Abstract
In this study kidneys were harvested from bred-for-research cats weighing 4 to 5 kg. General principles of donor bilateral nephrectomy en bloc with aorta, vena cava, renal vessels, and ureters were followed. After the harvest the grafts were placed in lactated Ringer slush. A cuff was prepared on the renal vein over a 10 French plastic tube. The aorta was divided and left in connection with the renal artery at each side. Twenty female checkered Flemish giant rabbits weighing 4.0–6.0 kg served as recipients. After pre-medication with 40 mg/kg of ketamine, anesthesia was maintained with repeated doses (every 10–15 min) of a 0.1-mL mixture of 5 parts ketamine and 1 part acepromazine diluted 50% in a normal saline. Arterial pressure, CVP, blood gases, and temperature were monitored. Through a limited midline incision a native left nephrectomy was performed. The venous anastomosis was performed with a cuff technique without clamping the vena cava (which causes severe hemodynamic instability); the anastomotic time was 2–3 min. The arterial anastomosis was performed with an end-to-side aorta-to-aorta anastomosis; the anastomotic time was 5 to 7 min. There were no episodes of venous or arterial thrombosis. The donor procedure took approximately 40 min, and the backtable preparation of the graft an additional 45 to 60 min. Preparation of the recipient for the anastomosis took 15 min and the anastomotic time (warm ischemia) was 13 ± 5 min. In this model suitable for xenograft research the duration of the surgery in the recipient has been greatly reduced because of (1) the previous backtable preparation of the graft, and (2) the cuff technique used for venous anastomosis. The present anesthesia regimen and careful hemodynamic monitoring were also important in the success of this model.
Animal research in transplantation is an essential tool to evaluate new technical procedures and pharmacological protocols, and to provide effective training in surgical skills. The availability of reproducible and appropriate transplant models in relatively small animals is important in the investigation of specific immunological processes (ie, in xenograft rejection) and to do so using adequately large numbers and at relatively low cost.
The rabbit represents an appropriate animal to use for transplantation research: it is easy to handle and its immunological characteristics and behavior have been fairly well characterized.1 Nonetheless, problems with anesthesia, hemodynamic monitoring, and certain technical difficulties with the handling of vessels in rabbits are limiting factors for certain transplant models.
In this paper we report our experience in developing a technique of orthotopic renal xenotransplantation in rabbits. The specific technical aspects that render this model reproducible and appropriate for research relating to hyperacute rejection and xenografting are presented.
Material and Methods
Animals
Twenty female checkered Flemish giant (CFG) rabbits weighing 4.0 to 6.2 kg were purchased from Zivic-Miller Laboratory (Allison Park, PA) and served as recipients. Fifteen female, bred for research cats, weighing 4 to 5 kg were purchased from Liberty Labs (Liberty Corner, NJ) and served as donors. All animals were housed in a central animal facility at the University of Pittsburgh and allowed to acclimatize for at least one week prior to experimentation. They received standard animal chow and water ad libitum, and were fasted for 24 h prior surgery.
Donor Nephrectomy and Backtable Preparation of the Grafts
The general principles of en bloc bilateral donor nephrectomy removing the aorta, vena cava, renal vessels, and ureters en masse were followed.2 The kidneys were flushed in situ, through an infrarenal aortic cannula, with 400–500 mL of cold (4°C) lactated Ringers. After harvest the grafts were placed in a large basin with lactated Ringers slush. Basic microsurgical techniques were then used at the backtable to separate both kidneys, and to fashion adequate vascular cuffs for anastomosis. Each donor was able to provide two technically suitable kidneys for transplantation.
The venous anastomoses were simplified and expedited by using a cuff vascular technique that has been previously described in several microsurgical models.3
A cuff was prepared for the vein anastomosis using a 0.3-cm segment of No. 10 polyethylene tubing. A small phalange was fashioned to allow easier introduction of the plastic cuff into the recipient renal vein.3 These cuffs were prepared on the renal vein of the left kidney, which is generally of adequate length, and on the infrarenal vena cava for the right kidney, which was used in connection with right renal vein to obtain adequate length.
The arterial cuffs were then prepared. After ligation of the celiac axis and the superior mesenteric artery, the aorta was divided, obliquely between the takeoffs of the renal arteries (Fig 1). A continuous polypropylene monofilament suture (7-O) was used on both sides (Fig 1a), unless there was sufficient space between the takeoff of the renal arteries to allow direct ligation and division of the aorta (Fig 1b). Thus, the upper aorta was left in continuity with the right renal artery and the lower aorta with the left. At the end of the preparation of the donor grafts all vessels were flushed with heparinized saline solution.
Figure 1.
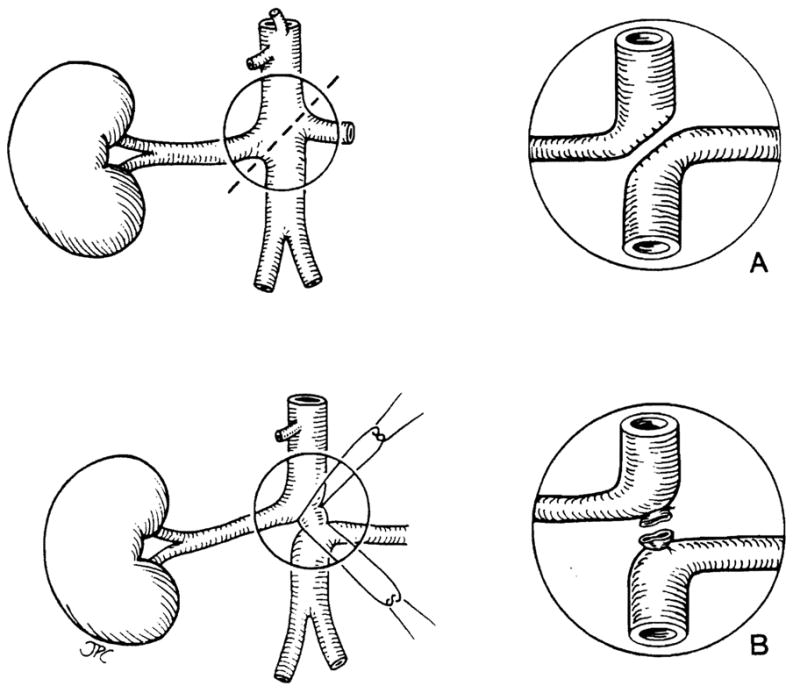
Surgical technique of renal arteries preparation on the backtable. (a) Oblique section of the aorta and reconstruction with running 7-O prolene; (b) direct ties on the aorta. In both techniques the aorta is left in connection with renal artery to facilitate the anastomosis on the recipient.
Recipient Anesthesia and Monitoring
Premedication consisted of 40 mg/kg body wt of ketamine (Ketalar-Parke-Davis) intramuscularly at 15–20 min before surgery. At this dosage a state of surgical anesthesia developed within 8–10 min and good muscle relaxation and spontaneous respiration was achieved in all animals.
The rabbit was then positioned on the operating table, and the auricular vein was cannulated with a 22-gauge catheter for administration of fluid and drugs. Anesthesia was maintained with repeated doses (every 10–15 min) of 0.1 mL of a mixture of five parts ketamine and one part acepromazine (PromAce-Aveco Co) diluted 50% in normal saline. The dosage was increased to 0.3 mL immediately prior to entering the peritoneum and then reduced to 0.1 mL for the remainder of the procedure. Approximately about 1000 mL of saline was administered during the surgical procedure and during the remaining postoperative observation period. Arterial pressure was monitored via the auricular artery of the opposite ear using a 20-gauge catheter. Central venous pressure (CVP) was monitored using a 16-gauge cannula in the external jugular vein, which was introduced via the internal facial vein. Both CVP and arterial lines were flushed with heparinized saline every 10 min.
Arterial blood samples can be drawn for blood gases, pH, and electrolytes before the skin incision (basal state), immediately prior to unclamping the aorta (unclamp minus 1 min), and 3–5 min after unclamping the aorta (unclamp + 5 min). Sodium bicarbonate, electrolytes, and oxygen via an open mask were administered as needed. Rectal temperature was monitored and a warming blanket was kept under the rabbit to maintain the body temperature at 37 °C or greater.
Surgical Procedure of Orthotopic Kidney Transplantation (KTx)
The peritoneal cavity was entered through a midline upper abdominal incision. The left lumbar fossa was exposed, the left renal vein carefully dissected free from the cava to the kidney hilum, and the adrenal vein ligated. A 4-O silk tie was placed around the renal vein and left untied at a point midway between the hilum and the takeoff of the adrenal vein.
The renal artery was also mobilized, and a satisfactory segment of infrarenal aorta was identified to facilitate aortic clamping during the arterial anastomosis. The renal artery and renal vein were then tied, the former close to its aortic takeoff and the latter close to the hilum. At that point the native kidney was removed.
A small clamp was then placed on the renal vein close to the vena cava. The renal vein was then opened and flushed with heparinized saline solution. At this time the graft was removed from the cold solution and placed in the left lumbar fossa. A microvascular cuff technique was used for the venous anastomosis; the previously prepared donor vein cuff was introduced into the opened end of the renal vein of the recipient and the 4-O silk tie encircling the vein was tied securing the plastic cuff (Fig 2).
Figure 2.
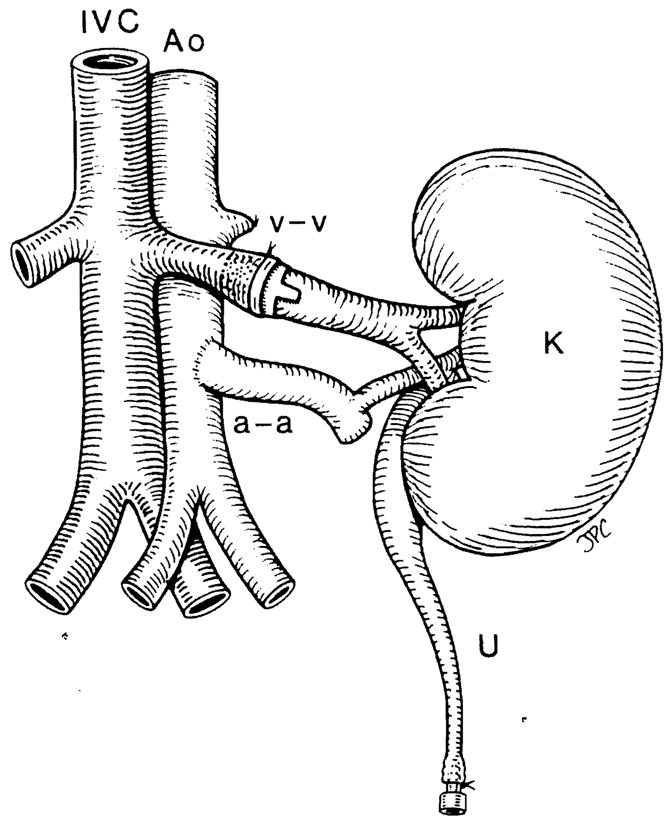
Renal xenograft in place in the recipient. Venous anastomosis (v-v) over a plastic cuff, arterial anastomosis (a-a) end-to-side with the aorta of the donor left connected with renal artery. IVC, inferior vena cava; AO, aorta; K, kidney; U, ureter cannulated over a stent for perioperative urine collection.
A Satinsky clamp was then placed on the aorta with either partial or complete occlusion, and an end-to-side aorta-to-aorta anastomosis was performed with a continuous 7-O polypropylene suture (Fig 2). The arterial clamp was released a few seconds prior to the vein clamp, allowing complete reperfusion of the kidney graft. The ureter was cannulated with a 20- to 22-gauge catheter and approximated to the skin for urine collection and monitoring.
The skin and the fascia were approximated using interrupted sutures; anesthesia and monitoring were maintained as described above. A small peritoneal window was prepared to inspect the kidney by observing changes in color and consistency of the xenograft, and to perform open biopsies during the subsequent 3 h.
Thereafter, the animals were sacrificed with euthanasia solution (Euthanasia, Schering Co) and the kidneys were removed for pathological examination.
Results
Anesthesia and Monitoring
The results of the several hemodynamic and metabolic parameters evaluated at predetermined time points of the procedure are presented in Table 1.
Table 1.
Cat-to-Rabbit Model: Intraoperative Monitoringa
| Basal Status | Aorta Unclamp |
||
|---|---|---|---|
| −1 min | +5 min | ||
| BP | 92.0 ± 11.9 | 88.0 ± 13.1 | 84.5 ± 9.1 |
| CVP | 7.3 ± 3.2 | 6.6 ± 3.8 | 7.1 ± 3.7 |
| t (°C) | 37.5 ± 0.8 | 37.0 ± 0.7 | 36.7 ± 0.7 |
| pH | 7.26 ± 0.04 | 7.24 ± 0.04 | 7.24 ± 0.08 |
| pO2 | 85.5 ± 11.6 | 88.3 ± 14.5 | 94.9 ± 14.5 |
| pCO2 | 33.8 ± 4.1 | 38.8 ± 5.0 | 36.2 ± 8.4 |
| BE | −10.8 ± 2.1 | −11.1 ± 1.7 | −11.2 ± 3.3 |
| Na | 142.7 ± 4.4 | 143.8 ± 4.7 | 143.3 ± 4.5 |
| K+ | 3.1 ± 0.3 | 2.8 ± 0.3 | 3.1 ± 0.1 |
| Ca2+ | 1.23 ± 0.3 | 1.11 ± 0.2 | 1.05 ± 0.2 |
BP, Blood pressure; CVP, central venous pressure; t, temperature.
The systemic blood pressure always dropped after unclamping the aorta and was associated with a fall in CVP. However, the blood pressure was always maintained above 84 ± 9.1 mm Hg, with complete recovery of CVP after graft reperfusion and during the following 3 h of observation. This well-documented hypotensive tendency4,5 could be easily controlled by an increase in fluid administration while monitoring CVP, and by rapid correction of any metabolic imbalances. Rapid anastomosis time and vessel unclamping were also very important.
With standardization of the anesthesia protocol, there were no operative mortalities. Spontaneous breathing was always achieved along with good muscle relaxation and analgesia. The administration of O2 via an open circuit during and after the aortic clamping avoided endotracheal intubation. Blood oxygenation improved during the course of the procedure.
Technical Results
The end-to-end venous anastomosis over a previously prepared donor cuff was generally performed in 2–3 min, while the arterial end-to-side anastomosis required 5 – 7 min.
After standardization of the operative technique there were no episodes of venous or arterial thrombosis in the 15 consecutive experiments here. It should be noted that the use of heparin in these experiments was restricted only to the heparin–saline flushes of the vessel stumps during the actual anastomoses. The donor weight (cats of about 4 kg) allowed a good size match with the native rabbit kidney and vessels. In fact, all kidneys of this size perfused well with normal consistency and color within 2–3 min after unclamping.
The donor procedure took approximately 45 min. The backtable preparation of the graft took an additional 45–60 min and preparation of the recipient for the anastomoses required 15 min, while the anastomosis time (warm ischemia time of the graft) was 13 ± 5 min.
Xenograft Function
Progressive rejection occurred in all kidneys. Changes in color and consistency (progressively cyanotic and firm grafts), decrease in urine output, or presence of hematuria were considered clinical parameters of rejection. The histological criteria for a diagnosis of hyperacute rejection was thrombosis of 10% or more of the glomeruli, with or without acute tubular necrosis (ATN). The clinical and histological findings of this cat-to-rabbit xenotransplantation model are presented in Table 2.
Table 2.
Xenograft Survival
| Start Rejection (min) | Complete Rejection (min) | Urine Output (ml/h) |
Onset of Histologic Evidence | |||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | ||||
| Cat-to-rabbit (20 cases) | 40 ± 10 | 84.3 ± 5.2 | 0.22 ± 0.12 | 0.05 ± 0.06 | — | >45–60 min picture of HAR showed. Low percentage of glomerular thrombosis |
HAR, Hyperacute rejection.
Clinical evidence of rejection occurred after 40 ± 10 min in the experiments reported here, while the histological onset of rejection occurred at least 20–30 min after the clinical diagnosis.
The histological features in the cat-to-rabbit transplants up to 40 min after reperfusion of the graft, demonstrated normal glomeruli with varying degrees of tubular vacuolization or ATN in all but one of the experiments (Fig 3). In the one experiment (one donor and two recipients), 10–14% of the glomeruli in each of the kidneys in the two recipients contained thrombi at 30 min after reperfusion. After 40 min one-third of the kidneys exhibited thrombi in 6–12% of the glomeruli, with clear hyperacute rejection only by clinical criteria (Fig 4). The remainder of the specimens taken at 1, 2, and 3 h after revascularization showed thrombi in 4–6% of the glomeruli and findings highly suspicious but not totally diagnostic for hyperacute rejection (HAR). Eosinophils were frequently noted in all specimens, and polymorphonuclear leukocytes and necrosis in only one specimen.
Figure 3.
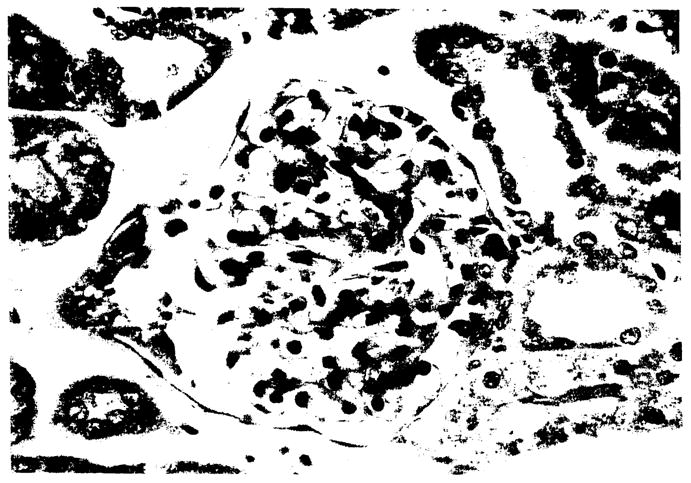
Cat-to-rabbit: Baseline biopsy after reperfusion showing a normal glomerulus. (H & E × 560)
Figure 4.

Cat-to-rabbit: Biopsy at 1 h showing thrombi in capillary loops (arrows) and vacuolization of tubules. (H & E × 560)
Discussion
The rabbit is increasingly becoming a model of choice for training purposes in microvascular surgery and more importantly for research in the biological sciences.4–6 Ease of handling, relatively low cost, and a good understanding of this species pathophysiology are some of the factors responsible for the widespread acceptance of this animal. It is especially important for transplantation research to have available well-standardized and reproducible animal models, particularly for investigating complex processes, such as hyperacute rejection, where a relatively large study population is required.7
Many previous reports have described the frustrations of similar models in rabbits.8–19 In this report we present a detailed accounting of the operative anesthesia and surgical techniques, especially the vascular anastomoses, which address many of the problems and which are required to make this model successful.
The weight of the recipient should be considered one of the contributing factors to the successful results reported here. The rabbits (checkered Flemish giants; 4.0–6.2 kg) weighed more than the rabbits described in previous reports (2.0–4.5 kg). The skeletal size of the rabbit is considered more important than the actual weight but in our experience the large animals facilitated the anesthetic and operative management, and allowed repeat follow-up studies.
Many anesthetic protocols for rabbits have been reported, 10–18 reflecting the difficulties in defining reproducible techniques to manage the animals intraoperatively.
Barbiturates (Pentobarbital, Nembutal, Methoexidone) had been used in most of the reported experiments, sometimes in combination with an inhalant agent (N2O/O2) through a nose cone. The precise dosage of barbiturates in rabbit anesthesia had never been definitely established, and different breeds of rabbits may respond differently to the same dosage.5 Moreover, barbiturates may induce hypotension, and some authors14 describe the use of 5 mL of saline with each injection of drug to counteract the drop in the blood pressure. Halothane (fluothane) mixed with oxygen has also been used quite often, but generally in homemade circuit apparatus, to remove carbon dioxide and to adjust the air–anesthetic mixture concentration. Halothane and N2O/O2 can also be delivered after intubation of the animals to facilitate a prolonged and stable anesthesia.17 Both of these methods require extensive experience and more sophisticated equipment.
The mixture of ketamine (5 parts) and acepromazine (1 part) diluted 1:1 with saline used in the present series for iv injection consistently gave good results in terms of analgesia and muscle relaxation and allowed spontaneous respiration. In the series of experiments reported here, there were no deaths due to overanesthesia. Ketamine has previously been used for anesthesia in rabbits, both for induction to facilitate intubation and as a single anesthetic agent in short-duration surgical procedures.4,5 Acepromazine has been reported to provide adequate anesthesia in rabbits as well, when followed by methoxyflurane inhalation.5 The protocol reported here has been used extensively in our animal research laboratory for the last two years to anesthetize rabbits for all types of surgical procedures, and has generally been considered to be easy to handle (0.1 mL iv every 10–15 min) and has almost never been associated with serious complications. Similar to the experience of others, 4,17,18 we found intramuscular premedication of the animals to be quite useful.
Both sensitivity to anesthesia and occlusion of the major vessels by the placement of surgical clamps have been responsible factors for the hypotension that is always encountered in rabbits. The duration of surgery in the recipient is certainly important, and it has been greatly reduced in our series because of the initial backtable preparation of the graft.
By preparing the donor organ as much as possible at the backtable in iced Ringer’s slush, the anastomotic time and the duration and simplicity of the entire procedure is shortened significantly. We have found the cuff technique for the venous anastomosis quite reliable; it should be noted that the cuff was prepared in the donor vessel instead of in the recipient renal vein, as previously reported.4,13,17 The donor aorta was left in continuity with the renal artery on the backtable, and the arterial anastomosis was performed end to side to the recipient aorta. Using this technique a good match for size and thickness can be obtained between the two vessels, thus facilitating the performance of the anastomoses and restricting the clamp time of the recipient aorta to no more than 7 min. No episodes of arterial or venous thrombosis occurred in the present series.
A fall in the arterial blood pressure and the CVP routinely occurred after aortic clamping, but we found this event quite easy to control with fluid infusion. In contrast to previous reports,4 we observed a greater and a more prolonged hypotension in the recipients in which the inferior vena cava (IVC) was clamped. Therefore, this method was abandoned and the use of the cuff technique for venous anastomosis without any interference to the flow in the IVC was adapted.
Prolonged occlusion of the infrarenal aorta in the rabbit usually gives rise to a spinal cord ischemia with a neurological impairment of a degree strictly dependent upon the duration of occlusion.20,21 This sequence of events, which has been extensively investigated in recent years, is responsible for the neurological damage (flaccid paralysis, paraplegia) occurring after aortic occlusion for KTx reported in previous studies.10,14,18 This particular complication could not be evaluated in the present experiments since the animals remained under anesthesia for the duration of the study and were sacrificed at the end of the experiment. However, in our experiments the aortic occlusion was never longer than 7 min, and thus the probability of permanent neurologic impairment is negligible.20
The features of xenograft or hyperacute rejection in this experiment were quite similar to those previously reported.22,25 The low incidence of glomerular thrombosis in the rejection process of cat-to-rabbit transplantation has already been reported25 and attributed to two differences in the fibrinolytic activity between the two species. It may be that a more definite picture of classical hyper-acute rejection could occur if the observation period was extended beyond 3 h, even in presence of early and clear clinical evidence of HAR.
The model described in this report is suitable and quite appropriate for studies of the pathophysiology of HAR and the events in xenotransplants, and moreover, for the evaluation of different methods of therapeutic interaction (ie, pharmacologic agents, immunological manipulation) that could abrogate this process.
In conclusion, a superior model of orthotopic kidney transplantation in the rabbit is reported. The high incidence of anesthetic and surgical complications usually reported in the rabbit have been overcome by extensive preparation of the grafts on the bench prior to implantation, which has resulted in a shorter aortic occlusion and operative time in the recipient, and in the avoidance inferior vena caval clamping. A reliable anesthetic protocol in conjunction with careful hemodynamic and metabolic monitoring of the animals has resulted in a safe and reproducible procedure.
In our hands this model has been particularly useful in evaluating renal xenotransplantation, and will surely be applicable to many other research aspects in transplantation and experimental surgery.
Acknowledgments
Supported by research grants from the Veterans Administration and Project Grant AM 29961 from the National Institutes of Health, Bethesda, Maryland. Dr Mazzaferro is the recipient of the Davis-Geck Fellowship Award 1988 at the Department of Surgery, University of Pittsburgh.
References
- 1.Sittisombut N, Knight KL. Rabbit major histocompatibility complex. J Immunology. 1986;136:1871–1875. [PubMed] [Google Scholar]
- 2.Starzl TE, Hakala TR, Shaw BR, Jr, et al. A flexible procedure for multiple cadaveric organ procurement. Surg Gynecol Obstet. 1984;158:223–230. [PMC free article] [PubMed] [Google Scholar]
- 3.Kamada K. Technique in the rat (cuff preparation) In: Calne RY, editor. Liver Transplantation. 2. London: Grone & Stratton; 1987. pp. 25–34. [Google Scholar]
- 4.Dunn DC. Orthotopic renal transplantation in the rabbit. Transplantation. 1976;22:427–433. doi: 10.1097/00007890-197611000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan HM, Timmons EH. Principles and application of rabbit anesthesia. In: Kapplan HM, Timmons EH, editors. The Rabbit. A Model for the Principles of Mammalian Physiology and Surgery. New York: Academic Press; 1979. pp. 41–50. [Google Scholar]
- 6.Peleg H, Rao UNMA, Emrich LJ. The rabbit as an experimental animal for tracheal surgery. J Surg Oncol. 1988;37:49–51. doi: 10.1002/jso.2930370114. [DOI] [PubMed] [Google Scholar]
- 7.Makowka L, Miller C, ChapChap P, et al. Prolongation of pig-to-dog renal xenograft survival by modification of the inflammatory mediator response. Ann Surg. 1987;206:482–495. doi: 10.1097/00000658-198710000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nathan P, Gonzales EE, Fowler R, Jr, et al. A method for transplantation of the rabbit kidney. Proc Soc Exp Biol Med. 1961;107:51–55. doi: 10.3181/00379727-107-26530. [DOI] [PubMed] [Google Scholar]
- 9.McDonald JC, Fukuda A. Rabbit renal homografts, I. Technique for graft to the neck. Surgery. 1966;59:1156–1160. [PubMed] [Google Scholar]
- 10.Bayuk JM, Schmidlapp CJ. Kidney transplantation in the rabbit. A method for the study of transplantation immunology. Invest Urol. 1967;4:378–388. [PubMed] [Google Scholar]
- 11.Klassen J, Milgrom F. The role of humoral antibodies in the rejection of renal homograft by rabbits. Transplantation. 1969;8:566–575. doi: 10.1097/00007890-196911000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Klassen J, Milgrom F. Studies on cortical necrosis in renal grafts. Transpl Proc. 1971;3:598–601. [PubMed] [Google Scholar]
- 13.Heron I. Kidney transplantation in the rabbit. A new method. Acta Path Microbiol Scand (Sect A) 1970;78:90–95. doi: 10.1111/j.1699-0463.1970.tb02134.x. [DOI] [PubMed] [Google Scholar]
- 14.Lund B. Renal transplantation in the rabbit: Surgical technique. Acta Path Microbiol Scand (Sect A) 1970;78:85–89. doi: 10.1111/j.1699-0463.1970.tb02133.x. [DOI] [PubMed] [Google Scholar]
- 15.Overton JH, Owen ER. The successful replacement of minute arteries. Surgery. 1970;68:713–723. [PubMed] [Google Scholar]
- 16.Holter AR, McKearn, Neu MR, et al. Renal transplantation in the rabbit, I. Development of a model for the study of hyperacute rejection and immunological enhancement. Transplantation. 1972;13:244–249. [PubMed] [Google Scholar]
- 17.Green CJ. Rabbit renal autografts as an organ preservation model. Lab Animals. 1973;7:1–11. doi: 10.1258/002367773781005879. [DOI] [PubMed] [Google Scholar]
- 18.Jacobsen IA. Renal transplantation in the rabbit: A model for preservation studies. Lab Animals. 1978;12:63–70. doi: 10.1258/002367778780953198. [DOI] [PubMed] [Google Scholar]
- 19.Ito S, Camussi G, Tetta C, et al. Hyperacute renal allograft rejection in the rabbit. The role of platelet-activating factor and of cationic proteins derived from polymorphonuclear leukocytes and from platelets. Lab Invest. 1984;51:148–161. [PubMed] [Google Scholar]
- 20.Ball TD, Lundy EF, Zelenock GB, et al. Effect of lodoxamide tromethamine on paraplegia that occurs after infrarenal aortic occlusion in the rabbit. J Vasc Surg. 1987;6:572–577. [PubMed] [Google Scholar]
- 21.Zivin JA, DeGirolami U. Spinal cord infarction: A highly reproducible stroke model. Stroke. 1980;11:200–202. doi: 10.1161/01.str.11.2.200. [DOI] [PubMed] [Google Scholar]
- 22.Starzl TE, Porter KA, Husberg BS, et al. Hyperacute rejection. Current Problems in Surgery: Renal Homotransplantation Part I. 1974:37–39. [PubMed] [Google Scholar]
- 23.Starzl TE, Porter KA, Husberg BS, et al. Mechanism of rejection of renal homograft in sensitized recipients. Current Problems in Surgery: Renal Homotransplantation Part II. 1974:28–30. [Google Scholar]
- 24.Starzl TE, Boehmig HJ, Amemiya H, et al. Clotting changes including disseminated intravascular coagulation during rapid renal homograft rejection. N Eng J Med. 1970;283:383–390. doi: 10.1056/NEJM197008202830801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kemp E, Kemp G, Jacobsen A, et al. Prolongation of survival of renal xenografts by infusion of donor blood. Acta Path Microbiol Scand (Sect A) 1977;85:267–269. doi: 10.1111/j.1699-0463.1977.tb00426.x. [DOI] [PubMed] [Google Scholar]


