Abstract
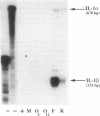
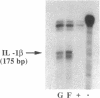
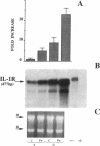
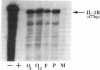
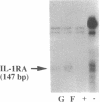
To delineate the scope of the human intraovarian IL-1 system we used a solution hybridization/RNase protection assay to test for expression of the genes encoding IL-1, its type I receptor (IL-1R), and its receptor antagonist (IL-1RA). IL-1 transcripts were not detected in whole ovarian material from days 4 or 12 of an unstimulated menstrual cycle but transcripts (IL-1 beta much greater than IL-11 alpha) were detected in preovulatory follicular aspirates from gonadotropin-stimulated cycles. Concurrently obtained peripheral monocytes did not contain IL-1 beta transcripts but macrophage-depleted follicular aspirates did, thus implicating the granulosa cells as the site of IL-1 expression. IL-1R transcripts were detected in RNA from whole ovaries and follicular aspirates but not in RNA from peripheral monocytes. IL-1RA transcripts were detected in whole ovarian material as well as in macrophage-free follicular aspirates. Cultured human granulosa and theca cells did not contain mRNA for IL-1 beta or IL-1RA but did contain mRNA for IL-1R. Treatment of cell cultures with forskolin (25 microM) induced IL-1 beta transcripts in granulosa but not theca cells. Forskolin also increased the basal levels of IL-1R transcripts in both granulosa and theca cells but did not induce IL-RA transcripts in either cell type. Taken together, these findings reveal the existence of a complete, highly compartmentalized, hormonally dependent intraovarian IL-1 system replete with ligands, receptor, and receptor antagonist.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arend W. P., Welgus H. G., Thompson R. C., Eisenberg S. P. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Invest. 1990 May;85(5):1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auron P. E., Webb A. C., Rosenwasser L. J., Mucci S. F., Rich A., Wolff S. M., Dinarello C. A. Nucleotide sequence of human monocyte interleukin 1 precursor cDNA. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7907–7911. doi: 10.1073/pnas.81.24.7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomsztyk K., Sims J. E., Stanton T. H., Slack J., McMahan C. J., Valentine M. A., Dower S. K. Evidence for different interleukin 1 receptors in murine B- and T-cell lines. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8034–8038. doi: 10.1073/pnas.86.20.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonin P. D., Chiou W. J., McGee J. E., Singh J. P. Two signal transduction pathways mediate interleukin-1 receptor expression in Balb/c3T3 fibroblasts. J Biol Chem. 1990 Oct 25;265(30):18643–18649. [PubMed] [Google Scholar]
- Carter D. B., Deibel M. R., Jr, Dunn C. J., Tomich C. S., Laborde A. L., Slightom J. L., Berger A. E., Bienkowski M. J., Sun F. F., McEwan R. N. Purification, cloning, expression and biological characterization of an interleukin-1 receptor antagonist protein. Nature. 1990 Apr 12;344(6267):633–638. doi: 10.1038/344633a0. [DOI] [PubMed] [Google Scholar]
- Chizzonite R., Truitt T., Kilian P. L., Stern A. S., Nunes P., Parker K. P., Kaffka K. L., Chua A. O., Lugg D. K., Gubler U. Two high-affinity interleukin 1 receptors represent separate gene products. Proc Natl Acad Sci U S A. 1989 Oct;86(20):8029–8033. doi: 10.1073/pnas.86.20.8029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayer J. M., de Rochemonteix B., Burrus B., Demczuk S., Dinarello C. A. Human recombinant interleukin 1 stimulates collagenase and prostaglandin E2 production by human synovial cells. J Clin Invest. 1986 Feb;77(2):645–648. doi: 10.1172/JCI112350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and its biologically related cytokines. Adv Immunol. 1989;44:153–205. doi: 10.1016/s0065-2776(08)60642-2. [DOI] [PubMed] [Google Scholar]
- Eisenberg S. P., Evans R. J., Arend W. P., Verderber E., Brewer M. T., Hannum C. H., Thompson R. C. Primary structure and functional expression from complementary DNA of a human interleukin-1 receptor antagonist. Nature. 1990 Jan 25;343(6256):341–346. doi: 10.1038/343341a0. [DOI] [PubMed] [Google Scholar]
- Espey L. L. Ovulation as an inflammatory reaction--a hypothesis. Biol Reprod. 1980 Feb;22(1):73–106. doi: 10.1095/biolreprod22.1.73. [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Mori T., Taii S., Yasuda K. Interleukin-1 inhibits luteinization of porcine granulosa cells in culture. Endocrinology. 1988 Jan;122(1):367–369. doi: 10.1210/endo-122-1-367. [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Taii S., Yasuda K., Takakura K., Mori T. Inhibitory effects of interleukin-1 on luteinizing hormone-stimulated adenosine 3',5'-monophosphate accumulation by cultured porcine granulosa cells. Endocrinology. 1989 Jul;125(1):136–143. doi: 10.1210/endo-125-1-136. [DOI] [PubMed] [Google Scholar]
- Fukuoka M., Yasuda K., Taii S., Takakura K., Mori T. Interleukin-1 stimulates growth and inhibits progesterone secretion in cultures of porcine granulosa cells. Endocrinology. 1989 Feb;124(2):884–890. doi: 10.1210/endo-124-2-884. [DOI] [PubMed] [Google Scholar]
- Gottschall P. E., Katsuura G., Arimura A. Interleukin-1 beta is more potent than interleukin-1 alpha in suppressing follicle-stimulating hormone-induced differentiation of ovarian granulosa cells. Biochem Biophys Res Commun. 1989 Sep 15;163(2):764–770. doi: 10.1016/0006-291x(89)92288-2. [DOI] [PubMed] [Google Scholar]
- Gottschall P. E., Katsuura G., Dahl R. R., Hoffmann S. T., Arimura A. Discordance in the effects of interleukin-1 on rat granulosa cell differentiation induced by follicle-stimulating hormone or activators of adenylate cyclase. Biol Reprod. 1988 Dec;39(5):1074–1085. doi: 10.1095/biolreprod39.5.1074. [DOI] [PubMed] [Google Scholar]
- Gottschall P. E., Katsuura G., Hoffmann S. T., Arimura A. Interleukin 1: an inhibitor of luteinizing hormone receptor formation in cultured rat granulosa cells. FASEB J. 1988 Jun;2(9):2492–2496. doi: 10.1096/fasebj.2.9.3131173. [DOI] [PubMed] [Google Scholar]
- Gottschall P. E., Uehara A., Hoffmann S. T., Arimura A. Interleukin-1 inhibits follicle stimulating hormone-induced differentiation in rat granulosa cells in vitro. Biochem Biophys Res Commun. 1987 Dec 16;149(2):502–509. doi: 10.1016/0006-291x(87)90396-2. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Glaister D., Chen E., Goeddel D. V., Pennica D. Two interleukin 1 genes in the mouse: cloning and expression of the cDNA for murine interleukin 1 beta. J Immunol. 1986 Dec 1;137(11):3644–3648. [PubMed] [Google Scholar]
- Horuk R., Huang J. J., Covington M., Newton R. C. A biochemical and kinetic analysis of the interleukin-1 receptor. Evidence for differences in molecular properties of IL-1 receptors. J Biol Chem. 1987 Dec 5;262(34):16275–16278. [PubMed] [Google Scholar]
- Hurwitz A., Payne D. W., Packman J. N., Andreani C. L., Resnick C. E., Hernandez E. R., Adashi E. Y. Cytokine-mediated regulation of ovarian function: interleukin-1 inhibits gonadotropin-induced androgen biosynthesis. Endocrinology. 1991 Sep;129(3):1250–1256. doi: 10.1210/endo-129-3-1250. [DOI] [PubMed] [Google Scholar]
- Jayson M. I. Use of sequential analysis to assess patient preference for local skin anaesthesia during knee aspiration. Br J Rheumatol. 1985 Feb;24(1):118–119. doi: 10.1093/rheumatology/24.1.118. [DOI] [PubMed] [Google Scholar]
- Kasson B. G., Gorospe W. C. Effects of interleukins 1, 2 and 3 on follicle-stimulating hormone-induced differentiation of rat granulosa cells. Mol Cell Endocrinol. 1989 Mar;62(1):103–111. doi: 10.1016/0303-7207(89)90118-4. [DOI] [PubMed] [Google Scholar]
- Khan S. A., Schmidt K., Hallin P., Di Pauli R., De Geyter C., Nieschlag E. Human testis cytosol and ovarian follicular fluid contain high amounts of interleukin-1-like factor(s). Mol Cell Endocrinol. 1988 Aug;58(2-3):221–230. doi: 10.1016/0303-7207(88)90158-x. [DOI] [PubMed] [Google Scholar]
- Kupper T. S., Ballard D. W., Chua A. O., McGuire J. S., Flood P. M., Horowitz M. C., Langdon R., Lightfoot L., Gubler U. Human keratinocytes contain mRNA indistinguishable from monocyte interleukin 1 alpha and beta mRNA. Keratinocyte epidermal cell-derived thymocyte-activating factor is identical to interleukin 1. J Exp Med. 1986 Dec 1;164(6):2095–2100. doi: 10.1084/jem.164.6.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Loukides J. A., Loy R. A., Edwards R., Honig J., Visintin I., Polan M. L. Human follicular fluids contain tissue macrophages. J Clin Endocrinol Metab. 1990 Nov;71(5):1363–1367. doi: 10.1210/jcem-71-5-1363. [DOI] [PubMed] [Google Scholar]
- Lowe W. L., Jr, Roberts C. T., Jr, Lasky S. R., LeRoith D. Differential expression of alternative 5' untranslated regions in mRNAs encoding rat insulin-like growth factor I. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8946–8950. doi: 10.1073/pnas.84.24.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March C. J., Mosley B., Larsen A., Cerretti D. P., Braedt G., Price V., Gillis S., Henney C. S., Kronheim S. R., Grabstein K. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985 Jun 20;315(6021):641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- Matsushima K., Akahoshi T., Yamada M., Furutani Y., Oppenheim J. J. Properties of a specific interleukin 1 (IL 1) receptor on human Epstein Barr virus-transformed B lymphocytes: identity of the receptor for IL 1-alpha and IL 1-beta. J Immunol. 1986 Jun 15;136(12):4496–4502. [PubMed] [Google Scholar]
- McAllister J. M., Kerin J. F., Trant J. M., Estabrook R. W., Mason J. I., Waterman M. R., Simpson E. R. Regulation of cholesterol side-chain cleavage and 17 alpha-hydroxylase/lyase activities in proliferating human theca interna cells in long term monolayer culture. Endocrinology. 1989 Oct;125(4):1959–1966. doi: 10.1210/endo-125-4-1959. [DOI] [PubMed] [Google Scholar]
- McAllister J. M., Mason J. I., Byrd W., Trant J. M., Waterman M. R., Simpson E. R. Proliferating human granulosa-lutein cells in long term monolayer culture: expression of aromatase, cholesterol side-chain cleavage, and 3 beta-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1990 Jul;71(1):26–33. doi: 10.1210/jcem-71-1-26. [DOI] [PubMed] [Google Scholar]
- Nakamura Y., Kato H., Terranova P. F. Interleukin-1 alpha increases thecal progesterone production of preovulatory follicles in cyclic hamsters. Biol Reprod. 1990 Aug;43(2):169–173. doi: 10.1095/biolreprod43.2.169. [DOI] [PubMed] [Google Scholar]
- Padilla S. L., Bayati J., Garcia J. E. Prognostic value of the early serum estradiol response to leuprolide acetate in in vitro fertilization. Fertil Steril. 1990 Feb;53(2):288–294. doi: 10.1016/s0015-0282(16)53283-x. [DOI] [PubMed] [Google Scholar]
- Polan M. L., Daniele A., Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988 Jun;49(6):964–968. [PubMed] [Google Scholar]
- Polan M. L., Loukides J., Nelson P., Carding S., Diamond M., Walsh A., Bottomly K. Progesterone and estradiol modulate interleukin-1 beta messenger ribonucleic acid levels in cultured human peripheral monocytes. J Clin Endocrinol Metab. 1989 Dec;69(6):1200–1206. doi: 10.1210/jcem-69-6-1200. [DOI] [PubMed] [Google Scholar]
- Sims J. E., Acres R. B., Grubin C. E., McMahan C. J., Wignall J. M., March C. J., Dower S. K. Cloning the interleukin 1 receptor from human T cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8946–8950. doi: 10.1073/pnas.86.22.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. F., Jr, Kueppers F. R., Young P. R., Lee J. C. A rapid and quantitative method for the determination of interleukin-1 alpha and -beta mRNA expression in human monocytes and macrophages. J Immunol Methods. 1989 Mar 31;118(2):265–272. doi: 10.1016/0022-1759(89)90015-x. [DOI] [PubMed] [Google Scholar]
- Takakura K., Taii S., Fukuoka M., Yasuda K., Tagaya Y., Yodoi J., Mori T. Interleukin-2 receptor/p55(Tac)-inducing activity in porcine follicular fluids. Endocrinology. 1989 Aug;125(2):618–623. doi: 10.1210/endo-125-2-618. [DOI] [PubMed] [Google Scholar]
- Watson M. L., Lewis G. P., Westwick J. Increased vascular permeability and polymorphonuclear leucocyte accumulation in vivo in response to recombinant cytokines and supernatant from cultures of human synovial cells treated with interleukin 1. Br J Exp Pathol. 1989 Feb;70(1):93–101. [PMC free article] [PubMed] [Google Scholar]
- Yaron I., Meyer F. A., Dayer J. M., Yaron M. Human recombinant interleukin-1 beta stimulates glycosaminoglycan production in human synovial fibroblast cultures. Arthritis Rheum. 1987 Apr;30(4):424–430. doi: 10.1002/art.1780300410. [DOI] [PubMed] [Google Scholar]
- Yoshimura Y., Wallach E. E. Studies of the mechanism(s) of mammalian ovulation. Fertil Steril. 1987 Jan;47(1):22–34. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]