Abstract
Experiments using live dissociated carotid body (CB) cells for patch clamping, [Ca++]i or other measurements require positive identification of the cell being recorded. At present, cell morphology is usually employed, but several cell types within the carotid body evidence similar morphologic characteristics. Therefore, we sought to develop a method utilizing a vital dye to identify glomus cells before and during experiments that require live cells, such as patch clamp studies. It was previously reported that the binding sites for peanut agglutinin (PNA) were highly expressed by all neuroendocrine-derivatives of the sympathoadrenal neural crest, including glomus cells, small, intensely fluorescent cells, PC-12 cells, and adrenal chromaffin cells in situ (Katz et al. 1995). By utilizing the binding characteristics of galactose-specific lectin peanut agglutinin (PNA) on the outer cell membrane, we tested the possibility that the fluoresceinated PNA may preferentially bind to CB glomus cells. The results to date show: (1) Rhodamine tagged PNA (Rhod-PNA) binds to the live dissociated glomus cells in less than one hour incubation and can be visualized in superfused cells; (2) Rhod-PNA labeled cells are perfectly matched with tyrosine hydroxylase (TH) positive glomus cells; (3) Rhod-PNA did not interfere with Fura-2 for Ca++ imaging; (4) Rhod-PNA bound to glomus cells in [Ca++]i studies does not affect O2 response of glomus cells. Thus fluoresceinated PNA may be a useful marker for live CB glomus studies, without adversely affecting their physiologic response.
Keywords: PNA, Peanut agglutinin, Lectin, CB, Glomus cells, Marker, O2 sensing, TH, Ca++ imaging, Double immunostaining
1 Introduction
The carotid body (CB) is a small neural crest derived neuroendocrine organ sensing blood O2, CO2, and pH level. CB chemoreceptor glomus cells increase [Ca++]i in response to low oxygen levels and their O2 responses are fully developed during first two weeks of postnatal life (Carroll and Kim 2005) in rats.
The CB consists of four principle components: cell clusters, blood vessels, connective tissue, and nerve fibers (Izal-Azcarate et al. 2008). Chemoreceptor glomus cells and sustentacular cells comprise ~60% of total CB volume (Gonzalez et al. 1994, 1995, Lopez-Barneo et al. 2001). After enzymatic dissociation, CB cells consist of several types: glomus cells, sustentacular cells, fibroblasts etc. Usually, glomus cells are identified based on cell size, shape, and occurrence in clusters. However, clear identification is uncertain since different cells may have similar morphologic characteristics. It is known that tyrosine hydroxylase (TH) and synaptophysine are markers for glomus cells and glial fibrillary acid protein (GFAP), S-100 protein and vimentin (Kameda 1996, Kameda 2005, Yamamoto et al. 2006) are markers for sustentacular cells. However, these markers are for intracellular proteins and are only useful in permeabilized cells. Experiments using live dissociated carotid body (CB) cells for patch clamping, [Ca++]i or other measurements require methods for live CB cell identification. In addition, live cell tagging must not interfere with the normal physiological responses of the cells. Therefore, we sought to develop a method to identify live dissociated glomus cells before and during experiments.
It was reported that the binding sites for peanut agglutinin (PNA) were highly expressed by all neuroendocrine-derivatives of the sympathyo-adrenal neural crest, including glomus cells, small, intensely fluorescent cells, PC-12 cells, and adrenal chromaffin cells in situ (Katz et al. 1995). Since galactose-specific lectin peanut agglutinin (PNA) (Lotan et al. 1977) is bound on the outer cell membrane, we investigated the possibility that the fluoresceinated PNA may bind to the membrane surface of CB glomus cells Although fluoresceinated PNA may bind to CB cells, several questions must be addressed: (1) whether the fluoresceinated-PNA bound CB cells are exclusively TH positive cells, (2) whether the fluoresceinated-PNA bound CB cells are viable after PNA treatment, and (3) whether PNA binding causes any adverse physiological effects on CB O2 sensing.
In order to address the first concern, cultured dissociated CB cells were treated with rhodamine labeled PNA (Rhod-PNA) or biotin labeled PNA (Biotin-PNA) for one hour, and double-stained with TH antibodies. Secondly, the calcium responsiveness of Fura-2 labeled, Rhod-PNA treated cells was tested using high [K+]o extracellular solution (20 mM K+). Thirdly, alterations in physiologic responsiveness was tested in Fura-2, Rhod-PNA treated CB cells by stimulation with hypoxia or anoxia.
The results suggest that the fluoresceinated PNA is an efficient cell surface marker for live CB glomus cells with no adverse physiological effects.
2 Methods
2.1 Preparation of Dissociated CB Cells and Immunocytochemistry
Carotid bodies (CB) isolated from newborn Sparague-Dawley rats were dissociated with enzyme mixture consisting trypsin and collagenase (~0.5 mg/ml), and plated on poly-D-lysine coated glass coverslips. After culturing for 3–5 hours, the cells were treated with biotinylated-PNA or Rhod-PNA in complete CB medium (25 μg/ml: Vector) for 1 hour in cell culture incubator at 37°C. Then, the CB cells were fixed with 4% paraformaldehyde and treated with 0.4% triton X-100 in PBS. After treating with blocking solution (5% non-fat dry milk), cells were labeled with Alexa 546 tagged streptoavidin (1:200) for 2 hours at room temperature (RT). For double labeling, anti-TH (1:1000) was treated for 5–6 hours and labeled with Alexa flour 488 tagged secondary antibodies (1:500) for 2 hours at RT. After a final wash, the coverslips were mounted on a glass slide with Prolong Gold antifade reagent with DAPI (Invitrogen). Cells are observed under Nikon TE-2000 at 400X and the images were taken and merged with NIS Element software.
2.2 Labeling Live CB Cells with Rhodamine-PNA (Rhod-PNA) and Intracellular Ca++ ([Ca++]i) Measurement
For labeling live CB glomus cells with Rhod-PNA, CB cells were treated with Rhod-PNA in complete CB medium (25 μg/ml:Vector) for 1 hour, 37°C. Rhod-PNA treated CB cells were washed several times with warm PBS to remove unbound Rhod-PNA. [Ca++]i was measured by quantitative fluorescence imaging using fura-2. Rhod-PNA treated cells were loaded with fura-2, by incubation for 30 minutes at 37°C (Molecular Probes). Cells were exposed to 20mM K+ extracellular solution, hypoxia (0% O2), or anoxia (0% O2 with 0.5 mM dithionite). Fura-2 fluorescent emission was measured at 510 nm in response to alternating excitation at 340 and 380 nm. Images were acquired and stored using a Nikon TE2000 microscope and CCD (CoolSNAP HQ2) camera under Metafluor software (Molecular Devices). For each coverslip, the background light levels were determined and subtracted, from each image before measurement of the fluorescence intensity ratio at 340 nm/380 nm.
3 Results
3.1 Identification of PNA Bound Cells as Glomus Cells
To assess initially the binding ability of PNA to dissociated CB cells, Biotin-PNA or Rhod-PNA was added into cultured CB cells at 37°C. Within one hour, staining was positive for biotin- PNA and Rhod-PNA. After 3 hours incubation, cells were fixed in 4% paraformaldehyde and visualized with Alexa 546 streptoavidin. As shown in Fig. 1A CB cells from a P16 rat demonstrated positive binding for biotin-PNA. In order to identify the cell type that was labeled, cells were double labeled with glomus cell marker, TH. As shown in Fig. 1A–C, the cells labeled with biotin-PNA or TH were identical. This suggests that PNA specifically labels glomus cells. Biotin-PNA labeling on glomus cells is cell specific, because PNA did not labeled every CB cell as shown in Fig. 1E. The cells labeled only with DAPI in Fig. 1E were stained with neither biotin-PNA nor TH antibody and marked with arrows in Fig. 1F.
Fig. 1.
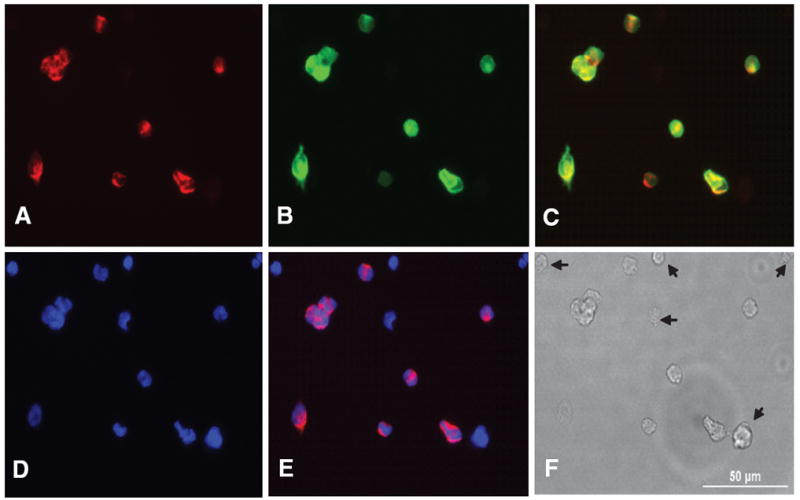
Dissociated CB cells were incubated with biotinylated PNA and labeled with Alexa 546 tagged streptoavidin (A) and double labeled by TH with Alexa 488 secondary antibody (B). PNA and TH are overlaid (C). The nucleus are stained with DAPI contained mounting medium (D) and PNA stained image is overlaid over DAPI (E). The CB cells labeled with PNA are exactly matched with cell labeled with TH. Thus PNA only labels type I cells and the cells marked with arrows on DIC (F) are not type I cells. A scale bar (50 μm) in DIC (F)
3.2 Identification of PNA Labeled Cells as O2 Sensing Glomus Cells
Biotin-PNA and Rhod-PNA did not appear to affect viability or cell responsiveness. The CB cells treated with Rhod – PNA still responded to. hypoxia in Ca++ imaging studies. In order to identify the PNA positive cells as functioning glomus cells, PNA treated CB cells were tested [Ca++]i response to acute hypoxia (0% O2) or anoxia (0% O2 + 0.5 mM dithionite). Both clusters (Fig. 2D) and single cells (Fig. 2E) on which Rhod-PNA was bound increased [Ca++]i in response to 20 mM K+, 0% O2 hypoxia, and anoxia as shown in Fig. 2. Non-PNA labeled cells did not show any [Ca++]i changes in response to hypoxia or anoxia. Thus, PNA labeled cells are O2 sensing CB glomus cells.
Fig. 2.
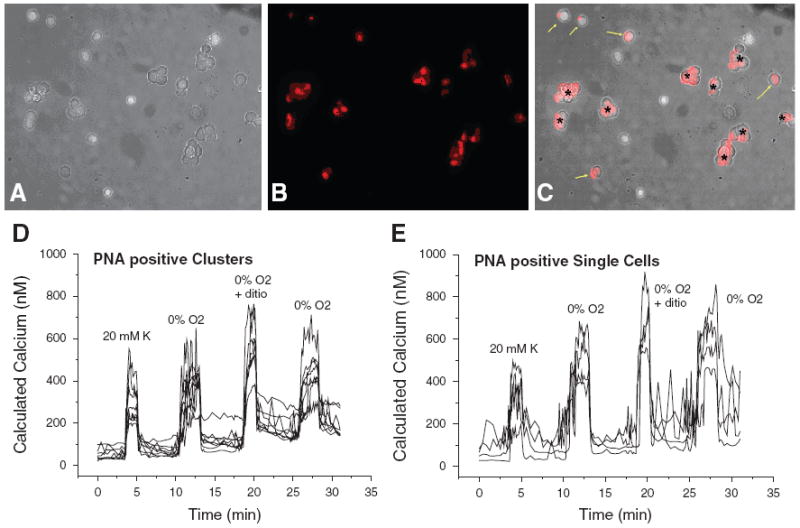
Dissociated 16 days old CB cells were treated with Rho-PNA for 1 hr. (A) DIC of dissociated CB cells, (B) Rhod-PNA labeled CB cells, (C) Rhod-PNA labeled cells were overlaid on DIC image. Intracellular Ca2+ increases in response to 20 mM K , 0% O2, and anoxia (with 0.5 mM dithionite [dithio]) in Rhod-PNA positive clusters marked asterisk (*) on C (D) and in Rhod PNA positive single cells marked yellow arrows in C (E)
3.3 No Adverse Physiological Effect of PNA Binding on O2 Sensing
To find out whether PNA binding adversely affects O2 sensing ability, the response to 20 mM K+, hypoxia, and anoxia in PNA bound CB cells were compared to Non-PNA treated CB glomus cells. The averaged [Ca++]i increase in response to 20 mM K+ and 0% hypoxia showed that there are no significant differences between PNA treated and not treated CB cells. Thus, adding fluoresceinated PNA to identify glomus cells before/during physiological experiments requiring real time and live CB cells does not have any adverse physiological effects.
4 Discussion
Identification of cell types, and, in particular, glomus cells is essential for studying O2 sensing mechanisms. The present results demonstrate that fluoresceinated PNA, Biotin-PNA and Rhod-PNA, bind to CB glomus cells within one hour of exposure. By co-immunostaining for TH, fluoresceinated PNA-bound cells were identified as exclusively glomus chemoreceptor cells. In addition, PNA binding produced no detectable alteration in the calcium response to 20 mM K+, 0% O2 hypoxia, and anoxia.
Therefore these preliminary results suggest that the fluoresceinated PNA is an efficient cell surface marker for live CB glomus cells to identify the live dissociated CB glomus chemoreceptor cells before and during experiments.
Acknowledgments
This study was supported by grants from the National Institutes of Health (RO1 HL54621) and CUMG grant from University of Arkansas for Medical Sciences.
References
- Carroll JL, Kim I. Postnatal development of carotid body glomus cell O2 sensitivity. Respir Physiol Neurobiol. 2005;149:201–215. doi: 10.1016/j.resp.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Almaraz L, Obeso A, Rigual R. Carotid body chemoreceptors: from natural stimuli to sensory discharges. Physiol Rev. 1994;74:829–98. doi: 10.1152/physrev.1994.74.4.829. [DOI] [PubMed] [Google Scholar]
- Gonzalez C, Lopez-Lopez JR, Obeso A, Perez-Garcia MT, Rocher A. Cellular mechanisms of oxygen chemoreception in the carotid body. Respir Physiol. 1995;102:137–147. doi: 10.1016/0034-5687(95)00069-0. [DOI] [PubMed] [Google Scholar]
- Izal-Azcarate A, Belzunegui S, Sebastian WS, Garrido-Gil P, Vazquez-Claverie M, Lopez B, Marcilla I, Luquin MA. Immunohistochemical characterization of the rat carotid body. Respir Physiol Neurobiol. 2008;161:95–99. doi: 10.1016/j.resp.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Kameda Y. Immunoelectron microscopic localization of vimentin in sustentacular cells of the carotid body and the adrenal medulla of guinea pigs. J Histochem Cytochem. 1996;44:1439–1449. doi: 10.1177/44.12.8985136. [DOI] [PubMed] [Google Scholar]
- Kameda Y. Mash1 is required for glomus cell formation in the mouse carotid body. Dev Biol. 2005;283:128–139. doi: 10.1016/j.ydbio.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Katz DM, White ME, Hall AK. Lectin binding distinguishes between neuroendocrine and neuronal derivatives of the sympathoadrenal neural crest. J Neurobiol. 1995;26:241–252. doi: 10.1002/neu.480260208. [DOI] [PubMed] [Google Scholar]
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- Lotan R, Beattie G, Hubbell W, Nicolson GL. Activities of lectins and their immobilized derivatives in detergent solutions. Implications on the use of lectin affinity chromatography for the purification of membrane glycoproteins. Biochemistry. 1977;16:1787–1794. doi: 10.1021/bi00628a004. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Taniguchi K. Expression of tandem P domain K+ channel, TREK-1, in the rat carotid body. J Histochem Cytochem. 2006;54:467–472. doi: 10.1369/jhc.5A6755.2005. [DOI] [PubMed] [Google Scholar]


