Abstract
Although methotrexate is widely used in clinical practice there remains significant lack of understanding of its mechanisms of action and the factors that contribute to the variability in toxicity and response seen clinically. In addition to differences in drug administration, factors that affect pharmacokinetics and pharmacodynamics such as genetic variation may explain individual differences in drug biotransformation. However, the pediatric population has an additional factor to consider, namely the ontogeny of gene expression which may result in variation throughout growth and development. We review the current understanding of methotrexate biotransformation and the concept of ontogeny, with further discussion of how to implement a developmental pharmacogenomics approach in future studies.
1. Introduction
Methotrexate (MTX) is the most common second-line therapeutic agent used to treat Juvenile Idiopathic Arthritis (JIA) worldwide. Regardless of age or disease subtype, considerable interindividual variability in clinical response and adverse reactions exists with MTX, and thus far, there have been no predictive variables for outcomes in patients taking this medication. Since the onset of clinical response may take months to manifest, the risk to benefit ratio early in treatment is altered, as there is risk for toxicity for several weeks to months before knowing if the medication has resulted in a clinical benefit. Side effects may compromise efficacy due to patient noncompliance, clinician dose adjustment, or discontinuation even if the drug eventually is medically effective. Medication dose alteration or discontinuation in the face of active disease is unacceptable when the alternatives for therapy in childhood arthritis are few and poorly studied in this population. By utilizing pharmacogenomic principles and a personalized therapeutic strategy, we hope to improve efficacy and prevent adverse drug reactions in children taking MTX to treat JIA.
2. Developmental Pharmacogenetics
When exploring the variability in response and toxicity to any medication used in children, a concept often overlooked is ontogeny [1]. The effects of development can be applied at every level of drug disposition and response. These effects range from differences in gastric pH [2, 3] and gastric emptying [4] which may affect absorption, to changes in circulating plasma proteins with age that may affect drug distribution [5]. Developmental changes in phase I drug biotransformation and phase II conjugating enzyme expression have the potential to alter drug metabolism [6], and developmental differences in glomerular filtration rates [7] will affect drug excretion in children compared to adults. Common drug biotransformation pathways are also shared with endogenous compounds involved in growth and development, such as testosterone, cortisol, and vitamin D3 [8], so it is not surprising that some of these pathways may be affected by the rapid growth and maturation of the pediatric patient, for example, during infancy and puberty. The developmental expression of these pathways at different rates or trajectories may also lead to variability in drug disposition and response.
Although pharmacogenetics appropriately strives to identify the correct dose of the correct drug for the correct person, the impact of development on an individual's response to a drug must be taken into account [1]. Genotyping an individual for variations that affect function is an important step to understanding variability in outcomes; however, knowing if and when that gene is expressed is a concept important to fully understanding genotype-phenotype relationships in children [9, 10]. An approach to investigating hypotheses related to drug outcomes in children can be guided by the following questions [9].
What gene products are quantitatively important in the disposition (absorption, distribution, metabolism, and excretion) of the drug in question?
For each gene product, what is the developmental trajectory for the acquisition of functional activity?
Is allelic variation in the gene(s) of interest associated with any functional consequences in vivo?
Is there any evidence that allelic variation affects the developmental trajectory of the drug disposition phenotype?
What is the developmental context in which the gene(s) of interest is/are operating?
This process is also relevant for genes/gene products involved in drug response. Partnered with the understanding of genetic variation in an individual, appreciating the changes in gene expression throughout growth and development will allow us to manage the complexity of therapeutics in children. We strive to individualize therapy for children rather than extrapolate from adult experience, which traditionally has been the norm.
3. JIA Background
Juvenile Idiopathic Arthritis (JIA), formerly termed Juvenile Chronic Arthritis (JCA) or Juvenile Rheumatoid Arthritis (JRA) is one of the most common chronic diseases of childhood, and is an important cause of morbidity and disability in children. It affects an estimated 300,000 children in the United States. This disease is characterized by idiopathic peripheral arthritis with an immunoinflammatory pathogenesis, thought to be triggered by an external antigen [11]. There is also speculation of a genetic predisposition for the disease [12–15]. JIA has a heterogeneous phenotypic expression, and includes several disease subtypes, whose classification continues to be revised and validated by clinicians worldwide [15].
Despite differences in disease expression between adults and children, like in many pediatric diseases, children are treated with generally the same armamentarium of drugs used to treat RA in adults. With the advent of Disease Modifying Antirheumatic Drugs (DMARDs), the philosophy of treatment changed from simple pain control to prevention of erosions and long-term damage of the joints. Methotrexate (MTX), a folic acid antagonist, was approved by the Food and Drug Administration for the treatment of RA in 1988, and several uncontrolled descriptive studies suggested, and a randomized placebo-controlled double blind clinical trial demonstrated the effectiveness of MTX in children with JIA [16–21]. MTX has subsequently become the most common second-line therapeutic agent used to treat Juvenile Idiopathic Arthritis (JIA) worldwide.
Although the collective clinical experience with MTX has been vast, there are still unanswered questions about its mechanism of action, and considerable interindividual variability in clinical response and adverse reactions exists [22–24]. Thus far, there have been few predictors for efficacy or toxicity in pediatric patients taking this medication, and clinicians essentially dose by trial and error. Factors that could contribute to this variability are extensive, and some are unique to the pediatric population. We would like to explore the potential sources of variability that may contribute to outcomes on MTX in JIA.
4. Cellular Effects of MTX
Methotrexate, a folic acid analog and potent inhibitor of several enzymes within the folate pathway, has been used in low doses for the treatment of rheumatic disease over the last several decades. For rheumatic conditions, the dose range in pediatrics spans 10-fold, ranging from 0.1 mg/kg/dose to 1 mg/kg/dose, administered on a weekly basis. Options include oral and subcutaneous administration, but intramuscular and intravenous administration are possible, although less practical in the outpatient setting. Before being taken into the body, contributors to variability that cannot be overlooked include patient compliance, differences in administered dose, and route of administration. Children, who are a fraction of the weight of their adult counterparts, are dosed with the same absolute MTX dose, despite their smaller size. Although attention has been brought to this phenomenon, there continues to be little understanding of why children appear to require, relative to body weight, higher doses of MTX or how these doses are tolerated. Serum MTX concentrations have not been found useful to predict response or toxicity with little correlation with dose or outcome [25–27].
MTX has been best studied at the cellular level. It is known that MTX acts as a folate antagonist, entering the cells primarily through the reduced folate carrier (RFC/SLC19A1) [28]. Once intracellular, MTX is bioactivated to a polyglutamated form by folylpolyglutamyl synthase (FPGS), which enhances the pharmacological activity and intracellular retention of MTX [29]. In the RA and pediatric oncology literature, current evidence indicates that the enzymatic addition of glutamate residues to the MTX molecule in vivo (polyglutamation/MTXglun) is critical for pharmacologic activity by increasing the intracellular concentration of the drug and increasing its affinity for its therapeutic targets, thereby allowing more opportunity for its inhibitory effects to be exerted upon its target enzymes [29–31]. The initial target of MTX to be identified was dihydrofolate reductase (DHFR), which forms tetrahydrofolate, a precursor required for one carbon donation for synthesis of thymidylate, purines, methionine and serine, remethylation of homocysteine to form methionine, and provision of methyl donors for multiple methyltransferase enzymes [29]. Inhibition of thymidylate synthetase (TYMS), both directly and indirectly via depletion of tetrahydrofolate, leads to inhibition of pyrimidine biosynthesis with a resultant antiproliferative effect [30]. The interruption of DNA synthesis was thought to be the basis for rapidly dividing cell death in cancer cells. Subsequently, the list of target genes has been extended to include amino-imidazole carboxamide ribonucleotide (AICAR) transformylase (gene name, ATIC), which inhibits de novo purine synthesis and promotes the accumulation of AICAR ribotide, inhibiting adenosine deaminase and leading to a build up of adenosine, a potent anti-inflammatory agent [32, 33]. Adenosine's effect is also mediated by adenosine A2 receptors (ADORA2), which are present on neutrophils, monocytes, lymphocytes and basophils, generally suppressing the immune function of these cells [32]. Gamma glutamyl hydrolase (GGH), the enzyme responsible for glutamate removal from MTX, transforms MTX into a form that can be effluxed from the cell by the ATP-binding cassette (ABC) family of transporters.
5. The Role of Methotrexate Polyglutamation
Due to the rapid decline in serum drug concentrations, serum MTX concentrations are of limited utility in determining appropriate dosing or management of this medication [25–27]. On the other hand, RBC folate concentrations are established during erythropoiesis and represent the average folate status over the preceding 120 days [34]. By extension, MTX concentrations in RBCs represent a reasonable surrogate biomarker of average drug exposure over a similar period of time. In vitro studies have revealed that the cellular response to folate deprivation is associated with increased expression of FPGS and decreased expression of ABCG2 [35], suggesting that the adaptive cellular response to low folate involves increased polyglutamation to promote the retention of folate. Homozygosity for the variant allele of SLC19A1 (80A/A genotype) has been associated with increased concentrations of intracellular MTXglun compared to heterozygous or WT genotypes in RA patients [33]. Although the data are limited, these examples reveal that allelic variation in these genes resulting in increased or decreased activity may be associated with inter-individual variability in intracellular MTXglun.
Recent associations between MTXglun and clinical outcomes have also been reported in the adult rheumatology literature [33, 36, 37]. Higher levels of “long chain MTXglun” (defined in adults as MTXglu3 or greater, parent MTX is MTXglu1) were associated with a number of improved response measures in RA. The relationship between MTXglun and side effects of the medication has not been established [38]. In children, experience is limited to a single study reporting a total of 38 JIA patients, and no relationship between intracellular total MTXglun concentrations and likelihood of response was apparent [39]. As individual MTX polyglutamates differ with respect to their inhibitory effects on target enzymes and inhibition is modulated by folate polyglutamates [40], it is likely that multiple as yet unidentified factors contribute to variability in the relationship between MTXglun concentrations and efficacy and/or toxicity.
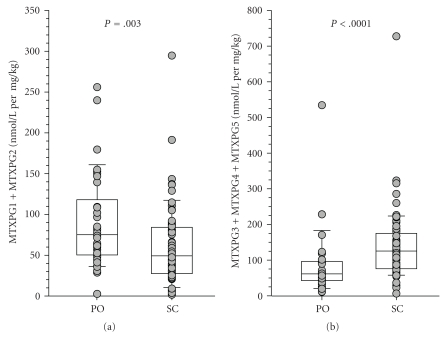
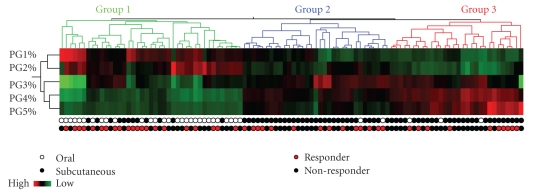
In order to better identify factors that may contribute to the inconsistencies in response and toxicity to MTX, we sought to characterize the extent of variability of intracellular MTXglun concentrations in our JIA patient population, and to investigate variables that may contribute to MTXglun variability. We have measured intracellular MTXglun concentrations in a cohort of 104 JIA patients. In this cohort, total intracellular MTXglun (MTXgluTOT, the sum of all individual MTXglun) concentrations varied 40-fold with a mean of 85.4 ± 48.8 nmol/L. Concentrations of MTXglu1-7 were measured individually and as a percentage of each patient's MTXgluTOT. MTXglu3 was the most prominent subtype identified, comprising 42% of MTXgluTOT, and was most highly correlated with MTXgluTOT (r = 0.96) [41]. Route was a significant predictor of MTXglu1-5 subtype. Higher concentrations of MTXglu1+2 were observed in patients receiving oral doses of MTX, whereas higher concentrations of MTXglu3-5 were observed in patients receiving subcutaneous doses of MTX (P <.0001), even after correcting for dose. (Figure 1) [41]. These findings were also supported further by hierarchical clustering, which revealed distinct clusters of patients with higher proportions of MTXglu1+2 and a second cluster of patients in whom MTXglu3–5 predominated (Figure 2). After controlling for MTX dose, subjects with higher proportions of MTXglu1+2 were more likely to be receiving oral MTX (P < .0001). Those with higher proportions of MTXglu3-5 were more likely to be receiving subcutaneous MTX (P = .0097) [42]. In agreement with Dolezalova and colleagues, we did not find a strong association of MTXglun concentrations (total or long chain) with MTX response (unpublished data), but we are actively investigating associations with clinical outcomes such as GI toxicity and hepatic enzyme elevation. Our experience demonstrates that MTXglun concentrations can be reliably measured in children and are extensively variable [41], yet the contributors to this variability are not fully explored.
Figure 1.
Comparison of MTXglun subgroups between oral (PO) and subcutaneous (SC) routes of administration. (a) Short chain polyglutamates (MTXglu1+2) were more predominant with PO administration, and (b) higher concentrations of long chain polyglutamates (MTXglu3-5) were more predominant with SC administration. Box and whisker plots are superimposed on data from individual patients. Boxes include the median and interquartile range, and whiskers indicate the 10th and 90th percentiles.
Figure 2.
Hierarchical clustering of the proportions of individual MTX polyglutamate subtype (PG1-5%) ordered by total intracellular MTX concentrations (MTXgluTOT) in 104 individual patients with JIA. Red denotes high concentrations, and green denotes low concentrations. Proportions of short chain MTXglu1 + 2 (PG1% and PG2%) cluster together and proportions of long chain MTXglu3-5 (PG3%, PG4%, PG5%) cluster together as subgroups. Each column represents an individual patient who has received MTX subcutaneously (black circles), or orally (white circles) and has active arthritis (black circles), or inactive arthritis (red circles) at the time of the blood draw.
6. Methotrexate Pharmacogenetics and Clinical Outcomes
Several studies have investigated the association of folate pathway pharmacogenetics and clinical outcomes with, at times, conflicting results. In genes associated with the cellular uptake and retention of MTX, there have been investigations studying allelic variations in influx transporters, (RFC/SLC19A1), efflux transporters (ABCB1 and ABCC2), as well as enzymes responsible for glutamation and deglutamation, (GGH and FPGS). Previous investigations have found no association between SNPs in RFC/SLC19A1 and clinical outcomes in RA patients [43, 44]. Alternatively, upregulation of the efflux transporter ABCG2 protein expression has been associated with MTX resistance in cancer cells [45], but no associations with SNPs evaluated in FPGS and clinical effects of MTX have been observed [44]. On the other hand, variations in GGH have shown conflicting associations with toxicity, however, a potential association with MTX response [33, 44].
Early work focused on genes directly involved in the methionine remethylation cycle, specifically methylenetetrahydrofolate reductase (MTHFR). Certain polymorphisms in the MTHFR gene have been associated with greater clinical improvement (MTHFR 1298AA and MTHFR 677CC genotypes) while MTHFR 1298C and MTHFR 677T alleles have been associated with an increased risk for toxicity [43, 46, 47]. In a retrospective cohort study in JIA, patients heterozygous for the MTHFR 677C/T genotype also exhibited adverse effects more frequently than homozygous 677C/C genotype [48], strengthening the adult association. Additionally, 5-methyltetrahydrofolate homocysteine methyltransferase (MTR) 2756GG was found to be overrepresented in pediatric osteosarcoma patients suffering GI toxicity after being treated with high dose MTX [49], and it has been associated with toxicity with low dose MTX in an adult RA cohort [50], supporting the potential for a similar effect in children with JIA.
Genes within the adenosine pathway responsible for de novo purine synthesis have begun to receive attention from investigators following initial reports of the anti-inflammatory effects of the genes and enzymes within this pathway. Favorable clinical response has been associated with polymorphisms in adenosine monophosphate deaminase (AMPD1 34T allele), the ATIC 347CC genotype and inosine triphosphate pyrophosphatase (ITPA 94CC) [51]. In a recently reported JIA cohort, presence of allelic variation in 2 ATIC SNPs (rs12995526 and rs4673990) and ITPA (rs2295553) were associated with increased risk for lack of response [52]. An increased risk of adverse effects was noted in ATIC 347G allele carriers [51]. Further work investigating the adenosine receptor 2a gene (ADORA2a) revealed several polymorphisms associated with GI toxicity in an adult RA cohort [53]. Polymorphisms in this pathway carry the potential to effect outcomes of arthritis patients treated with MTX. However, available pediatric experience suggests that the ontogeny of genes in the purine biosynthesis and adenosine response pathways may warrant further investigation.
Evaluation of the pyrimidine pathway has focused on TYMS, primarily a tandem repeat sequence in the 5′-UTR of the TYMS gene with enhancing function as well as a 6-bp deletion sequence in the 3′-UTR [54]. There have been data suggesting individuals homozygous for 2 tandem repeats had lower disease activity and better MTX response than patients with a third repeat [33]. There have also been data suggesting there is an association with the side effect of alopecia [55]. TYMS genetic variants have been also used in combination with other genes including MTHFR, ATIC, SLC191A, and SHMT to develop genomic indices, in an attempt to take into account the complexities of the cycle and gene-gene interactions that may contribute to understanding drug response and toxicity [33, 38, 55]. This concept makes more clinical sense than a single SNP approach, as a system as important as the folate cycle is likely protected by redundancy and intricate feedback regulation such that the function of this critically important pathway is not subject to serious disruption by variation in single genes.
Thus far, there has been very little investigation into genetic variation within the folate pathway in relation to drug response in JIA. Being that this pathway is critical for growth and development by contributing to the synthesis of DNA precursors and regulation of gene expression through methylation of cytosine and other methyltransferase reactions, it is likely that alterations in this pathway may have functional consequence, and if ontogeny plays a role, there may be more consequence during times of rapid growth and development. Thus far, the ontogeny of the folate pathway during postnatal growth and development has not been investigated. Investigating this pathway will require a better understanding of gene-gene interactions within the pathway, as well as the baseline folate status and the cell's response to shifts in supply and demand during periods of active growth and development as well as perturbation by a drug such as MTX.
7. Systematc Approach to Address Methotrexate Disposition and Response in JIA
With extensive 40-fold variability in intracellular MTXglun concentrations and distinct patterns of MTX polyglutamation highly associated with route of drug administration, one may consider absorption and cellular transport as key contributors to this observed variability. Intestinal influx transporters include the solute carrier (SLC) transporters, such as SLC19A1 (also known as the reduced folate carrier (RFC)) and the proton coupled folate transporter/heme carrier protein-1 (PCFT; SLC46A1) and folate receptors (FRα, FRβ, and FRγ) [56, 57]. The importance of these transporters, specifically PCFT has been exemplified by the autosomal recessive disorder Hereditary Folate Malabsorption (HFM) where loss of function mutations in these families have resulted in impaired folate absorption leading to severe folate deficiency and impaired folate transport into the CNS [57–59]. Further work has demonstrated that PCFT also transports MTX, although less avidly than folate and has demonstrated transporter inhibition by anionic compounds, including sulfasalzine. In addition, proton-pump inhibitors also inhibit PCFT transport function [59, 60], with the potential for clinically important consequences given that they often are coadministered with MTX in RA patients. In mice fed a low folate diet, expression of these transporters in the small intestine is increased [61], suggesting an adaptive process.
Efflux transporters include the ATP-binding cassette protein family of transporters (ABC transporters), which transport either back into the lumen (ABCG2/BCRP, ABCC2/MRP-2, ABCC4/MRP-4, and ABCB1/MDR1), or through the basolateral membrane into the blood (ABCC1/MRP-1, ABCC3/MRP-3, ABCC5/MPR-5) [56]. The overlapping and compensatory functions of these efflux transporters make investigation of their function complex. GI toxicity has been reported in ABCC1/MRP1 (−/−) knock out mice, found highly expressed in the small intestine[62]. In pediatric ALL patients, the presence of at least one variant ABCC2-24 C > T allele resulted in much higher MTX AUC in female patients [63]. Additionally, in RA patients, increased MTX toxicity was noted in subjects carrying SNPs in the ABCB1 and ABCC2 genes [64, 65]. Variation in the function of intestinal transporters, such as PCFT, may result in differences in MTX bioavailability, and may play a part in explaining why children, whose body surface area and weight are much less than adults, still require a similar MTX dose as adults to maintain an appropriate level of disease control. The combination and relation of both influx and efflux transporter function will likely need to be better elucidated before final associations with genotype and phenotype can be made.
After being absorbed from the gut, MTX is further metabolized in the liver to its primary metabolite 7-OH-MTX, and influx and efflux transporters within the liver can also be contributors to interpatient variability in systemic availability and response to the drug, especially when considering the inherent risk for hepatotoxicity. The role of hepatic specific transporters such as Organic Anion Transporter Polypeptides (OATP1B1/SLCO1B1, OATP1B3/SLCO1B3) have received more attention with the reported influence of the SLCO1B1*5 haplotype (c.51T > C) on statin pharmacokinetics and toxicity [66]. OATP1B1 is located at the sinusoidal membrane of hepatocytes, and its transcript has been detected in hepatocytes [67]. It has been shown to transport MTX in vitro [68], and in pediatric patients with ALL, variations in OATP1B1 were associated with clearance of MTX as well as GI toxicity [67]. Variations in the genotype of hepatic efflux transporters such as ABCB1/MDR-1 and ABCC2/MRP-2 may also play a role in drug response and toxicity. In ABCC2-deficient rats, MTX biliary excretion was significantly reduced [69]. In ABCC2/ABCC3/ABCG2 (−/−) deficient mice, a dramatic increase in MTX and 7OH-MTX concentrations are found in the liver, as well as prolonged systemic exposure to both compounds after MTX administration compared to single gene knockout mice [59]. There is currently a knowledge deficit regarding the ontogeny of these transporters. If dysfunction in efflux transporters such as ABCC2, ABCC3, and ABCG2 leads to prolonged systemic exposure and elevated liver concentrations of MTX, perhaps more effective or efficient function of these transporters in children allow high doses to be tolerated and hepatic toxicity to be minimal.
The complexity involved in predicting the cellular effects of MTX is only amplified by the multiple steps required to complete the journey to the cellular folate cycle, and recognizing that cell- and tissue-specific differences in cellular uptake and retention processes exist. The role of ontogeny adds a layer of complexity to an already intricate network of genes. Very little is known about differences in gene expression with age within the network during normal growth and development, let alone after perturbation following administration of MTX. Extensive variability in outcomes and intracellular MTXglun concentrations in our pediatric JIA population, as well as the observation that children need and tolerate the same absolute doses of the drug routinely prescribed to adults, begs the question: what role does ontogeny play?
8. Conclusions
Although MTX is widely used in clinical practice—in a number of disease entities, at different doses and by different routes of administration—there remains significant lack of understanding of its mechanisms of action and the factors that contribute to the variability in toxicity and response seen clinically. Given the time lag between initiation of treatment and the first indication of patient response, this knowledge is essential to determine a priori the probability of beneficial therapeutic response and also take into consideration the probability of toxicity so that the best informed clinical decisions can be made. In addition to differences in drug administration, (i.e., dose, route, compliance) factors that affect pharmacokinetics and pharamcodynamics such as genetic variation may explain individual differences in drug biotransformation. However, the pediatric population has an additional factor to consider, namely, the ontogeny of gene expression, which may invariably affect the relative expression of genes within the pathway as one carbon resources which are allocated to the different functions of the folate cycle (purine and pyrimidine biosynthesis, homocysteine remethylation to methionine, one carbon donor for methyltransferases) during periods of dynamic change in folate supply and demand. By taking into account not only the genes in question that may affect drug disposition, but also the developmental trajectory of genes involved in drug response, we may begin to better understand what makes children different, and identify and prevent unique adverse drug reactions in this population. Future areas of study in this area may include investigating the developmental expression of tissue specific transporters, which may explaining the higher rate of subcutaneous administration and the higher doses of MTX used in children compared to adults. Additionally, having a better understanding of how cellular folate concentrations and patterns change with age may also help explain the variation seen in MTX response. With newer techniques and a developmentally aware approach, we aim to successfully individualize therapy for our pediatric patients with JIA.
References
- 1.Leeder JS. Translating pharmacogenetics and pharmacogenomics into drug development for clinical pediatrics and beyond. Drug Discovery Today. 2004;9(13):567–573. doi: 10.1016/S1359-6446(04)03129-0. [DOI] [PubMed] [Google Scholar]
- 2.Agunod M, Yamaguchi N, Lopez R, Luhby AL, Glass GBJ. Correlative study of hydrochloric acid, pepsin, and intrinsic factor secretion in newborns and infants. The American Journal of Digestive Diseases. 1969;14(6):400–414. doi: 10.1007/BF02239360. [DOI] [PubMed] [Google Scholar]
- 3.Moazam F, Kirby WJ, Rodgers BM, McGuigan JE. Physiology of serum gastrin production in neonates and infants. Annals of Surgery. 1984;199(4):389–392. doi: 10.1097/00000658-198404000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta M, Brans YW. Gastric retention in neonates. Pediatrics. 1978;62(1):26–29. [PubMed] [Google Scholar]
- 5.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. New England Journal of Medicine. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 6.Hines RN. The ontogeny of drug metabolism enzymes and implications for adverse drug events. Pharmacology and Therapeutics. 2008;118(2):250–267. doi: 10.1016/j.pharmthera.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Johnson TR, Moore WM, Jeffries JE, editors. Children are Different: Developmental Physiology. 2nd edition. Columbus, Ohio, USA: Ross laboratories; 1978. [Google Scholar]
- 8.Leeder JS, Pearce RE, Gaedigk A, Modak A, Rosen DI. Evaluation of a [13C]-dextromethorphan breath test to assess CYP2D6 phenotype. Journal of Clinical Pharmacology. 2008;48(9):1041–1051. doi: 10.1177/0091270008319709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leeder JS. Developmental pharmacogenetics: a general paradigm for application to neonatal pharmacology and toxicology. Clinical Pharmacology and Therapeutics. 2009;86(6):678–682. doi: 10.1038/clpt.2009.195. [DOI] [PubMed] [Google Scholar]
- 10.Leeder JS, Kearns GL, Spielberg SP, van den Anker J. Understanding the Relative Roles of Pharmacogenetics and Ontogeny in Pediatric Drug Development and Regulatory Science. Journal of Clinical Pharmacology. doi: 10.1177/0091270009360533. E pub Feb 11, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Cassidy JT, Petty RE, Laxer RM. Textbook of Pediatric Rheumatology. 5th edition. Philadelphia, Pa, USA: Saunders; 2005. [Google Scholar]
- 12.Prahalad S, Glass DN. A comprehensive review of the genetics of juvenile idiopathic arthritis. Pediatric Rheumatology. 2008;6, article no. 11 doi: 10.1186/1546-0096-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffin TA, Barnes MG, Ilowite NT, et al. Gene expression signatures in polyarticular juvenile idiopathic arthritis demonstrate disease heterogeneity and offer a molecular classification of disease subsets. Arthritis and Rheumatism. 2009;60(7):2113–2123. doi: 10.1002/art.24534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes MG, Grom AA, Thompson SD, et al. Subtype-specific peripheral blood gene expression profiles in recent-onset juvenile idiopathic arthritis. Arthritis and Rheumatism. 2009;60(7):2102–2112. doi: 10.1002/art.24601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petty RE, Southwood TR, Manners P, et al. International league of associations for rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. Journal of Rheumatology. 2004;31(2):390–392. [PubMed] [Google Scholar]
- 16.Truckenbrodt H, Hafner R. Methotrexate therapy in juvenile rheumatoid arthritis: a retrospective study. Arthritis and Rheumatism. 1986;29(6):801–807. doi: 10.1002/art.1780290616. [DOI] [PubMed] [Google Scholar]
- 17.Speckmaier M, Findeisen J, Woo P, et al. Low-dose methotrexate in systemic onset juvenile chronic arthritis. Clinical and Experimental Rheumatology. 1989;7(6):647–650. [PubMed] [Google Scholar]
- 18.Wallace CA, Sherry DD, Mellins ED, Aiken RP. Predicting remission in juvenile rheumatoid arthritis with methotrexate treatment. Journal of Rheumatology. 1993;20(1):118–122. [PubMed] [Google Scholar]
- 19.Rose CD, Singsen BH, Eichenfield AH, Goldsmith DP, Athreya BH. Safety and efficacy of methotrexate therapy for juvenile rheumatoid arthritis. Journal of Pediatrics. 1990;117(4):653–659. doi: 10.1016/s0022-3476(05)80709-7. [DOI] [PubMed] [Google Scholar]
- 20.Halle F, Prieur AM. Evaluation of methotrexate in the treatment of juvenile chronic arthritis according to the subtype. Clinical and Experimental Rheumatology. 1991;9(3):297–302. [PubMed] [Google Scholar]
- 21.Giannini EH, Brewer EJ, Kuzmina N, et al. Methotrexate in resistant juvenile rheumatoid arthritis—results of the U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. New England Journal of Medicine. 1992;326(16):1043–1049. doi: 10.1056/NEJM199204163261602. [DOI] [PubMed] [Google Scholar]
- 22.Ruperto N, Murray KJ, Gerloni V, et al. A randomized trial of parenteral methotrexate comparing an intermediate dose with a higher dose in children with juvenile idiopathic arthritis who failed to respond to standard doses of methotrexate. Arthritis and Rheumatism. 2004;50(7):2191–2201. doi: 10.1002/art.20288. [DOI] [PubMed] [Google Scholar]
- 23.Lambert CM, Sandhu S, Lochhead A, Hurst NP, McRorie E, Dhillon V. Dose escalation of parenteral methotrexate in active rheumatoid arthritis that has been unresponsive to conventional doses of methotrexate: a randomized, controlled trial. Arthritis and Rheumatism. 2004;50(2):364–371. doi: 10.1002/art.20167. [DOI] [PubMed] [Google Scholar]
- 24.Cohen S, Cannon GW, Schiff M, et al. Two-year, blinded, randomized, controlled trial of treatment of active rheumatoid arthritis with leflunomide compared with methotrexate. Arthritis and Rheumatism. 2001;44(9):1984–1992. doi: 10.1002/1529-0131(200109)44:9<1984::AID-ART346>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 25.Lafforgue P, Monjanel-Mouterde S, Durand A, Catalin J, Acquaviva P-C. Lack of correlation between pharmacokinetics and efficacy of low dose methotrexate in patients with rheumatoid arthritis. Journal of Rheumatology. 1995;22(5):844–849. [PubMed] [Google Scholar]
- 26.Ravelli A, Di Fuccia G, Molinaro M, et al. Plasma levels after oral methotrexate in children with juvenile rheumatoid arthritis. Journal of Rheumatology. 1993;20(9):1573–1577. [PubMed] [Google Scholar]
- 27.Albertioni F, Flato B, Seideman P, et al. Methotrexate in juvenile rheumatoid arthritis. Evidence of age dependent pharmacokinetics. European Journal of Clinical Pharmacology. 1995;47(6):507–511. doi: 10.1007/BF00193703. [DOI] [PubMed] [Google Scholar]
- 28.Matherly LH, Goldman ID. Membrane transport of folates. Vitamins and Hormones. 2003;66:403–456. doi: 10.1016/s0083-6729(03)01012-4. [DOI] [PubMed] [Google Scholar]
- 29.Chabner BA, Allegra CJ, Curt GA. Polyglutamation of methotrexate. Is Methotrexate a prodrug? Journal of Clinical Investigation. 1985;76(3):907–912. doi: 10.1172/JCI112088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allegra CJ, Chabner BA, Drake JC. Enhanced inhibition of thymidylate synthase by methotrexate polyglutamates. Journal of Biological Chemistry. 1985;260(17):9720–9726. [PubMed] [Google Scholar]
- 31.Schroder H, Fogh K. Methotrexate and its polyglutamate derivatives in erythrocytes during and after weekly low-dose oral methotrexate therapy of children with acute lymphoblastic leukemia. Cancer Chemotherapy and Pharmacology. 1988;21(2):145–149. doi: 10.1007/BF00257362. [DOI] [PubMed] [Google Scholar]
- 32.Cronstein BN, Naime D, Ostad E. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. Journal of Clinical Investigation. 1993;92(6):2675–2682. doi: 10.1172/JCI116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dervieux T, Furst D, Lein DO, et al. Polyglutamation of methotrexate with common polymorphisms in reduced folate carrier, aminoimidazole carboxamide ribonucleotide transformylase, and thymidylate synthase are associated with methotrexate effects in rheumatoid arthritis. Arthritis and Rheumatism. 2004;50(9):2766–2774. doi: 10.1002/art.20460. [DOI] [PubMed] [Google Scholar]
- 34.Herbert V. Making sense of laboratory tests of folate status: folate requirements to sustain normality. American Journal of Hematology. 1987;26(2):199–207. doi: 10.1002/ajh.2830260211. [DOI] [PubMed] [Google Scholar]
- 35.Ifergan I, Shafran A, Jansen G, Hooijberg JH, Scheffer GL, Assaraf YG. Folate deprivation results in the loss of breast cancer resistance protein (BCRP/ABCG2) expression: a role for BCRP in cellular folate homeostasis. Journal of Biological Chemistry. 2004;279(24):25527–25534. doi: 10.1074/jbc.M401725200. [DOI] [PubMed] [Google Scholar]
- 36.Dervieux T, Furst D, Lein DO, et al. Pharmacogenetic and metabolite measurements are associated with clinical status in patients with rheumatoid arthritis treated with methotrexate: results of a multicentred cross sectional observational study. Annals of the Rheumatic Diseases. 2005;64(8):1180–1185. doi: 10.1136/ard.2004.033399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dervieux T, Kremer J, Lein DO, et al. Contribution of common polymorphisms in reduced folate carrier and gamma-glutamylhydrolase to methotrexate polyglutamate levels in patients with rheumatoid arthritis. Pharmacogenetics. 2004;14(11):733–739. doi: 10.1097/00008571-200411000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Dervieux T, et al. Gene-gene interactions in folate and adenosine biosynthesis pathways affect methotrexate efficacy and tolerability in rheumatoid arthritis. Pharmacogenet Genomics. 2009;19(12):935–944. doi: 10.1097/FPC.0b013e32833315d1. [DOI] [PubMed] [Google Scholar]
- 39.Doležalová P, Krijt J, Chládek J, Němcová D, Hoza J. Adenosine and methotrexate polyglutamate concentrations in patients with juvenile arthritis. Rheumatology. 2005;44(1):74–79. doi: 10.1093/rheumatology/keh401. [DOI] [PubMed] [Google Scholar]
- 40.Baggott JE, Vaughn WH, Hudson BB. Inhibition of 5-aminoimidazole-4-carboxamide ribotide transformylase, adenosine deaminase and 5′-adenylate deaminase by polyglutamates of methotrexate and oxidized folates and by 5-aminoimidazole-4-carboxamide riboside and ribotide. Biochemical Journal. 1986;236(1):193–200. doi: 10.1042/bj2360193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker ML, Van Haandel L, Gaedigk R, et al. Analysis of intracellular methotrexate polyglutamates in patients with juvenile idiopathic arthritis: effect of route of administration on variability in intracellular methotrexate polyglutamate concentrations. Arthritis and Rheumatism. 2010;62(6):1803–1812. doi: 10.1002/art.27434. [DOI] [PubMed] [Google Scholar]
- 42.Becker ML, Gaedigk R, van Haandel L, Stobaugh J, Leeder JS. Clinical and genetic predictors of methotrexate polyglutamate patterns in JIA. Clinical Pharmacology and Therapeutics. 87 supplement 1, S68, 2010. [Google Scholar]
- 43.Wessels JAM, De Vries-Bouwstra JK, Heijmans BT, et al. Efficacy and toxicity of methotrexate in early rheumatoid arthritis are associated with single-nucleotide polymorphisms in genes coding for folate pathway enzymes. Arthritis and Rheumatism. 2006;54(4):1087–1095. doi: 10.1002/art.21726. [DOI] [PubMed] [Google Scholar]
- 44.van der Straaten RJHM, Wessels JAM, de Vries-Bouwstra JK, et al. Exploratory analysis of four polymorphisms in human GGH and FPGS genes and their effect in methotrexate-treated rheumatoid arthritis patients. Pharmacogenomics. 2007;8(2):141–150. doi: 10.2217/14622416.8.2.141. [DOI] [PubMed] [Google Scholar]
- 45.Volk EL, Schneider E. Wild-type breast cancer resistance protein (BCRP/ABCG2) is a methotrexate polyglutamate transporter. Cancer Research. 2003;63(17):5538–5543. [PubMed] [Google Scholar]
- 46.Van Ede AE, Laan RFJM, Blom HJ, et al. The C677T mutation in the methylenetetrahydrofolate reductase gene: a genetic risk factor for methotrexate-related elevation of liver enzymes in rheumatoid arthritis patients. Arthritis and Rheumatism. 2001;44(11):2525–2530. doi: 10.1002/1529-0131(200111)44:11<2525::aid-art432>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 47.Berkun Y, Levartovsky D, Rubinow A, et al. Methotrexate related adverse effects in patients with rheumatoid arthritis are associated with the A1298C polymorphism of the MTHFR gene. Annals of the Rheumatic Diseases. 2004;63(10):1227–1231. doi: 10.1136/ard.2003.016337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmeling H, Biber D, Heins S, Horneff G. Influence of methylenetetrahydrofolate reductase polymorphisms on efficacy and toxicity of methotrexate in patients with juvenile idiopathic arthritis. Journal of Rheumatology. 2005;32(9):1832–1836. [PubMed] [Google Scholar]
- 49.Patiño-García A, Zalacaín M, Marrodán L, San-Julián M, Sierrasesúmaga L. Methotrexate in pediatric osteosarcoma: response and toxicity in relation to genetic polymorphisms and dihydrofolate reductase and reduced folate carrier 1 expression. Journal of Pediatrics. 2009;154(5):688–693. doi: 10.1016/j.jpeds.2008.11.030. [DOI] [PubMed] [Google Scholar]
- 50.Dervieux T, Greenstein N, Kremer J. Pharmacogenomic and metabolic biomarkers in the folate pathway and their association with methotrexate effects during dosage escalation in rheumatoid arthritis. Arthritis and Rheumatism. 2006;54(10):3095–3103. doi: 10.1002/art.22129. [DOI] [PubMed] [Google Scholar]
- 51.Wessels JAM, Kooloos WM, De Jonge R, et al. Relationship between genetic variants in the adenosine pathway and outcome of methotrexate treatment in patients with recent-onset rheumatoid arthritis. Arthritis and Rheumatism. 2006;54(9):2830–2839. doi: 10.1002/art.22032. [DOI] [PubMed] [Google Scholar]
- 52.Hinks A, Halima M, Martin P, et al. Genetic polymorphisms in key methotrexate pathway genes associated with response to MTX treatment in juvenile idiopathic arthritis. Arthritis and Rheumatism. 2009;60(supplement 10):p. S233. [Google Scholar]
- 53.Hider SL, Thomson W, Mack LF, Armstrong DJ, Shadforth M, Bruce IN. Polymorphisms within the adenosine receptor 2a gene are associated with adverse events in RA patients treated with MTX. Rheumatology. 2008;47(8):1156–1159. doi: 10.1093/rheumatology/ken182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ranganathan P. An update on pharmacogenomics in rheumatoid arthritis with a focus on TNF-blocking agents. Current Opinion in Molecular Therapeutics. 2008;10(6):562–567. [PubMed] [Google Scholar]
- 55.Weisman M, Furst D, Park G, et al. Risk genotypes in folate-dependent enzymes profile rheumatoid arthritis patients with side effects to methotrexate therapy. Arthritis and Rheumatism. 2005;52, supplement:p. 1950. doi: 10.1002/art.21573. [DOI] [PubMed] [Google Scholar]
- 56.Oostendorp RL, Beijnen JH, Schellens JHM. The biological and clinical role of drug transporters at the intestinal barrier. Cancer Treatment Reviews. 2009;35(2):137–147. doi: 10.1016/j.ctrv.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 57.Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Reviews in Molecular Medicine. 2009;11:p. e4. doi: 10.1017/S1462399409000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu A, Jansen M, Sakaris A, et al. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell. 2006;127(5):917–928. doi: 10.1016/j.cell.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 59.Vlaming ML, et al. Abcc2 (Mrp2), Abcc3 (Mrp3), and Abcg2 (Bcrp1) are the main determinants for rapid elimination of methotrexate and its toxic metabolite 7-hydroxymethotrexate in vivo. Molecular Cancer Therapeutics. 2009;8(12):3350–3359. doi: 10.1158/1535-7163.MCT-09-0668. [DOI] [PubMed] [Google Scholar]
- 60.Yuasa H, Inoue K, Hayashi Y. Molecular and functional characteristics of proton-coupled folate transporter. Journal of Pharmaceutical Sciences. 2009;98(5):1608–1616. doi: 10.1002/jps.21515. [DOI] [PubMed] [Google Scholar]
- 61.Liu M, Ge Y, Cabelof DC, et al. Structure and regulation of the murine reduced folate carrier gene: identification of four noncoding exons and promoters and regulation by dietary folates. Journal of Biological Chemistry. 2005;280(7):5588–5597. doi: 10.1074/jbc.M412662200. [DOI] [PubMed] [Google Scholar]
- 62.Kato S, Ito K, Kato Y, et al. Involvement of multidrug resistance-associated protein 1 in intestinal toxicity of methotrexate. Pharmaceutical Research. 2009;26(6):1467–1476. doi: 10.1007/s11095-009-9858-6. [DOI] [PubMed] [Google Scholar]
- 63.Rau T, Erney B, Göres R, Eschenhagen T, Beck J, Langer T. High-dose methotrexate in pediatric acute lymphoblastic leukemia: impact of ABCC2 polymorphisms on plasma concentrations. Clinical Pharmacology and Therapeutics. 2006;80(5):468–476. doi: 10.1016/j.clpt.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 64.Ranganathan P, Culverhouse R, Marsh S, et al. Methotrexate (MTX) pathway gene polymorphisms and their effects on MTX toxicity in caucasian and african american patients with rheumatoid arthritis. Journal of Rheumatology. 2008;35(4):572–579. [PubMed] [Google Scholar]
- 65.Takatori R, Takahashi KA, Tokunaga D, et al. ABCB1 C3435T polymorphism influences methotrexate sensitivity in rheumatoid arthritis patients. Clinical and Experimental Rheumatology. 2006;24(5):546–554. [PubMed] [Google Scholar]
- 66.Niemi M. Transporter pharmacogenetics and statin toxicity. Clinical Pharmacology and Therapeutics. 2009;87(1):130–133. doi: 10.1038/clpt.2009.197. [DOI] [PubMed] [Google Scholar]
- 67.Trevino LR, et al. Germline genetic variation in an organic anion transporter polypeptide associated with methotrexate pharmacokinetics and clinical effects. Journal of Clinical Oncology. 2009;27(35):5972–5978. doi: 10.1200/JCO.2008.20.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abe T, Unno M, Onogawa T, et al. LST-2, a human liver-specific organic anion transporter, determines methotrexate sensitivity in gastrointestinal cancers. Gastroenterology. 2001;120(7):1689–1699. doi: 10.1053/gast.2001.24804. [DOI] [PubMed] [Google Scholar]
- 69.Masuda M, I’izuka Y, Yamazaki M, et al. Methotrexate is excreted into the bile by canalicular multispecific organic anion transporter in rats. Cancer Research. 1997;57(16):3506–3510. [PubMed] [Google Scholar]