMP activity [34–36]. It has been reported that green tea polyphenol, epigallocatechin-3-gallate (EGCG) inhibits the expression of MMPs by attenuating the phosphorylation of ASK-1 and MAPkinase pathway [37]. In addition, treatment of UV-irradiated cells with 4-hydroxypanduratin A, isolated from Kaempferia pandurata Roxb, leads to decreased MMP-1 expression by inhibiting c-Fos and c-Jun phosphorylation and MAP kinase pathway activation [38]. In this study, TCLW significantly inhibited UVB-induced p-ERK, p-JNK, and phosphorylated p38 expression at doses of 10, 25, and 5 μg/mL, respectively. It has been shown that UV irradiation induces p38 activation [34]. Furthermore, it has been reported that UVB irradiation induces ERK, JNK, and p38 activation via production of ROS [39, 40]. Green tea polyphenol, EGCG, suppresses UV-induced oxygen stress-mediated MAP kinase phosphorylation pathway [41]. The flavonoid, fisetin inhibits UV-induced MAP kinase expression and NF-κB signal transduction [42, 43]. In our study, TCLW exhibited potent antioxidant activity, inhibited the expression of MAP kinases and MMPs, and activated type I procollagen. Those findings indicate that TCLW is a potential agent for the treatment or prevention of photodamage. TCLW hampered the activation of MAPKs, and, therefore, may downregulate the down stream proteins c-Fos and c-Jun, as well as the transcription factor AP-1 and protooncogenes involved in photodamage (Figure 9). The effects of TCLW on transcription of c-Fos, c-Jun, and AP-1 need further study.
Figure 9.
The mechanisms of TCLW on UVB-induced photodamage.
5. Conclusions
TCLW inhibits UVB-induced ROS, phosphorylation of p38, JNK, and ERK, and attenuates the expression of MMP-1, -3, -9, thereby elevating type I procollagen synthesis. TCLW, therefore, could be a potential antiphotodamage agent.
Acknowledgments
This study was partly sponsored by the China Medical University (CMU96-142, CMU97-270), Taichung and the National Science Council (NSC97-2320-B-039-040-MY3 and NSC98-2622-B-039-002-CC3), Taipei, Taiwan.
References
- 1.Lin C-C, Chen Y-L, Lin JM, Ujiie T. Evaluation of the antioxidant and hepatoprotective activity of Terminalia catappa . American Journal of Chinese Medicine. 1997;25(2):153–161. doi: 10.1142/S0192415X97000172. [DOI] [PubMed] [Google Scholar]
- 2.Chen P-S, Li J-H. Chemopreventive effect of punicalagin, a novel tannin component isolated from Terminalia catappa, on H-ras-transformed NIH3T3 cells. Toxicology Letters. 2006;163(1):44–53. doi: 10.1016/j.toxlet.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 3.Chu S-C, Yang S-F, Liu S-J, Kuo W-H, Chang Y-Z, Hsieh Y-S. In vitro and in vivo antimetastatic effects of Terminalia catappa L. leaves on lung cancer cells. Food and Chemical Toxicology. 2007;45(7):1194–1201. doi: 10.1016/j.fct.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Kinoshita S, Inoue Y, Nakama S, Ichiba T, Aniya Y. Antioxidant and hepatoprotective actions of medicinal herb, Terminalia catappa L. from Okinawa Island and its tannin corilagin. Phytomedicine. 2007;14(11):755–762. doi: 10.1016/j.phymed.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Tan GT, Pezzuto JM, Kinghorn AD, Hughes SH. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. Journal of Natural Products. 1991;54(1):143–154. doi: 10.1021/np50073a012. [DOI] [PubMed] [Google Scholar]
- 6.Lin C-C, Hsu Y-F, Lin T-C. Effects of punicalagin and punicalin on carrageenan-induced inflammation in rats. American Journal of Chinese Medicine. 1999;27(3-4):371–376. doi: 10.1142/S0192415X99000422. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y-L, Kuo Y-H, Shiao M-S, Chen C-C, Ou J-C. Flavonoid glycosides from Terminalia catappa L. Journal of the Chinese Chemical Society. 2000;47(1):253–256. [Google Scholar]
- 8.Mau J-L, Ko P-T, Chyau C-C. Aroma characterization and antioxidant activity of supercritical carbon dioxide extracts from Terminalia catappa leaves. Food Research International. 2003;36(1):97–104. [Google Scholar]
- 9.Fan YM, Xu LZ, Gao J, et al. Phytochemical and antiinflammatory studies on Terminalia catappa . Fitoterapia. 2004;75(3-4):253–260. doi: 10.1016/j.fitote.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 10.Gao J, Tang X, Dou H, Fan Y, Zhao X, Xu Q. Hepatoprotective activity of Terminalia catappa L. leaves and its two triterpenoids. Journal of Pharmacy and Pharmacology. 2004;56(11):1449–1455. doi: 10.1211/0022357044733. [DOI] [PubMed] [Google Scholar]
- 11.Jenkins G. Molecular mechanisms of skin ageing. Mechanisms of Ageing and Development. 2002;123(7):801–810. doi: 10.1016/s0047-6374(01)00425-0. [DOI] [PubMed] [Google Scholar]
- 12.Gelse K, Pöschl E, Aigner T. Collagens—structure, function, and biosynthesis. Advanced Drug Delivery Reviews. 2003;55(12):1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Fisher GJ, Wang Z, Datta SC, Varani J, Kang S, Voorhees JJ. Pathophysiology of premature skin aging induced by ultraviolet light. The New England Journal of Medicine. 1997;337(20):1419–1428. doi: 10.1056/NEJM199711133372003. [DOI] [PubMed] [Google Scholar]
- 14.Rittié L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Research Reviews. 2002;1(4):705–720. doi: 10.1016/s1568-1637(02)00024-7. [DOI] [PubMed] [Google Scholar]
- 15.Fisher GJ, Datta SC, Talwar HS, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996;379(6563):335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 16.Shin JY, Hur W, Wang JS, et al. HCV core protein promotes liver fibrogenesis via up-regulation of CTGF with TGF-β1. Experimental and Molecular Medicine. 2005;37(2):138–145. doi: 10.1038/emm.2005.19. [DOI] [PubMed] [Google Scholar]
- 17.Wen K-C, Lin S-P, Yu C-P, Chiang H-M. Comparison of puerariae radix and its hydrolysate on stimulation of hyaluronic acid production in NHEK cells. American Journal of Chinese Medicine. 2010;38(1):143–155. doi: 10.1142/S0192415X10007725. [DOI] [PubMed] [Google Scholar]
- 18.Fisher GJ, Talwar HS, Lin J, Voorhees JJ. Molecular Mechanisms of Photoaging in Human Skin in Vivo and Their Prevention by All-Trans Retinoic Acid. Photochemistry and Photobiology. 1999;69(2):154–157. doi: 10.1562/0031-8655(1999)069<0154:mmopih>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 19.Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Seminars in Cancer Biology. 2001;11(2):143–152. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 20.Siddhuraju P, Becker K. Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. Journal of Agricultural and Food Chemistry. 2003;51(8):2144–2155. doi: 10.1021/jf020444+. [DOI] [PubMed] [Google Scholar]
- 21.Lee B-C, Lee SY, Lee HJ, et al. Anti-oxidative and photo-protective effects of coumarins isolated from Fraxinus chinensis . Archives of Pharmacal Research. 2007;30(10):1293–1301. doi: 10.1007/BF02980270. [DOI] [PubMed] [Google Scholar]
- 22.Bae J-T, Sim G-S, Kim J-H, Pyo H-B, Yun J-W, Lee B-C. Antioxidative activity of the hydrolytic enzyme treated Sorbus commixta Hedl. and its inhibitory effect on matrix metalloproteinase-1 in UV irradiated human dermal fibroblasts. Archives of Pharmacal Research. 2007;30(9):1116–1123. doi: 10.1007/BF02980246. [DOI] [PubMed] [Google Scholar]
- 23.Meeran SM, Akhtar S, Katiyar SK. Inhibition of UVB-induced skin tumor development by drinking green tea polyphenols is mediated through DNA repair and subsequent inhibition of inflammation. Journal of Investigative Dermatology. 2009;129(5):1258–1270. doi: 10.1038/jid.2008.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee Y-R, Noh E-M, Kwon K-B, et al. Radix clematidis extract inhibits UVB-induced MMP expression by suppressing the NF-κB pathway in human dermal fibroblasts. International Journal of Molecular Medicine. 2009;23(5):679–684. doi: 10.3892/ijmm_00000180. [DOI] [PubMed] [Google Scholar]
- 25.Yang B, Ji C, Kang J, Chen W, Bi Z, Wan Y. Trans-zeatin inhibits UVB-induced matrix metalloproteinase-1 expression via MAP kinase signaling in human skin fibroblasts. International Journal of Molecular Medicine. 2009;23(4):555–560. doi: 10.3892/ijmm_00000164. [DOI] [PubMed] [Google Scholar]
- 26.Mandal M, Mandal A, Das S, Chakraborti T, Chakraborti S. Clinical implications of matrix metalloproteinases. Molecular and Cellular Biochemistry. 2003;252(1-2):305–329. doi: 10.1023/a:1025526424637. [DOI] [PubMed] [Google Scholar]
- 27.Hwang T-L, Leu Y-L, Kao S-H, Tang M-C, Chang H-L. Viscolin, a new chalcone from Viscum coloratum, inhibits human neutrophil superoxide anion and elastase release via a cAMP-dependent pathway. Free Radical Biology and Medicine. 2006;41(9):1433–1441. doi: 10.1016/j.freeradbiomed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Masuda M, Murata K, Fukuhama A, et al. Inhibitory effects of constituents of Morinda citrifolia seeds on elastase and tyrosinase. Journal of Natural Medicines. 2009;63(3):267–273. doi: 10.1007/s11418-009-0328-6. [DOI] [PubMed] [Google Scholar]
- 29.Oh H-I, Shim J-S, Gwon S-H, Kwon H-J, Hwang J-K. The effect of xanthorrhizol on the expression of matrix metalloproteinase-1 and type-I procollagen in ultraviolet-irradiated human skin fibroblasts. Phytotherapy Research. 2009;23(9):1299–1302. doi: 10.1002/ptr.2768. [DOI] [PubMed] [Google Scholar]
- 30.Moon H-I, Lee J, Chung JH. The effect of erythrodiol-3-acetate on the expressions of matrix metalloproteinase-1 and type-1 procollagen caused by ultraviolet irradiated cultured primary old aged human skin fibroblasts. Phytomedicine. 2006;13(9-10):707–711. doi: 10.1016/j.phymed.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 31.Tanaka K, Hasegawa J, Asamitsu K, Okamoto T. Magnolia ovovata extract and its active component magnolol prevent skin photoaging via inhibition of nuclear factor κB. European Journal of Pharmacology. 2007;565(1-3):212–219. doi: 10.1016/j.ejphar.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 32.Philips N, Conte J, Chen Y-J, et al. Beneficial regulation of matrixmetalloproteinases and their inhibitors, fibrillar collagens and transforming growth factor-β by Polypodium leucotomos, directly or in dermal fibroblasts, ultraviolet radiated fibroblasts, and melanoma cells. Archives of Dermatological Research. 2009;301(7):487–495. doi: 10.1007/s00403-009-0950-x. [DOI] [PubMed] [Google Scholar]
- 33.Yang S-F, Chen M-K, Hsieh Y-S, et al. Antimetastatic effects of Terminalia catappa L. on oral cancer via a down-regulation of metastasis-associated proteases. Food and Chemical Toxicology. 2010;48(4):1052–1058. doi: 10.1016/j.fct.2010.01.019. [DOI] [PubMed] [Google Scholar]
- 34.Jinlian L, Yingbin Z, Chunbo W. p38 MAPK in regulating cellular responses to ultraviolet radiation. Journal of Biomedical Science. 2007;14(3):303–312. doi: 10.1007/s11373-007-9148-4. [DOI] [PubMed] [Google Scholar]
- 35.Shim J-S, Kwon Y-Y, Han Y-S, Hwang J-K. Inhibitory effect of panduratin A on UV-induced activation of mitogen-activated protein kinases (MAPKs) in dermal fibroblast cells. Planta Medica. 2008;74(12):1446–1450. doi: 10.1055/s-2008-1081352. [DOI] [PubMed] [Google Scholar]
- 36.Yang B, Ji C, Kang J, Chen W, Bi Z, Wan Y. Trans-zeatin inhibits UVB-induced matrix metalloproteinase-1 expression via MAP kinase signaling in human skin fibroblasts. International Journal of Molecular Medicine. 2009;23(4):555–560. doi: 10.3892/ijmm_00000164. [DOI] [PubMed] [Google Scholar]
- 37.Bae J-Y, Choi J-S, Choi Y-J, et al. (-)Epigallocatechin gallate hampers collagen destruction and collagenase activation in ultraviolet-B-irradiated human dermal fibroblasts: involvement of mitogen-activated protein kinase. Food and Chemical Toxicology. 2008;46(4):1298–1307. doi: 10.1016/j.fct.2007.09.112. [DOI] [PubMed] [Google Scholar]
- 38.Shim J-S, Han Y-S, Hwang J-K. The effect of 4-hydroxypanduratin A on the mitogen-activated protein kinase-dependent activation of matrix metalloproteinase-1 expression in human skin fibroblasts. Journal of Dermatological Science. 2009;53(2):129–134. doi: 10.1016/j.jdermsci.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 39.Chang H, Oehrl W, Elsner P, Thiele JJ. The role of H2O2 as a mediator of UVB-induced apoptosis in keratinocytes. Free Radical Research. 2003;37(6):655–663. doi: 10.1080/1071576031000094907. [DOI] [PubMed] [Google Scholar]
- 40.Peus D, Pittelkow MR. Reactive oxygen species as mediators of UVB-induced mitogen-activated protein kinase activation in keratinocytes. Current problems in dermatology. 2001;29:114–127. doi: 10.1159/000060661. [DOI] [PubMed] [Google Scholar]
- 41.Katiyar SK, Afaq F, Azizuddin K, Mukhtar H. Inhibition of UVB-induced oxidative stress-mediated phosphorylation of mitogen-activated protein kinase signaling pathways in cultured human epidermal keratinocytes by green tea polyphenol ( - )-epigallocatechin-3-gallate. Toxicology and Applied Pharmacology. 2001;176(2):110–117. doi: 10.1006/taap.2001.9276. [DOI] [PubMed] [Google Scholar]
- 42.Mantena SK, Katiyar SK. Grape seed proanthocyanidins inhibit UV-radiation-induced oxidative stress and activation of MAPK and NF-κB signaling in human epidermal keratinocytes. Free Radical Biology and Medicine. 2006;40(9):1603–1614. doi: 10.1016/j.freeradbiomed.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 43.Yao K, Zhang L, Zhang YD, Ye PP, Zhu N. The flavonoid, fisetin, inhibits UV radiation-induced oxidative stress and the activation of NF-κB and MAPK signaling in human lens epithelial cells. Molecular Vision. 2008;14:1865–1871. [PMC free article] [PubMed] [Google Scholar]