Abstract
BACKGROUND AND PURPOSE
The compound NS5806 increases the transient outward current (Ito) in canine ventricular cardiomyocytes and slows current decay. In human and canine ventricle, Ito is thought to be mediated by KV4.3 and various ancillary proteins, yet, the exact subunit composition of Ito channels is still debated. Here we characterize the effect of NS5806 on heterologously expressed putative Ito channel subunits and other potassium channels.
EXPERIMENTAL APPROACH
Cloned KV4 channels were co-expressed with KChIP2, DPP6, DPP10, KCNE2, KCNE3 and KV1.4 in Xenopus laevis oocytes or CHO-K1 cells.
KEY RESULTS
NS5806 increased KV4.3/KChIP2 peak current amplitudes with an EC50 of 5.3 ± 1.5µM and significantly slowed current decay. KCNE2, KCNE3, DPP6 and DPP10 modulated KV4.3 currents and the response to NS5806, but current decay was slowed only in complexes containing KChIP2. The effect of NS5806 on KV4.2 was similar to that on KV4.3, and current decay was only slowed in presence of KChIP2. However, for KV4.1, the slowing of current decay by NS5806 was independent of KChIP2. KV1.4 was strongly inhibited by 10 µM NS5806 and KV1.5 was inhibited to a smaller extent. Effects of NS5806 on kinetics of currents generated by KV4.3/KChIP2/DPP6 with KV1.4 in oocytes could reproduce those on cardiac Ito in canine ventricular myocytes. KV7.1, KV11.1 and Kir2 currents were unaffected by NS5806.
CONCLUSION AND IMPLICATIONS
NS5806 modulated KV4 channel gating depending on the presence of KChIP2, suggesting that NS5806 can potentially be used to address the molecular composition as well as the physiological role of cardiac Ito.
Keywords: Ito, transient outward potassium current, KV4.3, KV1.4, KChIP2, DPP6, KCNE, NS5806
Introduction
Epi- and midmyocardial cells from larger mammals have a prominent phase 1 repolarization of the action potential due to the presence of a Ca2+-independent transient outward potassium current (Ito). In human and canine hearts, Ito is principally mediated by the α-subunit KV4.3, but other channels such as KV1.4 may be involved (Dixon et al., 1996; Akar et al., 2004; channel nomenclature follows Alexander et al., 2009). However, heterologously expressed KV4.3 channels do not reproduce the kinetics of native cardiac Ito and KV4.3 has been demonstrated to assemble with various ancillary β-subunits in cardiac tissue; the most prominent of which is K Channel Interaction Protein 2 (KChIP2). KChIP2 belongs to a large family of cytosolic Ca2+– sensing proteins that contain up to four putative Ca2+ binding EF-hands. KChIP2 increases KV4.3 current density by facilitating trafficking, slowing inactivation and by accelerating recovery kinetics (An et al., 2000). Interestingly, the expression of KChIP2 mRNA is more abundant in epi- and midmyocardium than in endocardium and this differential expression of KChIP2 has been suggested to underlie the transmural Ito gradient (Rosati et al., 2001; 2003; Calloe et al., 2009b). Besides KChIP2, several other proteins expressed in ventricular tissue have been shown to interact with KV4.3. Dipeptidyl-peptidase (DDP) 6 can facilitate KV4.3 trafficking, accelerate KV4.3 inactivation (Nadal et al., 2003) and increase single channel conductance of KV4.2 (Kaulin et al., 2009). Co-expression of KV4.3 with KChIP2 and DPP6 in heterologous systems results in currents with kinetics similar to those of Ito in human ventricular myocytes (Radicke et al., 2005).
Members of the KCNE β-subunit family have been shown to modulate KV4.3 currents (Zhang et al., 2001; Lundby and Olesen, 2006; Radicke et al., 2006). KCNE2 may be a promising candidate for the human Ito channel complex as co-expression of KV4 channels with KCNE2 induced an overshoot of peak current during recovery from inactivation (Zhang et al., 2001; Radicke et al., 2006), comparable to that described for Ito in human epicardial myocytes (Wettwer et al., 1994). However, other studies have shown that KCNE2 is expressed in low quantities in ventricular tissue compared with Purkinje tissue (Pourrier et al., 2003) suggesting KCNE2 is important mainly in the conduction system of the heart (Sanguinetti and Tristani-Firouzi, 2006). KCNE3 has been shown to inhibit KV4.3 currents (Lundby and Olesen, 2006) and mutations in KCNE3 resulting in less inhibition of KV4.3 current have been linked to the Brugada syndrome (Delpón et al., 2008) and atrial fibrillation (Lundby et al., 2008). Furthermore, heterologously expressed KV4.3 channels are modulated by KVβ, KChAP and NaVβ accessory subunits (Deschenes and Tomaselli, 2002) but their physiological roles have yet to be determined.
KV4 currents are selectively inhibited by several spider toxins that modify gating kinetics, including the Heteropoda venatoria toxins (Sanguinetti et al., 1997) HpTX2 (Zarayskiy et al., 2005) and HpTX3 (Brahmajothi et al., 1999), the Phrixotrichus auratus toxins PaTx1 and PaTx2 (Diochot et al., 1999) and the Theraphosa leblondi toxins TLx1-3 (Ebbinghaus et al., 2004). In common with other A-type KV channels, KV4.3 is blocked by 4-aminopyridine (4-AP) in millimolar range concentrations (Wang et al., 1995). Ito is blocked by several sodium channel blockers, including flecainide (Radicke et al., 2008) and quinidine (Wang et al., 1995), and several calcium channel blockers, including nifedipine (Hatano et al., 2003; Bett et al., 2006). We have recently added an Ito activator, NS5806, to this list of compounds affecting Ito and described the effect of NS5806 on canine ventricular wedge preparations (Calloe et al., 2009a) as well as on native Ito in isolated cells from canine left ventricular epi-, mid- and endocardium (Calloe et al., 2009b). We found that 10 µM NS5806 increased the magnitude of Ito, slowed current decay, induced a negative shift in steady-state inactivation and accelerated recovery from inactivation for native Ito.
In the present study, we characterized the effects of NS5806 on heterologously expressed putative Ito channel subunits. NS5806 enhanced peak currents for all KV4 channels and affected channel gating. In the presence of KChIP2, NS5806 slowed the decay of KV4.2 and KV4.3 currents significantly, whereas it had little effect in the absence of KChIP2. Co-expression of KV4.3 with and without KChIP2 with DPP6, DPP10, KCNE2 or KCNE3 β-subunits corroborated that NS5806 only slowed current decay of channel complexes containing KChIP2. Besides the effects on KV4 channels, NS5806 inhibited KV1.4 and KV1.5 mediated currents independently of the presence of KChIP2. Effects of NS5806 on currents generated by KV4.3/KChIP2/DPP6 with KV1.4 in oocytes could reproduce those on cardiac Ito in canine ventricular myocytes
Methods
NS5806
NS5806 (1-[2,4-dibromo-6-(1H-tetrazol-5-yl)-phenyl]-3-(3,5-bis-trifluoromethyl-phenyl)-urea) was synthesized at NeuroSearch A/S (Ballerup, Denmark) by reaction of 6-cyano-2,4-dibromoaniline with sodium azide to form the respective 2,4-dibromo-6-tetrazolylaniline, which was condensed with 3,5-bis-trifluoromethyl-phenylisocyanate to provide the final product, NS5806. NS5806 was dissolved in DMSO to give a concentrated stock solution of 30 mM. The final DMSO concentration never exceeded 0.1%, and at this concentration DMSO did not influence the electrical properties of the cells.
Molecular biology
α-subunits
cDNAs coding for human (h) Kv4.1 (NM_004979) and hKv4.2 (NM_012281) were a kind gift from D. Isbrandt (U Hamburg, Germany). cDNA coding for hKv4.3 (short isoform, NM_172198) and hKv1.5 (NM_002234) were kindly provided by O. Pongs (U Hamburg, Germany). hKv4.3 cDNA was subcloned into the expression vector pXOOM, Kv1.5 was cloned into pXOON. hKv4.1 and hKv4.2 were PCR-amplified and cloned into the expression vector pGEM-HEJuel. cDNA encoding hKv1.4 (NM_002233) was amplified from EST HU_p940D11203D (ImaGenes, Berlin, Germany) and cloned into pGEM-HEJuel.
Ancillary subunits
hKChIP2.1 (NM_173192) was amplified from IMAGE clone 2430271 and subcloned into pXOOM. cDNA coding for hDPP6 iso 2 (NM_001936) and hDPP10 iso 1 (NM_020868) were kindly provided by E. Wettwer (TU Dresden, Germany), PCR-amplified and cloned into pGEM-HEJuel. KCNE2 (NM_172201) in pSGEM and hKCNE3 (NM_005472) in pXOOM have been described previously (Lundby and Olesen, 2006). All constructs were verified by sequencing.
Heterologous expression in Xenopus laevis oocytes
All animal care and experimental procedures were in accordance with Danish National Committee for Animal Studies guidelines. Female Xenopus laevis frogs were anesthetized (2 g·L−1 Tricaine; Sigma) and ovarian lobes cut off through a small abdominal incision. The oocytes were manually dissected into smaller groups and defolliculated using collagenase (Type 1, Sigma-Aldrich) for 1 h. Oocytes were kept in Kulori solution (mM); NaCl 90, KCl 4, MgCl2 1, CaCl2 1, HEPES 5, pH 7.4 with NaOH at 19°C for 24 h before injection of cRNA. cRNA was prepared from linearized plasmid DNA using the T7 mMessage mMachine kit (Ambion) according to the manufacturer's instructions. 50 nl containing 0.5 ng KV4 cRNA and ancillary subunits added in a 1:1 molar ratio was injected using a Nanoject microinjector (Drummond Scientific, Broowell, PA, USA). KV4.3/KChIP2 and KV1.4 channels were co-expressed in a 1:1 current ratio corresponding to 0.15 ng KV4.3 + 0.15 ng KChIP2 + 1.3 ng KV1.4 pr oocyte. The oocytes were kept in Kulori solution at 19°C, which was changed daily and currents were recorded after 2 to 3 days.
Two-electrode voltage clamp
Recordings were at room temperature in Kulori solution using a two-electrode voltage-clamp amplifier (Dagan CA-1B; Chicago, IL, USA). Borosilicate glass recording electrodes (Module Ohm, Denmark) were fabricated using a DMZ-Universal Puller (Zeitz Instruments, Munich, Germany) and had a resistance of 0.5 to 1 MΩ when filled with 3 M KCl.
CHO-K1 cell culture and transfection
CHO-K1 cells were transiently transfected with hKV4.3 (0.5 µg to a 25 cm2 cell flask), hKChIP2.1, hDPP6 in a 1:3:3 molar ratio using Lipofectamine and Plus Reagent according to manufacturer's instruction (Gibco, Invitrogen). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM, Substrate Department, University of Copenhagen, Denmark) supplemented with 10% fetal calf serum (Gibco, Invitrogen) and 40 mg·L−1 L-proline at 37°C in 5% CO2.
Electrophysiological recordings of transiently transfected CHO-K1
Whole-cell currents were recorded using an EPC-10 amplifier (HEKA Electronics, Lambrecht, Germany). Data were sampled with Pulse software (HEKA Electronics) and analysed with IGOR software (Wavemetrics, Lake Oswego, OR, USA). The series resistance (Rs) was compensated 80 % and did not exceed 3 MΩ. Electrodes were pulled from borosilicate glass capillaries (Module Ohm, Herlev, Denmark) and had tip resistances between 1.5 and 2.5 MΩ. For the KV4.3/KChIP2/DPP6 experiments shown in Figure 1, recording conditions were identical to those previously used for measuring native Ito (Calloe et al., 2009a). Cells were superfused with a HEPES buffer of the following composition (mM): NaCl 126, KCl 5.4, MgCl2 1.0, CaCl2 2.0, HEPES 10 and glucose 11, pH adjusted to 7.4 with NaOH. The patch pipette solution had the following composition (mM): K-aspartate 90, KCl 30, glucose 5.5, MgCl2 1.0, EGTA 5, MgATP 5, HEPES 5, NaCl 10, pH = 7.2 with KOH. In myocytes, Ito was recorded in the presence of 300 µM Cd2+ which was used to block ICaL. We therefore included 300 µM Cd2+ in the present study to allow a more direct comparison.
Figure 1.
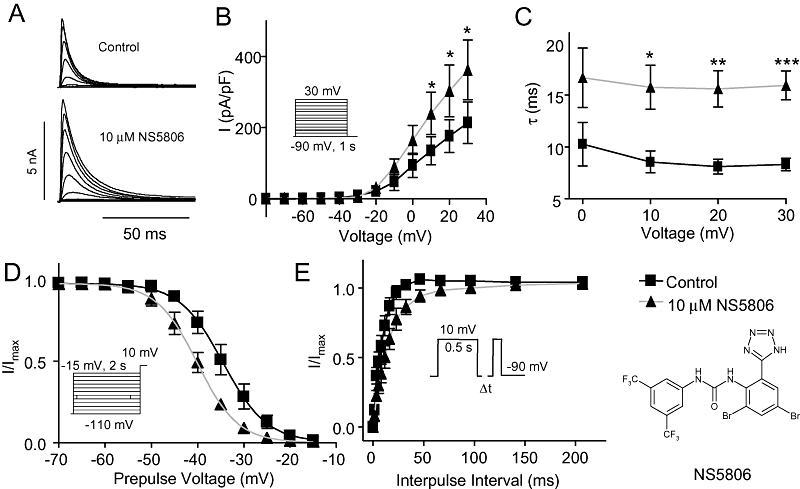
Effect of NS5806 on KV4.3/KChIP2/DPP6. KV4.3/KChIP2/DPP6 were transiently expressed in CHO-K1 cells and currents were measured in the absence and in the presence of 10 µM NS5806. (A) Representative recordings of KV4.3/KChIP2/DPP6 currents elicited by the protocol shown in panel B. (B) Relation between peak current density and voltage, n= 5. (C) Mono-exponential functions were fitted to the current decays, and the time constants (τ) are shown as a function of voltage, n= 7). (D) Steady-state inactivation of KV4.3/KChIP2/DPP6 currents. Normalized tail current amplitudes recorded at +10 mV are plotted as a function of the prepulse potential and Boltzmann equations are fitted to the data, n= 5. (E) Time-dependent release from inactivation. KV4.3/KChIP2/DPP6 currents were activated by the depicted two-pulse protocol. Current amplitudes at the second test pulse were normalized to that of the first test pulse and plotted as a function of the inter-pulse interval, and a single exponential equation was fitted to the data, n= 6). *P < 0.05, **P < 0.01, ***P < 0.001; significantly different in the absence and presence of NS5806. CHO-K1, Chinese hamster ovary cells; DPP, dipeptidyl-peptidase; KChIP2, K channel interacting protein 2.
For CHO-K1 cells expressing KV4.3/KChIP2/DPP6, the membrane capacitance was 14.0 ± 2.2 pF and the mean peak current 7.5 ± 2.0 nA. For the concentration-response experiments on KV4.3/KChIP2 (Figure 3), a standard extracellular NaCl solution consisted of (mM): NaCl 140, KCl 4, CaCl2 2, MgCl2 1, HEPES 10, pH = 7.4 adjusted with NaOH was used and the intracellular solution contained (mM): KCl 110, KOH/EDTA 31/10, CaCl2 5.17, MgCl2 1.42, HEPES 10, MgATP 4, pH = 7.2 with KOH. The membrane capacitance was 14.9 ± 1.4 pF and the mean peak current in control 7.1 ± 1.4 nA at +20 mV. All experiments were performed at 37 ± 1°C.
Figure 3.
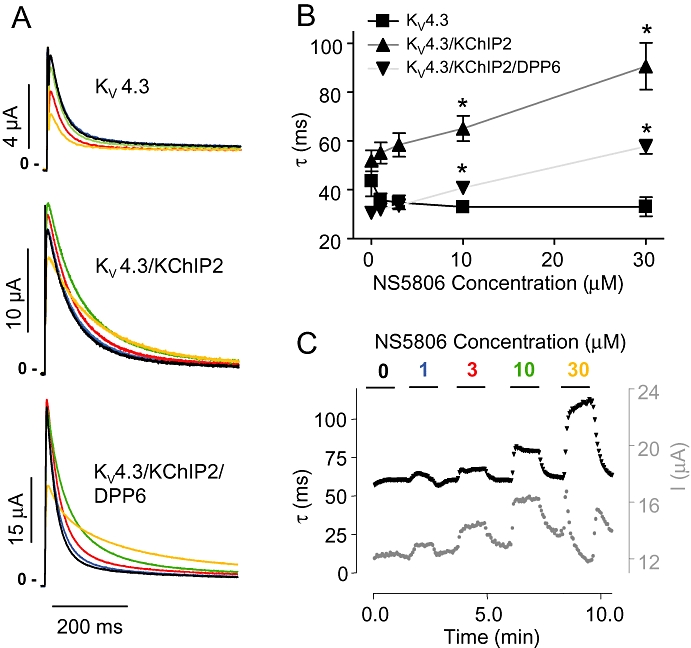
Concentration dependent effect of NS5806 on KV4.3, KChIP2 and DPP6 in Xenopus laevis oocytes. KV4.3, KChIP2 and/or DPP6 were expressed in Xenopus laevis oocytes. Currents were repeatedly activated from −80 mV by steps to +40 mV. (A) Representative recordings of KV4.3, KV4.3/KChIP2 and KV4.3/KChIP2/DPP6 currents in presence of 0 (black), 1 (blue), 3 (red) 10 (green) or 30 (orange) µM NS5806. (B) Single exponential functions were fitted to the first 150 ms of KV4.3, KV4.3/KChIP2 and KV4.3/KChIP2/DDP6 current decays and the time constants (τ) are plotted as a function of NS5806 concentration (n= 6–8). *P < 0.05, significantly different in the absence and presence of NS5806. (C) KV4.3/KChIP2 time-course experiment. The black trace shows the effect of increasing concentrations of NS5806 on KV4.3/KChIP2 peak current decay (τ; lefthand Y axis) and the grey trace shows the effect on the peak current amplitude (I, righthand Y axis), representative of n= 6. DPP, dipeptidyl-peptidase; KChIP2, K channel interacting protein 2.
Statistics
Mean ± SEM are shown. Statistical significance was evaluated by Student's t-test and one way anova with Dunnett's post test as appropriate using GraphPad Prism (GraphPad Software, San Diego, CA, USA).
Results
Effect of NS5806 on KV4.3/KChIP2/DPP6 channels expressed in CHO-K1 cells
KV4.3 together with KChIP2 and DPP6 produces a current resembling native Ito (Radicke et al., 2005). As an initial basis for comparison, we co-expressed KV4.3/KChIP2/DPP6 in CHO-K1 cells and recorded currents in solutions identical to those previously used for native Ito measurements (Calloe et al., 2009a). As we found with native Ito, 10 µM NS5806 induced a 65% increase of KV4.3/KChIP2/DPP6 peak current amplitudes (Figure 1A and B). The time constant of decay (τ) of Ito was significantly slowed as reflected in 80% increase in τ-values over a range of voltages (Figure 1C). Steady-state gating parameters were evaluated using a pre-pulse-test pulse voltage clamp protocol. The peak current following a 2 s prepulse was normalized to the maximum current and plotted as a function of prepulse voltage to obtain the availability of channels. A Boltzmann curve was fitted to the data and the mid-inactivation voltage (V1/2) was determined. NS5806 induced a significant left-shift in mid-inactivation from −34.5 ± 0.6 mV to −40 ± 0.4 mV (Figure 1D), indicating a tendency for a larger fraction of channels being in the inactivated state in presence of NS5806, as observed for native Ito (Calloe et al., 2009b).
Time-dependent recovery from inactivation of KV4.3/KChIP2/DPP6 was evaluated by a two-pulse protocol with increasing interpulse intervals. The fraction of recovered current was plotted as a function of interpulse interval and an exponential equation fitted to the data. NS5806 significantly slowed recovery of KV4.3/KChIP2/DPP6 current, with time constants of 9.3 ± 0.6 ms prior to NS5806 application versus 16.4 ± 1.2 ms after application of 10 µM NS5806. These results were opposite to the effect of NS5806 on Ito recorded in isolated canine ventricular cardiomyocytes where recovery of Ito was faster in the presence of NS5806.
Concentration-dependence of the effect of NS5806 on KV4.3/KChIP2/DDP6 channels
To characterize NS5806, we initially tested the concentration-dependent effect of NS5806 on peak current amplitude and time course of current inactivation of Kv4.3/KChIP2 expressed in CHO-K1 cells. NS5806 increased peak-current amplitudes concentration-dependently with an EC50 value of 5.3 ± 1.5 µM and the time course of inactivation (τ) was slowed with an EC50 value of 25.4 ± 1.1 µM (Figure 2).
Figure 2.
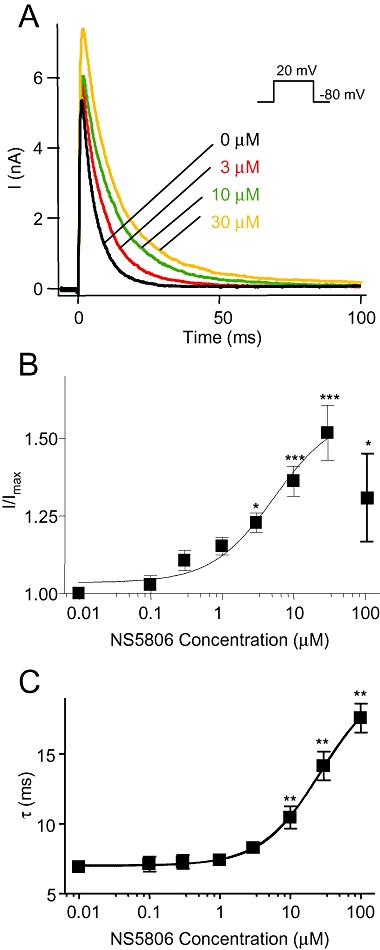
Concentration dependent effect of NS5806 on KV4.3/KChIP2 in CHO-K1 cells. KV4.3 and KChIP2 were transiently expressed in CHO-K1 cells and measured in presence of 0 to 100 µM NS5806 as indicated in the figure. (A) Representative current recordings at different NS5806 concentrations. (B) Peak current amplitudes normalized to current amplitudes in absence of drug and plotted as function of NS5806 concentration. A sigmoidal dose-response curve was fitted to the data in the range from 0–30 µM and an EC50 of 5.3 ± 1.5 µM with and a Hill slope of 1.1 ± 0.05 and minimum and maximum values of 1.04 ± 0.02 and 1.6 ± 0.06 respectively. (C) Effect of different NS5806 concentrations on Kv4.3/KChIP2 decay. The time-constants, τ, were plotted as function of NS5806 concentration and revealed an EC50 of 25.4 ± 1.1 µM. The Hill slope was 1.08 ± 0.06 with minimum and maximum values of 7.0 ± 0.1 ms and 20.0 ± 0.5 ms respectively (n= 7–12). *P < 0.05, **P < 0.01, ***P < 0.001; significantly different in the absence and presence of NS5806. CHO-K1, Chinese hamster ovary cells; KChIP2, K channel interacting protein 2.
To further test the effect of NS5806 on different ion channels and multiple combinations of ion channel subunits we used Xenopus laevis oocytes as cRNA encoding ion channel subunits can be injected directly into the oocytes, ensuring better control over subunit ratios than the transfection procedure of mammalian cell lines. In this series of experiments, KV4.3 was expressed in Xenopus laevis oocytes in absence or presence of KChIP2 and DPP6 and currents measured in presence of 0 to 30 µM NS5806 (Figure 3A). Interestingly, the KV4.3 peak current amplitude was reduced by NS5806, whereas NS5806 caused a minor increase in KV4.3/KChIP2 and KV4.3/KChIP2/DPP6 peak current amplitude as well as a pronounced slowing of current decay. In contrast to KV4.3/KChIP2/DPP6 expressed in CHO-K1 cells and native Ito (Calloe et al., 2009a,b;) the effect of NS5806 on KV4.3/KChIP2 peak current amplitude in Xenopus laevis oocytes was minor. The slowing of current decay was concentration-dependent and qualitatively similar with and without DPP6. For KV4.3 expressed alone, there was no effect on the decay even at higher concentrations of NS5806 (Figure 3B). A sigmoidal concentration-response curve was fitted to KV4.3/KChIP2 current decay and an EC50 18.8 ± 2.0 µM was calculated. For all subunit combinations, the effect of NS5806 was rapid and fully reversible, as illustrated by the representative KV4.3/KChIP2 time-course experiment (Figure 3C).
Effect of NS5806 on KV4 family channels in presence or absence of KChIP2
To investigate if NS5806 affected other members of the KV4 family and whether this effect also depended on KChIP2, all KV4 family α-subunits were expressed with and without KChIP2 in oocytes. Currents were activated by depolarizing voltage steps from a holding potential of −100 mV. For KV4.2 and KV4.3, NS5806 had no effect on current amplitude, in contrast to the results presented for KV4.3 in Figure 3, where a holding potential of −80 mV was used. For all KV4 channels, co-expression with KChIP2 resulted in higher current amplitudes and a significantly slower current decay (Figure 4 and Table 1). NS5806 had no effect on current decay of KV4.2 and KV4.3, whereas it markedly slowed the decay in presence of KChIP2 (Figure 4B and C). For KV4.1, NS5806 slowed current decay both in presence and absence of KChIP2 (Figure 4A).
Figure 4.
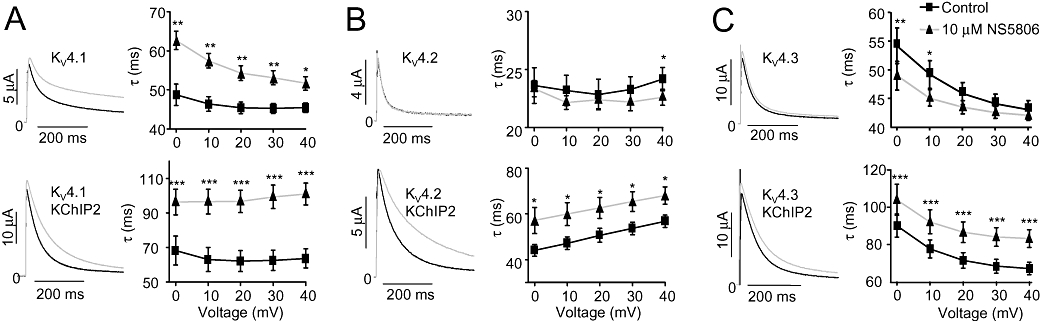
Effect of NS5806 on KV4 channels in presence or absence of KChIP2. KV4 channels were expressed with and without KChIP2 in Xenopus laevis oocytes and currents were elicited from a holding potential of −100 mV by a step to +40 mV in absence and presence of 10 µM NS5806. The first 150 ms of current decay was fitted to a mono-exponential function, and the time constants (τ) are shown as a function of voltage. (A) Representative KV4.1 (n= 5) and KV4.1/KChIP2 currents (n= 6) and τ-values as a function of voltage before and after application of drug. (B) Representative KV4.2 (n= 6) and KV4.2/KChIP2 (n= 10) currents and τ-values as a function of voltage. (C) Representative KV4.3 (n= 8) and KV4.3/KChIP2 (n= 8) currents and τ-values as a function of voltage. *P < 0.05, **P < 0.01, ***P < 0.001; significantly different in the absence and presence of NS5806. KChIP2, K channel interacting protein 2.
Table 1.
Effect of 10 µM NS5806 on Kv4 channels in absence or presence of KChIP2
| Kv4.1 | Kv4.1/KChIP2 | Kv4.2 | Kv4.2/KChIP2 | Kv4.3 | Kv4.3/KChIP2 | |
|---|---|---|---|---|---|---|
| Peak current (µA) control | 12.2 ± 1.1 | 23.6 ± 1.3 | 1.9 ± 0.3 | 6.6 ± 1.4 | 13.8 ± 1.2 | 23.2 ± 1.2 |
| 10 µM NS5806 | 12.6 ± 1.0 (5) | 27.0 ± 1.3 (6)*** | 2.0 ± 0.3 (6) | 7.5 ± 1.3 (10)* | 15.3 ± 1.4 (11) | 26.9 ± 1.3 (9)*** |
| Decay τ (ms) control | 45.4 ± 1.2 | 63.6 ± 5.5 | 24.2 ± 1.1 (6) | 56.8 ± 2.7 | 44.2 ± 1.9 | 67.2 ± 3.4 |
| 10 µM NS5806 | 51.7 ± 1.8 (5)* | 101 ± 6.2 (8)*** | 22.6 ± 0.7 (6)* | 68.1 ± 3.8 (10)* | 43.5 ± 1.5 (11) | 83.2 ± 4.7 (9)*** |
| Area (µA·ms–1) control | 814 ± 70 | 2260 ± 181 | 66 ± 9.8 | 378 ± 74 | 762 ± 63.9 | 1450 ± 103 |
| 10 µM NS5806 | 1250 ± 109 (5)** | 3150 ± 166 (8)*** | 67 ± 10 (6) | 533 ± 101 (10)** | 825 ± 84 (11) | 1890 ± 142 (8)** |
| V1/2 (mV) control | −60.8 ± 0.85 | −44.7 ± 0.7 | −65.2 ± 1.1 | −57.3 ± 0.9 | −55.4 ± 0.5 | −48.6 ± 0.4 |
| 10 µM NS5806 | −75 ± 1.8 (5)*** | −59.5 ± 0.5 (8)*** | −75.7 ± 1.9 (6)*** | −67.1 ± 1.3 (10)*** | −66.0 ± 1.0 (10)*** | −54.0 ± 1.1 (8)** |
| Recovery τ (ms) control | 80.8 ± 3.5 | 8.0 ± 2.8 | 110 ± 29 | 12.6 ± 3.0 | 95.8 ± 2 | 15.9 ± 0.5 |
| 10 µM NS5806 | 205 ± 31 (5)*** | 37.1 ± 10 (8)*** | 202 ± 54 (6)** | 26.4 ± 1.8 (10)*** | 221 ± 8 (8)*** | 23.7 ± 0.48 (8)*** |
KV4 channels expressed with and without KChIP2 (1:1 molar ratio) in Xenopus laevis oocytes. Currents were activated from a holding potential of −100 mV by a +40 mV step before and during application of 10 µM NS5806 and peak current amplitudes, time constants (τ) of current decay and total charge movement for the first 150 ms after complete activation was measured. Steady-state mid-inactivation (V1/2) was evaluated from a holding potential of −100 mV by a series of 2 s prepulses from −100 to +40 mV followed by a +40 mV step. Boltzmann equations were fitted to normalized tail current amplitudes plotted as a function of the prepulse potential. Time constants (τ) for recovery from inactivation was determined by a two-pulse protocol; from a holding potential of −80 mV currents were activated by two test potentials to +40 mV with increasing interpulse time. The peak current at the second pulse was normalized to the first pulse and single exponential functions were fitted to the data points. The different KV4 α-subunits were recorded on different days; however, the experiments in absence and presence of KChIP2 were performed in parallel and thus current amplitude and biophysical characteristics can be directly compared.
P < 0.05,
P < 0.01
P < 0.001; significantly different in the absence and presence of NS5806.
KChIP2, K channel interacting protein 2.
Slowing of KV4/KChIP2 current decay resulted in an increase in total charge movement in presence of the drug, as assessed by the area under the current traces (Table 1 and Supplementary Figures S1 and 2). To evaluate steady-state mid-inactivation (V1/2) of KV4 and KV4/KChIP2 channels, peak tail-currents following a 2 s prepulse were normalized to the maximal current and a Boltzmann equation was fitted to the data. For all channel complexes, 10 µM NS5806 caused a prominent left-shift in V1/2 (Tables 1 and 2 and Supplementary Figures S1 and S2), and as illustrated for KV4.3 and KV4.3/KChIP2 in Figure 5A and B. This negative shift in V1/2 explains the disparate effects of NS5806 on KV4.3 peak currents (Figures 3B and 4C, Tables 1 and 2) when using −80 mV or −100 mV as holding potential. As −80 mV is close to physiological resting membrane potentials, we used −80 mV as holding in the following studies. Recovery from inactivation for KV4 and KV4/KChIP2 channels expressed in Xenopus laevis oocytes was addressed by a standard two-pulse protocol and was found to be slowed by 10 µM NS5806 for all KV4 and KV4/KChIP2 channels (Table 1, Figure 5C and D and Supplementary Figures S1 and 2).
Table 2.
Effect of 10 µM NS5806 on Kv4.3 co-expressed with different subunits
| Kv4.3+ | − | DPP6 | KCNE2 | KCNE3 | DPP6/KCNE3 | KChIP2/DPP10 |
|---|---|---|---|---|---|---|
| Peak current (µA) control | 14.4 ± 2.0 | 20.4 ± 1.4 | 12.1 ± 1.9 | 1.8 ± 0.18 | 37.8 ± 6.8 | 14 ± 1.4 |
| 10 µM NS5806 | 6.6 ± 1.3 (8)** | 11.0 ± 1.7 (9)*** | 7.5 ± 1.5 (6)** | 1.3 ± 0.28 (8)* | 23.6 ± 5.8 (8)** | 10.4 ± 1.1 (5)** |
| Decay τ (ms) control | 42.4 ± 2.1 | 37.7 ± 2.7 | 66.4 ± 13.8 | 55.1 ± 4.7 | 31.0 ± 1.0 | 17.6 ± 0.8 |
| 10 µM NS5806 | 45.6 ± 3.6 (8) | 28.8 ± 0.73 (9)* | 52.4 ± 7–3 (6) | 48.1 ± 1.9 (4) | 28.2 ± 7.7 (8)* | 11.9 ± 0.5 (5)*** |
| Area (µA·ms–1) control | 783 ± 102 | 939 ± 120 | 959 ± 251 | 176 ± 45 | 1415 ± 311 | 409 ± 23 |
| 10 µM NS5806 | 367 ± 59 (8)*** | 501 ± 88 (9)*** | 408 ± 88 (6)** | 169 ± 49 (4) | 877 ± 20 (9)** | 188 ± 194 (5)*** |
| V1/2 (mV) control | −56.8 ± 1.3 | −75.2 ± 0.14 | −59.6 ± 0.9 | −60.1 ± 0.57 | −78.6 + 0.3 | −72.8 ± 0.5 |
| 10 µM NS5806 | −80.7 ± 2.7 (8)*** | −82.2 ± 0.14 (8)*** | −79.9 ± 1.1 (6)*** | −78.4 ± 1.1 (8)*** | −81.7 ± 0.2 (9)*** | −80.6 ± 0.4 (5)*** |
| Recovery τ (ms) control | 480 ± 71 | 159 ± 3.8 | 235 ± 13.4 | 543 ± 24 | 176 ± 1.9 | 93.9 ± 2.1 |
| 10 µM NS5806 | 1459 ± 10 (8)*** | 239 ± 2.2 (5)*** | 492 ± 94.6 (6)*** | 1237 ± 123 (8)*** | 252 ± 2.4 (10)*** | 86.6 ± 6.3 (5) |
| Kv4.3+ | KChIP2 | KChIP2/DPP6 | KChIP2/KCNE2 | KChIP2/KCNE3 | KChIP2/DPP6/KCNE3 | KChIP2/DPP6/KCNE2 |
|---|---|---|---|---|---|---|
| Peak current (µA) control | 33.9 ± 2.9 | 33.1 ± 8.5 | 13.9 ± 1.47 | 1.8 ± 0.36 (5) | 24.7 ± 3.9 | 33.9 ± 1.3 |
| 10 µM NS5806 | 35.9 ± 3.1 (7)** | 33.0 ± 9.5 (9) | 10.7 ± 1.09 (9)*** | 1.5 ± 0.36 (5)* | 25.3 ± 4.0 (9) | 25.9 ± 1.4 (5)** |
| Decay τ (ms) control | 65.5 ± 6.3 | 33.6 ± 1.6 | 72.0 ± 6.5 | 187 ± 38 | 37.0 ± 2.1 | 44.3 ± 3.0 |
| 10 µM NS5806 | 90.0 ± 14.9 (7)*** | 45.3 ± 1.0 (10)*** | 78.0 ± 6.2 (9)* | 199 ± 100(5) | 42.1 ± 1.3 (9)* | 44.9 ± 1.9 (5) |
| Area (µA·ms–1) control | 2011 ± 219 | 1111 ± 304 | 1140 ± 98 | 195 ± 86 | 1190 ± 224 | 1804 ± 310 |
| 10 µM NS5806 | 2702 ± 316 (7)*** | 1752 ± 498 (10)* | 822 ± 101 (9)* | 153 ± 84 (5) | 1622 ± 325 (9)** | 1616 ± 98 (5)* |
| V1/2 (mV) control | −50.2 ± 0.7 | −63 ± 0.4 | −52.44 ± 1.3 | −60.4 ± 1.3 (5) | −71.4 ± 0.6 | −70.2 ± 0.4 |
| 10 µM NS5806 | −58.9 ± 0.7 (7)*** | −69 ± 0.3 (4)*** | −71.9 ± 2.2 (9)*** | −79.9 ± 1.5 (5)*** | −77.8 ± 0.43 (9)*** | −78.2 ± 0.2 (5)*** |
| Recovery τ (ms) control | 76.6 ± 2 | 42.6 ± 1.8 | 189.1 ± 5.5 | 522 ± 56 (5) | 62.6 ± 1.6 | 68.6 ± 1.1 |
| 10 µM NS5806 | 274 ± 8.0 (7)*** | 66.8 ± 3.1 (8)*** | 547 ± 151 (9)*** | 1304 ± 134 (5)*** | 99.1 ± 1.6 (5)*** | 106 ± 4.5 (5)*** |
KV4.3 channels expressed with different accessory subunits in Xenopus laevis oocytes. Currents were activated from a holding potential of −80 mV by a +40 mV step before and during application of 10 µM NS5806 and peak current amplitudes, time constants (τ) of current decay and total charge movement for the first 150 ms after complete activation was measured. Steady-state mid-inactivation (V1/2) was evaluated from a holding potential of −100 mV by a series of 2 s prepulses from −100 to +40 mV followed by a +40 mV step. Boltzmann equations were fitted to normalized tail current amplitudes plotted as a function of the prepulse potential. Time constants (τ) for recovery from inactivation was determined by a two-pulse protocol; from a holding potential of −80 mV currents were activated by two test potentials to +40 mV with increasing interpulse time. The peak current at the second pulse was normalized to the first pulse and single exponential functions were fit to the data points. The different KV4.3 β-subunit combinations were recorded on different days; however, the experiments in absence and presence of KChIP2 were in all cases performed in parallel and thus current amplitude and biophysical characteristics can be directly compared.
P < 0.05,
P < 0.01,
P < 0.001; significantly different in the absence and presence of NS5806.
DPP, dipeptidyl-peptidase.
Figure 5.
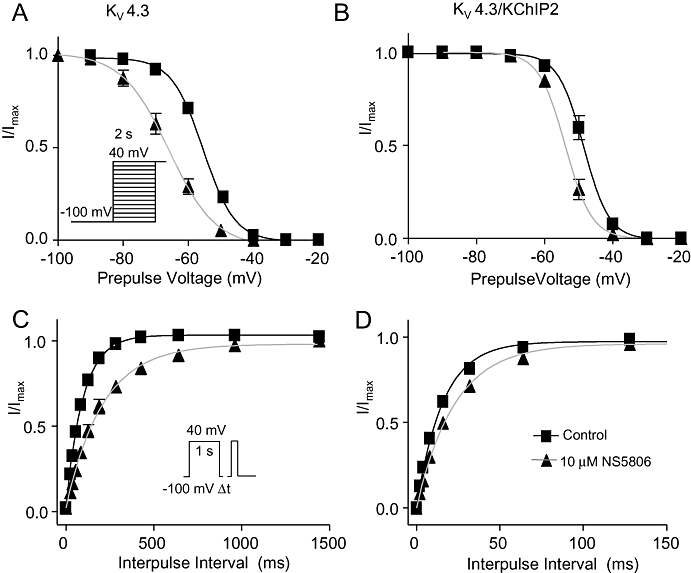
Effect of NS5806 on steady-state inactivation and time dependent recovery of KV4.3 and KV4.3/KChIP2 channels. KV4.3 and KV4.3/KChIP2 were expressed in Xenopus laevis oocytes. Steady-state inactivation was evaluated before (squares) and after application of 10 µM NS5806 (triangles). Normalized tail current amplitudes plotted as a function of the prepulse potential for currents elicited by the depicted voltage-clamp protocol and Boltzmann equations were fitted to the data. (A) Steady-state inactivation of KV4.3, n= 10. (B) Steady-state inactivation of KV4.3/KChIP2, n= 9. Time-dependent recovery from inactivation at −100 mV was addressed by a two-pulse protocol with an increasing inter-pulse interval as shown. The peak current at the second pulse was normalized to the current at the first pulse and a single exponential equation was fitted to the data. (C) Time-dependent recovery of KV4.3, n= 8. (D) Time-dependent recovery of KV4.3/KChIP2, n= 8. KChIP2, K channel interacting protein 2.
The negative shift in mid-inactivation V1/2 suggests that NS5806 increases closed-state inactivation. Closed-state inactivation of KV4.3 and for KV4.3/KChIP2 expressed in Xenopus laevis oocytes was addressed by a modified double-pulse protocol (Bahring et al., 2001). After the first test-pulse, inactivation was completely released by a hyperpolarizing step to −100 mV followed by a step to a voltage below the activation threshold (−50 mV) allowing for onset of closed-state inactivation for increasing time intervals before application of a second test pulse. The current amplitude at the second pulse was plotted as a function of interpulse interval and a mono-exponential decay function was fitted to the data (Figure 6). Both in the presence and absence of KChIP2, NS5806 significantly accelerated the onset of closed-state inactivation. For KV4.3, the time constant (τ) of closed-state inactivation was 701 ± 203 ms before and 164 ± 29 ms after application of 10 µM NS5806 and for KV4.3/KChIP2, τ= 838 ± 338 ms before, and 310 ± 80 ms after application of NS5806.
Figure 6.
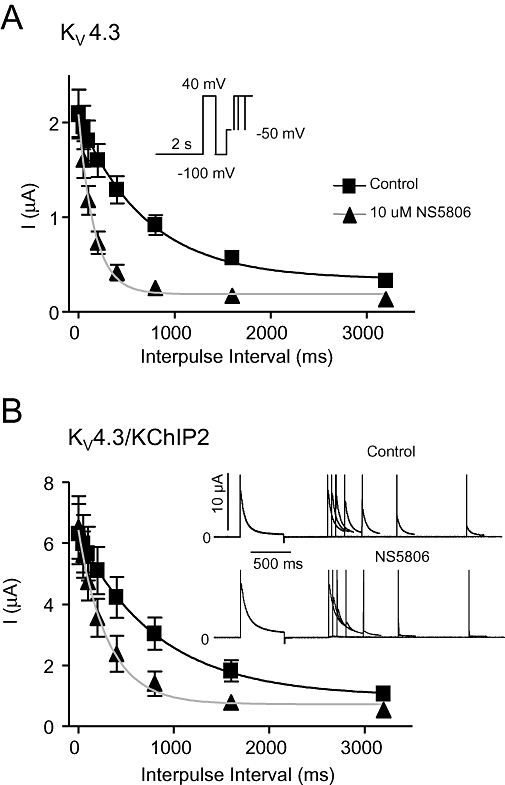
Effect of NS5806 on closed-state inactivation of KV4.3 and KV4.3/KChIP2 channels. KV4.3 and KV4.3/KChIP2 were expressed in Xenopus laevis oocytes. Closed-state inactivation was evaluated before and after application of 10 µM NS5806. Currents were activated from a holding of −100 mV by a brief +40 mV step. Inactivation was completely released by a −100 mV step and followed by a step at a voltage below the activation threshold (−50 mV) at increasing time-intervals before currents were activated by a second +40 mV step. Current amplitude at the second pulse is plotted as a function of pulse interval time for KV4.3, n= 9 (A) and for KV4.3/KChIP2, n= 5 (B). Representative KV4.3/KChIP2 currents are shown in panel B. KChIP2, K channel interacting protein 2.
Effect of NS5806 on KV4.3 expressed with various ancillary subunits
For KV4.3/KChIP2 currents in CHO-K1 cells and in Xenopus laevis oocytes, the time dependent recovery from inactivation was slowed by NS5806 (Figure 1E and Figure 5C and D); however, for native Ito the recovery was accelerated by 10 µM NS5806 (Calloe et al., 2009a). As ancillary subunits can dramatically change the effect of exogenous compounds (Bett and Rasmusson, 2008), we wondered if the difference between Ito and KV4.3/KChIP2 currents could be due to presence of additional ancillary subunits in the native Ito channel complex. In the next series of experiments we evaluated the effect of 10 µM NS5806 on KV4.3 expressed with and without KChIP2 and the putative Ito ancillary subunits DPP6, DPP10, KCNE2 and KCNE3 and the results are summarized in Table 2.
Comparing the control recordings revealed that DPP6 and DPP10 increased KV4.3 and KV4.3/KChIP2 currents and accelerated current decay. KCNE2 inhibited KV4.3 and KV4.3/KChIP2 current amplitude as also reported by Radicke et al. (2006). In agreement with our previous findings (Lundby and Olesen, 2006; Delpón et al., 2008), KCNE3 reduced both KV4.3 and KV4.3/KChIP2 currents. Interestingly, it appeared that this inhibition of current could be abolished by co-expressing KV4.3/KCNE3 with DPP6 (Table 2). Addressing the effect of NS5806 on the different subunit combinations, we found NS5806 exclusively caused a slowing of the current decay (τ) in channel complexes encompassing KChIP2. Thus, KChIP2 expression appears to be central for the effect of NS5806 on current decay and suggests that KChIP2 is part of the native Ito channel. Co-expression of KV4.3/KChIP2 or KV4.3/KChIP2/DPP6 with KCNE2 resulted in a marked slowing in recovery from inactivation following application of NS5806. These results are opposite to the effects seen in canine Ito. NS5806 accelerated KV4.3/KChIP2/DPP10 current decay suggesting that DPP10 is an unlikely candidate for the native Ito channel. For all subunit combinations tested, application of NS5806 slowed recovery from inactivation, in sharp contrast to the acceleration of recovery observed in native Ito channels.
Effect of NS5806 on other cardiac potassium channels
The effect of 10 µM NS5806 was further tested on α-subunits mediating other important repolarizing cardiac potassium currents. KV1.4 and KV1.5 were expressed in Xenopus laevis oocytes in the presence or absence of KChIP2. For KV1.4, 10 µM NS5806 inhibited more than 80% of the current (Figure 7A). The effect on the related KV1.5 channel was smaller and mainly on the sustained current (Figure 7C). For both KV1.4 and KV1.5, co-expression with KChIP2 did not affect basal currents or the response to NS5806 (Figure 7B and D). There was no effect of NS5806 on currents generated by KV11.1, KV7.1 and Kir2.1-3 channels (Supplementary Figures S3–5).
Figure 7.
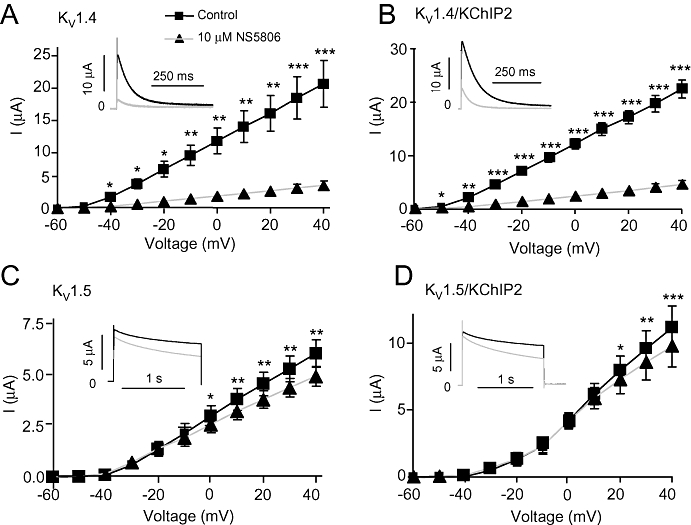
Effect of NS5806 on KV1.4 and KV1.5 channels in presence or absence of KChIP2. KV1.4 and KV1.5 were expressed with and without KChIP2 in Xenopus laevis oocytes. Currents were activated from a holding potential of −80 mV to voltage steps from −100 mV to +40 mV before (squares) and after application of 10 µM NS5806 (triangles). Peak current amplitudes as function of voltage and representative current traces are shown for KV1.4, n= 5 (A), KV1.4/KChIP2, n= 5 (B), Kv1.5, n= 9 (C) and KV1.5/KChIP2, n= 10 (D). *P < 0.05, **P < 0.01, ***P < 0.001; significantly different in the absence and presence of NS5806. KChIP2, K channel interacting protein 2.
Are other α-subunits contributing to canine ventricular Ito?
Ito measured in isolated cells from canine left ventricular epi- and midmyocardium recovers from inactivation with a bi-exponential time-course. Besides increasing currents and accelerating the recovery, the time-course of the recovery is changed from bi-exponential to mono-exponential in the presence of NS5806 (Calloe et al., 2009b).This might be explained by NS5806 enhancing a fast recovering current and inhibiting a slower recovering current. Thus, to test how NS5806 would affect a current generated by a mixture of the slowly recovering KV1.4 (Supplementary Figure S6A) and the fast recovering KV4.3/KChIP2/DPP6 channels, we co-expressed the channel constructs in Xenopus laevis oocytes in a 1:1 current ratio. Peak-current amplitude was unaffected by 10 µM NS5806 (Figure 8A), current decay was slowed (Figure 8B) and there was a significant left shift in steady-state mid-nactivation V1/2, from −53.0 ± 0.8 mV to −67.7 ± 1.3 mV (Figure 8C). Recovery from inactivation was addressed as previously described and the fraction of recovered current was plotted as a function of interpulse time and a bi-exponential function was fitted to the data (Figure 8D and E). For control, the recovery showed a slow and a fast phase, τ1= 20.1 ± 2.6 ms (relative weight of the pre-exponential factor, A1= 0.30 ± 0.04) and τ2= 1155 ± 448 ms (relative weight of the pre-exponential factor, A2= 0.70 ± 0.03) respectively. In the presence of NS5806 the reactivation time course was markedly faster with τ1= 26.0 ± 1.9 ms (A1= 0.95 ± 0.04) and τ2= 411 ± 242 ms (A2= 0.05 ± 0.03). In the presence of NS5806, the pre-exponential factor of the slow component was small and the reactivation could be fitted with a mono-exponential equations with τ= 30.8 ± 1.2 ms. Thus, the recovery of the KV4.3/KChIP2/DPP6/KV1.4 current from inactivation was qualitatively similar to that of native Ito in the absence and presence of NS5806. Similar results were found for KV4.3/KChIP2/KV1.4 (Supplementary Figure S6B).
Figure 8.
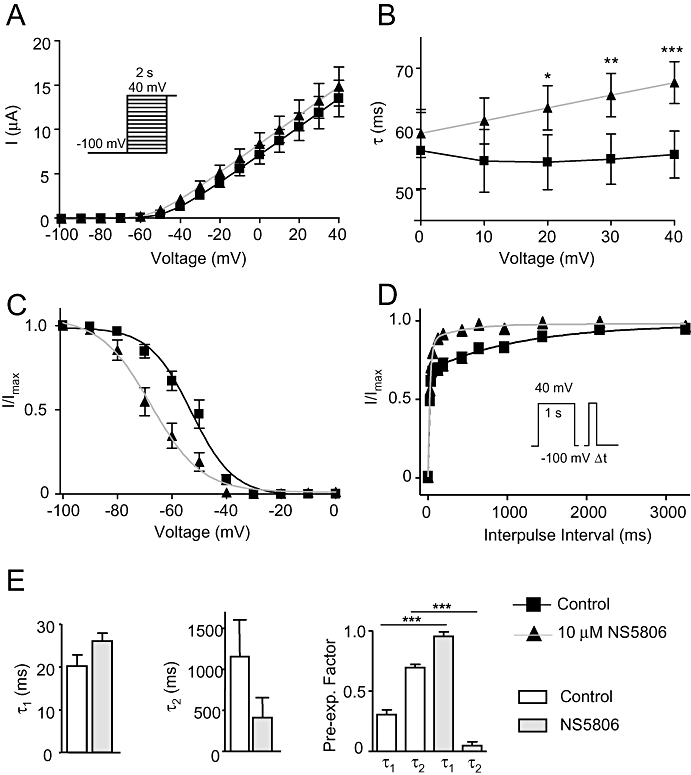
Effect of NS5806 on KV4.3/KChIP2/DPP6 co-expressed with KV1.4 channels. KV4.3/KChIP2/DPP6 and KV1.4 were co-expressed in Xenopus laevis oocytes in a 1:1 current ratio. Currents were elicited using the depicted voltage protocol and measured in the absence and in the presence of 10 µM NS5806. (A) Relation between peak current density and voltage, n= 8. (B) Single exponential equations were fitted to the current decays, and the time constants (τ) are shown as a function of voltage, n= 8. (C) Steady-state inactivation of KV4.3/KChIP2/DPP6/KV1.4 currents. Normalized tail current amplitudes are plotted as a function of the prepulse potential and Boltzmann equations are fitted to the data, n= 8. (D) Time-dependent recovery from inactivation. KV4.3/KChIP2/DPP6/KV1.4 currents were activated by a two-pulse protocol. Current amplitudes at the second test pulse were normalized to that of the first test pulse and plotted as a function of the inter-pulse interval and double-exponential equations were fitted to the data, n= 7. (E) Bar graph showing the time-constants of KV4.3/KChIP2/DPP6/KV1.4 recovery from inactivation, τ1 and τ2 as well as pre-exponential factors. *P < 0.05, **P < 0.01, ***P < 0.001; significantly different in the absence and presence of NS5806. DPP, dipeptidyl-peptidase; KChIP2, K channel interacting protein 2.
Discussion and conclusion
Effects of NS5806 on KV4.3 channel gating
NS5806 increased peak current amplitude of Kv4.3/KChIP2 expressed in CHO-K cells with an EC50 of 5.3 ± 1.5 µM and slowed the time course of current inactivation (τ) with an EC50 of 25.4 ± 1.1 µM. For Kv4.3/KChIP2 expressed in Xenopus laevis oocytes, the effect of the compound was mainly on current decay. Ancillary subunits can modify channel gating and may induce conformational changes that can affect access or affinity for exogenous compounds (Bett and Rasmusson, 2008) and the effect of NS5806 on current decay appeared to be linked to KChIP2. KChIP2 is a cytosolic protein that interacts with the intracellular N-termini of KV4 subunits (Callsen et al., 2005); however, NS5806 also affected KV4 channels in the absence of KChIP2, as evident from the shift in mid-inactivation, suggesting the binding site is on the α-subunit.
The negative shift in the mid-inactivation observed for all subunit combinations tested indicates that NS5806 increased closed-state inactivation, which was confirmed in a set of experiments addressing the onset of closed-state inactivation for KV4.3 in the presence and absence of KChIP2. For KV4.3 and KV4.3/KChIP2, NS5806 increased the fraction of channels that entered closed-state inactivation.
Recovery from closed-state inactivation has been suggested to be the limiting factor for KV4 channel recovery (Bahring et al., 2001; Amadi et al., 2007). In agreement, we show that the time-dependent recovery of KV4 channels was slowed in the presence of NS5806. The faster onset of closed state inactivation and slower recovery from inactivation results in a larger fraction of KV4 channels being in an inactivated state, mainly in the absence of KChIP2. Thus, NS5806 modified Kv4.3/KChIP2 gating in several ways that inhibit current. This dual mode of activation and inhibition may explain why 100 µM NS5806 increased Kv4.3/KChIP2 peak-currents less than 30 µM did in CHO-K1 cells. However, it should be emphasized that in canine ventricular cardiomyocytes and in CHO-K1 cells, the prominent effect of 10 µM NS5806 was to increase the size of current. The mechanisms behind the observed increase in peak current are unknown, for KV4.3/KChIP2 channels it could be due to slowed inactivation; however, this is an unlikely mechanism for the increase in Kv4.3 currents in the absence of KChIP2 as current decay is unaffected in the absence of KChIP2 and the exact mechanism of drug binding and how drug binding is transmitted to a response is not known.
Differential effect of NS5806 on KV4.3 and KV4.3/KChIP2 channels – can NS5806 be used to discriminate the molecular composition of native Ito?
We have previously characterized the effect of NS5806 on native canine Ito in isolated cardiomyocytes. For mid- and epicardial cells, NS5806 increased Ito peak current, slowed current decay, caused a negative shift in the steady-state mid-inactivation and accelerated the time dependent recovery from inactivation. As an initial basis for comparison, KV4.3 was co-expressed with KChIP2 in CHO-K1 cells and Xenopus laevis oocytes (Calloe et al., 2009b). In neither expression system did the measured current recapitulate native Ito fully, in terms of the response to NS5806. Interestingly KV4.3 current decay was slowed when KChIP2 was co-expressed, but unaffected in the absence of KChIP2.
As KV4.3 co-expressed with KChIP2 and DPP6 in heterologous systems has been demonstrated to result in currents with kinetics similar to those of Ito in human ventricular cardiomyocytes (Radicke et al., 2005), we first co-expressed KV4.3 with KChIP2 and DPP6 in CHO-K1 cells in the present study. Currents were measured using similar solutions as for native Ito. Similar to native Ito, peak current density was increased by NS5806, the current decay slowed and mid-inactivation V1/2 shifted to more negative voltages. However, in contrast to the effect on native Ito, the time dependent recovery from inactivation was markedly slowed for KV4.3/KChIP2/DPP6 currents in the presence of NS5806.
We therefore tested the effect of NS5806 on KV4.3 expressed with KChIP2 and other ancillary subunits in Xenopus laevis oocytes. In agreement with our previous observations, current decay was slowed by NS5806 only for KV4.3 co-expressed with KChIP2, independently on the presence of other ancillary subunits. This implies that NS5806 potentially can be used to investigate the molecular composition of native Ito channels. Furthermore, for KV4.3 channels expressed in the absence of KChIP2, NS5806 may inhibit currents at physiological resting membrane potentials due to the negative shift in the steady-state mid-inactivation (Figure 5 and Table 2).
NS5806 induced a marked increase in peak Ito and slowed Ito decay in canine mid- and epicardial Ito (Calloe et al., 2009a,b;) suggesting that KChIP2 is an integral part of the Ito channel in canine ventricle. However, none of the tested ancillary subunit combinations completely reconstituted native canine mid- and epicardial Ito with regard to the effect of NS5806. For native Ito, the recovery from inactivation was accelerated by NS5806, but for all subunit combinations tested, recovery was slowed by NS5806.
Besides the various ancillary subunits that have been implied to contribute to native Ito, other channel-forming subunits have also been suggested; for other species, including humans, KV1.4 has been reported to contribute to Ito (Patel and Campbell, 2005). Previous studies have found KV1.4 mRNA (Dixon et al., 1996; Rosati et al., 2001) and KV1.4 protein (Akar et al., 2004) in the canine left ventricle. However, the functional role of KV1.4 and Ito, slow in canine tissue is uncertain. Akar and colleagues found no appreciable contribution of KV1.4 current to canine ventricular Ito (Akar et al., 2004). Interestingly, for canine mid- and epicardial Ito, application of NS5806 accelerated the recovery but also changed the kinetics from bi-exponential to mono-exponential (Calloe et al., 2009b). It was tempting to speculate whether this change in recovery kinetics could be due to enhancement of a fast recovering current component in combination with inhibition of a slow recovering component of Ito. To test this hypothesis, the fast recovering KV4.3/KChIP2/DPP6 channels were co-expressed with slowly recovering KV1.4 channels in Xenopus laevis oocytes. The current generated by KV4.3/KChIP2/DPP6/KV1.4 recovered from inactivation with a bi-exponential time-course (Figure 8), similar to native Ito. In addition, NS5806 accelerated the recovery and changed the time-course from bi-exponential to mono-exponential. This suggests that KV1.4 or some other slowly recovering current could contribute to native canine Ito. Whether this is the case in vivo remains to be determined. NS5806 slowed KV4.3/KChIP2/DPP6/KV1.4 current decay yet did not affect peak current amplitude. We consistently observed that NS5806 had little effect on amplitude of currents recorded in Xenopus laevis oocytes whereas current amplitudes were increased when similar constructs were expressed in CHO-K1 or HEK-293 cells (data not shown). Increasing the temperature to 37°C for the oocytes experiments and replacing the extracellular solution with that used for native Ito recordings did not alter the effect of NS5806 on KV4.3/KChIP2 peak current amplitudes in Xenopus laevis oocytes (data not shown) suggesting that the observed difference was due to intrinsic differences between expression systems.
Comparison with other Ito affecting compounds
The effects of several compounds on KV4.3 have been reported to be modulated by co-expression of ancillary subunits. The IKr blocker tedisamil inhibits KV4.3/KChIP2 with different IC50 values depending on co-expression with KCNE1, KCNE2 or DPP6 due to differential modulation of current kinetics (Radicke et al., 2009). Similarly for the class 1C anti-arrhythmic agent flecainide, the IC50 values for the peak current amplitudes of KV4.3/KChIP2 are dependent on co-expression with KCNE1, KCNE2 or DPP6 (Radicke et al., 2008). The inhibition of KV4.3 current by the local anesthetic bupivacaine is reduced by co-expression of KChIP2 (Solth et al., 2005). However, for these compounds the differences in drug action due to ancillary subunits are so subtle that they limit their usefulness to investigate the molecular constituents of native Ito or IA channel complexes. The calcium channel blocker nifedipine is an open-pore blocker of KV4.3 and KV4.3/KChIP2 channels when used in high concentrations (150 µM). Interestingly, the time-dependent recovery of KV4.3 is markedly slowed by nifedipine whereas in the presence of KChIP2 the recovery is unaffected by the compound (Bett et al., 2006). This suggests that nifedipine can be used to identify the presence of KChIP2 in native channels; however, the effect on ICaL may be an issue in some preparations. For NS5806, a small inhibiting effect on ICaL and INa in isolated canine ventricular cells was observed (Calloe et al., 2009a), and in the present work we found that NS5806 inhibits KV1.4 and KV1.5 currents. Nevertheless, even with these caveats in mind, NS5806 provides a tool to address the physiological role and the molecular composition of Ito and IA.
Limitations of this study
In the present study we compared the effect of NS5806 on heterologously expressed putative human Ito channel subunits. The results were compared with results obtained in a previous paper where we characterized the effect of NS5806 on canine ventricular Ito (Calloe et al., 2009b). Canine Ito has a faster current decay and recovers slower from inactivation than human Ito. Additionally, human Ito recovery follows a single exponential time course, whereas canine Ito follows a biexponential time course (Akar et al., 2004). We cannot exclude the possibility that the observed discrepancies between the effect of NS5806 on the heterologously expressed channels in this study and native canine Ito are due to species differences. However, more importantly, the expression systems may lack important regulatory factors, additional ancillary subunits, etc. found in native cells and the molecular composition of the native Ito channel remains speculative. Further Xenopus laevis oocyte experiments were performed at room temperature which slows the kinetic parameters compared with currents measured at 37°C. However, for screening as well as for expression of multiple constructs, the oocytes are preferable to mammalian cell lines.
In conclusion, NS5806 activates native Ito and KV4 channels heterologously expressed with KChIP2 in both Xenopus laevis oocytes and CHO-K1 cells. The differential effect of NS5806 in the absence and presence of KChIP2 suggests that NS5806 besides providing an experimental model of the Brugada syndrome can be used as a tool to address the physiological role of Ito and IA and potentially to identify the molecular components of the channels mediating the currents in vivo; however, this should be tested by complementary approaches.
Acknowledgments
The authors wish to thank Dr Morten B. Thomsen for valuable discussion, Trine Christensen for technical assistance and NeuroSearch A/S Ballerup, Denmark for providing NS5806.
This work was supported by grants from the Danish Cardiovascular Research Academy (DaCRA) and the Danish Heart Foundation (AL), the Danish National Research Foundation (NS, SPO), The Novo Nordisk Foundation (TJ), The Lundbeck Foundation (TJ), Carlsberg Foundation (KC), the American Health Assistance Foundation to JMC.
Conflicts of Interest Morten Grunnet is employed by NeuroSearch A/S and Søren-Peter Olesen is a consultant to the company.
Glossary
Abbreviations
- CHO-K1
Chinese hamster ovary cells
- DPP
dipeptidyl-peptidase
- IA
A-type potassium current
- Ito
transient outward potassium current
- KChIP2
K channel interacting protein 2
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 KV4.1 with and without KChIP2 were expressed in Xenopus laevis oocytes. Currents were elicited by protocols shown in Figure 5 in the absence (black) and presence of 10 μM NS5806 (red). (A) Peak current amplitude as a function of voltage, n = 5. (B) Total charge movement evaluated as area under current trace during the first 150 ms after complete activation, n = 5. (C) Steady-state mid-inactivation. Current amplitudes at this step was normalized, plotted as a function of the pre-pulse potential and fitted to Boltzmann equation. For KV4.1, V1/2 was −61 ± 0.4 mV in the absence and −75 ± 0.8 mV in the presence of NS5806, n = 5. For KV4.1/KChIP2, V1/2 was −45 ± 0.2 mV in the absence and −59 ± 0.2 mV in the presence of NS5806, n = 6. (D) The time constant of recovery was determined by a two-pulse protocol. The peak current at the second pulse was normalized to the first pulse and the data points obtained were fit to a single exponential function. For KV4.1, τ = 109 ± 3 ms in the absence and τ = 492 ± 24 ms in the presence of NS5806, n = 6. For KV4.1/KChIP2, τ = 16.8 ± 1.3 ms in the absence and τ = 52.8 ± 3.3 ms in the presence of NS5806, n = 5.
Figure S2 KV4.2 with and without KChIP2 were expressed in Xenopus laevis oocytes. Currents were elicited by protocols shown in Figure 5 in the absence (black) and presence of 10 μM NS5806 (red). (A) Peak current amplitude as a function of voltage, n = 6. (B) Total charge movement evaluated as area under current trace during the first 150 ms after complete activation, n = 6. (C) Steady-state mid-inactivation. Current amplitudes at this step was normalized, plotted as a function of the pre-pulse potential and fitted to Boltzmann equation, For KV4.2, V1/2 was −65 ± 0.5 mV in the absence and −76 ± 0.8 mV in the presence of NS5806, n = 6. For KV4.2/KChIP2, V1/2 was −57 ± 0.3 mV in the absence and −67 ± 0.4 mV in the presence of NS5806, n = 10. (D) The time constant of recovery was determined by a two-pulse protocol. The peak current at the second pulse was normalized to the first pulse and the data points obtained were fitted to a single exponential function. For KV4.2, τ = 176 ± 12 ms in the absence and τ = 328 ± 16 ms in the presence of NS5806, n = 5. For KV4.2/KChIP2, τ = 27.6 ± 1.1 ms in the absence and τ = 44.2 ± 1.9 ms in the presence of NS5806, n = 10.
Figure S3 KV11.1 was expressed in Xenopus laevis oocytes. Currents were elicited from a holding potential of −80 mV by a series of steps from −80 to +40 mV in 10 mV increments. Tails current were measured at −60 mV. Recordings were made in the absence (black) and presence of 10 μM NS5806 (red). (A) Representative currents of n = 5 (B) Steady-state current amplitude plotted as a function of voltage, n = 5. (C) Peak tail-current amplitude as a function of voltage, n = 5.
Figure S4 KV7.1 was expressed in Xenopus laevis oocytes. Currents were elicited from a holding potential of −80 mV by a series of steps from −80 to +40 mV in 10 mV increments. Tails current were measured at −60 mV. Recordings were made in the absence (black) and presence of 10 μM NS5806 (red). (A) Representative currents of n = 5 (B) Peak-current amplitude as a function of voltage, n = 5.
Figure S5 Kir2.1, 2.2 or 2.3 were expressed in Xenopus laevis oocytes. From a holding potential of 0 mV, currents were activated a ramp protocol from −120 mV to +50 mV. Recordings were made in the absence (black) and presence of 10 μM NS5806 (red). (A) Representative recordings of Kir2.1, n = 5. (B) Representative recordings of Kir2.2, n = 5. (C) Representative recordings of Kir2.3, n = 5.
Figure S6 Time-dependent recovery from inactivation of KV1.4 and KV4.3/KChIP2 co-expressed with KV1.4 in Xenopus laevis oocytes. The time constant of recovery was determined by a two-pulse protocol. The peak current at the second pulse was normalized to the first pulse and the data points obtained were fitted to a single exponential function before (black) and after application of 10 μM NS5806 (red). (A) Reactivation of KV1.4 in the absence of NS5806 showed a bi-exponential time course with τ1 = 286 ± 125 ms and τ2 = 2055 ± 798 ms pre-exponential factors of 0.65 ± 0.12 and 0.35 ± 0.002 for controls. In the presence of NS5806 a mono-exponential function could be fitted to the data with τ = 11 297 ± 3945 ms, n = 5. (B) KV4.3/KChIP2 co-expressed with KV1.4 followed a bi-exponential time course for reactivation with τ1 = 40 ± 11ms and τ2 = 1384 ± 260 ms with pre-exponential factors of 0.8 ± 0.05 and 0.2 ± 0.01 respectively. In the presence of NS5806, τ1 = 79 ± 10 ms and τ2 = 6906 ± 1450 ms with pre-exponential factors of 0.96 ± 1.4 and 0.04 ± 0.001, respectively, n = 7.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Akar FG, Wu RC, Deschenes I, Armoundas AA, Piacentino V, III, Houser SR, et al. Phenotypic differences in transient outward K+ current of human and canine ventricular myocytes: insights into molecular composition of ventricular Ito. Am J Physiol Heart Circ Physiol. 2004;286:H602–H609. doi: 10.1152/ajpheart.00673.2003. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (4th edn) 2009;158(Suppl. 1):S1–S254. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadi CC, Brust RD, Skerritt MR, Campbell DL. Regulation of Kv4.3 closed state inactivation and recovery by extracellular potassium and intracellular KChIP2b. Channels (Austin) 2007;1:305–314. doi: 10.4161/chan.5017. [DOI] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, et al. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Bahring R, Boland LM, Varghese A, Gebauer M, Pongs O. Kinetic analysis of open- and closed-state inactivation transitions in human Kv4.2 A-type potassium channels. J Physiol. 2001;535:65–81. doi: 10.1111/j.1469-7793.2001.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett GC, Rasmusson RL. Modification of K+ channel-drug interactions by ancillary subunits. J Physiol. 2008;586:929–950. doi: 10.1113/jphysiol.2007.139279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett GC, Morales MJ, Strauss HC, Rasmusson RL. KChIP2b modulates the affinity and use-dependent block of Kv4.3 by nifedipine. Biochem Biophys Res Commun. 2006;340:1167–1177. doi: 10.1016/j.bbrc.2005.12.135. [DOI] [PubMed] [Google Scholar]
- Brahmajothi MV, Campbell DL, Rasmusson RL, Morales MJ, Trimmer JS, Nerbonne JM, et al. Distinct transient outward potassium current (Ito) phenotypes and distribution of fast-inactivating potassium channel alpha subunits in ferret left ventricular myocytes. J Gen Physiol. 1999;113:581–600. doi: 10.1085/jgp.113.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloe K, Cordeiro JM, Di Diego JM, Hansen RS, Grunnet M, Olesen SP, et al. A transient outward potassium current activator recapitulates the electrocardiographic manifestations of Brugada syndrome. Cardiovasc Res. 2009a;81:686–694. doi: 10.1093/cvr/cvn339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloe K, Soltysinska E, Jespersen T, Lundby A, Antzelevitch C, Olesen SP, et al. Differential effects of the transient outward K(+) current activator NS5806 in the canine left ventricle. J Mol Cell Cardiol. 2009b doi: 10.1016/j.yjmcc.2009.07.017. doi: 10.1016/j.yjmcc.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callsen B, Isbrandt D, Sauter K, Hartmann LS, Pongs O, Bahring R. Contribution of N- and C-terminal Kv4.2 channel domains to KChIP interaction [corrected] J Physiol. 2005;568:397–412. doi: 10.1113/jphysiol.2005.094359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delpón E, Cordeiro JM, Núñez L, Bloch-Thomsen PE, Guerchicoff A, Pollevick GD, et al. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ Arrhythmia Electrophysiol. 2008;1:209–218. doi: 10.1161/CIRCEP.107.748103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes I, Tomaselli GF. Modulation of Kv4.3 current by accessory subunits. FEBS Lett. 2002;528:183–188. doi: 10.1016/s0014-5793(02)03296-9. [DOI] [PubMed] [Google Scholar]
- Diochot S, Drici MD, Moinier D, Fink M, Lazdunski M. Effects of phrixotoxins on the Kv4 family of potassium channels and implications for the role of Ito1 in cardiac electrogenesis. Br J Pharmacol. 1999;126:251–263. doi: 10.1038/sj.bjp.0702283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon EJ, Shi W, Wang H-S, McDonald C, Yu H, Wymore RS, et al. Role of the Kv4.3 K+ channel in ventricular muscle. A molecular correlate for the transient outward current. Circ Res. 1996;79:659–668. doi: 10.1161/01.res.79.4.659. [DOI] [PubMed] [Google Scholar]
- Ebbinghaus J, Legros C, Nolting A, Guette C, Celerier ML, Pongs O, et al. Modulation of Kv4.2 channels by a peptide isolated from the venom of the giant bird-eating tarantula Theraphosa leblondi. Toxicon. 2004;43:923–932. doi: 10.1016/j.toxicon.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Hatano N, Ohya S, Muraki K, Giles W, Imaizumi Y. Dihydropyridine Ca2+ channel antagonists and agonists block Kv4.2, Kv4.3 and Kv1.4 K+ channels expressed in HEK293 cells. Br J Pharmacol. 2003;139:533–544. doi: 10.1038/sj.bjp.0705281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulin YA, De Santiago-Castillo JA, Rocha CA, Nadal MS, Rudy B, Covarrubias M. The dipeptidyl-peptidase-like protein DPP6 determines the unitary conductance of neuronal Kv4.2 channels. J Neurosci. 2009;29:3242–3251. doi: 10.1523/JNEUROSCI.4767-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby A, Olesen SP. KCNE3 is an inhibitory subunit of the Kv4.3 potassium channel. Biochem Biophys Res Commun. 2006;346:958–967. doi: 10.1016/j.bbrc.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Lundby A, Ravn LS, Svendsen JH, Hauns S, Olesen SP, Schmitt N. KCNE3 mutation V17M identified in a patient with lone atrial fibrillation. Cell Physiol Biochem. 2008;21:47–54. doi: 10.1159/000113746. [DOI] [PubMed] [Google Scholar]
- Nadal MS, Ozaita A, Amarillo Y, Vega-Saenz de ME, Ma Y, Mo W, et al. The CD26-related dipeptidyl aminopeptidase-like protein DPPX is a critical component of neuronal A-type K+ channels. Neuron. 2003;37:449–461. doi: 10.1016/s0896-6273(02)01185-6. [DOI] [PubMed] [Google Scholar]
- Patel SP, Campbell DL. Transient outward potassium current, ‘Ito’, phenotypes in the mammalian left ventricle: underlying molecular, cellular and biophysical mechanisms. J Physiol. 2005;569:7–39. doi: 10.1113/jphysiol.2005.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourrier M, Zicha S, Ehrlich J, Han W, Nattel S. Canine ventricular KCNE2 expression resides predominantly in Purkinje fibers. Circ Res. 2003;93:189–191. doi: 10.1161/01.RES.0000084851.60947.B5. [DOI] [PubMed] [Google Scholar]
- Radicke S, Cotella D, Graf EM, Ravens U, Wettwer E. Expression and function of dipeptidyl-aminopeptidase-like protein 6 as a putative beta-subunit of human cardiac transient outward current encoded by Kv4.3. J Physiol. 2005;565:751–756. doi: 10.1113/jphysiol.2005.087312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radicke S, Cotella D, Graf EM, Banse U, Jost N, Varro A, et al. Functional modulation of the transient outward current Ito by KCNE beta-subunits and regional distribution in human non-failing and failing hearts. Cardiovasc Res. 2006;71:695–703. doi: 10.1016/j.cardiores.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Radicke S, Vaquero M, Caballero R, Gomez R, Nunez L, Tamargo J, et al. Effects of MiRP1 and DPP6 beta-subunits on the blockade induced by flecainide of Kv4.3/KChIP2 channels. Br J Pharmacol. 2008;154:774–786. doi: 10.1038/bjp.2008.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radicke S, Cotella D, Sblattero D, Ravens U, Santoro C, Wettwer E. The transmembrane beta-subunits KCNE1, KCNE2, and DPP6 modify pharmacological effects of the antiarrhythmic agent tedisamil on the transient outward current I (to) Naunyn Schmiedebergs Arch Pharmacol. 2009;379:617–626. doi: 10.1007/s00210-008-0389-1. [DOI] [PubMed] [Google Scholar]
- Rosati B, Pan Z, Lypen S, Wang HS, Cohen I, Dixon JE, et al. Regulation of KChIP2 potassium channel beta subunit gene expression underlies the gradient of transient outward current in canine and human ventricle. J Physiol. 2001;533:119–125. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosati B, Grau F, Rodriguez S, Li H, Nerbonne JM, McKinnon D. Concordant expression of KChIP2 mRNA, protein and transient outward current throughout the canine ventricle. J Physiol. 2003;548:815–822. doi: 10.1113/jphysiol.2002.033704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Tristani-Firouzi M. hERG potassium channels and cardiac arrhythmia. Nature. 2006;440:463–469. doi: 10.1038/nature04710. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Johnson JH, Hammerland LG, Kelbaugh PR, Volkmann RA, Saccomano NA, et al. Heteropodatoxins: peptides isolated from spider venom that block Kv4.2 potassium channels. Mol Pharmacol. 1997;51:491–498. [PubMed] [Google Scholar]
- Solth A, Siebrands CC, Friederich P. Inhibition of Kv4.3/KChIP2.2 channels by bupivacaine and its modulation by the pore mutation Kv4.3V401I. Anesthesiology. 2005;103:796–804. doi: 10.1097/00000542-200510000-00018. [DOI] [PubMed] [Google Scholar]
- Wang Z, Fermini B, Nattel S. Effects of flecainide, quinidine, and 4-aminopyridine on transient outward and ultrarapid delayed rectifier currents in human atrial myocytes. J Pharmacol Exp Ther. 1995;272:184–196. [PubMed] [Google Scholar]
- Wettwer E, Amos GJ, Posival H, Ravens U. Transient outward current in human ventricular myocytes of subepicardial and subendocardial origin. Circ Res. 1994;75:473–482. doi: 10.1161/01.res.75.3.473. [DOI] [PubMed] [Google Scholar]
- Zarayskiy VV, Balasubramanian G, Bondarenko VE, Morales MJ. Heteropoda toxin 2 is a gating modifier toxin specific for voltage-gated K+ channels of the Kv4 family. Toxicon. 2005;45:431–442. doi: 10.1016/j.toxicon.2004.11.015. [DOI] [PubMed] [Google Scholar]
- Zhang M, Jiang M, Tseng GN. minK-related peptide 1 associates with Kv4.2 and modulates its gating function: potential role as beta subunit of cardiac transient outward channel? Circ Res. 2001;88:1012–1019. doi: 10.1161/hh1001.090839. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


