Abstract
Background and purpose:
F15599, a novel 5-hydroxytryptamine (5-HT)1A receptor agonist with 1000-fold selectivity for 5-HT compared with other monoamine receptors, shows antidepressant and procognitive activity at very low doses in animal models. We examined the in vivo activity of F15599 at somatodendritic autoreceptors and postsynaptic 5-HT1A heteroreceptors.
Experimental approach:
In vivo single unit and local field potential recordings and microdialysis in the rat.
Key results:
F15599 increased the discharge rate of pyramidal neurones in medial prefrontal cortex (mPFC) from 0.2 µg·kg−1 i.v and reduced that of dorsal raphe 5-hydroxytryptaminergic neurones at doses >10-fold higher (minimal effective dose 8.2 µg·kg−1 i.v.). Both effects were reversed by the 5-HT1A antagonist (±)WAY100635. F15599 did not alter low frequency oscillations (∼1 Hz) in mPFC. In microdialysis studies, F15599 increased dopamine output in mPFC (an effect dependent on the activation of postsynaptic 5-HT1A receptors) with an ED50 of 30 µg·kg−1 i.p., whereas it reduced hippocampal 5-HT release (an effect dependent exclusively on 5-HT1A autoreceptor activation) with an ED50 of 240 µg·kg−1 i.p. Likewise, application of F15599 by reverse dialysis in mPFC increased dopamine output in a concentration-dependent manner. All neurochemical responses to F15599 were prevented by administration of (±)WAY100635.
Conclusions and implications:
These results indicate that systemic administration of F15599 preferentially activates postsynaptic 5-HT1A receptors in PFC rather than somatodendritic 5-HT1A autoreceptors. This regional selectivity distinguishes F15599 from previously developed 5-HT1A receptor agonists, which preferentially activate somatodendritic 5-HT1A autoreceptors, suggesting that F15599 may be particularly useful in the treatment of depression and of cognitive deficits in schizophrenia.
Keywords: 5-HT1A receptors; dopamine; pyramidal neurones; depression; prefrontal cortex; schizophrenia; microdialysis, local field potential
Introduction
5-hydroxytryptamine (5-HT)1A receptors are widely expressed in mammalian brain. They are located on the soma and dendrites of 5-hydroxytryptaminergic neurones, where they have an autoreceptor role, and are also located in pyramidal and GABAergic neurones of the neocortex and limbic system (Pazos and Palacios, 1985; Pompeiano et al., 1992; Kia et al., 1996; Riad et al., 2000; Martinez et al., 2001; Cruz et al., 2004; Santana et al., 2004), where they mediate inhibitory actions of 5-HT (Andrade and Nicoll, 1987; Blier and de Montigny, 1987; Innis and Aghajanian, 1987; Araneda and Andrade, 1991; Puig et al., 2005).
5-HT1A receptors play a major role in the antidepressant action of selective 5-HT re-uptake inhibitors and other 5-HT-enhancing drugs. 5-HT1A autoreceptor activation by the increased extracellular 5-HT induced by re-uptake or MAO inhibition in the median and dorsal raphe nuclei (DR) limits the neurochemical and clinical actions of these antidepressant drugs (Artigas et al., 1996; 2001;). On the other hand, the activation of postsynaptic 5-HT1A receptors is a characteristic of several types of antidepressant drugs (Haddjeri et al., 1998; Blier and Ward, 2003).
Although no. 5-HT1A receptor subtypes have been identified, 5-HT1A receptor subpopulations exhibit contrasting properties. Thus, 5-HT1A agonists such as 8-OH-DPAT and azapirones, preferentially activate raphe somatodendritic 5-HT1A autoreceptors (Sprouse and Aghajanian, 1987; see Blier and Ward, 2003 for review). This regional selectivity has been interpreted as resulting from the existence of a receptor reserve in the raphe (Meller et al., 1990; Cox et al., 1993), which limits the potential antidepressant usefulness of existing 5-HT1A agonists. Indeed, their administration first elicits a loss of 5-hydroxytryptaminergic tone on all other postsynaptic receptors, due to activation of somatodendritic 5-HT1A autoreceptors, which also results in insufficient activation of postsynaptic 5-HT1A receptors. However, 5-HT1A agonists may be useful as adjuvant therapies for the treatment of negative symptoms and cognitive deficits in schizophrenia as they increase dopamine (DA) release in prefrontal cortex (PFC) as a result of the activation of postsynaptic 5-HT1A receptors (Millan, 2000; Rollema et al., 1997; 2000; Ichikawa et al., 2001; Díaz-Mataix et al., 2005). Clinical support for this assertion has been generated using the partial agonist tandospirone to improve cognitive function in schizophrenia patients treated with antipsychotics (Sumiyoshi et al., 2001a, b; see Meltzer and Sumiyoshi, 2008 for review).
F15599 (3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-pyrimidin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl)-methanone) is a novel 5-HT1A receptor agonist with >1000-fold selectivity for this receptor compared with other known monoamine receptors, transporters and enzymes (Newman-Tancredi et al., 2009). F15599 is highly effective in the forced swim test, a measure of antidepressant-like properties (ED50-100 µg·kg−1 p.o.) (Assiéet al., 2010) suggesting a strong postsynaptic action. Moreover, it shows procognitive activity [attenuation of phencyclidine (PCP)-induced deficits in working memory] at 160 µg·kg−1 i.p. (Auclair et al., 2007; Pierre Fabre, unpublished observations). However, while the in vitro and ex vivo actions of F15599 reveal an agonist profile at 5-HT1A receptors located in different brain regions that distinguish it from other agonists and chemical congeners (Buritova et al., 2009; Newman-Tancredi et al., 2009), nothing is known about its auto/heteroreceptor selectivity in vivo. Hence, in the present study the in vivo effect of F15599 on pre- and postsynaptic 5-HT1A receptors was examined using single unit extracellular recordings and microdialysis in rat brain.
Methods
Electrophysiological studies
Animals
Male albino Wistar rats (230–300 g; Iffa Credo, Lyon, France) were kept in a controlled environment (12 h light–dark cycle and 22 ± 2°C room temperature) with food and water provided ad libitum. Animal care followed the European Union regulations (O.J. of E.C. L358/1 18/12/1986) and was approved by the Institutional Animal Care and Use Committee. Stereotaxic co-ordinates (in mm) were taken from bregma and duramater according to the atlas of Paxinos and Watson (1998). The total number of rats used in the electrophysiological experiments was 50.
Single unit recordings
We examined the responses elicited by F15599 on 5-hydroxytryptaminergic neurones of the DR and on pyramidal neurones of the medial PFC (mPFC). For 5-hydroxytryptaminergic neurones, recordings were made as described in Celada et al. (2001) and Romero et al. (2003). Pyramidal neurones were recorded as described in Puig et al. (2003) and Kargieman et al. (2007). Rats were administered chloral hydrate (400 mg·kg−1 i.p.) and positioned in a David Kopf stereotaxic frame. Thereafter, chloral hydrate was continuously administered i.p. at 50–70 mg·kg−1·h−1 using a perfusion pump.
Neurones were recorded extracellularly with glass micropipettes filled with 2 M NaCl. Impedance was between 6 and 12 MΩ. Single unit recordings were amplified with a Neurodata IR283 (Cygnus Technology Inc., Delaware Water Gap, PA), postamplified and filtered with a Cibertec amplifier and computed on-line using a DAT 1401plus interface system Spike2 software (Cambridge Electronic Design, Cambridge, UK). Local field potentials (LFP) were obtained by on-line band-pass filtering the signal from the recording electrode, as described (Kargieman et al., 2007).
For recordings in the DR, a burr hole was drilled over lambda and the sagittal sinus was ligated, cut and deflected. Descents were carried out along the midline. 5-HT neurones were typically recorded 4.7–6.6 mm below the brain surface and were identified according to previously described electrophysiological criteria (Wang and Aghajanian, 1977).
Pyramidal neurones in the mPFC were recorded as follows. Bipolar stimulating electrodes were implanted in the VTA (AP −5.8, L −0.4, DV −8.2) for the antidromic stimulation of pyramidal neurones projecting to midbrain. All recorded units were identified as pyramidal neurones by antidromic activation from VTA and collision extinction with spontaneously occurring spikes as described previously (Puig et al., 2003). Descents in mPFC were carried out at AP +3.2–3.4, L −0.5 to −1.0, DV −1.0 to −4.0 below the brain surface. Basal firing activity was recorded for at least 5 min and then, one or increasing drug doses were administered i.v. every 3 min, followed by the 5-HT1A receptor antagonist (±)WAY100635 when appropriate.
At the end of the experiments, rats were killed by an anaesthetic overdose. The placement of the stimulating electrodes was verified histologically. After being perfused with 10% formalin solution (Sigma), the brains were post-fixed, coronally sectioned (80 µm) and stained with Neutral Red. The data from rats with stimulating electrodes outside VTA were not included in the analysis.
Data analysis
Changes in discharge rate were quantified by averaging the values in the third minute after each drug injection. Neurones were considered to respond to drugs if firing rate was altered ±30% from baseline. Burst analysis was carried out using the method of Laviolette et al. (2005).
Power spectra were constructed using Fast Fourier Transformations (FFT) of 1 min signal intervals (same as discharge rate) with a resolution of 0.3 Hz (FFT size of 8192). Data are given as area under the curve (AUC) of the power spectrum between 0.3 and 4 Hz, expressed as percentage of pre-drug values.
Drug effects were assessed using Student's t-test or one-way anova for independent or repeated measures, as appropriate. Data are expressed as the mean ± SEM. Statistical significance has been set at the 95% confidence level.
Microdialysis studies
Animals
Male Sprague-Dawley rats (OFA, Charles River, France), weighing 240–260 g upon arrival were group housed (three rats per cage), under controlled conditions (12/12 light/dark cycle: lights on 07h00min; ambient temperature 21 ± 1°C; humidity 55 ± 5%), with rat food (A04, SAFE, Augy, France) and filtered (0.2 µm pore diameter) tap water freely available. Animals were housed and tested in an Association for the Assessment and Accreditation of Laboratory Animal Care International (AAALAC Itl.)-accredited facility in strict compliance with all applicable regulations and the protocols were carried out in compliance with French regulations and with local Ethical Committee guidelines for animal research. The total number of rats used in the microdialysis experiments was 145.
Microdialysis procedure
Rats were anaesthetized with chloral hydrate (400–500 mg·kg−1, i.p.) or isoflurane. A guide cannula with a dummy probe was stereotaxically implanted into the mPFC, stereotaxic coordinates: AP +3.0 mm, L +0.8 mm, DV −1.7 mm or the hippocampus: AP −4.8 mm, L +4.6 mm, DV −4.6 mm, from bregma and skull surface. Following surgery and recovery from anaesthesia, animals were returned to their home cages. At the end of the day, each rat was placed in a microdialysis cage. On the following day, the dummy probe was replaced by a microdialysis probe (3 mm length, 0.5 mm diameter, CMA, Microdialysis AB). The probe was continuously perfused (1.1 µL·min−1) with artificial CSF (aCSF) containing 1 µM citalopram for the measurement of 5-HT. Samples were collected every 20 min with the first four samples used for baseline. For the experiments involving systemic administration of the compounds, saline or (±)WAY100635 was injected s.c., followed by i.p. administration of saline or F15599 40 min later. For the experiments involving local perfusion, saline was injected s.c and 40 min later, F15599 was added to the perfusion medium for the concentration-response experiment. For the antagonism (±)WAY100635 (or aCSF) was delivered through the dialysis probe and 40 min later, F15599 was added to the perfusion medium. Samples were collected for 140 min after administration or beginning of the perfusion of the agonist. At the end of the experiment, rats were killed by anaesthetic overdose (pentobarbital 160 mg·kg−1, i.p.) and the brain was removed, frozen and cut in a cryomicrotome (Jung Frigocut 2800) to verify the placement of the probe.
Analytical procedure
Analysis of 5-HT and DA was performed by HPLC, as described by Assiéet al. (2005). The HPLC column was a reverse phase (Merck, Lichrocart 125-2, Superspher 100 RP-18). The mobile phase was pumped at 0.2 mL·min−1 (HPLC-118 solvent module, Beckman Coulter Inc., Fullerton, CA, USA). DA or 5-HT was electrochemically detected with a glassy carbon working electrode kept at +0.64 V versus Ag/AgCl reference electrode (DECADE detector, ANTEC Leyden BV, Leiden, the Netherlands). Data were acquired using a Beckman 32 Karats system. Concentrations of DA or 5-HT in the brain dialysates were estimated by comparing peak areas with those of external standards of known concentration of each neurotransmitter. The limit of detection (three times baseline noise) was approximately 1 fmol·20 µL−1 sample.
Data analysis
Dialysate 5-HT or DA concentrations are given as fmol·20 min−1 fraction and expressed in the figures as percentages of baseline. Statistical analysis was carried out using repeated-measures anova using treatment and time as variables. The mean % AUC values for the 140 min period after administration of F15599 were analysed by one-way anova followed by Dunnett's test (GraphPad Prism, San Diego, CA, USA). Mean % AUC values were used to estimate the ED50 value of F15599 by linear interpolation between the two doses that increased DA or decreased 5-HT levels with amounts bordering 50% (vehicle control as 0% and maximal effect of the compound as 100%). The antagonist effect was analysed by comparing the overall effect of the agonist with its effect at the same dose in the presence of (±)WAY100635 using Student's t-test.
Drugs
F15599 (3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-pyrimidin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl)-methanone) tosylate salt was from Pierre Fabre Medicament. (±)WAY100635 (N-[2-[4-(2-methoxyphenyl)-1-piperazinyl]ethyl]-N-(2-pyridinyl)cyclohexanecarboxamide) hydrochloride was from RBI (Natick, MA). Stock solutions were prepared and aliquots were stored at −20°C. Working solutions were prepared daily by dilution in saline at the appropriate concentrations and injected i.v. (up to 1 mL·kg−1) through the femoral vein. Doses are expressed as weight of free bases.
Chloral hydrate was purchased from Acros (Geel, Belgium), pentobarbital sodium from Ceva Santé Animale (Libourne, France), isoflurane from Baxter SA (Paurenas, France). Citalopram hydrobromide was kindly donated by Lundbeck (Copenhagen, Denmark). (±)WAY100635 and F15599 (fumarate salt) were synthesized at the Centre de Recherche Pierre Fabre. The doses of compounds are expressed as the weight of the free base. (±)WAY100635 and F15599 were dissolved in distilled water; the injection volume was 0.1 mL·kg−1.
All drug and molecular target nomenclature conforms to The British Journal of Pharmacology's ‘Guide to Receptors and Channels’ (Alexander et al., 2008).
Results
Effect of F15599 on the activity of DR 5-hydroxytryptaminergic neurones
We examined the effect of F15599 on 5-hydroxytryptaminergic neurones (n= 11; one per rat) of the DR in the range 0.2–20 µg·kg−1 i.v. At the higher doses, F15599 decreased the activity of all 5-HT neurones. Most neurones were fully inhibited after the administration of 5–10 µg·kg−1 i.v. (corresponding to 8.2–18.2 µg·kg−1 i.v. cumulative dose). Figure 1 shows examples of the suppressant effect of F15599 on DR 5-HT neurones. Figure 2A shows the dose–response relationship for all neurones examined. One-way anova indicated a significant effect of F15599 (F5,46= 13.5; P < 0.00001) with significant post-hoc differences (Newman–Keuls test) at the doses of 8.2 and 18.2 µg·kg−1 vs. baseline. One-way repeated measures anova of the subset of neurones that received all F15599 doses (n= 7) also revealed a significant effect of treatment (F5,30= 19.1; P < 0.00001) with significant post-hoc differences (Newman–Keuls test) at the doses of 8.2 and 18.2 µg·kg−1 vs. baseline. The minimal effective dose for the inhibition of DR 5-HT neurones was 8.2 µg·kg−1 i.v.
Figures 1.
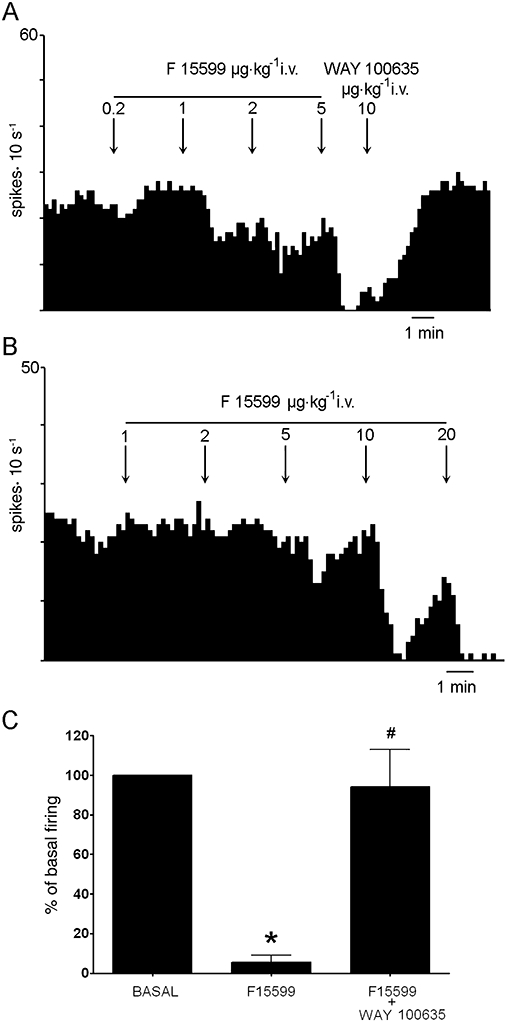
(A, B) Integrated firing rate histogram showing the effect of the i.v. administration of F15599 on the activity of DR 5-HT neurones recorded extracellularly in chloral hydrate anaesthetized rats. The activity of these neurones was completely suppressed at 8.2 and 38 µg·kg−1 i.v. (cumulative doses) respectively. (A) Shows the reversal of the inhibition induced by F15599 by the subsequent administration of the 5-HT1A receptor antagonist (±)WAY100635. (C) Histogram showing the reversal by (±)WAY100635 (5–50 µg·kg−1 i.v) of the maximal effect on DR 5-HT cell firing induced by F15599. Data are means ± SEM of five neurones. *P < 0.05 vs. baseline; #P < 0.05 vs.maximal inhibition; Newman–Keuls test post-anova.
Figure 2.
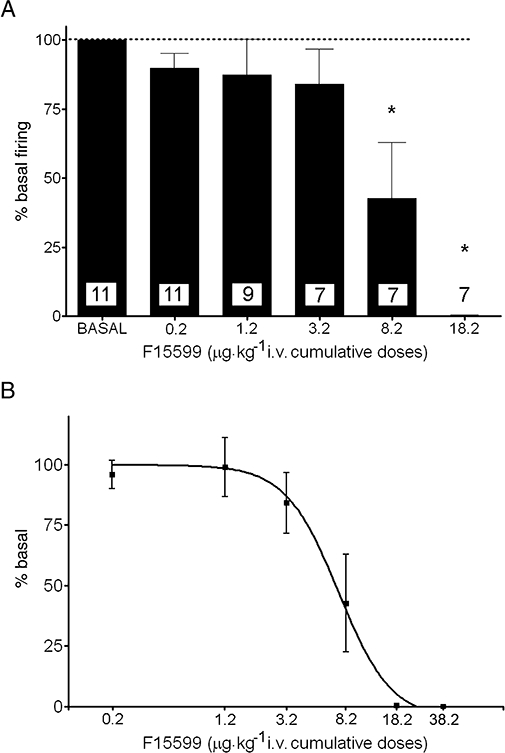
(A) Dose–response relationship of the effect of F15599 (0.2–38.2 µg·kg−1 i.v., cumulative doses) on the discharge rate of 5-HT neurones in the DR. Data (means ± SEM) are expressed as percentages of baseline. The number of neurones in each group is shown in the columns. The complete suppression of firing at the higher dose (38.2 µg·kg−1) is not shown. (B) Semi-log plot between cumulative F15599 dose (0.2–38.2 µg·kg−1 i.v.) and the suppressant effect on DR 5-HT neurones receiving all F15599 doses (n= 7). The ED50 was 7.3 µg·kg−1 i.v. *P < 0.05 vs. baseline, Newman–Keuls test post-anova.
ED50 values for the suppression of 5-HT cell firing have been calculated for the neurones of rats receiving all F15599 doses (n= 7) and for all rats receiving one or more doses of F15599 (n= 11). The corresponding values (given as cumulative doses) are 7.3 µg·kg−1 i.v. (all doses) and 7.4 µg·kg−1 i.v. (all rats) (Figure 2B).
The inhibition produced by F15599 was reversed in all cases examined (n= 5) by the subsequent administration of the selective 5-HT1A receptor antagonist (±)WAY100635 (5–50 µg·kg−1 i.v) (F2,8= 24.1; P < 0.0005) (Figure 1A and C).
Effect of F15599 on the activity of pyramidal neurones of the mPFC
After pilot experiments had revealed a marked enhancement of the firing rate of pyramidal neurones in the mPFC at the lowest does used for DR recordings (0.2 µg·kg−1), two sets of experiments were carried out. In the first experiment we examined the effect of this dose of F15599 and its reversal by (±)WAY-100635. A total of 15 neurones were recorded (1 neurone per rat) of which 11 were excited, two were inhibited and two remained unaltered by F15599 (Figure 3). The overall effect of this dose was a significant increase in the discharge rate to 210 ± 33% of baseline (P < 0.005, Student's paired t-test; n= 15). We attempted to reverse the action of 0.2 µg·kg−1 F15599 in a subset of five neurones. One-way anova indicated a significant effect of the treatment (F2,8= 11.45; P < 0.005) with significant post-hoc differences (Newman–Keuls) between F15599 and baseline and between F15599 and F15599+(±)WAY100635 (Figure 3C).
Figures 3.
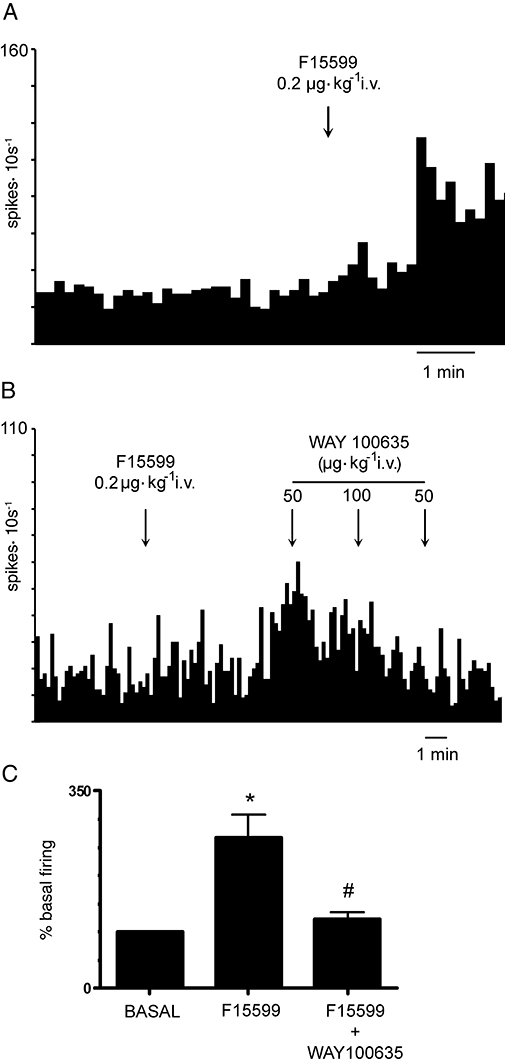
(A, B) Examples of the increase in discharge rate produced in mPFC pyramidal neurones by the i.v. administration of 0.2 µg·kg−1 F15599. Note the reversal of F15599 effect by the subsequent administration of (±)WAY100635 (50–200 µg·kg−1) in (B). (C) Shows the average effect produced by 0.2 µg·kg−1 F15599 and its reversal by the subsequent administration of (±)WAY100635 (n= 5). *P < 0.01 vs. basal; #P < 0.01 vs. F15599.
In a second set of experiments we examined the response of pyramidal neurones to increasing doses of F15599 (0.2–18.2 µg·kg−1 i.v., cumulative doses). From the pyramidal neurones examined (n= 16), 11 were excited and five were inhibited by F15599, with an overall excitatory effect of F15599. Figure 4 shows representative examples of neurones excited and inhibited by increasing doses of F15599. The minimal effective dose for the activation of mPFC pyramidal neurones was 0.2 µg·kg−1 i.v.
Figure 4.
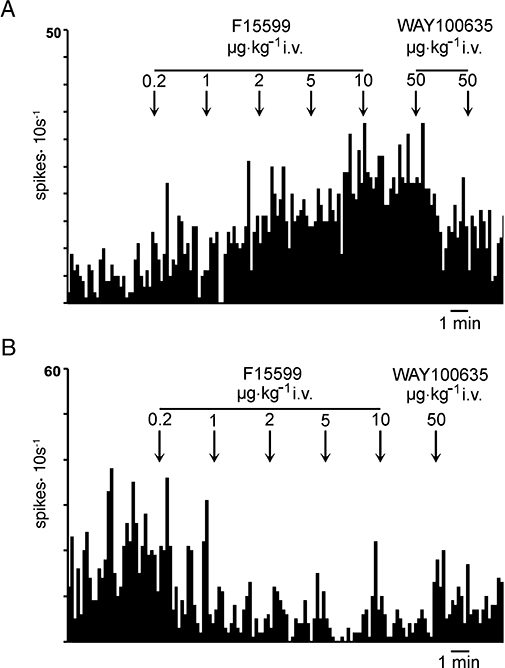
Examples of the effect of cumulative doses of F15599 on the discharge rate of pyramidal neurones of the mPFC. (A) This neurone was excited by F15599 at all doses examined. The maximal effect was attained after the administration of 10 µg·kg−1 (18.2 µg·kg−1 i.v., cumulative dose) and was antagonized by the subsequent administration of (±)WAY100635 (50–100 µg·kg−1 i.v.). (B) A pyramidal neurone inhibited by low doses (0.2–1 µg·kg−1 i.v.) of F15599 with an apparent maximal inhibition at 8.2 µg·kg−1 i.v.; this inhibition was also reversed by (±)WAY100635.
Data from the neurones used in both experiments were pooled and analysed together. For neurones excited by F15599, anova indicated a significant effect of the treatment (F5,63= 9,79, P < 0.00001) with significant post-hoc (Newman–Keuls) differences between the doses (i.v.) of 0.2 µg·kg−1, 2 µg·kg−1, 8.2 µg·kg−1 and 18.2 µg·kg−1 vs. baseline (Figure 5A).
Figure 5.
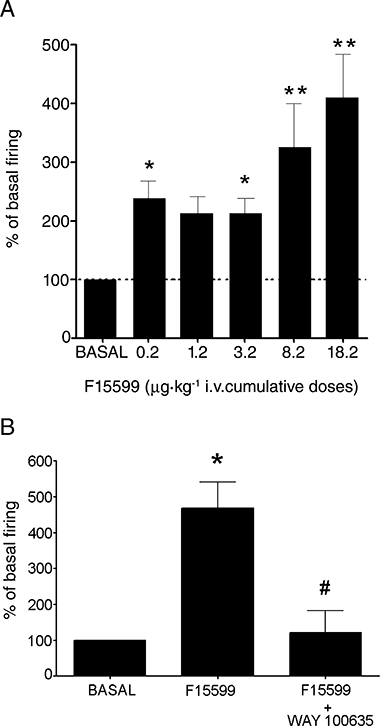
(A) Histogram showing the excitatory effect produced by F15599 on the firing rate of mPFC pyramidal neurones. Data correspond to neurones in the two experiments conducted (see text) which are 22, 20, 10, 7, 6 and 5 neurones for baseline, 0.2, 1.2, 3.2, 8.2 and 18.2 µg·kg−1 i.v. F15599 (cumulative doses) respectively. (B) Histogram showing the reversal by the 5-HT1A receptor antagonist WAY-100635 of the maximal excitatory effect of F15599 on mPFC pyramidal neurones (n= 4). *P < 0.05 vs. baseline; **P < 0.001 vs. baseline; #P < 0.02 vs. maximal effect of F15599; Newman–Keuls test post-anova.
As observed for the 0.2 µg·kg−1 dose, F15599-induced excitations at higher doses (average 15.7 µg·kg−1 i.v.) were also reversed by the subsequent administration of (±)WAY100635 (50–100 µg·kg−1 i.v) in all cases examined (F2,6= 12.14, P < 0.01, n= 4) (Figure 5B).
The maximal inhibitory effect of F15599 was noted at a dose of 18.2 µg·kg−1 (50 ± 26% of baseline). anova revealed a non-significant effect of the treatment (F5,19= 1.55; P= 0.23), possibly due to the lower number of neurones inhibited compared with those excited by F15599.
F15599 had a minor impact on the burst firing of activated mPFC pyramidal neurones. From all the variables examined, only the number of spikes per burst was significantly affected, but only with a small effect size (c. 10%).
Effect of F15599 on slow cortical oscillations
The non-competitive NMDA receptor antagonist PCP and the preferential 5-HT2A receptor agonist 1-[2,5-dimethoxy-4-iodophenyl-2-aminopropane] (DOI) increase PFC pyramidal cell discharge and reduce low frequency (delta) cortical oscillations (0.3–4 Hz) (Kargieman et al., 2007; Celada et al., 2008). Given the increase in pyramidal discharge induced by F15599, we examined whether this effect was accompanied by an alteration of low frequency oscillations.
Spikes were discharged in synchrony with active phases of LFP under baseline conditions and after F15599 administration. This occurred when spikes were fired as single events or as spike trains (not shown). However, the administration of F15599 did not alter the power of low frequency cortical oscillations (0.3–4 Hz) in the whole dose range examined (n.s., one-way anova).
Extracellular concentrations of 5-HT in the ventral hippocampus
The mean basal extracellular level of 5-HT in ventral hippocampus was 41.6 ± 2.7 fmol·20 µL−1 (n= 36). None of the treatment groups differed from controls (34.5 ± 5.9 fmol·20 µL−1) although the F15599 160 µg·kg−1 group (57.6 ± 9.2 fmol·20 µL−1) was significantly greater than the WAY+F15599 630 µg·kg−1 group (27.7 ± 6.6 fmol·20 µL. fmol·20 µL−1; P < 0.05) (post-hoc analysis).
Administration of F15599 (40–2500 µg·kg−1) dose-dependently decreased extracellular 5-HT during the 140 min period after injection (Figure 6, top left panel). Two-way anova for repeated measures indicated a significant effect of treatment (F6,29= 23.3, P < 0.0001), time (F6,165= 6.24, P < 0.0001) and treatment-time interaction (F36,165= 2.02, P= 0.02). Post-hoc analysis by the contrasts method indicated a significant global effect at 160 (P= 0.0016), 630 and 2500 µg·kg−1 (P < 0.0001). (±)WAY100635 (160 µg·kg−1), when given alone, did not significantly alter extracellular 5-HT (Figure 6 left middle panel). The effects of 630 µg·kg−1 F15599 were significantly reversed by (±)WAY100635 (P < 0.0001).
Figure 6.
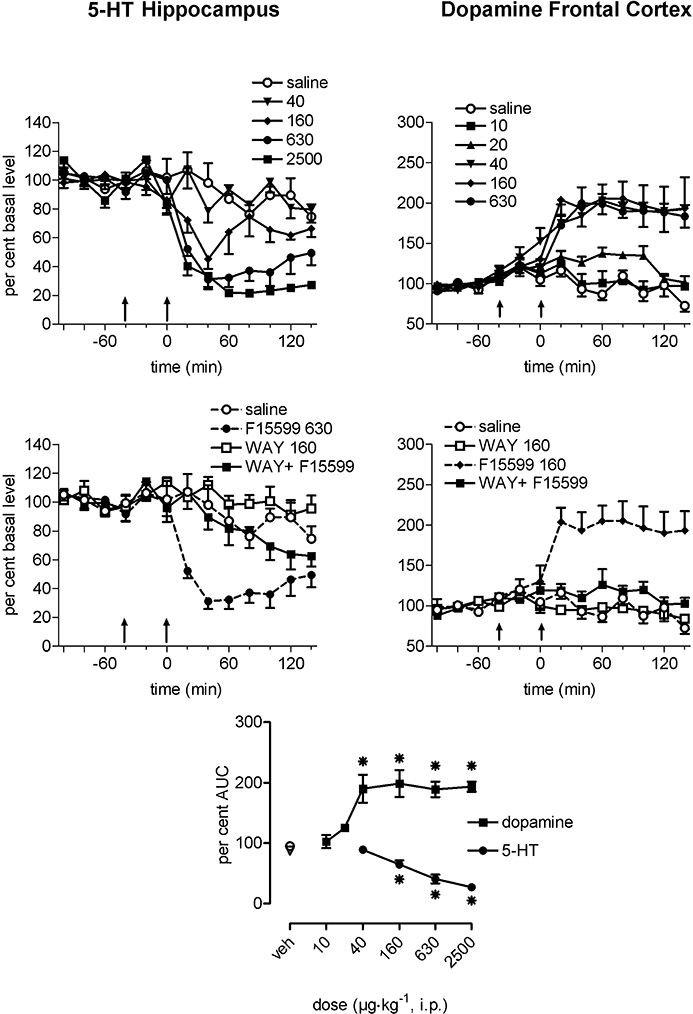
Effects of i.p. administration of F15599 on extracellular 5-HT in the ventral hippocampus (left panels) and on dopamine levels in the medial prefrontal cortex (right panels) of freely moving rats. Data are expressed as a percentage of the mean absolute amount of neurotransmitter in the four samples collected before treatment. The first arrow indicates injection of saline or (±)WAY100635 (160 µg·kg−1 s.c., middle panels), the second arrow indicates administration of saline or F15599. The bottom panel represents mean area under the curve (AUC) for 140 min after administration of the compound. Results are mean ± SEM of five to seven animals per group. Note: to avoid overloading the top-right panel, the dose of 2500 µg·kg−1 is not shown. AUC dose–response data were analysed using one-way anova followed by Dunnett's test (*P < 0.05 compared with corresponding control animals).
The effect of F15599, analysed by the mean AUC data for the 140 min period after administration, was significant at the doses of 160, 630, 2500 µg·kg−1, compared with controls (P < 0.05, F4,25= 24.48, one-way anova followed by Dunnett's test; Figure 6, bottom panel). The ED50 value of F15599, calculated by linear interpolation, was 240 µg·kg−1. (±)WAY100635 significantly reversed the effects of F15599 630 µg·kg−1 (Student's t-test).
Extracellular concentration of DA in the mPFC
Systemic administration of F15599
The mean basal extracellular level of DA in mPFC was 6.00 ± 0.36 fmol·20 µL−1 (n= 45). One-way anova indicated no significant difference between groups F8,44= 1.15, P= 0.36.
Systemic administration of F15599 (10–2500 µg·kg−1, i.p.) dose-dependently increased extracellular DA during the 140 min period after injection with a maximal increase of approximately 200% of basal level at the dose of 40 µg·kg−1 (Figure 6 top right panel). Two-way anova for repeated measures indicated a significant effect of treatment (F8,36= 13.2, P < 0.0001), time (F6,216= 2.97, P= 0.0084) and treatment × time interaction (F48,216= 1.70, P= 0.006). The method of contrasts indicated a significant global effect at doses 40–2500 µg·kg−1 (P < 0.0001). (±)WAY100635, when given alone, did not significantly alter extracellular DA (Figure 6 right middle panel). The effects of F15599 (160 µg·kg−1) were significantly reversed by (±)WAY100635 (160 µg·kg−1) (P < 0.0001).
The overall increase in DA, analysed by the AUC data for the 140 min period after drug injection, was significant from 40–2500 µg·kg−1 F15599 (P < 0.05, F7,37= 10.35, one-way anova followed by Dunnett's test; Figure 6 bottom panel). The ED50 value of F15599, calculated by linear interpolation was 30 µg·kg−1. (±)WAY100635 significantly reversed the effects of F15599 160 µg·kg−1 (Student's t-test).
Local perfusion of F15599 through the dialysis probe
The mean basal extracellular level of DA was 7.19 ± 0.35 fmol·20 µL−1 (n= 57). One-way anova indicated no significant differences between groups for the concentration-response data (F5,22= 1.29, P= 0.32) nor for the antagonism experiments (F5,33= 0.89, P= 0.50).
Local perfusion of F15599 through the dialysis probe in the mPFC (1–1000 µM) increased extracellular DA in a concentration-dependent manner during the 140 min period examined. The maximal increase was approximately 470% of basal level at 1000 µM (Figure 7, top panel). The two-way anova for repeated measures indicated a significant effect of treatment (F5,17= 6.02, P= 0.0022) but no significant effect of time (F6,102= 0.87, P= 0.52) nor treatment-time interaction (F30,102= 1.10, P= 0.36). Post-hoc analysis by the contrasts method indicated a significant global effect at the concentrations 300 µM (P= 0.048) and 1000 µM (P= 0.0001).
Figure 7.

Effects of local perfusion of F15599 in the medial prefrontal cortex on extracellular dopamine levels and antagonism by (±)WAY100635. Data are expressed as a percentage of the mean absolute amount of dopamine in the four samples collected before treatment. Top panel: the effects of different concentrations of F15599 administered through the dialysis probe from time 0 (bar), the arrow indicates saline administration (s.c.). Middle and bottom panels: the antagonist effects of (±)WAY100635 100 and 300 µM, administered through the dialysis probe, on the increase in extracellular dopamine induced by 300 µM F15599. The bar indicates the presence of compounds in the perfusion medium, the arrow indicates the addition of F15599 to the perfusion medium. Results are mean ± SEM of three to eight animals per group.
(±)WAY100635 (100 and 300 µM), perfused through the dialysis probe 40 min before and together with F15599 300 µM prevented the increase in extracellular DA induced by F15599 (Figure 7, middle and bottom panels). The two-way anova for repeated measures indicated a significant effect of treatment (F5,28= 5.64, P= 0.001) and time (F6,168= 3.48, P= 0.0029) but not a significant interaction (F30,168= 1.34, P= 0.12). Post-hoc analysis by the contrasts method indicated a significant global effect at 300 µM F15599 (P= 0.0036) and a significant antagonist effect of (±)WAY100635 100 µM (P= 0.018) and 300 µM (P= 0.0003).
Discussion
The electrophysiological results from the present study indicate that the selective 5-HT1A receptor agonist F15599 has a preferential action on cortical pyramidal compared with 5-hydroxytryptaminergic neurones in the rat brain. This regional selectivity is also supported by microdialysis experiments showing a greater sensitivity of cortical DA vs. hippocampal 5-HT output to the effects of F15599. Based on current knowledge on 5-HT1A receptor function, this regional selectivity may be attributed to the preferential activation by F15599 of postsynaptic 5-HT1A receptors. Overall, F15599 appears to be about one order of magnitude more active at postsynaptic than at somatodendritic 5-HT1A autoreceptors.
To the best of our knowledge, F15599 is the first selective 5-HT1A agonist shown to have a preferential postsynaptic action. F15599 increased pyramidal discharge in mPFC to ∼200% of baseline at the lowest dose assayed, 0.2 µg·kg−1 i.v., whereas most 5-hydroxytryptaminergic neurones were only inhibited by the administration of 8.2–18.2 µg·kg−1 i.v. (cumulative doses). Both F15599-induced responses were due to the exclusive activation of 5-HT1A receptors, as indicated by the in vitro selectivity of the compound and by the reversal of its actions by (±)WAY100635.
Because mPFC 5-HT1A receptors control DA neurones in the VTA and are involved in the mechanism of action of atypical antipsychotic drugs (Rollema et al., 1997; Millan, 2000; Bantick et al., 2001; Díaz-Mataix et al., 2005), we examined the actions of F15599 on the activity of pyramidal neurones in the mPFC projecting to VTA
Given the inhibitory nature of 5-HT1A receptors on neuronal activity (Andrade and Nicoll, 1987; Innis and Aghajanian, 1987; Araneda and Andrade, 1991; Puig et al., 2005), the increase in mPFC pyramidal discharge elicited by the systemic administration of 5-HT1A receptor agonists (Borsini et al., 1995; Hajós et al., 1999; Díaz-Mataix et al., 2006; this work) may seem paradoxical, as (i) microiontophoretic application of these compounds at large concentrations in mPFC inhibits the discharge of putative pyramidal neurones (Ashby et al., 1994; Rueter and Blier, 1999); (ii) high doses of 8-OH-DPAT inhibit mPFC pyramidal neurones (Hajós et al., 1999); (iii) physiological release of 5-HT inhibits the activity of mPFC pyramidal neurones through 5-HT1A receptors (Hajós et al., 2003; Amargós-Bosch et al., 2004; Puig et al., 2005); and (iv) there is in vitro evidence of inhibition of mPFC pyramidal cells by 5-HT1A agonists (Tanaka and North, 1993). Conceivably, this excitatory effect may result from an action on cortical networks involving the activation of 5-HT1A receptors in local GABAergic interneurones (Santana et al., 2004) and the subsequent disinhibition of pyramidal neurones. Although this possibility requires further testing, it is supported by the existence of molecular and functional differences between neurotransmitter receptors expressed by GABAergic and glutamatergic cells (Jonas et al., 1994). Likewise, differences in the composition and response of NMDA receptor subtypes have been shown (Hollmann and Heinemann, 1994; Plant et al., 1997) and a differential response of pyramidal and GABAergic neurones in mPFC to MK-801 have been reported (Homayoun and Moghaddam, 2007). Although there are no molecular differences in the structure of 5-HT1A autoreceptors and postsynaptic 5-HT1A receptors, functional differences may exist in the coupling to different transduction mechanisms, to the cellular receptor reserve or in cellular sensitivity to the opening of K+ channels, among others. Hence, the response of pyramidal neurones to 8-OH-DPAT in rat mPFC is changed by local blockade of GABAA receptors (Lladó-Pelfort et al., 2009). Further work is required to determine whether this also occurs for F15599.
Electrophysiological studies comparing the sensitivity of auto- (raphe) and postsynaptic (hippocampus) 5-HT1A receptors have previously indicated a greater sensitivity of the former receptors to the inhibitory actions of 5-HT and exogenous agonists (Sprouse and Aghajanian, 1987, 1988; Dong et al., 1997; Blier and Ward, 2003). Using similar experimental approaches, the selective 5-HT1A agonists 8-OH-DPAT and BAY × 3702 (repinotan), were found to have ED50 values around 1 µg·kg−1 i.v. for suppression of 5-HT cell firing (Blier and de Montigny, 1987; Dong et al., 1998; Hajós et al., 1999; Casanovas et al., 2000; Romero et al., 2003). However, similar or higher doses are required to increase the discharge of cortical neurones (Borsini et al., 1995; Hajós et al., 1999; Díaz-Mataix et al., 2006).
This preferential selectivity for 5-HT1A autoreceptors was attributed to a receptor reserve in the raphe (Meller et al., 1990; Cox et al., 1993). This concept was partly supported by in vitro observations indicating that the agonist/antagonist profile of 5-HT1A receptor agents varied depending on the cellular density of 5-HT1A receptors (Hoyer and Boddeke, 1993).
Delta (0.3–4 Hz) oscillations recorded extracellularly in anaesthetized animals are similar to the physiological changes in cortical activity during slow-wave sleep (Steriade et al., 1993). These oscillations play an important role in the disconnection of the brain from external afferent sources, information processing and memory consolidation during sleep (Marshall et al., 2006). Particular attention has been paid in recent years to slow oscillations (≤1 Hz), which appear to reflect “up” and “down” states of cortical networks (Sanchez-Vives and McCormick, 2000). This slow oscillation is a major component of delta waves. Unlike the NMDA receptor antagonist, PCP and the 5-HT2A receptor agonist, DOI, which markedly suppress slow oscillatory activity in the mPFC (Kargieman et al., 2007; Celada et al., 2008), F15599, did not alter slow cortical oscillations despite inducing similar changes in pyramidal discharge. Thus, F15599 does not disrupt the strict temporal pattern of slow oscillatory activity seen in basal conditions. Although we did not determine whether F15599 could normalize the loss of slow oscillatory activity induced by PCP, F15599 has been shown to attenuate the deficits in working memory induced by this drug (Auclair et al. 2007 and unpublished observations), a property that may be related to the divergent influence of F15599 and PCP on PFC networks. This property suggests that F15599 might be also useful as add-on therapy in the treatment of cognitive deficits in schizophrenia.
Microdialysis data also support a preferential postsynaptic action of F15599. The 5-HT and DA output in hippocampus and mPFC are used as neurochemical indicators of the activation of somatodendritic autoreceptors and postsynaptic 5-HT1A receptors respectively. Some studies support the view that 5-HT release in ventral hippocampus depends on the activation of somatodendritic 5-HT1A autoreceptors (Hutson et al., 1989; Adell et al., 1993; McQuade and Sharp, 1997). However, as the activity of DR 5-HT neurones is also dependent on mPFC 5-HT1A receptor activation (Ceci et al., 1994; Hajós et al., 1999; Celada et al., 2001), it is uncertain whether hippocampal 5-HT release may be influenced to some extent by postsynaptic 5-HT1A receptor activation, as happens with that in mPFC (Casanovas et al., 1999; Celada et al., 2001).
On the other hand, DA output in mPFC is dependent on the activation of cortical postsynaptic 5-HT1A receptors, possibly located on or close to pyramidal neurones projecting to the ventral tegmental area (Díaz-Mataix et al., 2005; Bortolozzi et al., 2007). This assertion is further supported by the increase in DA output produced by the local application of F15599 in mPFC.
F15599 was more potent at promoting DA release in mPFC (postsynaptic 5-HT1A receptor-mediated effect) than at suppressing hippocampal 5-HT release (5-HT1A autoreceptor effect), with calculated ED50 values of 30 and 240 µg·kg−1 i.p. respectively. Interestingly, despite the different strain of rats and the routes of administration, the ratio between the suppression of 5-HT release and cell firing (autoreceptor effect) was similar to that between activation of pyramidal cell firing and DA release (postsynaptic 5-HT1A receptor effect).
The cellular bases responsible for this regional in vivo selectivity of F15599 are not fully understood. In cell lines stably expressing 5-HT1A receptors, this agent preferentially stimulated the phosphorylation of extracellular signal-regulated kinase (ERK1/2) compared with other signalling pathways linked to 5-HT1A receptors, such as G-protein activation, receptor internalization or inhibition of cAMP accumulation. 5-HT and (+)8-OH-DPAT displayed a different rank order of potency for these responses (Newman-Tancredi et al., 2009). In addition, F15599 stimulated [35S]-GTPγS binding, ERK1/2 phosphorylation and c-fos expression more potently in frontal cortex than in the midbrain raphe, compared with other agonists (Buritova et al., 2009; Newman-Tancredi et al., 2009). Thus, a distinctive ‘signalling signature’ for specific intracellular transduction responses may underlie the brain region specificity of F15599. Furthermore, in microPET experiments, [18F]-F15599 preferentially labelled frontal cortex 5-HT1A receptors in rat and cat (Lemoine et al., 2008; 2010;), which may add to the preferential postsynaptic action of F15599.
Thus, F15599 shows a preferential postsynaptic activity in cortical vs. raphe 5-HT1A receptors, a property that may underlie the effects of very low doses of this drug in animal models of depression and cognitive function, suggesting that F15599 may overcome the existing limitations of 5-HT1A receptor agonists in the treatment of psychiatric disorders.
Acknowledgments
Work supported by grants SAF 2007-62378, FIS PI060264 and Pierre Fabre Médicament. L.L.-P. is supported by a JAE fellowship from CSIC. P.C. is supported by the Researcher Stabilization Program of the Health Department of the Generalitat de Catalunya. We acknowledge Véronique Ravailhe and Christelle Benas for technical assistance with microdialysis experiments and Mónica Gutiérrez and Verónica Paz for electrophysiological experiments.
Glossary
Abbreviations:
- DR
dorsal raphe nucleus
- F15599
3-chloro-4-fluorophenyl-(4-fluoro-4-{[(5-methyl-pyrimidin-2-ylmethyl)-amino]-methyl}-piperidin-1-yl)-methanone
- LFP
local field potential
- mPFC
medial prefrontal cortex
Conflicts of interest
None.
References
- Adell A, Carceller A, Artigas F. In vivo brain dialysis study of the somatodendritic release of serotonin in the raphe nuclei of the rat. Effects of 8-hydroxy-2-(di-n-propylamino)tetralin. J Neurochem. 1993;60:1673–1681. doi: 10.1111/j.1471-4159.1993.tb13390.x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 3rd edition (2008 revision) Br J Pharmacol. 2008;153:S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amargós-Bosch M, Bortolozzi A, Puig MV, Serrats J, Adell A, Celada P, et al. Co-expression and in vivo interaction of serotonin1A and serotonin2A receptors in pyramidal neurons of prefrontal cortex. Cereb Cortex. 2004;14:281–299. doi: 10.1093/cercor/bhg128. [DOI] [PubMed] [Google Scholar]
- Andrade R, Nicoll RA. Pharmacologically distinct actions of serotonin on single pyramidal neurons of the rat hippocampus recorded in vitro. J Physiol. 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araneda R, Andrade R. 5-Hydroxytryptamine2 and 5-hydroxytryptamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. doi: 10.1016/0306-4522(91)90128-b. [DOI] [PubMed] [Google Scholar]
- Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. doi: 10.1016/S0166-2236(96)10037-0. [DOI] [PubMed] [Google Scholar]
- Artigas F, Celada P, Laruelle M, Adell A. How does pindolol improve antidepressant action? Trends Pharmacol Sci. 2001;22:224–228. doi: 10.1016/s0165-6147(00)01682-5. [DOI] [PubMed] [Google Scholar]
- Ashby CR, Edwards E, Wang RY. Electrophysiological evidence for a functional interaction between 5 and HT(1A) and 5-HT(2A) receptors in the rat medial prefrontal cortex: an iontophoretic study. Synapse. 1994;17:173–181. doi: 10.1002/syn.890170306. [DOI] [PubMed] [Google Scholar]
- Assié MB, Ravailhe V, Faucillon V, Newman-Tancredi A. Contrasting contribution of 5-HT1A receptor activation to neurochemical profile of novel antipsychotics: frontocortical dopamine and hippocampal serotonin release in rat brain. J Pharmacol Exp Ther. 2005;315:265–272. doi: 10.1124/jpet.105.087163. [DOI] [PubMed] [Google Scholar]
- Assié M-B, Bardin L, Auclair AL, Carilla-Durand E, Depoortere R, Koek W, et al. F15599, a highly selective postsynaptic 5-HT1A receptor agonist: in vivo profile in behavioural models of antidepressant and serotonergic activity. Int J Neuropsychopharmacol. 2010 doi: 10.1017/S1461145709991222. DOI: 10.1017/s1461145709991222. [DOI] [PubMed] [Google Scholar]
- Auclair A, Bardin L, Depoortère R, Newman-Tancredi A. F15599, A 5-HT1A Agonist That Preferentially Targets Post-Synaptic Receptors in the Frontal Cortex. III) Activity in Rodent Models of Cognition. 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience; 2007. Online. 170.24. [Google Scholar]
- Bantick RA, Deakin JFW, Grasby PM. The 5-HT1A receptor in schizophrenia: a promising target for novel atypical neuroleptics? J Psychopharmacol. 2001;15:37–46. doi: 10.1177/026988110101500108. [DOI] [PubMed] [Google Scholar]
- Blier P, de Montigny C. Modification of 5-HT neuron properties by sustained administration of the 5-HT1A agonist gepirone: electrophysiological studies in the rat brain. Synapse. 1987;1:470–480. doi: 10.1002/syn.890010511. [DOI] [PubMed] [Google Scholar]
- Blier P, Ward NM. Is there a role for 5-HT1A agonists in the treatment of depression? Biol Psychiatry. 2003;53:193–203. doi: 10.1016/s0006-3223(02)01643-8. [DOI] [PubMed] [Google Scholar]
- Borsini F, Ceci A, Bietti G, Donetti A. BIM17, a 5-HT1A receptor agonist/5-HT2A receptor antagonist directly activates postsynaptic 5-HT inhibitory responses in the rat cerebral cortex. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:283–290. doi: 10.1007/BF00168558. [DOI] [PubMed] [Google Scholar]
- Bortolozzi A, Díaz-Mataix L, Toth M, Celada P, Artigas F. In vivo actions of aripiprazole on serotonergic and dopaminergic systems in rodent brain. Psychopharmacology. 2007;191:745–758. doi: 10.1007/s00213-007-0698-y. [DOI] [PubMed] [Google Scholar]
- Buritova J, Berrichon G, Cathala C, Colpaert F, Cussac D. Region-specific changes in 5-HT1A agonist-induced Extracellular signal-Regulated Kinases 1/2 phosphorylation in rat brain: a quantitative ELISA study. Neuropharmacology. 2009;56:350–361. doi: 10.1016/j.neuropharm.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Casanovas JM, Hervas I, Artigas F. Postsynaptic 5-HT1A receptors control 5-HT release in the rat medial prefrontal cortex. NeuroReport. 1999;10:1441–1445. doi: 10.1097/00001756-199905140-00010. [DOI] [PubMed] [Google Scholar]
- Casanovas JM, Berton O, Celada P, Artigas F. In vivo actions of the selective 5-HT1A receptor agonist BAY x 3702 on serotonergic cell firing and release. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:248–254. doi: 10.1007/s002100000291. [DOI] [PubMed] [Google Scholar]
- Ceci A, Baschirotto A, Borsini F. The inhibitory effect of 8-OH-DPAT on the firing activity of dorsal raphe serotoninergic neurons in rats is attenuated by lesion of the frontal cortex. Neuropharmacology. 1994;33:709–712. doi: 10.1016/0028-3908(94)90177-5. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Casanovas JM, Guillazo G, Artigas F. Control of dorsal raphe serotonergic neurons by the medial prefrontal cortex: involvement of 5-HT1A, GABAA, and glutamate receptors. J Neurosci. 2001;21:9917–9929. doi: 10.1523/JNEUROSCI.21-24-09917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Díaz-Mataix L, Artigas F. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol Psychiatry. 2008;64:392–400. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Cox RF, Meller E, Waszczak BL. Electrophysiological evidence for a large receptor reserve for inhibition of dorsal raphe neuronal firing by 5-HT1A agonists. Synapse. 1993;14:297–304. doi: 10.1002/syn.890140407. [DOI] [PubMed] [Google Scholar]
- Cruz DA, Eggan SM, Azmitia EC, Lewis DA. Serotonin1A receptors at the axon initial segment of prefrontal pyramidal neurons in schizophrenia. Am J Psychiatry. 2004;161:739–742. doi: 10.1176/appi.ajp.161.4.739. [DOI] [PubMed] [Google Scholar]
- Díaz-Mataix L, Scorza MC, Bortolozzi A, Toth M, Celada P, Artigas F. Involvement of 5-HT1A receptors in prefrontal cortex in the modulation of dopaminergic activity: role in atypical antipsychotic action. J Neurosci. 2005;25:10831–10843. doi: 10.1523/JNEUROSCI.2999-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Mataix L, Artigas F, Celada P. Activation of pyramidal cells in rat medial prefrontal cortex projecting to ventral tegmental area by a 5-HT1A receptor agonist. Eur Neuropsychopharmacol. 2006;16:288–296. doi: 10.1016/j.euroneuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Dong J, de Montigny C, Blier P. Effect of acute and repeated versus sustained administration of the 5-HT1A receptor agonist ipsapirone: electrophysiological studies in the rat hippocampus and dorsal raphe. Naunyn Schmiedebergs Arch Pharmacol. 1997;356:303–311. doi: 10.1007/pl00005055. [DOI] [PubMed] [Google Scholar]
- Dong J, de Montigny C, Blier P. Full agonistic properties of BAY x 3702 on presynaptic and postsynaptic 5-HT1A receptors electrophysiological studies in the rat hippocampus and dorsal raphe. J Pharmacol Exp Ther. 1998;286:1239–1247. [PubMed] [Google Scholar]
- Haddjeri N, Blier P, de Montigny C. Long-term antidepressant treatments result in a tonic activation of forebrain 5-HT1A receptors. J Neurosci. 1998;18:10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M, Hajos-Korsok E, Sharp T. Role of the medial prefrontal cortex in 5-HT1A receptor-induced inhibition of 5-HT neuronal activity in the rat. Br J Pharmacol. 1999;126:1741–1750. doi: 10.1038/sj.bjp.0702510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M, Gartside SE, Varga V, Sharp T. In vivo inhibition of neuronal activity in the rat ventromedial prefrontal cortex by midbrain-raphe nuclei: role of 5-HT1A receptors. Neuropharmacology. 2003;45:72–81. doi: 10.1016/s0028-3908(03)00139-4. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27:11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Boddeke HW. Partial agonists, full agonists, antagonists: dilemmas of definition. Trends Pharmacol Sci. 1993;14:270–275. doi: 10.1016/0165-6147(93)90129-8. [DOI] [PubMed] [Google Scholar]
- Hutson PH, Sarna GS, O'Connell MT, Curzon G. Hippocampal 5-HT synthesis and release in vivo is decreased by infusion of 8-OH-DPAT into the nucleus raphe dorsalis. Neurosci Lett. 1989;100:276–280. doi: 10.1016/0304-3940(89)90698-8. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O'Laughlin IA, Meltzer HY. 5-HT2A and D-2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Innis RB, Aghajanian GK. Pertussis toxin blocks 5-HT1A and GABAB receptor-mediated inhibition of serotonergic neurons. Eur J Pharmacol. 1987;143:195–204. doi: 10.1016/0014-2999(87)90533-4. [DOI] [PubMed] [Google Scholar]
- Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Kargieman L, Santana N, Mengod G, Celada P, Artigas F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc Natl Acad Sci USA. 2007;104:14843–14848. doi: 10.1073/pnas.0704848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kia HK, Miquel MC, Brisorgueil MJ, Daval G, Riad M, El Mestikawy S, et al. Immunocytochemical localization of serotonin(1A) receptors in the rat central nervous system. J Comp Neurol. 1996;365:289–305. doi: 10.1002/(SICI)1096-9861(19960205)365:2<289::AID-CNE7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J Neurosci. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine L, Verdurand M, Vacher B, Blanc E, Le Bars D, Newman-Tancredi A, et al. Synthesis, fluorine-18 labelling and radio-pharmacological evaluation of F15599, a novel 5-HT1A receptor agonist. J Nucl Med. 2008;49(Suppl. 1):285P. doi: 10.1007/s00259-009-1274-y. [DOI] [PubMed] [Google Scholar]
- Lemoine L, Verdurand M, Vacher B, Blanc E, Le Bars D, Newman-Tancredi A, et al. [18F]F15599, a novel 5-HT1A receptor agonist, as a radioligand for PET neuroimaging. Eur J Nucl Med Mol Imaging. 2010;37:594–605. doi: 10.1007/s00259-009-1274-y. [DOI] [PubMed] [Google Scholar]
- Lladó-Pelfort L, Santana N, Artigas F, Celada P. Postsynaptic 5-HT1A Receptors Are Involved in the Excitatory Action of 8-OH-DPAT on Prefrontal Cortex Pyramidal Neurons. 748.2. 2009 Neuroscience Meeting Planner. Chicago, IL: Society for Neuroscience; 2009. Online. [Google Scholar]
- McQuade R, Sharp T. Functional mapping of dorsal and median raphe 5-hydroxytryptamine pathways in forebrain of the rat using microdialysis. J Neurochem. 1997;69:791–796. doi: 10.1046/j.1471-4159.1997.69020791.x. [DOI] [PubMed] [Google Scholar]
- Marshall L, Helgadottir H, Molle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Martinez D, Hwang D, Mawlawi O, Slifstein M, Kent J, Simpson N, et al. Differential occupancy of somatodendritic and postsynaptic 5HT1A receptors by pindolol: a dose-occupancy study with [11C]WAY 100635 and positron emission tomography in humans. Neuropsychopharmacology. 2001;24:209–229. doi: 10.1016/S0893-133X(00)00187-1. [DOI] [PubMed] [Google Scholar]
- Meller E, Goldstein M, Bohmaker K. Receptor reserve for 5-hydroxytryptamine-mediated inhibition of serotonin synthesis: possible relationship to anxiolytic properties of 5-hydroxytriptamine agonists. Mol Pharmacol. 1990;37:231–237. [PubMed] [Google Scholar]
- Meltzer HY, Sumiyoshi T. Does stimulation of 5-HT1A receptors improve cognition in schizophrenia? Behav Brain Res. 2008;195:98–102. doi: 10.1016/j.bbr.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Improving the treatment of schizophrenia: focus on serotonin 5-HT1A receptors. J Pharmacol Exp Ther. 2000;295:853–861. [PubMed] [Google Scholar]
- Newman-Tancredi A, Martel JC, Assié MB, Buritova J, Lauressergues E, Cosi C, et al. Signal transduction and functional selectivity of F15599, a preferential post-synaptic 5-HT receptor agonist. Br J Pharmacol. 2009;156:338–353. doi: 10.1111/j.1476-5381.2008.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1998. [Google Scholar]
- Pazos A, Palacios JM. Quantitative autoradiographic mapping of serotonin receptors in the rat brain. I. Serotonin-1 receptors. Brain Res. 1985;346:205–230. doi: 10.1016/0006-8993(85)90856-x. [DOI] [PubMed] [Google Scholar]
- Plant T, Schirra C, Garaschuk O, Rossier J, Konnerth A. Molecular determinants of NMDA receptor function in GABAergic neurones of rat forebrain. J Physiol. 1997;399:47–63. doi: 10.1113/jphysiol.1997.sp021910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J Neurosci. 1992;12:440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig MV, Celada P, Diaz-Mataix L, Artigas F. In vivo modulation of the activity of pyramidal neurons in the rat medial prefrontal cortex by 5-HT2A receptors: relationship to thalamocortical afferents. Cereb Cortex. 2003;13:870–882. doi: 10.1093/cercor/13.8.870. [DOI] [PubMed] [Google Scholar]
- Puig MV, Artigas F, Celada P. Modulation of the activity of pyramidal neurons in rat prefrontal cortex by raphe stimulation in vivo: involvement of serotonin and GABA. Cereb Cortex. 2005;15:1–14. doi: 10.1093/cercor/bhh104. [DOI] [PubMed] [Google Scholar]
- Riad M, Garcia S, Watkins KC, Jodoin N, Doucet E, Langlois X, et al. Somatodendritic localization of 5-HT1A and preterminal axonal localization of 5-HT1B serotonin receptors in adult rat brain. J Comp Neurol. 2000;417:181–194. [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Zorn SH. Clozapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Eur J Pharmacol. 1997;338:R3–R5. doi: 10.1016/s0014-2999(97)81951-6. [DOI] [PubMed] [Google Scholar]
- Rollema H, Lu Y, Schmidt AW, Sprouse JS, Zorn SH. 5-HT1A receptor activation contributes to ziprasidone-induced dopamine release in the rat prefrontal cortex. Biol Psychiatry. 2000;48:229–237. doi: 10.1016/s0006-3223(00)00850-7. [DOI] [PubMed] [Google Scholar]
- Romero L, Celada P, Martín-Ruiz R, Díaz-Mataix L, Mourelle M, Delgadillo J, et al. Modulation of serotonergic function in rat brain by VN2222, a serotonin reuptake inhibitor and 5-HT1A receptor agonist. Neuropsychopharmacology. 2003;28:445–456. doi: 10.1038/sj.npp.1300062. [DOI] [PubMed] [Google Scholar]
- Rueter LE, Blier P. Electrophysiological examination of the effects of sustained flibanserin administration on serotonin receptors in rat brain. Br J Pharmacol. 1999;126:627–638. doi: 10.1038/sj.bjp.0702344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Vives MV, McCormick DA. Cellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat Neurosci. 2000;3:1027–1034. doi: 10.1038/79848. [DOI] [PubMed] [Google Scholar]
- Santana N, Bortolozzi A, Serrats J, Mengod G, Artigas F. Expression of 5-HT1A and 5-HT2A receptors in pyramidal and GABAergic neurons of the rat prefrontal cortex. Cereb Cortex. 2004;14:1100–1109. doi: 10.1093/cercor/bhh070. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Electrophysiological responses of serotonergic dorsal raphe neurons to 5-HT1A and 5-HT1B agonists. Synapse. 1987;1:3–9. doi: 10.1002/syn.890010103. [DOI] [PubMed] [Google Scholar]
- Sprouse JS, Aghajanian GK. Responses of hippocampal pyramidal cells to putative serotonin 5-HT1A and 5-HT1B agonists: a comparative study with dorsal raphe neurons. Neuropharmacology. 1988;27:707–715. doi: 10.1016/0028-3908(88)90079-2. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Nohara S, Yamashita I, Kurachi M, Sumiyoshi C, et al. Enhancement of cognitive performance in schizophrenia by addition of tandospirone to neuroleptic treatment. Am J Psychiatry. 2001a;158:1722–1725. doi: 10.1176/appi.ajp.158.10.1722. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Matsui M, Yamashita I, Nohara S, Kurachi M, Uehara T, et al. The effect of tandospirone, a serotonin1A agonist, on memory function in schizophrenia. Biol Psychiatry. 2001b;49:861–868. doi: 10.1016/s0006-3223(00)01025-8. [DOI] [PubMed] [Google Scholar]
- Tanaka E, North RA. Actions of 5 hydroxytryptamine on neurons of the rat cingulate cortex. J Neurophysiol. 1993;69:1749–1757. doi: 10.1152/jn.1993.69.5.1749. [DOI] [PubMed] [Google Scholar]
- Wang RY, Aghajanian GK. Antidromically identified serotonergic neurons in the rat midbrain raphe: evidence for collateral inhibition. Brain Res. 1977;132:186–193. doi: 10.1016/0006-8993(77)90719-3. [DOI] [PubMed] [Google Scholar]


