Abstract
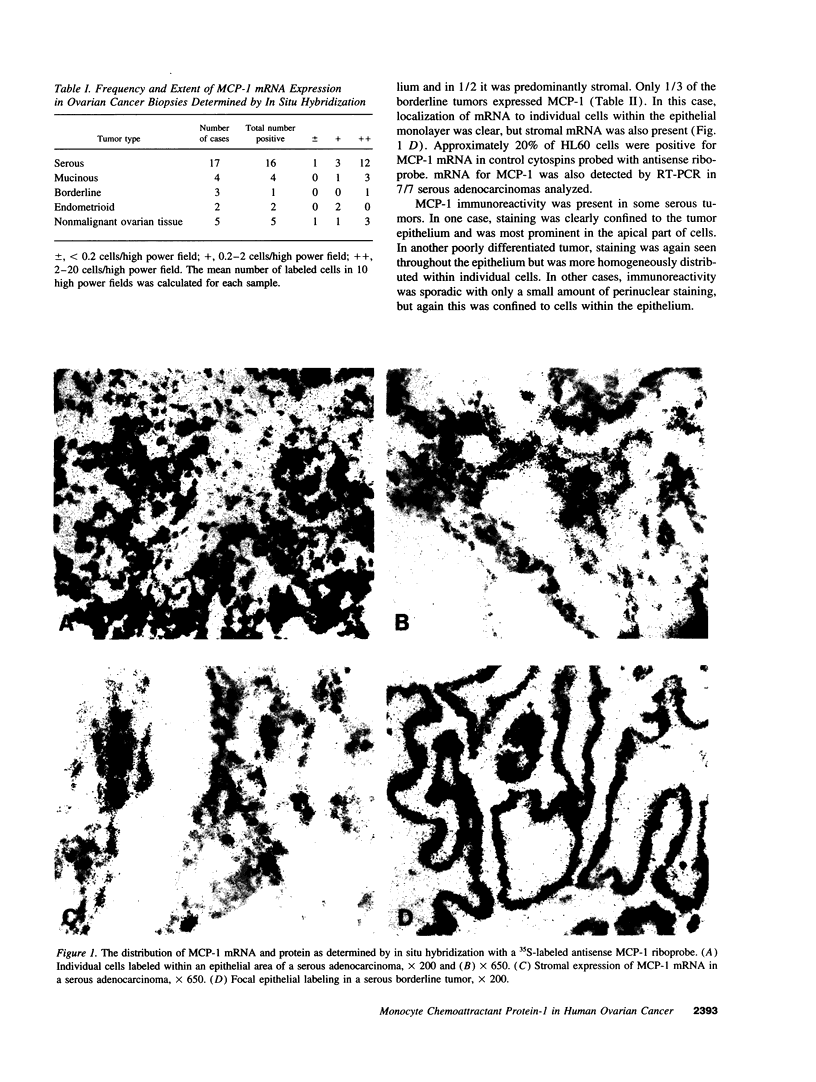
Chemokines may control the macrophage infiltrate found in many solid tumors. In human ovarian cancer, in situ hybridization detected mRNA for the macrophage chemokine monocyte chemoattractant protein-1 (MCP-1) in 16/17 serous carcinomas, 4/4 mucinous carcinomas, 2/2 endometrioid carcinomas, and 1/3 borderline tumors. In serous tumors, mRNA expression mainly localized to the epithelial areas, as did immunoreactive MCP-1 protein. In the other tumors, both stromal and epithelial expression were seen. All tumors contained variable numbers of cells positive for the macrophage marker CD68. MCP-1 mRNA was also detected in the stroma of 5/5 normal ovaries. RT-PCR demonstrated mRNA for MCP-1 in 7/7 serous carcinomas and 6/6 ovarian cancer cell lines. MCP-1 protein was detected by ELISA in ascites from patients with ovarian cancer (mean 4.28 ng/ml) and was produced primarily by the cancer cells. Human MCP-1 protein was also detected in culture supernatants from cell lines and in ascites from human ovarian tumor xenografts which induce a peritoneal monocytosis in nude mice. We conclude that the macrophage chemoattractant MCP-1 is produced by epithelial ovarian cancer and that the tumor cells themselves are probably a major source. MCP-1 may contribute to the accumulation of tumor-associated macrophages, which may subsequently influence tumor behavior.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. H., Shaw S. Leucocyte-endothelial interactions and regulation of leucocyte migration. Lancet. 1994 Apr 2;343(8901):831–836. doi: 10.1016/s0140-6736(94)92029-x. [DOI] [PubMed] [Google Scholar]
- Allavena P., Bianchi G., Zhou D., van Damme J., Jílek P., Sozzani S., Mantovani A. Induction of natural killer cell migration by monocyte chemotactic protein-1, -2 and -3. Eur J Immunol. 1994 Dec;24(12):3233–3236. doi: 10.1002/eji.1830241249. [DOI] [PubMed] [Google Scholar]
- Balkwill F. R., Lee A., Aldam G., Moodie E., Thomas J. A., Tavernier J., Fiers W. Human tumor xenografts treated with recombinant human tumor necrosis factor alone or in combination with interferons. Cancer Res. 1986 Aug;46(8):3990–3993. [PubMed] [Google Scholar]
- Bottazzi B., Ghezzi P., Taraboletti G., Salmona M., Colombo N., Bonazzi C., Mangioni C., Mantovani A. Tumor-derived chemotactic factor(s) from human ovarian carcinoma: evidence for a role in the regulation of macrophage content of neoplastic tissues. Int J Cancer. 1985 Aug 15;36(2):167–173. doi: 10.1002/ijc.2910360207. [DOI] [PubMed] [Google Scholar]
- Bottazzi B., Polentarutti N., Acero R., Balsari A., Boraschi D., Ghezzi P., Salmona M., Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983 Apr 8;220(4593):210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- Bottazzi B., Polentarutti N., Balsari A., Boraschi D., Ghezzi P., Salmona M., Mantovani A. Chemotactic activity for mononuclear phagocytes of culture supernatants from murine and human tumor cells: evidence for a role in the regulation of the macrophage content of neoplastic tissues. Int J Cancer. 1983 Jan 15;31(1):55–63. doi: 10.1002/ijc.2910310110. [DOI] [PubMed] [Google Scholar]
- Brunda M. J., Sulich V., Wright R. B., Palleroni A. V. Tumoricidal activity and cytokine secretion by tumor-infiltrating macrophages. Int J Cancer. 1991 Jul 9;48(5):704–708. doi: 10.1002/ijc.2910480513. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Colotta F., Borré A., Wang J. M., Tattanelli M., Maddalena F., Polentarutti N., Peri G., Mantovani A. Expression of a monocyte chemotactic cytokine by human mononuclear phagocytes. J Immunol. 1992 Feb 1;148(3):760–765. [PubMed] [Google Scholar]
- Diamond J. R., Kees-Folts D., Ding G., Frye J. E., Restrepo N. C. Macrophages, monocyte chemoattractant peptide-1, and TGF-beta 1 in experimental hydronephrosis. Am J Physiol. 1994 Jun;266(6 Pt 2):F926–F933. doi: 10.1152/ajprenal.1994.266.6.F926. [DOI] [PubMed] [Google Scholar]
- Fidler I. J., Schroit A. J. Synergism between lymphokines and muramyl dipeptide encapsulated in liposomes: in situ activation of macrophages and therapy of spontaneous cancer metastases. J Immunol. 1984 Jul;133(1):515–518. [PubMed] [Google Scholar]
- Furutani Y., Nomura H., Notake M., Oyamada Y., Fukui T., Yamada M., Larsen C. G., Oppenheim J. J., Matsushima K. Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF). Biochem Biophys Res Commun. 1989 Feb 28;159(1):249–255. doi: 10.1016/0006-291x(89)92430-3. [DOI] [PubMed] [Google Scholar]
- Graves D. T., Jiang Y. L., Williamson M. J., Valente A. J. Identification of monocyte chemotactic activity produced by malignant cells. Science. 1989 Sep 29;245(4925):1490–1493. doi: 10.1126/science.2781291. [DOI] [PubMed] [Google Scholar]
- Haskill S., Becker S., Fowler W., Walton L. Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. Br J Cancer. 1982 May;45(5):728–736. doi: 10.1038/bjc.1982.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kacinski B. M., Carter D., Mittal K., Yee L. D., Scata K. A., Donofrio L., Chambers S. K., Wang K. I., Yang-Feng T., Rohrschneider L. R. Ovarian adenocarcinomas express fms-complementary transcripts and fms antigen, often with coexpression of CSF-1. Am J Pathol. 1990 Jul;137(1):135–147. [PMC free article] [PubMed] [Google Scholar]
- Larsen C. G., Zachariae C. O., Oppenheim J. J., Matsushima K. Production of monocyte chemotactic and activating factor (MCAF) by human dermal fibroblasts in response to interleukin 1 or tumor necrosis factor. Biochem Biophys Res Commun. 1989 May 15;160(3):1403–1408. doi: 10.1016/s0006-291x(89)80160-3. [DOI] [PubMed] [Google Scholar]
- Leibovich S. J., Polverini P. J., Shepard H. M., Wiseman D. M., Shively V., Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987 Oct 15;329(6140):630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- Malik S. T., Griffin D. B., Fiers W., Balkwill F. R. Paradoxical effects of tumour necrosis factor in experimental ovarian cancer. Int J Cancer. 1989 Nov 15;44(5):918–925. doi: 10.1002/ijc.2910440529. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Tumor-associated macrophages in neoplastic progression: a paradigm for the in vivo function of chemokines. Lab Invest. 1994 Jul;71(1):5–16. [PubMed] [Google Scholar]
- Martinet N., Beck G., Bernard V., Plenat F., Vaillant P., Schooneman F., Vignaud J. M., Martinet Y. Mechanism for the recruitment of macrophages to cancer site. In vivo concentration gradient of monocyte chemotactic activity. Cancer. 1992 Aug 15;70(4):854–860. doi: 10.1002/1097-0142(19920815)70:4<854::aid-cncr2820700422>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Mussoni L., Riganti M., Acero R., Erroi A., Conforti G., Mantovani A., Donati M. B. Macrophages associated with murine tumours express plasminogen activator activity. Int J Cancer. 1988 Feb 15;41(2):227–230. doi: 10.1002/ijc.2910410212. [DOI] [PubMed] [Google Scholar]
- Naylor M. S., Stamp G. W., Balkwill F. R. Investigation of cytokine gene expression in human colorectal cancer. Cancer Res. 1990 Jul 15;50(14):4436–4440. [PubMed] [Google Scholar]
- Naylor M. S., Stamp G. W., Davies B. D., Balkwill F. R. Expression and activity of MMPS and their regulators in ovarian cancer. Int J Cancer. 1994 Jul 1;58(1):50–56. doi: 10.1002/ijc.2910580110. [DOI] [PubMed] [Google Scholar]
- Naylor M. S., Stamp G. W., Foulkes W. D., Eccles D., Balkwill F. R. Tumor necrosis factor and its receptors in human ovarian cancer. Potential role in disease progression. J Clin Invest. 1993 May;91(5):2194–2206. doi: 10.1172/JCI116446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelken N. A., Coughlin S. R., Gordon D., Wilcox J. N. Monocyte chemoattractant protein-1 in human atheromatous plaques. J Clin Invest. 1991 Oct;88(4):1121–1127. doi: 10.1172/JCI115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdenakker G., Froyen G., Fiten P., Proost P., Van Damme J. Human monocyte chemotactic protein-3 (MCP-3): molecular cloning of the cDNA and comparison with other chemokines. Biochem Biophys Res Commun. 1993 Mar 15;191(2):535–542. doi: 10.1006/bbrc.1993.1251. [DOI] [PubMed] [Google Scholar]
- Peri G., Milanese C., Matteucci C., Ruco L., Zhou D., Sozzani S., Coletta I., Mantovani A. A new monoclonal antibody (5D3-F7) which recognizes human monocyte-chemotactic protein-1 but not related chemokines. Development of a sandwich ELISA and in situ detection of producing cells. J Immunol Methods. 1994 Sep 14;174(1-2):249–257. doi: 10.1016/0022-1759(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Polverini P. J., Leibovich S. J. Induction of neovascularization in vivo and endothelial proliferation in vitro by tumor-associated macrophages. Lab Invest. 1984 Dec;51(6):635–642. [PubMed] [Google Scholar]
- Sciacca F. L., Stürzl M., Bussolino F., Sironi M., Brandstetter H., Zietz C., Zhou D., Matteucci C., Peri G., Sozzani S. Expression of adhesion molecules, platelet-activating factor, and chemokines by Kaposi's sarcoma cells. J Immunol. 1994 Nov 15;153(10):4816–4825. [PubMed] [Google Scholar]
- Takeshima H., Kuratsu J., Takeya M., Yoshimura T., Ushio Y. Expression and localization of messenger RNA and protein for monocyte chemoattractant protein-1 in human malignant glioma. J Neurosurg. 1994 Jun;80(6):1056–1062. doi: 10.3171/jns.1994.80.6.1056. [DOI] [PubMed] [Google Scholar]
- Takeya M., Yoshimura T., Leonard E. J., Kato T., Okabe H., Takahashi K. Production of monocyte chemoattractant protein-1 by malignant fibrous histiocytoma: relation to the origin of histiocyte-like cells. Exp Mol Pathol. 1991 Feb;54(1):61–71. doi: 10.1016/0014-4800(91)90044-x. [DOI] [PubMed] [Google Scholar]
- Underwood J. C. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br J Cancer. 1974 Dec;30(6):538–548. doi: 10.1038/bjc.1974.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme J., Decock B., Lenaerts J. P., Conings R., Bertini R., Mantovani A., Billiau A. Identification by sequence analysis of chemotactic factors for monocytes produced by normal and transformed cells stimulated with virus, double-stranded RNA or cytokine. Eur J Immunol. 1989 Dec;19(12):2367–2373. doi: 10.1002/eji.1830191228. [DOI] [PubMed] [Google Scholar]
- Ward B. G., Wallace K., Shepherd J. H., Balkwill F. R. Intraperitoneal xenografts of human epithelial ovarian cancer in nude mice. Cancer Res. 1987 May 15;47(10):2662–2667. [PubMed] [Google Scholar]
- Ylä-Herttuala S., Lipton B. A., Rosenfeld M. E., Särkioja T., Yoshimura T., Leonard E. J., Witztum J. L., Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura T., Yuhki N., Moore S. K., Appella E., Lerman M. I., Leonard E. J. Human monocyte chemoattractant protein-1 (MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett. 1989 Feb 27;244(2):487–493. doi: 10.1016/0014-5793(89)80590-3. [DOI] [PubMed] [Google Scholar]
- Zachariae C. O., Anderson A. O., Thompson H. L., Appella E., Mantovani A., Oppenheim J. J., Matsushima K. Properties of monocyte chemotactic and activating factor (MCAF) purified from a human fibrosarcoma cell line. J Exp Med. 1990 Jun 1;171(6):2177–2182. doi: 10.1084/jem.171.6.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ravenswaay Claasen H. H., Kluin P. M., Fleuren G. J. Tumor infiltrating cells in human cancer. On the possible role of CD16+ macrophages in antitumor cytotoxicity. Lab Invest. 1992 Aug;67(2):166–174. [PubMed] [Google Scholar]





