Abstract
BACKGROUND AND PURPOSE
Darbepoetin, a long-acting erythropoietin derivative, attenuates cardiomyocyte apoptosis and improves short-term (3 days) cardiac function, but the mechanisms responsible are unknown. We investigated potential mechanisms by which darbepoetin exerts cardioprotection following myocardial infarction in mice and the significance of the erythropoietin receptor (EPOR)–common β-chain (c-β-chain) heteroreceptor.
EXPERIMENTAL APPROACH
Mice underwent 60 min coronary occlusion followed by treatment with vehicle or a single dose of darbepoetin. Effects on gene expression, apoptosis and neutrophil accumulation in infarcted left ventricle were assessed 24 h later. Cardiac function, effects on vascularization and fibrosis were assessed 28 days later. The significance of EPOR–c-β-chain heteroreceptor was examined 28 days after infarction using mice deficient in c-β-chain.
KEY RESULTS
Twenty-four hours after darbepoetin, mRNAs encoding haeme oxygenase-1 (HO-1), iNOS and brain natriuretic peptide (BNP) were markedly elevated only in infarcted regions, and the frequency of apoptotic cells attenuated. Inflammation was also attenuated with reductions in neutrophil numbers. Darbepoetin also elevated mRNAs encoding angiogenic factors: placental growth factor, monocyte chemoattractant protein-1 and interleukin-1β. Twenty-eight days after treatment, CD31+ vessels in the infarct zone doubled and fibrosis reduced. Cardiac haemodynamics were improved. Darbepoetin also improved cardiac haemodynamics in c-β-chain-deficient mice, increased HO-1 and iNOS expression and vessel numbers and attenuated fibrosis.
CONCLUSIONS AND IMPLICATIONS
Darbepoetin stimulates expression of haeme oxygenase, iNOS, BNP and angiogenic factors specifically in infarcted left ventricle that attenuates inflammation, apoptosis and fibrosis; elevate vessel numbers; and improve cardiac function. The EPOR–c-β-chain heteroreceptor is not essential for these effects.
Keywords: darbepoetin, myocardial infarction, function and repair, haeme oxygenase-1, iNOS, inflammation, angiogenic factors, erythropoietin–common β-chain heteroreceptor
Introduction
The mortality and morbidity of acute myocardial infarction remain a major public health challenge with a significant percentage of surviving patients being severely disabled despite advances in thrombolysis and angioplasty. This disability is the consequence of grossly inadequate cardiac repair resulting in the loss of cardiomyocytes, poor vascularization and excessive collagen accumulation. Any improvement in salvaging damaged tissue would represent a major advance in therapy for myocardial infarction. Recent studies aimed at improving healing have focused on stem cells, stem cell mobilization by agents such as granulocyte colony-stimulating factor (G-CSF) and treatment with erythropoietin (EPO) (Hanlon et al., 2005; Kanellakis et al., 2006; Nelson et al., 2009). EPO is a glycoprotein hormone secreted by the kidney in response to hypoxia, which enhances erythropoiesis. Its effects are mediated via a specific cellular receptor erythropoietin receptor (EPOR), a member of the type 1 superfamily of single transmembrane cytokine receptors (Constantinescu et al., 1999). Although still controversial (Um et al., 2007), it has been suggested that EPO, in addition to signalling via a dimeric receptor for tissue protection, signals via a second receptor system consisting of EPOR and common β-chain (c-β-chain) (Brines et al., 2004). Recombinant EPO protects tissues from damage and has significant therapeutic value in rodent models such as ischaemia–reperfusion injury in kidneys (Yang et al., 2003), stroke (Wang et al., 2004) and diabetic neuropathy (Bianchi et al., 2004).
Darbepoetin is a structural analogue of EPO differing from EPO in five amino acids, resulting in addition of two extra N-linked oligosaccharides to the molecule. This results in a fourfold lower affinity for EPOR. However, in vivo, a longer plasma half-life more than compensates for the reduced affinity. Darbepoetin also exerts significant tissue protective effects. It confers behavioural and histological neuroprotection after focal cerebral ischaemia (Belayev et al., 2005), protects the kidney against the detrimental effects of ischaemia (Johnson et al., 2006), podocytes from injury by puromycin (Eto et al., 2007) and attenuates hepatocellular apoptosis induced by lipopolysaccharides (Le Minh et al., 2007). Darbepoetin also has significant effects on infarcted myocardium. In rats, it reduces infarct size, attenuates cardiomyocyte apoptosis and improves short-term (3 days) cardiac function (Gao et al., 2007). Its cardioprotective effects were reported to be dependent on p42/44 MAPK, p38MAPK, mitochondrial ATP-dependent potassium channels and sarcolemmal KATP channels (Baker et al., 2007). In contrast, in pigs, darbepoetin did not reduce infarct size, but limited fibrosis and preserved regional myocardial wall motion (Toma et al., 2007). Despite these studies, the spectrum of effects by which darbepoetin exerts protective effects on infarcted myocardium has yet to be defined. In addition, potential mechanisms and receptors utilized by darbepoetin to exert its cardioprotective effects after myocardial infarction are not known.
In the present study, we used a mouse cardiac ischaemia–reperfusion model to define the actions of darbepoetin. We confirmed its ability to attenuate apoptosis following reperfusion of ischaemic myocardium. This was associated with increased expression of inducible NOS (iNOS), haeme oxygenase-1 (HO-1) and brain natriuretic peptide (BNP). Treatment also reduced neutrophil accumulation in the infarct zone and increased the expression of angiogenic factors. Blood vessels in the infarct regions were also increased, fibrosis attenuated and cardiac function improved. We also demonstrated that the EPOR–c-β-chain is not required for darbepoetin to exert its cardioprotective effects.
Methods
Animals
C57Bl/6 mice were from AMREP Animal Services (AMREP, Melbourne, Victoria, Australia). Mice deficient in c-β-chain were obtained from the Walter and Eliza Hall Institute of Medical Research (Melbourne, VIC, Australia) and bred at AMREP Animal Services. All experiments were performed when the mice were 10 weeks of age. The mice were housed under conventional conditions with free access to food and water. All experiments were approved by the AMREP Animal Ethics Committee.
Surgical procedures
The mice were anaesthetized with a mixture of ketamine, xylazine and atropine, and intubated and ventilated as previously described (Kanellakis et al., 2006). A thoracotomy was performed in the left third intercostal space and the beating heart isolated. A 7-0 silk suture was passed under the left coronary artery at the inferior edge of the left atrium, and tied with a slipknot to produce occlusion. Air was then evacuated from the chest, the cavity closed and normal respiration restored. After 1 h of ischaemia, blood flow through the left coronary artery was re-established by pulling the slipknot (Kanellakis et al., 2006). Because c-β-chain-deficient mice were much more resistant to the detrimental effects of ischaemia–reperfusion injury than wild-type C57Bl/6 mice (see Results), these mice were also subjected to permanent coronary artery ligation. In this instance, the left coronary artery was permanently ligated. Sham-operated mice underwent identical surgical procedures except that the coronary arteries were not ligated. Darbepoetin or vehicle was administered 1 h after the coronary artery had been occluded.
Experimental groups
In the first experiments, five groups of C57Bl/6 mice (15 per group) were studied. A sham-operated group was treated 1 h after chest closure with a single dose of darbepoetin (30 µg·kg−1 i.v.). Another group subjected to ischaemia–reperfusion was treated immediately after reperfusion with i.v. saline, and three groups subjected to ischaemia–reperfusion injury were treated immediately after reperfusion with darbepoetin (0.3, 3.0 or 30 µg·kg−1 i.v.). Twenty-four hours after the initiation of reperfusion, five mice in each group were culled and their hearts excised for immunohistochemistry and molecular biological studies. All mice surviving for 28 days were subjected to cardiac haemodynamic studies, and then culled and hearts collected for histological/immunohistochemical studies. We also utilized four groups of mice deficient in c-β-chain, two of which were subjected to ischaemia–reperfusion and two to permanent coronary artery ligation. The mice subjected to ischaemia–reperfusion were treated as previously indicated, while those subjected to permanent ligation were treated upon chest closure either with saline (i.v.) or darbepoetin (30 µg·kg−1 i.v.); sham-operated mice deficient in c-β-chain were also included. Cardiac haemodynamics was measured 28 days after infarctions, and then the mice were culled and hearts collected for histological studies.
Cardiac haemodynamics
Cardiac haemodynamics were measured using a 1.4F Millar microtipped transducer catheter (Millar Instruments, Houston, TX, USA; Kanellakis et al., 2006). The mice were anaesthetized with pentobarbitone (8 mg·100 g−1 i.p.) and atropine (0.06 mg·100 g−1 i.p.), placed in a supine position, intubated and ventilated. The catheter was inserted via the right carotid artery into the aorta and advanced into the left ventricle. Resting aortic pressures, as well as left ventricular systolic pressure (LVSP) and LV + dp/dt(max) and LV − dp/dt(min), were recorded using a computer and ADInstruments software (ADlnstruments, Bella Vista, NSW, Australia; Kanellakis et al., 2006).
Cardiac fibrosis
After haemodynamic measurements, the hearts were arrested in diastole with 15% potassium chloride and fixed by immersion in 10% buffered formalin for 24–48 h. Three transverse slices from the base, mid-region and apex were embedded in paraffin. Each slice was then sectioned (6 mm thick) for histology and immunohistochemistry. The amount of collagen deposited in the left ventricle was measured as previously described after staining the sections with picrosirius red (Picrosirius Red F3BA, 0.1% solution in saturated aqueous picric acid), using an Optimus Bioscan 2 Imaging System (Thomas Optical Measurement System, Media Cybernetics, Sliver Spring, MD, USA; Kanellakis et al., 2006).
Immunohistochemistry
Paraffin sections were cleared in xylene, rehydrated and antigen retrieved by heating the sections in 0.01 M citrate buffer (pH 6.0) in a microwave for 8 min. After the slides had been incubated for a further 20 min, they were transferred to 0.1 M phosphate-buffered saline (PBS, pH 7.5). After peroxidase activity had been quenched, the sections were incubated in 10% horse serum for 30 min, washed in PBS and incubated for 1 h in PBS containing an anti-CD31 antibody (1–20; BD Pharmingen, Frankin Lakes, NJ, USA) (Raj et al., 2006). Following multiple washings, the sections were incubated with a biotinylated anti-rat antibody [in normal goat serum (NGS)/PBS], followed by incubation with streptavidin–horseradish peroxide complex. Antigen was visualized using DAB. Sections were counterstained with haematoxylin and CD31-positive vessels within the infarct zone counted.
Neutrophil accumulation, HO-1 and iNOS in the infarct zone 24 h after reperfusion or permanent ligation were also assessed by immunohistochemistry, using cardiac tissue embedded in OCT compound (Tissue-Tek, Sakura Finetek USA Inc, Torrence, CA, USA) and frozen (Raj et al., 2006). Briefly, cryostat-cut sections (6 µm) were fixed in cold (−20°C) acetone for 20 min. The sections were then sequentially incubated in 3% hydrogen peroxide in PBS, 10% NGS/PBS and biotin/avidin blocking reagents (Vector Laboratories, Burlingame, CA, USA). Thereafter, the sections were incubated (1 h) with a rat anti-mouse neutrophil antibody (1–50, Serotec, Raleigh, NC, USA) in NGS/PBS, rabbit polyclonal anti-HO-1 (1–50, Abcam, Cambridge, MA, USA), rabbit polyclonal anti-iNOS (1–50, Abcam) or corresponding non-immune IgG. After multiple washings, the sections were processed as above after the addition of the biotinylated anti-rat antibody. Neutrophils were quantified by counting.
Apoptotic [terminal deoxynucleotidyl transferase-mediated dUTP-X nick-end labelling (TUNEL)-positive] cells in infarct zones of C57Bl/6 mice 24 h after myocardial infarction were estimated using TUNEL (in situ cell death detection kit, Roche, Dee Why, NSW, Australia) as previously described (Kanellakis et al., 2009).
Real-time PCR
Total RNA was extracted from infarcted and non-infarcted left ventricle as previously described (Ward et al., 1997). Any contaminating DNA was removed by incubating these RNA extracts with 2 U DNase (Stratagene, La Jolla, CA, USA). Then, RNA was precipitated using isopropanol and washed in 70% ethanol prior to being dissolved in sterile water and quantification at 260 nm. RNA was then reverse transcribed using TaqMan Methodology (Applied Biosystems, Foster City, CA, USA) as described by the manufacturer. The cDNA (40 ng) was used for real-time PCR to determine the expression of each gene using Applied Biosystems SYBR Green PCR Mix and an ABI PRISM 7500 system. Each amplification was performed in duplicate and included internal controls for ribosomal 18s. Relative amounts of each mRNA for each gene in infarcted and non-infarcted LV from control and treated mice subjected to ischaemia–reperfusion injury were calculated using comparative CT values (Raj et al., 2006). Oligonucleotide primers were: iNOS, sense: 5′-GTGGAGAGATTTTGCATGACACTCT-3′ and antisense: 5′-ACCCCAAGCAAGACTTGGACTT-3′; hemoxygenase-1: sense: 5′-CAGGTGTCCAGAGAAGGCTTTAA-3′ and antisense: 5′-TGCGCTCTATCTCCTCTTCCA-3′; BNP, sense: 5′-CAGTCTCCAGAGCAATTCAAGATG-3′ and antisense: 5′-GAGCTGTCTCTGGGCCATTTC-3′; survivin, sense: 5′-GGCCCAGTGTTTTTTCTGCTT-3′ and antisense: 5′-GGGAGTGCTTTCTATGCTCCTCT-3′; Bcl-2, sense: 5′-CGGGAGAACAGGGTATGATAACC-3′ and antisense: 5′-ATCTCCAGCATCCCACTCGTA-3′; Bax, sense: 5′-TGGTTGCCCTCTTCTACTTTGC-3′ and antisense: 5′-TGATCAGCTCGGGCACTTTAGT-3′.
Gene arrays
RNA from infarcted and non-infarcted regions of hearts from C57Bl/6 mice was also subjected to gene expression profiling using Oligo GEArray Mouse Angiogenesis Microarrays (SABiosciences, Frederick, MD, USA). RNA was converted to biotinylated cRNA according to the manufacturer's directions, and hybridized to membrane-bound oligonucleotides representing 113 genes implicated in angiogenesis. After X-ray film development, data analyses were performed using the GEArray Expression Analysis Suite (SABiosciences).
Statistical analyses
Results are expressed as mean ± SEM. Comparisons between groups were carried out using one-way anova or Student's t-test. P < 0.05 was considered statistically significant.
Results
Darbepoetin and genes regulating apoptosis
We investigated whether a single injection of low- (0.3 µg·kg−1) or high- (30 µg·kg−1) dose darbepoetin could attenuate apoptosis in the infarct zone 24 h after ischaemia–reperfusion injury. Both low and high doses were equally effective in attenuating apoptotic cell numbers by nearly 80% (P < 0.05; Figure 1A). Because expression of HO-1, iNOS and BNP has been associated with protection of the heart against ischaemia (D'Souza et al., 2003; Jones and Bolli, 2006; Liu et al., 2006), we investigated whether darbepoetin also affected their expression. Darbepoetin treatment doubled the expression of HO-1 specifically in the infarcted region of the heart (P < 0.05; Figure 1A); mRNA levels encoding HO-1 in non-infarcted regions were unaffected (Figure 1A). These effects were apparent at low and high doses of darbepoetin. Similarly, darbepoetin also increased expression of iNOS by threefold (P < 0.05; Figure 1B), again only in the infarcted region of the heart with significant increases observed at all doses of darbepoetin. mRNA encoding BNP also doubled in the infarct region (P < 0.05; Figure 1B). In contrast, other anti-apoptotic factors, survivin and Bcl-2, were mostly unaffected, although mRNA encoding Bcl-2 and Bax was increased twofold at the lowest darbepoetin dose (Figure 1B).
Figure 1.
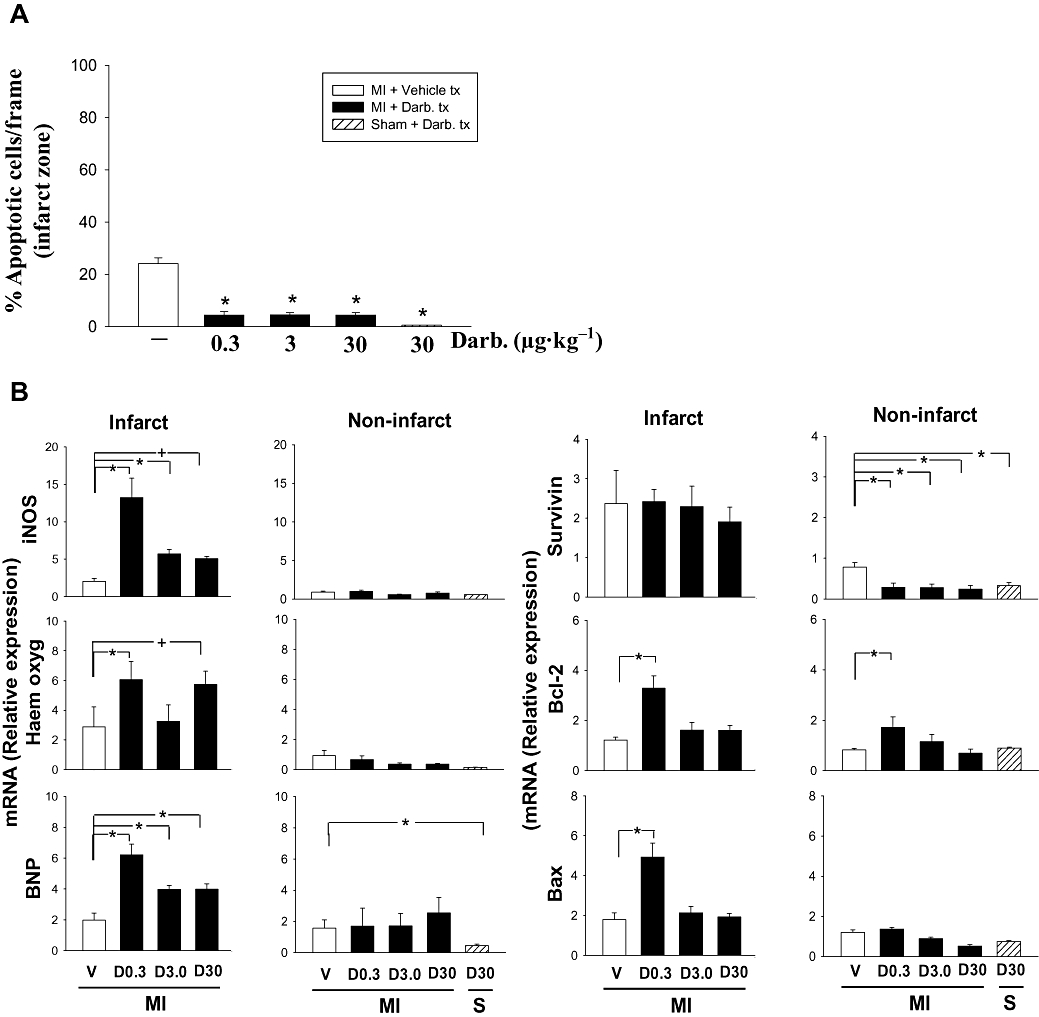
Darbepoetin, apoptosis and genes that regulate cell survival in infarct zone. (A) Apoptotic cells in the infarct zone 24 h after reperfusion determined using an ‘in situ Cell Death Detection Kit’ and direct cell counting, expressed as percentage of total cells in the infarct zone. (B) Total RNA isolated from infarct regions 24 h after reperfusion, and treatment with darbepoetin or vehicle was subjected to real-time PCR to quantify expression levels of genes that regulate responses to injury. Columns represent infarcted mice treated with vehicle (V) or darbepoetin (D; µg·kg−1) or sham-operated mice (S) treated with darbepoetin. All results are means ± SEM of five mice in each group. *P < 0.05 from vehicle-treated mice.
Darbepoetin and neutrophil accumulation
Neutrophils are known to be involved in murine myocardial ischaemia–reperfusion injury, releasing a variety of cytotoxic mediators augmenting cardiac necrosis and apoptosis (Vinten-Johansen, 2004). Consequently, we examined the effects of darbepoetin on neutrophil accumulation in the infarct zone 24 h after ischaemia–reperfusion. Both low and high doses of darbepoetin were equally effective in attenuating neutrophil accumulation, by approximately 50% (P < 0.05; Figure 2).
Figure 2.
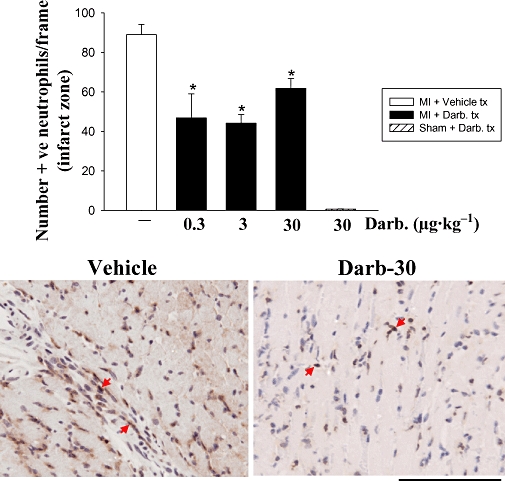
Darbepoetin and neutrophil accumulation in infarcted myocardium. Top: neutrophil numbers in the infarct zone were determined by counting immunopositive neutrophils in the infarct zone 24 h after reperfusion. Results are mean ± SEM of five mice in each group. *P < 0.05 from infarct zone of vehicle-treated group. Bottom: photomicrographs of neutrophils accumulating in the infarct zone of mice treated with vehicle or darbepoetin (30 µg·kg−1). Bar represents 100 µm.
Darbepoetin and genes regulating angiogenesis
EPO can induce angiogenesis in cultured heart tissue independently of endothelial progenitor cells (Jaquet et al., 2002). To determine whether darbepoetin treatment also increases vascularization in the infarct zone after ischaemia–reperfusion injury, we assessed vessel numbers. Infarcted regions of the left ventricle of mice treated with darbepoetin contained up to three times more CD31-positive vessels compared to untreated mice (P < 0.05; Figure 3A). This was apparent with both the low and high darbepoetin doses.
Figure 3.
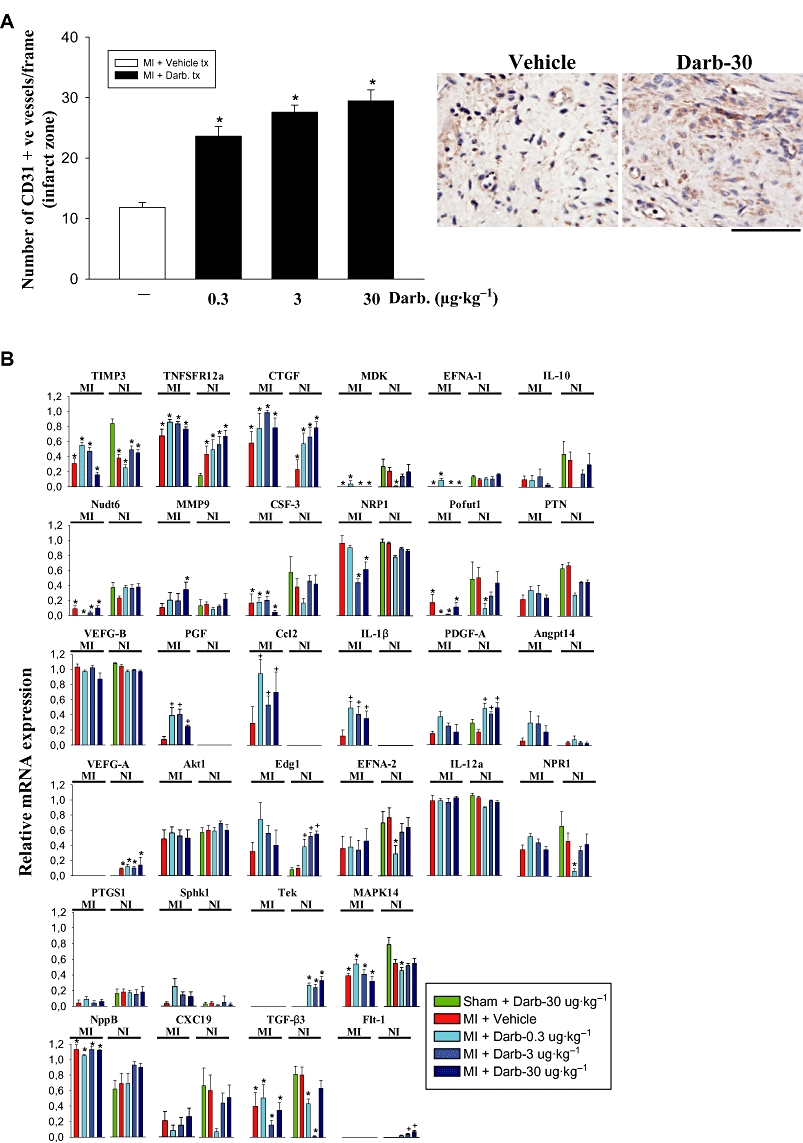
Darbepoetin affects vessels in infarct zone and angiogenic genes. (A) Left: CD31-positive vessels in infarcted region of myocardium 28 days after treatment with vehicle or darbepoetin. Right: Cd31-positive vessels in the infarct zone of mice treated with vehicle or darbepoetin (30 µg·kg−1). The bar represents 100 µm. (B) Expression of 28 angiogenic genes 24 h after myocardial infarction/sham operation and treatment with vehicle or darbepoetin. TNFSFR12a, tumour necrosis super family receptor 12a; CTGF, connective tissue growth factor; IL-10, interleukin-10; Nudt6, nudix (nucleoside diphosphate linked moiety X)-type motif 6; MMP9, matrix metalloproteinase-9; CSF-3, G-CSF; NRP1, neurophilin-1; Pofut 1, protein O-fucosyltransferase-1; PTN, pleiotrophin; CCL2, monocyte chemoattractant protein-1; Angpt14, angiopoietin-like-4; PTGS1, prostaglandin endoperoxide synthase-1; Sphk1, sphingosine kinase-1. All other genes follow standard accepted abbreviations. In (A), results are means ± SEM of five mice in each group. *P < 0.05 from sham-operated, darbepoetin-treated mice. In (B), results are means ± SEM of 10 mice in each group. *P < 0.05 from darbepoetin-treated, sham-operated mice; +P < 0.05 from infarcted, vehicle-treated mice.
We next investigated whether this increase in vessel numbers was associated with increased expression of angiogenic factors. We assessed its ability to stimulate expression of genes encoding angiogenic factors by analyzing mRNA obtained 24 h after treatment, using angiogenesis oligonucleotide gene arrays. Twenty-four hours after infarction, a number of negative regulators of angiogenesis were down-regulated in the infarct zone, including tissue inhibitor of metalloproteinase-3 (TIMP3), midkine (MDK), ephrin-A1 (EFNA-1) and IL-10, while natriuretic peptide precursor B was up-regulated (Figure 3B).
Darbepoetin increased expression of placental growth factor (PGF) 5- to 10-fold (P < 0.05), specifically in infarcted myocardium. Increases in PGF were apparent after low- and high-dose darbepoetin (Figure 3B). We were unable to detect its receptor Flt-1 in the infarct zone (Figure 3B), but neurophilin-1 (NRP1) was readily detectable (Figure 3B). Chemokine (C-C motif) ligand 2 (CCL2, MCP-1) was also elevated specifically in infarcted myocardium (P < 0.05; Figure 3B). Although best known for inducing monocyte/macrophage recruitment to sites of inflammation, CCL2 is also capable of stimulating endothelial cell chemotaxis and inducing new vessel formation (Salcedo et al., 2000). Darbepoetin also increased interleukin-1β (IL-1β) expression three- to fourfold (P < 0.05; Figure 3B), which can also exert pro-angiogenic effects (Naldini et al., 2006). In contrast, vascular endothelial growth factor (VEGF)-B expression was unaffected as was VEGF-A (Figure 3B). Interestingly, increased VEGF-A expression was only apparent in non-infarcted regions of the heart and not dependent on darbepoetin, but was associated with darbepoetin-mediated elevations in Flt-1 mRNA (Figure 3B). Platelet-derived growth factor (PDGF)-A chain mRNA was also up-regulated by darbepoetin, but only in non-infarcted regions of the heart as was TEK receptor tyrosine kinase (P < 0.05; Figure 3B).
Darbepoetin and fibrosis
Because the frequency of cardiomyocyte apoptosis was attenuated and BNP elevated by darbepoetin, we reasoned that this may result in less fibrosis. Accordingly, we investigated whether treatment with darbepoetin also attenuated fibrosis following cardiac ischaemia–reperfusion. Treatment with darbepoetin attenuated fibrosis by more than 50% (P < 0.05; Figure 4) and was apparent with low and high doses of darbepoetin.
Figure 4.
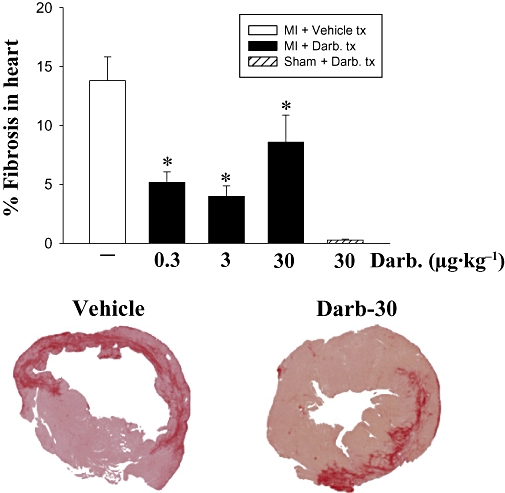
Darbepoetin and cardiac fibrosis. Fibrosis was assessed 28 days after ischaemia/sham operation and treatment with vehicle or darbepoetin. Top: cross-sections were stained with picrosirius red, and fibrosis expressed as % area stained with picrosirius red in each cross section. Results are means ± SEM from 10 mice in each group. *P < 0.05 from infarcted, vehicle-treated mice. Bottom: photomicrographs of cross sections of infarcted left ventricle stained with picrosirius red, demonstrating the extent of fibrosis in mice treated with vehicle or darbepoetin (30 µg·kg−1).
Darbepoetin improved cardiac haemodynamics
Because darbepoetin reduced inflammation and improved healing of infarcted hearts, we reasoned that this may also result in improved cardiac haemodynamics. Darbepoetin improved cardiac haemodynamics, increasing LVSP, LV + dp/dt and LV − dp/dt (P < 0.05; Figure 5), effects that were most apparent at intermediate and high doses of darbepoetin.
Figure 5.
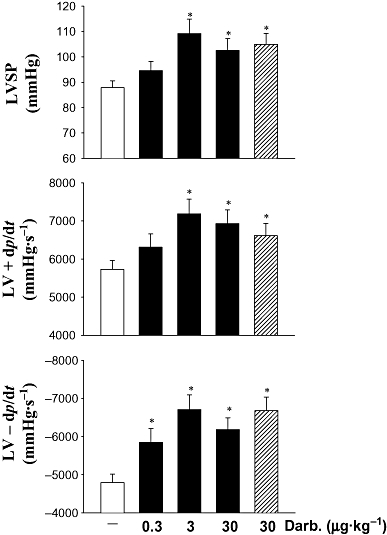
Cardiac haemodynamics following ischaemic injury. LVSP, and LV + dp/dt and LV − dp/dt were measured in anaesthetized C57Bl6 mice using an intraventricular Miller catheter. Results are means ± SEM of 10 mice in each group. *P < 0.05 from infarcted, vehicle-treated mice.
Darbepoetin and c-β-chain
It has been suggested that the tissue-protective effects of EPO might be dependent on its interaction with an EPO–c-β-chain heteroreceptor complex (Brines et al., 2004). To determine whether the cardioprotective effects of darbepoetin were dependent on an EPO–c-β-chain heteroreceptor complex, we evaluated its effects in c-β-chain-deficient mice. c-β-Chain-deficient mice were resistant to the detrimental effects of 1 h ischaemia followed by reperfusion. In these mice, LVSP, LV + dp/dt and LV − dp/dt were unaffected 28 days after ischaemia–reperfusion and similar to sham-operated c-β-chain-deficient mice (P for differences >0.05; Figure 6). Administration of darbepoetin to c-β-chain-deficient mice subjected to ischaemia–reperfusion did not further improve already normal cardiac haemodynamics (Figure 6). Consequently, we assessed its effects on c-β-chain-deficient mice after more severe cardiac damage, induced by permanently ligating the coronary artery. Following permanent coronary ligation, LVSP, LV + dp/dt and LV − dp/dt in the c-β-chain-deficient mice were reduced by 25–35% (all P < 0.05; Figure 6). In these mice, darbepoetin administered 1 h after the onset of cardiac ischaemia significantly (P < 0.05) increased LVSP 28 days later; LV + dp/dt and LV − dp/dt also tended to be increased (Figure 6). To determine whether other tissue responses were affected, we also assessed its effects on the expression of HO-1 and iNOS protein, as well as fibrosis and vessel numbers within the infarct border zone. Treatment of the c-β-chain-deficient mice with darbepoetin markedly increased expression of HO-1 and iNOS protein in cardiomyocytes within the infarct zone 24 h later (Figure 7). Treatment also resulted in 50% less cardiac fibrosis (P < 0.05; Figure 7) and significantly increased the number of CD31+ vessels within the infarct border zone 28 days later (P < 0.05; Figure 7).
Figure 6.
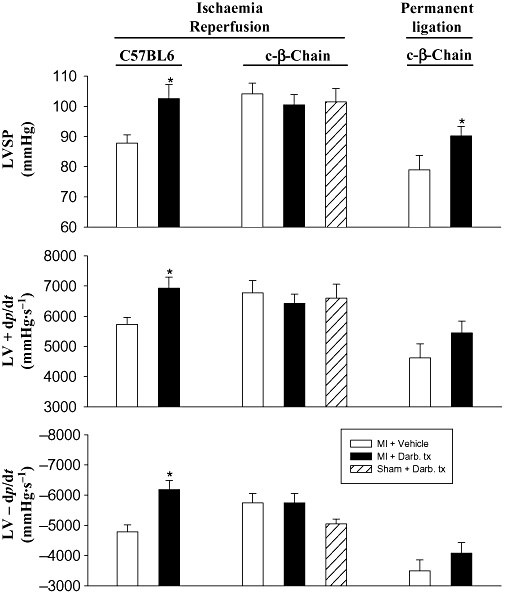
Effects of darbepoetin on cardiac haemodynamics (LVSP, LV + dp/dt and LV − dp/dt) 28 days after subjecting C57Bl6 and c-β-chain-deficient mice to 1 h of ischaemia followed by reperfusion and also c-β-chain-deficient mice to permanent coronary ligation. Results are means ± SEM of six to nine mice in each group. *P < 0.05 from vehicle-treated, infarcted mice.
Figure 7.
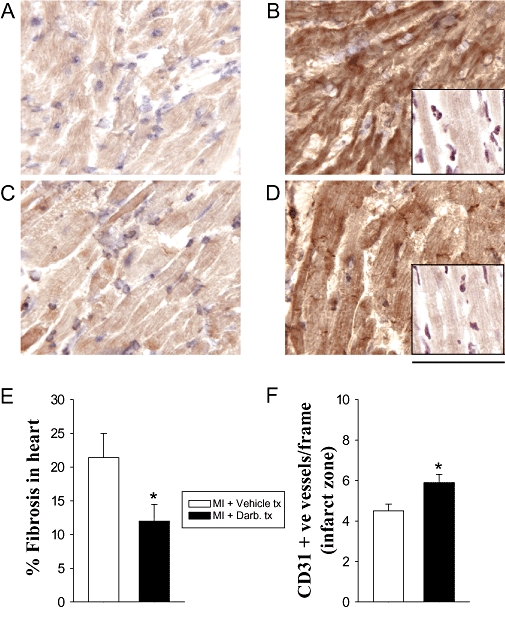
Effects of darbepoetin on the expression of HO-1 and iNOS in infarcted left ventricle of c-β-chain-deficient mice 24 h after permanent coronary ligation, and fibrosis and number of vessels 28 days later. (A) iNOS expression in infarcted left ventricle of vehicle-treated mice. (B) iNOS expression in infarcted left ventricle of darbepoetin-treated mice; insert: non-immune IgG control. (C) HO-1 expression in infarcted left ventricle of vehicle-treated mice. (D) HO-1 expression in infarcted left ventricle of darbepoetin-treated mice; insert: non-immune IgG control. (E) Left ventricular fibrosis (F) vessels in border infarct zone of left ventricle. Results are means ± SEM of six to nine mice in each group. *P < 0.05 from vehicle-treated, infarcted mice.
Discussion
Our results indicate that darbepoetin administration at the time of reperfusion initiates multiple cellular responses in the ischaemic heart, which contributes to its cardioprotective effects. This includes activation of anti-inflammatory and angiogenic genes: iNOS, BNP, HO-1, PGF, CCL2 and IL-1β. Their elevations were associated with reduced inflammation, reduced apoptosis and reduced fibrosis, as well as improved vascularization and cardiac function, effects that are independent of signalling via an EPOR–c-β-chain heteroreceptor complex.
Treatment with darbepoetin markedly elevated the expression of three cardioprotective genes specifically within the infarcted region of the heart: iNOS, HO-1 and BNP. Over-expression of iNOS protects against cardiac ischaemia–reperfusion injury by preventing mitochondrial permeability transition and attenuating free radical generation (West et al., 2008). NO generated by iNOS can also attenuate apoptosis by inhibiting caspase-3 via S-nitrosation (Rossig et al., 1999). While not specifically studied, it is likely that darbepoetin stimulates iNOS over-expression in the infarct zone via a STAT3 pathway. Darbepoetin activates STAT3 pathways in autoimmune cardiomyopathies (Mao et al., 2008), and STAT3 is crucial for up-regulation of iNOS (Ziesche et al., 2007). Our study suggests that B-type natriuretic peptide (BNP) may also contribute to darbepoetin's cardioprotective effects; darbepoetin up-regulated BNP expression specifically in the infarct zone. BNP is a cardiac anti-fibrotic factor in vivo and a local regulator of ventricular remodelling (Tamura et al., 2000).
Our finding that darbepoetin elevated HO-1 expression is consistent with its effects on iNOS expression; NO produced by iNOS stabilizes mRNAs and via this mechanism increases HO-1 (Kuwano et al., 2009). HO-1 is a phase II enzyme, a member of the family of heat shock protein that is associated with defence responses against oxidative and cellular stress, and also promotes angiogenesis (Dulak et al., 2008). It interacts with haeme to yield equimolar quantities of iron, biliverdin and carbon monoxide (Dulak et al., 2008). Increased carbon monoxide production is most likely the mechanism by which HO-1 exerts its cardioprotective, anti-apoptotic and anti-inflammatory effects. Carbon monoxide protects against cardiac ischaemia–reperfusion injury (Fujimoto et al., 2004), and the HO-1/carbon monoxide–biliverdin pathway down-regulates neutrophil rolling, adhesion and migration in acute inflammation (Freitas et al., 2006). This latter mechanism explains why darbepoetin attenuates neutrophil accumulation following cardiac ischaemia–reperfusion. HO-1-derived carbon monoxide also protects endothelial cells against apoptosis, by elevating NF-κB-inducible anti-apoptotic genes A20, c-IAP2 and manganese superoxide dismutase (Brouard et al., 2002). Whether such a mechanism also protects cardiomyocytes against apoptosis remains to be determined. Over-expression of cardiac HO-1 also attenuates fibrosis after myocardial infarction (Liu et al., 2006); hence, it is likely that the darbepoetin-induced over-expression of HO-1 also contributes to its anti-fibrotic effect after infarction.
Our study confirms earlier studies in pigs that darbepoetin can increase vessel number in the infarct zone (Toma et al., 2007). This increase could be due to a number of mechanisms including anti-apoptotic effects of darbepoetin on endothelial cells and/or increased angiogenesis stimulated by growth factors. Our gene array studies indicate that darbepoetin elevates the angiogenic factors, PGF, CCL2 and IL-1β, specifically within the infarct zone, while VEGF-A was elevated only within non-infarcted regions of the heart. Because Flt-1 was only elevated in non-infarcted regions of the heart, it is unlikely that PGF directly contributes to the increase in vessel number. It is possible that CCL2 and IL-1β contribute to vessel numbers within the infarct zone. CCL2 recruits macrophages to the infarct zone, as well as stimulating angiogenesis (Schwarz et al., 2004), while IL-1β induces angiogenesis independently of VEGF or FGF-2 (Naldini et al., 2006). Although adeno-associated adenoviral over-expression of HO-1 promotes vascularization in ischaemia heart, which is associated with increased expression of VEGF (Lin et al., 2008), the elevation in endogenous HO-1 induced by darbepoetin is unlikely to contribute to angiogenesis, as we did not observe any associated increase in VEGF.
Recently, non-erythropoietic derivatives of EPO have also been shown to be cardioprotective (Fiordaliso et al., 2005). Because these compounds do not bind to ‘classical’ homodimeric EPORs (Leist et al., 2004), it has been suggested that cardioprotection may be mediated via an EPOR–c-β-chain heteroreceptor; EPO failed to protect cultured cardiomyocytes deficient in the common β-subunit against staurosporin-induced apoptosis (Brines et al., 2004). Our studies with darbepoetin in mice deficient in the c-β-chain indicate that expression of the c-β-chain is not essential for its cardioprotective effects in vivo, because it still improved cardiac haemodynamics, stimulated the expression of HO-1 and iNOS, reduced fibrosis and increased vessel numbers in c-β-chain-deficient mice. Rather, our results are consistent with darbepoetin mediating its cardioprotective effects via the classical dimeric EPOR. In these studies, we unexpectedly found that c-β-chain-deficient mice are much more resistant to ischaemia–reperfusion injury than C57Bl6 mice, necessitating us to permanently ligate the coronary artery to significantly impair cardiac haemodynamics, so that we could assess the ability of darbepoetin to improve cardiac function. The reason for this resistance of c-β-chain-deficient mice requires further studies and may involve reductions in M1 macrophages and a change in the balance between inflammatory M1- and healing M2-macrophages, which accumulate in infarcted regions of the heart (Nahrendorf et al., 2007); c-β-chain is essential for GM-CSF-mediated induction of M1 macrophages (Leidi et al., 2009).
In conclusion, our results demonstrate that darbepoetin stimulates multiple cardioprotective mechanisms in infarcted myocardium to improve cardiac function. Its cardioprotective effects appear to be mediated via molecular mechanisms that attenuate apoptosis, inflammation and fibrosis, as well as increasing vessel numbers within the infarcted regions. These effects are not dependent on signalling via an EPO–c-β-chain heteroreceptor complex.
Acknowledgments
The study was supported by grants from the National Health and Medical Research Council of Australia (no. 472635) and Amgen Inc.
Glossary
Abbreviations
- Akt1
serine–threonine protein kinase encoded by the AKT1 gene
- Angpt14
angiopoietin-like-4
- BNP
brain natriuretic peptide
- Ccl2
chemokine (C-C motif) ligand 2
- CSF-3
colony-stimulating factor-3
- CT
point at which fluorescence crosses a threshold value
- CTGF
connective tissue growth factor
- CXCl9
chemokine (C-X-C motif) ligand 9
- dUTP
deoxyuridine triphosphate
- Edg1
functional sphingosine-1-phosphate receptor
- EFNA-1
ephrin-A1
- EFNA-2
ephrin-A2
- EPO
erythropoietin
- EPOR
erythropoietin receptor
- Flt-1
vascular endothelial growth factor receptor-1
- G-CSF
granulocyte colony-stimulating factor
- HO-1
haeme oxygenase-1
- IL
interleukin
- iNOS
inducible NOS
- KATP
adenosine triphosphate-sensitive potassium channels
- LVSP
left ventricular systolic pressure
- LV + dp/dt(max)
maximum rate of rise of left ventricular pressure
- LV – dp/dt(min)
maximum rate of fall in left ventricular pressure
- MAPK14
mitogen-activated protein kinase-14
- MDK
midkine
- MMP9
matrix metalloproteinase-9
- NF-κB
nuclear factor kappa light chain enhancer of activated B cells
- NPR1
natriuretic peptide receptor-A
- NRP1
neurophilin-1
- Nudt6
nucleoside diphosphate linked moiety X-type motif 6
- PDGF-A
platelet-derived growth factor-A
- PGF
placental growth factor
- Pofut1
protein O-fucosyltransferase-1
- PTGS1
prostaglandin endoperoxide synthase-1
- PTN
pleiotrophin
- P42/44 MAPK
p42/p44 mitogen-activated protein kinase
- P38MAPK
p38 mitogen-activated protein kinase
- Sphk1
sphingosine kinase-1
- STAT3
signal transducer and activation of transcription 3
- TIMP3
tissue inhibitor of metalloproteinase-3
- TNFSFR
tumour necrosis factor receptor superfamily
- Tek
angiopoietin-1 receptor
- TGF-b3
transforming growth factor-b3
- VEGF-A/B
vascular endothelial growth factor-A/B
Conflict of interest
All authors have no conflict of interest.
References
- Baker JE, Kozik D, Hsu AK, Fu X, Tweddell JS, Gross GJ. Darbepoetin alfa protects the rat heart against infarction: dose–response, phase of action, and mechanisms. J Cardiovasc Pharmacol. 2007;49:337–345. doi: 10.1097/FJC.0b013e318040cf81. [DOI] [PubMed] [Google Scholar]
- Belayev L, Khoutorova L, Zhao W, Vigdorchik A, Belayev A, Busto R, et al. Neuroprotective effect of darbepoetin alfa, a novel recombinant erythropoietic protein, in focal cerebral ischemia in rats. Stroke. 2005;36:1071–1076. doi: 10.1161/01.STR.0000160753.36093.da. [DOI] [PubMed] [Google Scholar]
- Bianchi R, Buyukakilli B, Brines M, Savino C, Cavaletti G, Oggioni N, et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc Natl Acad Sci USA. 2004;101:823–828. doi: 10.1073/pnas.0307823100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common beta-subunit heteroreceptor. Proc Natl Acad Sci USA. 2004;101:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouard S, Berberat PO, Tobiasch E, Seldon MP, Bach FH, Soares MP. Heme oxygenase-1-derived carbon monoxide requires the activation of transcription factor NF-kappa B to protect endothelial cells from tumor necrosis factor-alpha-mediated apoptosis. J Biol Chem. 2002;277:17950–17961. doi: 10.1074/jbc.M108317200. [DOI] [PubMed] [Google Scholar]
- Constantinescu SN, Ghaffari S, Lodish HF. The erythropoietin receptor: structure, activation and intracellular signal transduction. Trends Endocrinol Metab. 1999;10:18–23. doi: 10.1016/s1043-2760(98)00101-5. [DOI] [PubMed] [Google Scholar]
- D'Souza SP, Yellon DM, Martin C, Schulz R, Heusch G, Onody A, et al. B-type natriuretic peptide limits infarct size in rat isolated hearts via KATP channel opening. Am J Physiol Heart Circ Physiol. 2003;284:H1592–H1600. doi: 10.1152/ajpheart.00902.2002. [DOI] [PubMed] [Google Scholar]
- Dulak J, Deshane J, Jozkowicz A, Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto N, Wada T, Inagi R, Takano H, Shimizu A, Kato H, et al. Podocyte protection by darbepoetin: preservation of the cytoskeleton and nephrin expression. Kidney Int. 2007;72:455–463. doi: 10.1038/sj.ki.5002311. [DOI] [PubMed] [Google Scholar]
- Fiordaliso F, Chimenti S, Staszewsky L, Bai A, Carlo E, Cuccovillo I, et al. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia–reperfusion injury. Proc Natl Acad Sci USA. 2005;102:2046–2051. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas A, Alves-Filho JC, Secco DD, Neto AF, Ferreira SH, Barja-Fidalgo C, et al. Heme oxygenase/carbon monoxide–biliverdin pathway down regulates neutrophil rolling, adhesion and migration in acute inflammation. Br J Pharmacol. 2006;149:345–354. doi: 10.1038/sj.bjp.0706882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto H, Ohno M, Ayabe S, Kobayashi H, Ishizaka N, Kimura H, et al. Carbon monoxide protects against cardiac ischemia–reperfusion injury in vivo via MAPK and Akt–eNOS pathways. Arterioscler Thromb Vasc Biol. 2004;24:1848–1853. doi: 10.1161/01.ATV.0000142364.85911.0e. [DOI] [PubMed] [Google Scholar]
- Gao E, Boucher M, Chuprun JK, Zhou RH, Eckhart AD, Koch WJ. Darbepoetin alfa, a long-acting erythropoietin analog, offers novel and delayed cardioprotection for the ischemic heart. Am J Physiol Heart Circ Physiol. 2007;293:H60–H68. doi: 10.1152/ajpheart.00227.2007. [DOI] [PubMed] [Google Scholar]
- Hanlon PR, Fu P, Wright GL, Steenbergen C, Arcasoy MO, Murphy E. Mechanisms of erythropoietin-mediated cardioprotection during ischemia–reperfusion injury: role of protein kinase C and phosphatidylinositol 3-kinase signaling. FASEB J. 2005;19:1323–1325. doi: 10.1096/fj.04-3545fje. [DOI] [PubMed] [Google Scholar]
- Jaquet K, Krause K, Tawakol-Khodai M, Geidel S, Kuck KH. Erythropoietin and VEGF exhibit equal angiogenic potential. Microvasc Res. 2002;64:326–333. doi: 10.1006/mvre.2002.2426. [DOI] [PubMed] [Google Scholar]
- Johnson DW, Pat B, Vesey DA, Guan Z, Endre Z, Gobe GC. Delayed administration of darbepoetin or erythropoietin protects against ischemic acute renal injury and failure. Kidney Int. 2006;69:1806–1813. doi: 10.1038/sj.ki.5000356. [DOI] [PubMed] [Google Scholar]
- Jones SP, Bolli R. The ubiquitous role of nitric oxide in cardioprotection. J Mol Cell Cardiol. 2006;40:16–23. doi: 10.1016/j.yjmcc.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Kanellakis P, Slater NJ, Du XJ, Bobik A, Curtis DJ. Granulocyte colony-stimulating factor and stem cell factor improve endogenous repair after myocardial infarction. Cardiovasc Res. 2006;70:117–125. doi: 10.1016/j.cardiores.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Kanellakis P, Pomilio G, Walker C, Husband A, Huang JL, Nestel P, et al. A novel antioxidant 3,7-dihydroxy-isoflav-3-ene (DHIF) inhibits neointimal hyperplasia after vessel injury attenuating reactive oxygen species and nuclear factor-kappaB signaling. Atherosclerosis. 2009;204:66–72. doi: 10.1016/j.atherosclerosis.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Kuwano Y, Rabinovic A, Srikantan S, Gorospe M, Demple B. Analysis of nitric oxide-stabilized mRNAs in human fibroblasts reveals HuR-dependent heme oxygenase 1 upregulation. Mol Cell Biol. 2009;29:2622–2635. doi: 10.1128/MCB.01495-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidi M, Gotti E, Bologna L, Miranda E, Rimoldi M, Sica A, et al. M2 macrophages phagocytose rituximab-opsonized leukemic targets more efficiently than m1 cells in vitro. J Immunol. 2009;182:4415–4422. doi: 10.4049/jimmunol.0713732. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Le Minh K, Klemm K, Abshagen K, Eipel C, Menger MD, Vollmar B. Attenuation of inflammation and apoptosis by pre- and posttreatment of darbepoetin-alpha in acute liver failure of mice. Am J Pathol. 2007;170:1954–1963. doi: 10.2353/ajpath.2007.061056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HH, Chen YH, Chang PF, Lee YT, Yet SF, Chau LY. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. J Mol Cell Cardiol. 2008;45:44–55. doi: 10.1016/j.yjmcc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Liu X, Pachori AS, Ward CA, Davis JP, Gnecchi M, Kong D, et al. Heme oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and restores ventricular function. FASEB J. 2006;20:207–216. doi: 10.1096/fj.05-4435com. [DOI] [PubMed] [Google Scholar]
- Mao W, Iwai C, Liu J, Sheu SS, Fu M, Liang CS. Darbepoetin alfa exerts a cardioprotective effect in autoimmune cardiomyopathy via reduction of ER stress and activation of the PI3K/Akt and STAT3 pathways. J Mol Cell Cardiol. 2008;45:250–260. doi: 10.1016/j.yjmcc.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naldini A, Leali D, Pucci A, Morena E, Carraro F, Nico B, et al. Cutting edge: IL-1beta mediates the proangiogenic activity of osteopontin-activated human monocytes. J Immunol. 2006;177:4267–4270. doi: 10.4049/jimmunol.177.7.4267. [DOI] [PubMed] [Google Scholar]
- Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–416. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj T, Kanellakis P, Pomilio G, Jennings G, Bobik A, Agrotis A. Inhibition of fibroblast growth factor receptor signaling attenuates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2006;26:1845–1851. doi: 10.1161/01.ATV.0000227689.41288.5e. [DOI] [PubMed] [Google Scholar]
- Rossig L, Fichtlscherer B, Breitschopf K, Haendeler J, Zeiher AM, Mulsch A, et al. Nitric oxide inhibits caspase-3 by S-nitrosation in vivo. J Biol Chem. 1999;274:6823–6826. doi: 10.1074/jbc.274.11.6823. [DOI] [PubMed] [Google Scholar]
- Salcedo R, Ponce ML, Young HA, Wasserman K, Ward JM, Kleinman HK, et al. Human endothelial cells express CCR2 and respond to MCP-1: direct role of MCP-1 in angiogenesis and tumor progression. Blood. 2000;96:34–40. [PubMed] [Google Scholar]
- Schwarz ER, Meven DA, Sulemanjee NZ, Kersting PH, Tussing T, Skobel EC, et al. Monocyte chemoattractant protein 1-induced monocyte infiltration produces angiogenesis but not arteriogenesis in chronically infarcted myocardium. J Cardiovasc Pharmacol Ther. 2004;9:279–289. doi: 10.1177/107424840400900408. [DOI] [PubMed] [Google Scholar]
- Tamura N, Ogawa Y, Chusho H, Nakamura K, Nakao K, Suda M, et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc Natl Acad Sci USA. 2000;97:4239–4244. doi: 10.1073/pnas.070371497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma C, Letts DP, Tanabe M, Gorcsan J, 3rd, Counihan PJ. Positive effect of darbepoetin on peri-infarction remodeling in a porcine model of myocardial ischemia–reperfusion. J Mol Cell Cardiol. 2007;43:130–136. doi: 10.1016/j.yjmcc.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Um M, Gross AW, Lodish HF. A ‘classical’ homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell Signal. 2007;19:634–645. doi: 10.1016/j.cellsig.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang Z, Wang Y, Zhang R, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Ward MR, Agrotis A, Kanellakis P, Dilley R, Jennings G, Bobik A. Inhibition of protein tyrosine kinases attenuates increases in expression of transforming growth factor-beta isoforms and their receptors following arterial injury. Arterioscler Thromb Vasc Biol. 1997;17:2461–2470. doi: 10.1161/01.atv.17.11.2461. [DOI] [PubMed] [Google Scholar]
- West MB, Rokosh G, Obal D, Velayutham M, Xuan YT, Hill BG, et al. Cardiac myocyte-specific expression of inducible nitric oxide synthase protects against ischemia/reperfusion injury by preventing mitochondrial permeability transition. Circulation. 2008;118:1970–1978. doi: 10.1161/CIRCULATIONAHA.108.791533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Li C, Jung JY, Shin SJ, Choi BS, Lim SW, et al. Preconditioning with erythropoietin protects against subsequent ischemia–reperfusion injury in rat kidney. FASEB J. 2003;17:1754–1755. doi: 10.1096/fj.02-1191fje. [DOI] [PubMed] [Google Scholar]
- Ziesche E, Bachmann M, Kleinert H, Pfeilschifter J, Muhl H. The interleukin-22/STAT3 pathway potentiates expression of inducible nitric-oxide synthase in human colon carcinoma cells. J Biol Chem. 2007;282:16006–16015. doi: 10.1074/jbc.M611040200. [DOI] [PubMed] [Google Scholar]


