Abstract
To investigate whether electrophysiologic changes can detect the early onset and progress of intestinal rejection, changes in in vitro electrophysiologic function, intestinal histopathology, and Na–K-ATPase activity were studied in dogs. Adult mongrel dogs of both sexes, weighing 18–24 kg, were used for auto and allo small bowel transplantation. The entire small bowels, except for short segments at the proximal and distal ends, were switched between a pair of dogs (allograft). Animals receiving intestinal autotransplantation were used as controls. Allograft recipients were sacrificed 3, 4, 5, 7, or 9 days after transplantation, and autograft recipients were sacrificed 3, 7, or 14 days after transplantation. Immunosuppression was not used. Electrophysiologic measurements were done with an Ussing chamber. Histological analysis was performed blindly using whole thickness sections. Na–K–ATPase activity in the mucosal tissue, which is said to regulate the potential difference, was also measured. Potential difference, resistance, and Na–K-ATPase activity of the allograft intestine decreased with time and were significantly lower 7 and 9 days after transplantation compared to host intestine, normal intestine, and graft intestine of controls (autograft). Potential difference, resistance, and Na–K-ATPase activity of the native intestinal tissue and the autografts did not decrease with time. Detection of histologically mild rejection of the intestine, which is important for appropriate immunosuppressive treatment in clinical cases, could not be achieved based on electrophysiology or Na–K-ATPase activity. Deterioration of electrophysiologic function during rejection correlated with the histological rejection process and Na–K-ATPase activity; however, electrophysiology may not be a reliable tool for monitoring grafts, since it cannot detect early intestinal rejection.
Keywords: small bowel transplantation, Ussing chamber, electrophysiologic function, Na–K-ATPase, rejection
The prevention and early detection of rejection is crucial for successful clinical small bowel transplantation [1–4]. In the absence of any known biochemical markers of small bowel rejection, histopathological analysis of endoscope-guided mucosal biopsies has been recommended for the diagnosis and treatment of intestinal rejection [1,4,5]. Even though histological diagnosis of rejection has proven effective, a more objective and quantitative method is needed. The use of in vitro electrophysiologic study of the intestinal mucosal has been proposed for monitoring intestinal graft rejection [6–8]. Using an in vivo technique, Lee et al. [9] and Meijssen et al. [10] reported that electrophysiologic measurement of epithelial function paralleled both the clinical and morphologic parameters of rejection. However, it is not clear whether electrophysiologic function correlates with histological degree of rejection or whether the measurement of electrophysiologic function can accurately diagnose early histological rejection.
The measurement of potential difference of the intestine is based on the active ion transport of Na–K-ATPase at the basolateral membrane and is a sensitive reflection of changes in the electrical activity of the mucosal cells [9]. However, potential difference and Na–K-ATPase activity during rejection of the small intestine have never been measured simultaneously.
This study compared in vitro electrophysiologic function with the histology of the whole-thickness bowel and mucosal tissue Na–K-ATPase activity, and investigated whether electrophysiologic function is useful for monitoring intestinal graft rejection.
Materials and Methods
Adult male and female mongrel dogs weighing 18–24 kg were used.
Operative Procedure
Animals were fed with a low residual diet, and were given 1 g neomycin sulfate and 500 mg metronidazole daily for 5 days before small bowel transplantation. Animals were fasted from the evening prior to surgery. Under general anesthesia with halothane and 02:N02 (50:50), the entire small bowel, except for short segments of duodenum and ileum, was isolated on a vascular pedicle of the superior mesenteric artery (SMA) and vein (SMV). The small bowel was then removed and placed in slushed ice on the back table. The vasculature of the graft was perfused with 1 L cold lactated Ringer's solution containing 2000 U/L of heparin and 300 mg/L of neomycin sulfate. The lumen was flushed with 1 L of the same solution. Intestinal grafts (allografts) were exchanged between a pair of dogs. Vascular reconstruction was accomplished by end-to-end anastomoses of the donor SMA to the recipient SMA and the donor SMV to the recipient SMV. Small bowel continuity was restored by end-to-end anastomoses of the donor jejunum to the recipient duodenum and the donor ileum to the recipient ileum. All animals were treated with 1 g/day of Mandol intramuscularly for 3–5 days postoperatively. Lactated Ringer's solution (1–2 L) was given daily until the animals were eating and drinking normally (about 5 days). No immunosuppression was given.
The dogs were sacrificed on postoperative day (POD) 3 (n = 4), POD 4 (n = 3), POD 5 (n = 5), POD 7 (n = 7), or POD 9–12 (n = 4). Control animals undergoing intestinal autotransplantation were sacrificed on POD 3 (n = 5), POD 7 (n = 4), or POD 14 (n = 5). Normal values (POD 0) were obtained by taking samples under general anesthesia from untreated dogs.
Electrophysioiogicai Study
Electrophysiologic measurements were performed in essentially the same manner as described by Kirkmann et al. [6]. Isolated loops from both host and graft ileum were rinsed in cold lactated Ringer's solution to remove luminal contents. The mucosa and submucosa were separated from the seromuscular layer by blunt dissection. Graft and host ileal membranes, without Peyer's patches, were simultaneously mounted on a 13.5-mm-diameter Us sing chamber (World Precision Instruments Inc., Sarasota, FL, USA). When mounted in these chambers, each surface interfaced with a reservoir of continuously recirculated oxygenated (95% O2, 5% CO2) buffer solution at a pH of 7.4. The recirculating reservoirs were maintained at 37°C. Ten milliliters of perfusion solution, consisting of 120 mM NaCl, 4.7 mM KCl, 1.2 mM MgS04, 2.5 mM CaCl2, 25 mM NaHC03, 1.2 mM KH2P04, and 10 mM glucose, was placed in each chamber. Agar bridges separately connected the mucosal and submucosal compartments to voltage sensitive and current passing electrodes. Potential difference was directly measured, and resistance was calculated according to Ohm's law after voltage deflection measurements of transmucosal current (50 μA) were recorded. The voltage deflection measurements were obtained after a 20-min equilibration period. Data were obtained from a digital recorder.
Histological Study
Tissue samples (allograft and native jejunum and ileum, and allograft mesenteric lymph nodes) were obtained at the time of sacrifice for histological examination. Tissues were fixed with 10% buffered formalin, and embedded in paraffin. The tissues were then cut into 5-μm sections and stained with hematoxilin and eosin.
Histologically acute rejection was characterized by architectural changes of the mucosa, including villous blunting and ulceration, lymphocytic infiltration in the lamina propria, and epithelial damage with apoptotic cryptitis. The grades of rejection were defined according to the following criteria.
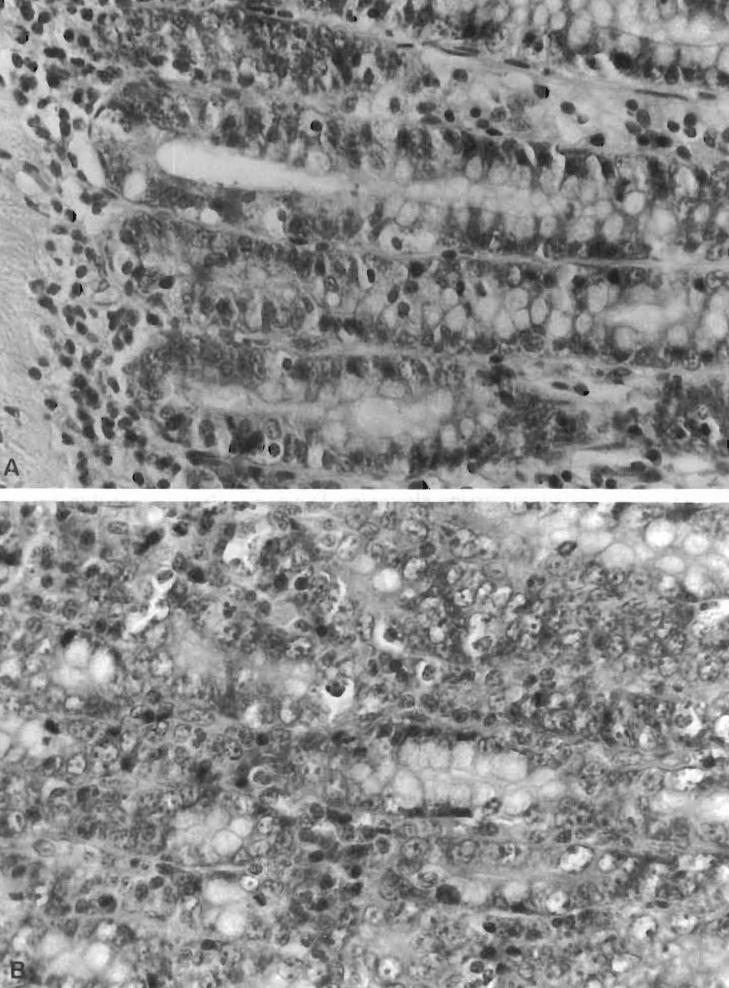
Grade 1: No to minimal changes. Preserved mucosal architecture, normal or slightly increased inflammatory cells in the lamina propria, and no evidence of epithelial damage (Figure 1A)
Grade 2: Mild rejection. Villous blunting, increased mononuclear cell infiltration, and occasional epithelial damage with apoptotic cryptitis (Figure 1B)
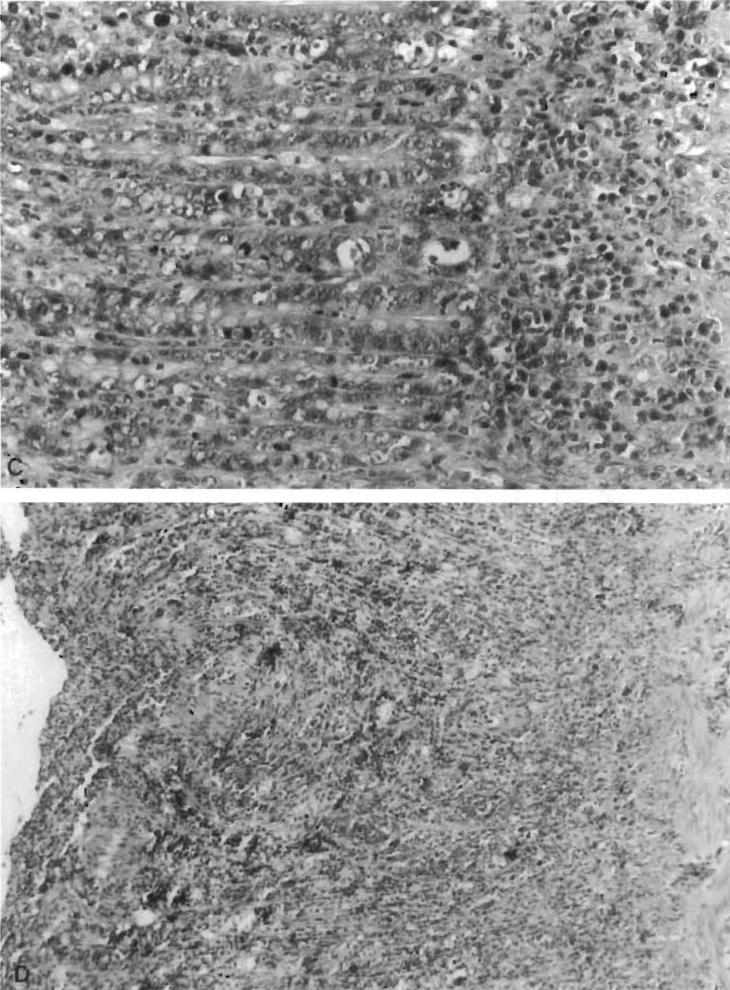
Grade 3: Moderate rejection. Ulcerations and more intestinal mucosal changes than in grade 2 (Figure 1C)
Grade 4: Severe rejection. Widespread sloughing of the mucosa, largely replaced by necrotic or granulation tissue accompanied by hemorrhage and abscess formation (Figure 1D)
Figure 1.
Histological criteria for intestinal rejection. Hematoxylin–eosin stains of intestinal mucosal sections. (A) No to minimal change (grade 1); note that the mucosal architecture is preserved, and there are normal or slightly increased inf1ammatory cells in the lamina propria. (B) Mild rejection (grade 2); villous blunting, and mononuclear cell infiltration. Histological criteria for intestinal rejection. Hematoxylin–eosin stains of intestinal mucosal sections. (C) Moderate rejection (grade 3); ulcerations and more prominent intestinal mucosal changes than seen in mild rejection. (D) Severe rejection (grade 4); widespread sloughing of the mucosa.
Biochemical Study
Graft mucosal samples for Na–K-ATPase were collected from the ileum by dividing the seromuscular layer, rinsing it with ice-cold saline, freezing it quickly, and then storing it in liquid nitrogen until sample preparation.
The tissue (0.2 g) was homogenized in a 3-mL mixture of 0.25 mM sucrose and 1 mM EDTA, pH 7.2, at 4°C. It was centrifuged at 7000 rpm for 15 min at 4°C. The supernatant (A) was separated from the pellet (A), and the pellet (A) was rehomogenized and recentrifuged at the same speed and temperature. The supernatant (B) from the second centrifugation was mixed with supernatant A and ultracentrifuged at 30,000 rpm for 1 h at 4°C. The resulting pellet (B) was resuspended with 1 mL of 0.25 M sucrose, 1 mM EDTA, 30 mM histidine, pH 7.2. The suspension was stored at −70°C until analysis. The microsome was heated slowly to room temperature and diluted 1:1 (v/v) with reagent (25 mM imidazole, 1 mM EDTA, deoxycholate 0.065% with 0.25–1.0 mg protein/mL, pH 7.2).
Na–K-ATPase activity was measured by the method described by Esmann [11]. Liberated organic phosphate was measured electrophotometrically, and enzyme activity was expressed as micromoles of inorganic phosphate liberated per milligram protein per hour.
Statistics
All data are expressed as the means ± standard errors of the mean. For statistical analysis of the electrophysiologic parameters and ATPase, a one-way analysis of variance (ANOVA) was performed and significance was determined using the Mann–Whitney U test. A p value of less than .05 was considered significant.
Results
Histopathology
Histological grading of intestinal tissues is shown in Table 1. No rejection was seen in any of the autograft animals and in 3 of the allograft animals. Mild, moderate, and severe rejection was seen in 8, 3, and 9 allograft intestines, respectively.
Table 1.
Histological grading of intestinal tissues
| Allografts |
Autografts |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| POD | 0 | 3 | 4 | 5 | 7 | 9 | 3 | 7 | 14 |
| (n) | (8) | (4) | (3) | (5) | (7) | (4) | (5) | (4) | (5) |
| Rejection | |||||||||
| No | 1 | 2 | 5 | 4 | 5 | ||||
| Mild | 3 | 3 | 2 | ||||||
| Moderate | 1 | 2 | |||||||
| Severe | 5 | 4 | |||||||
| Electrophysiology | |||||||||
| PD (mv) | 2.95 ± 0.20 | 2.83 ± 0.44 | 2.37 ± 0.55 | 1.94 ± 0.34 | 0.61 ± 0.45* | 0.28 ± 0.15* | 2.88 ± 0.41 | 2.10 ± 0.40 | 2.12 ± 0.37 |
| R (Ω/cm2) | 178.7 ± 10.3 | 146.6 ± 15.3 | 146.9 ± 21.2 | 123.6 ± 17.8 | 49.1 ± 21.6* | 34.3 ± 8.9* | 149.9 ± 14.2 | 156.6 ± 13.3 | 149.3 ± 14.5 |
| Biochemistry | |||||||||
| ATPase (μmol Pi/h mg protein–1) | 9.8 ± 0.3 | 9.5 ± 0.8 | 9.2 ± 2.0 | 11.9 ± 1.7 | 4.3 ± 1.6* | 1.9 ± 0.5* | 7.2 ± 0.3 | 8.5 ± 0.6 | 9.7 ± 1.5 |
Note. PD, potential difference; R, resistance.
p < .05 versus autograph values and postoperative day 0 values.
Submucosal and muscular layers of the intestinal graft were relatively devoid of congestive, hemorrhagic, or inflammatory insults in no-to-minimal change and mild rejection, but these insults were often seen in moderate and severe rejection. Mesenteric lymph nodes showed a relative widening of the perifollicular zone with infiltration of blastic lymphocytes as rejection progressed. Scarring or marked depletion of lymphoid tissue was not seen in Peyer's patches of the intestinal wall or mesenteric lymph nodes.
During periods of rejection, the jejunum and ileum of the allograft intestine showed similar microscopic changes in all animals; however, lymphoid follicles and Peyer's patches were seen more often in the ileum. All native intestinal tissues showed nonspecific, mild regenerative changes of the mucosa or a slight increase of chronic inflammatory cells in the lamina propria, irrespective of the type of grafting (allo- or auto-) or the date of sacrifice. There was no evidence of graft-versus-host disease in any animals.
Electrophysiology
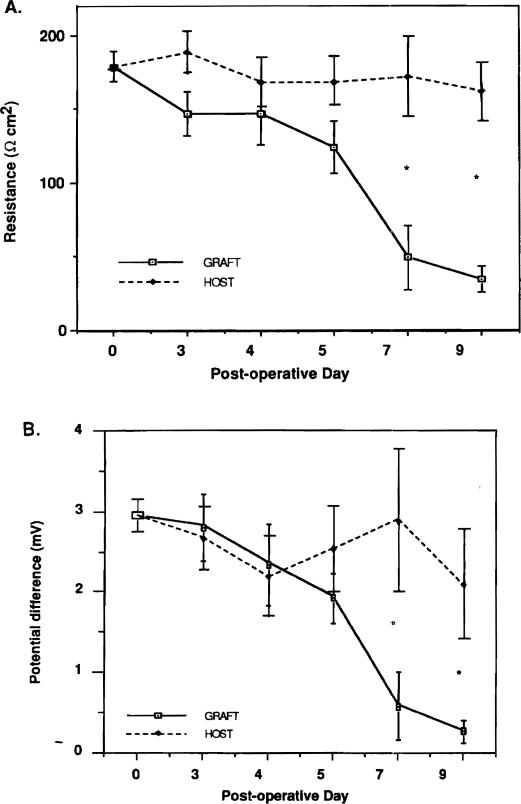
Spontaneous electrical potential difference and transepitheliai resistance of the host intestine after small bowel transplantation were essentially similar throughout the test period (Figure 2). Transepithelial resistance of the allograft decreased over time. Allograft resistance decreased from 178.7 ± 10.3 Q/cm2 on day 0 to 123.6 ± 17.8 Q/cm2 on day 5 (p < .05) and to 49.1 ± 21.6 Q/cm2 on day 7. In contrast, the resistance of the autograft controls did not significantly decrease with time. On postoperative days 7 and 9, the transepithelial resistance of the allograft was significantly lower than the trans epithelial resistance of the autograft controls on POD 7 and 14 (Table 1).
Figure 2.
(A) Comparison of the change in resistance of allograft and host intestine over time. (B) Comparison of the change in potential difference of allograft and host intestine over time. *p < .05, graft versus host.
The spontaneous potential difference of the allograft intestine also progressively diminished with time: from 2.95 ± 0.20 mV on day 0 to 0.61 ± 0.45 mV on day 7 (p < .05). Potential difference of autograft controls did not significantly decrease with time. On postoperative days 7 and 9, the allograft potential difference was significantly lower than control (Table 1). The resistance and potential difference of the allograft intestine was less than host intestine on days 7 and 9 (Figure 2).
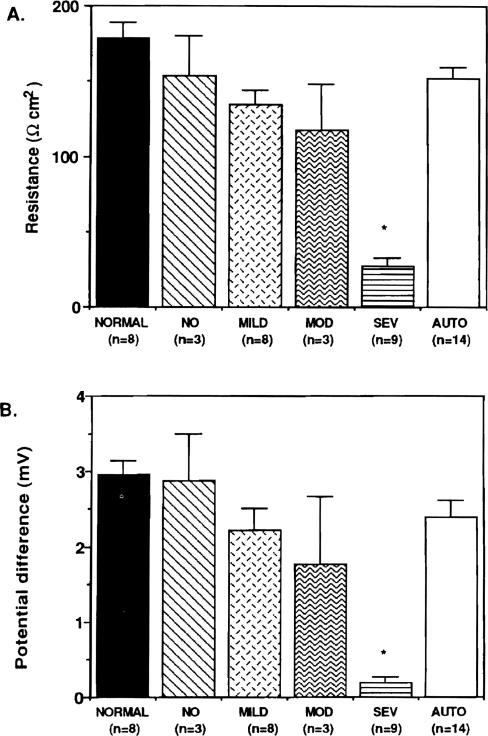
As shown in Figure 3, the spontaneous potential difference and the transepithelial resistance diminished as rejection became more severe; however, the decrease in potential difference and resistance was significant only during periods of severe rejection.
Figure 3.
(A) Comparison of graft resistance with histological degree of rejection. (B) Comparison of graft potential difference with histological degree of rejection. *p < .05, severe versus normal and auto.
Biochemistry
Changes in Na–K-A TPase activity were similar to the changes in potential difference and resistance. Na–K-ATPase activity of the allograft decreased from 9.8 ± 0.3 μmol Pi/h mg protein-1 on day 0 to 4.3 ± 1.6 μmol Pi/h mg protein-1 on day 7 (p < .05). Na–K-ATPase activity of the autograft did not decrease with time. Na–K-ATPase activity decreased as histologically diagnosed rejection worsened (Figure 4).
Figure 4.
Comparison of Na–K-ATPase with histological degree of rejection. *p < .05, severe versus normal and auto.
Discussion
Histology and functional studies (glucose absorption [13–15], maltose absorption [16], fatty acid absorption [17], and motility [18,19]) have been proposed to monitor intestinal rejection. Histological findings of intestinal rejection have been evaluated in great detail [7,20]. While random mucosal biopsies and full-thickness intestinal biopsies may be inappropriate for determining intestinal rejection [22,28], recent reports show that endoscope-guided mucosal biopsies are able to effectively diagnose early intestinal rejection after human small bowel transplantation [1,4,5].
While histology has been studied well, intestinal functional assessment with the Ussing chamber has received only limited attention [6–10]. Functionally, rejection is associated with impaired epithelial active ion transport as indicated by decreased potential difference and by diminished epithelial barrier function as reflected by decreased transepithelial resistance [6]. Studies have shown that potential difference across the mucosal membrane decreased with rejection in in vitro [6–8] and in vivo [9,10] models. Decreased potential difference is caused by a reduction of the overall enterocyte population or by a decrease in transport activity by the individual enterocyte [6,7]. The measurement of potential difference of the intestine is based on the active ion transport of Na–K-ATPase. Potential difference is a sensitive reflection of changes in the electrical activity of the mucosal cells [9]. In rat intestinal epithelial cells, more than 85% of the Na–K-ATPase is located in a plasma membrane fraction devoid of bmsh border, nuclei, and mitochondria [25]. The cell extrudes sodium from basolateral membrane into the plasma by active transport [24,26]. The specific activity of Na–K-ATPase is higher (three times higher in the jejunum and two times higher in the ileum) in the villus tip than in the crypt cells of the intestine [27].
Potential difference, resistance, and ATPase activity were compared with the histological degree of rejection, because the onset and progress of rejection in dogs are different than in rodents [12]. In the rodent model, rejection occurs and progresses at very predictable rates, while in the dog model, rejection occurs and progresses at very different rates from dog to dog. For example, no rejection was seen in one dog on POD 3 and in 2 dogs on POD 5; mild rejection was seen from POD 3 to POD 5; moderate rejection was seen from POD 5 to 7; and severe rejection was seen from POD 7 to 9. Mild rejection caused no significant changes in potential difference or ATPase activity, which suggests no villous cells impairment. Moderate rejection caused reductions in potential difference, resistance, and ATPase in one of three dogs. While the numbers are small, the decrease in potential difference, resistance, and ATPase may indicate damage to the villi. Severe rejection, with extensive mucosal sloughing and complete architecture loss, caused significant reductions in potential difference, resistance, and ATPase, thus indicating significant damage to the villi.
The functional results accurately paralleled the progression of histological rejection from moderate to severe, but did not show any functional changes associated with mild rejection. The lack of functional changes associated with mild rejection may be related to the site of rejection and location of Na–K-ATPase. Though the specific concentration of Na–K-ATPase is higher in the villous tips than in the crypt cells of the intestine, the crypts may be the initial target of rejection [6]. Thus, to detect mild rejection it may be necessary to examine the effect of rejection on crypt cell function by measuring electrical parameters of active chloride secretion, an index of crypt epithelial function [7].
A reliable and objective method for detecting mild rejection would be an important tool in human intestinal transplantation. Functional testing of the intestinal mucosa with the Ussing chamber was not able to detect mild rejection. In summary, our results suggest that deterioration of electrophysiologic function of rejecting intestinal grafts correlates with the rejection process; however, electrophysiology cannot detect early intestinal rejection. Therefore, electrophysiology may not be a reliable tool for monitoring intestinal rejection.
Acknowledgments
Aided by research grants from the Veterans Administration and Project Grant DK 29961 from the National Institutes of Health, Bethesda, MD, USA. The authors sincerely thank Steve Miller and Joyce Petrow for their assistance in the preparation of this manuscript.
References
- 1.Todo S, Tzakis AG, Abu-Elmagd K, et al. Intestinal transplantation in composite visceral grafts or alone. Ann Surg. 1992;216:223–234. doi: 10.1097/00000658-199209000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grant D, Wall W, Mimeault R, et al. Successful small bowel/liver transplantation. Lancet. 1990;335:181–184. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 3.Schroeder P, Goulet O, Lear PA. Small bowel transplantation: European experience. Lancet. 1990;336:110–111. doi: 10.1016/0140-6736(90)91621-g. [DOI] [PubMed] [Google Scholar]
- 4.Todo S, Tzakis AG, Abu-Elmagd K, et al. Cadaveric small bowel and small bowel-liver transplantation in humans. Transplantation. 1992;53:369–376. doi: 10.1097/00007890-199202010-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura K, Nalesnik M, Jaffe R, et al. Morphological monitoring of human small bowel allografts. Transplant Proc. 1993;25:1212. [PMC free article] [PubMed] [Google Scholar]
- 6.Kirkman RL, Lear PA, Madara JL, Tilney NL. Small intestine transplantation in the rat: immunology and function. Surgery. 1984;96:280–287. [PubMed] [Google Scholar]
- 7.Madara JL, Kirkman RL. Structural and functional evaluation of jejunal allograft rejection in rats and ameliorating effects of cyclosporin therapy. J Clin Invest. 1985;75:502–512. doi: 10.1172/JCI111726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee TK, Cardona MA, Kurkchusbache AG, et al. Mucosal glutamine utilization after small bowel transplantation: an electrophysiologic study. J Surg Res. 1992;52:605–614. doi: 10.1016/0022-4804(92)90137-o. [DOI] [PubMed] [Google Scholar]
- 9.Lee MD, Smith SD, Yunis EJ, Rowe MI. In vivo transmural potential difference: an early monitor of rejection in small bowel transplantation. J Pediatric Surg. 1989;24:767–770. doi: 10.1016/s0022-3468(89)80533-0. [DOI] [PubMed] [Google Scholar]
- 10.Meijssen MAC, Heineman E, De Bruin RWF, Ten Kate FJW, Marquet RL, Molenaar JC. Detection of canine intestinal allografts rejection in vivo electrophysiologic monitoring. Transplantation. 1991;51:955–959. doi: 10.1097/00007890-199105000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Esmann M. ATPase phosphatase activity of Na+, K+-ATPase: molar and specific activity, protein determination. Methods Enzymol. 1988;156:105–109. doi: 10.1016/0076-6879(88)56013-5. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimi F, Nakamura K, Zhu Y, et al. Canine total orthotopic small bowel transplantation under FK 506. Transplant Proc. 1991;23:3240–3242. [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen WB, Hardy MA, Quint J, State D. Absorptive function in canine jejunal autografts and allografts. Surgery. 1969;65:440–446. [PubMed] [Google Scholar]
- 14.Dennison AR, Collin J, Watkins RM, Millard PR, Morris PI. Segmental small intestinal allografts in the dog, I: morphological and functional indicates of rejection. Transplantation. 1987;44:474–478. doi: 10.1097/00007890-198710000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Nordgren S, Cohen Z, MacKenzie R, Finkelstein D, Greenberg GR, Langer B. Functional monitors of rejection in small intestinal transplants. Am J Surg. 1984;147:152–158. doi: 10.1016/0002-9610(84)90050-3. [DOI] [PubMed] [Google Scholar]
- 16.Billiar TR, Garberogio C, Schraut WH. Maltose absorption as an indicator of small intestinal allograft rejection. J Surg Res. 1984;37:75–82. doi: 10.1016/0022-4804(84)90164-1. [DOI] [PubMed] [Google Scholar]
- 17.Stanford WP, Hardy MA. Fatty acid absorption in jejunal autografts and allografts. Surgery. 1974;75:496–502. [PubMed] [Google Scholar]
- 18.Schamaun M. Electromyography to determine viability of injured small bowel segment. Surgery. 1967;62:899–909. [PubMed] [Google Scholar]
- 19.Schiller WR, Suriyapa C, Mutchler JHW, Gohara SF, Anderson MC. Motility changes associated with canine intestinal allografting. J Surg Res. 1973;15:379–384. doi: 10.1016/0022-4804(73)90107-8. [DOI] [PubMed] [Google Scholar]
- 20.Murase N, Demetris AJ, Kim DG, Todo S, Fung JJ, Starzl TE. Rejection of multivisceral allografts in rats: a sequential analysis with comparison to isolated orthotopic small bowel and liver grafts. Surgery. 1990;108:880–889. [PMC free article] [PubMed] [Google Scholar]
- 21.Rosemurgy AS, Schraut WH. Small bowel allografts: sequence of histologic changes in acute and chronic rejection. Am J Surg. 1986;151:470–475. doi: 10.1016/0002-9610(86)90106-6. [DOI] [PubMed] [Google Scholar]
- 22.Millard PR, Dennison A, Hughes DA, Collin J, Morris PJ. Morphology of intestinal allografts rejection and inadequacy of mucosal biopsy in its recognition. Br J Exp Pathol. 1986;67:687–698. [PMC free article] [PubMed] [Google Scholar]
- 23.Murase N, Demetris AJ, Matsuzaki T. Long survival in rats after multivisceral versus isolated small-bowel allotransplantation under FK506. Surgery. 1991;110:87–98. [PMC free article] [PubMed] [Google Scholar]
- 24.Nakahara S, Itoh H, Mibu R, Ikeda S, Nakayama F. Regional difference in intestinal adaptation after total colectomy as judged by the changes of mucosal Na-K-ATPase, cyclic AMP, and transmural potential difference. Dis Colon Rectum. 1988;31:523–528. doi: 10.1007/BF02553725. [DOI] [PubMed] [Google Scholar]
- 25.Quigley JP, Gotterer GS. Distribution of Na–K stimulated ATPase activity in rat intestinal mucosa. BBA. 1969;173:456–468. doi: 10.1016/0005-2736(69)90010-8. [DOI] [PubMed] [Google Scholar]
- 26.Charney AN, Donowitz M. Functional significance of intestinal Na–K-ATPase: in vivo ouabain inhibition. Am J Physiol. 1978;234:E629–E636. doi: 10.1152/ajpendo.1978.234.6.E629. [DOI] [PubMed] [Google Scholar]
- 27.Charney AN, Gots RE, Giannella RA. Na–K-stimulated adenosinetriphosphatase in isolated intestinal villus tip and crypt cells. BBA. 1974;367:265–270. doi: 10.1016/0005-2736(74)90083-2. [DOI] [PubMed] [Google Scholar]
- 28.Schmid T, Oberhuber G, Korozsi G, Klima G, Margreiter R. Histologic pattern of small bowel allograft rejection: mucosal biopsies do not provide sufficient information. Gastroenterology. 1989;96:1529–1532. doi: 10.1016/0016-5085(89)90522-2. [DOI] [PubMed] [Google Scholar]