Abstract
• Experience with the use of Orthoclone 0KT3 monoclonal antibody for the treatment of acute cellular rejection in a series of 130 human orthotopic liver transplantations is reviewed. Treatment was highly effective in reversing rejection, in reducing the rate of retransplantation, and in lowering patient mortality. 0KT3 was also useful for cyclosporine sparing in patients with poor renal function, hypertension, or CNS toxicity. There was a significant incidence of opportunistic infection associated with the use of 0KT3.
Keywords: OKT3, monoclonal antibody, rejection, liver transplantation
One-year survival after liver transplantation with conventional azathioprine high-dose steroid therapy was only 32% in the Denver series. This therapy was replaced by cyclosporine low-dose prednisone in the Denver-Pittsburgh liver transplant series in 1980 and I-year graft survival subsequently improved to 70%.1 However, approximately 20% of the patients who have undergone liver transplantation in Pittsburgh have required at least one more graft and over half of these retransplantations have been for irreversible allograft rejection.1 2
Polyclonal antilymphocyte agents (ALG) were introduced into clinical practice by Starzl et al.3 These agents were effective but clinical use was limited by toxicity, allergenicity, and the highly variable biological activity of ALG preparations.
Cosimi et al conducted the first successful clinical trials with Orthoclone OKT3 mouse anti-human T cell monoclonal antibody (Ortho Pharmaceuticals, Raritan, NJ) in renal transplantation.4 5 Encouraged by these results, in November 1984 we began experimental use of Orthoclone OKT3 for the treatment of acute cellular rejection in both cadaver kidney and liver transplant recipients. The results of this experience have been previously published6 8 We here review our experience with OKT3 in liver transplantation.
METHODS AND MATERIALS
Case Material
Eighteen liver transplant recipients with biopsy proven acute cellular rejection were included in an initial randomized tnal of steroids vOKT3. Because of the impressive response to OKT3 (Table 1), the randomized tnal was abandoned after this short senes and regular treatment with Orthoclone OKT3 of all patients with steroid resistant or early aggressive cellular rejection was instituted.
Table 1.
Initial Randomized Trial Comparing High-Dose Steroids to Orthoclone OKT3 for the Treatment of Biopsy Proven Acute Cellular Rejection
| No | Reversal | Rescue Needed |
Rescue Successful |
|
|---|---|---|---|---|
| OKT3 | 7 | 6 | — | — |
| Steroids | 11 | 4 | 7 | 6/7 |
The trial was abandoned after only 18 patients because of the obvious superiority of Orthoclone OKT3
Between November 1984 and December 1985 130 liver recipients were treated with OKT3. Mean age of the patients was 28.6 years and 57% were females. Donor-recipient ABO blood group compatibility was observed whenever possible, but lymphocytotoxic antibody crossmatching and HLA typing were done retrospectively and played no role in recipient selection. Results were compared with a historical control series of 237 patients transplanted under cyclosporine low-dose steroids with steroid therapy or polyclonal ALG for treatment of acute rejection.
Cyclosporine
An oral dose of cyclosporine (17.5 mg/kg) was administered five to six hours preoperatively when possible. Postoperatively. patients received intravenous (IV) cyclosporine (2 mg kg) two to three times per day. Oral administration was begun as soon as gastrointestinal (GI) tract function permitted, at a dose of 17.5 mg kg in two divided doses. IV and oral administration were continued until stable liver function and oral absorption were obtained. IV administration was then tapered. Blood levels were monitored daily using a whole blood radioimmunoassay method and were maintained in the 700 to 1.000 ng/mL range, renaJ function permitting.
Corticosteroids
Steroids were administered as an initial bolus of 1.000 mg methylprednisolone followed by a five-day burst beginning at 200 mg d in divided doses and reduced by 40 mgd until a maintenance dose of 20 mgd was reached. Doses were appropriately reduced for pediatric recipient. If rejecion was suspected. the 1.000 mg methylprednisolone bolus and five-day burst of steroids were repeated. However, if there was no response to steroids or in the case of severe, early rejection, the steroid therapy was aborted and therapy with Orthoclone OKT3 begun.
Method of OKT3 Administration
Orthoclone OKT3 was administered as a single daily 5 mg IV push over two to five minutes Smaller children received 2.5 mg Premedication included 50 mg IV diphenhydramine and 500 mg to 1.000 mg hydrocortisone administered one hour before administration of the first two doses of OKT3. Treatment was continued for ten to 14 days. All patients were carefulh observed for development of serious reactions to the drug but routine placement in an 1CU environment for administration of OKT3 was not necessary.
OKT3 levels were measured using an enzyme-linked immunosorbent assay (ELISA) technique and showed an excess of OKT3 throughout therapy. The development of anti-murine antibodies in recipients of OKT3 was also monitored in most patients. Results of these studies have been previously reported6
Analysis of Results
To analyze the results of treatment, patients were divided into three groups group 1. treatment with OKT3 started within nine days of transplantation, group 2, treatment with OKT3 started between ten and 90 days of transplantation; and group 3, treatment with OKT3 started more than 90 days after transplantation.
Group 1 patients were patients with poor early graft function uschemic injury, poor renal function necessitating cyclosporin sparing, or in rare instances, patients with early aggressive cellular rejection Group 2 patients were those most likely to have acute cellular rejection, and group 3 patients were those most likely to have a mixed pattern of acute and chrome rejection.
RESULTS
Randomized Trial
Eighteen patients, including seven treated with steroids and 11 treated with OKT3 were entered in an initial randomized trial. The results are summarized in Table 1. Only four of the 11 patients treated with steroids responded well and six of the seven grafts that failed to respond were rescued with OKT3. Because of the obvious superiority of OKT3 in this series, we felt that further randomized treatment was not justified.
OKT3 v Historical Controls
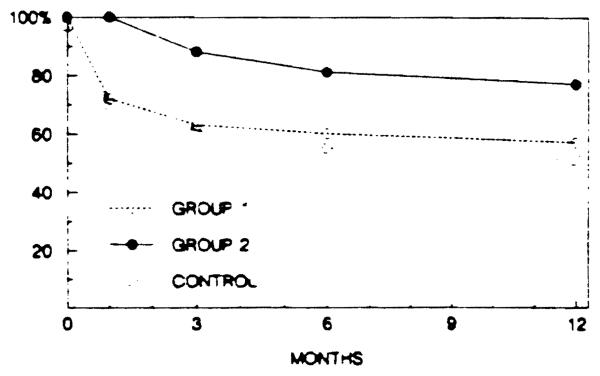
The results of treatment in the three OKT3 treatment groups are compared with the historical control series in Table 2 and Fig 1. The retransplantation rate in the historical controls was 22.2% but in OKT3 group 2 was only 6.87% (P < .05). Furthermore, the 1-year graft survival rate is higher in all three OKT3 treatment groups and is highest in group 2. Actuarial survival for group 2 patients was significantly better (P < .01) than for the controls (Fig 1).
Table 2.
Results of Therapy With Orthoclont OKT3 In Three Treatment Groups Compared With Historical Controls
| Percent Retransplantation |
Percent Graft Survival 1 Yr |
Percent Patient Survival |
||
|---|---|---|---|---|
| 6 Mo | 1 Yr | |||
| Control | 22.2 | 54.9 | 73.6 | 71.6 |
| n = 237 | ||||
| Overall | 15.9 | 64.4 | 82.9 | 75.1 |
| n = 130 | ||||
| Group 1 | 20.6 | 54.1 | 72.1 | 67.1 |
| n = 49 | ||||
| Group 2 | 6.8 | 76.7 | 86.7 | 79.2 |
| n = 65 | ||||
| Group 3 | 37.5 | 68.8 | 81.2 | 72.2 |
| n = 16 | ||||
Group 1 was treated zero to nine days after transplantation; group 2 was treated ten to 90 days after transplantation; and group 3 was treated more than 90 days after transplantation.
Fig 1.
Actuarial graft survival in group 1 and group 2 patients treated with Orthoclone OKT3 compared with historical controls treated with high dose steroids and/or polyclonal ALG. Survival of group 2 grafts is significantly better than control and group 1 grafts (P < .01).
Adverse Reactions and Complications
Side effects of therapy were common but usually self limited and tolerable. Detailed records of 72 consecutively treated patients were reviewed to assess side effects and are summarized in Table 3. GI side effects were the most common followed by fever and chills. None of these patients had to be withdrawn from the drug and there were no anaphylactic reactions. In fact, in our entire experience with OKT3, we have only observed one possible anaphylactic reaction in a patient treated for the third time with OKT3 who developed respiratory distress and required intubation. She promptly recovered and was extubated within 24 hours.
Table 3.
Side Effects in 72 Patients Treated With OKT3
| Nausea, vomiting, diarrhea | 22 |
| Pyrexia, chills | 19 |
| Flushing, diaphoresis | 7 |
| Hypotension | 6 |
| Tachycardia | 6 |
| Abnormal chest sounds | 6 |
| Hypertension | 5 |
| Weakness | 5 |
| Dyspnea | 4 |
| Edema | 4 |
| Lightheadedness | 4 |
| Chest pain | 4 |
| Headache | 3 |
| Rash | 2 |
| Cough | 2 |
| Anorexia | 1 |
Nearly all occurred within the first 48 hours of administration and subsided with subsequent doses. None of these patients had to be withdrawn from the drug.
Infectious complications have been common. Leucopenia (WBC < 4.0/mm3) suggestive of viral infection were observed in more than half of the patients and infections with cytomegalovirus, herpes virus, and pneumocystis were common and occasionally fatal.
CONCLUSIONS
Orthoclone OKT3 is a highly effective immunosuppressive agent for the treatment of acute cellular rejection in liver transplant recipients. It has been most effective when administered in the period ten to 90 days after transplantation when acute cellular rejection is most prone to occur, but it may be effective when administered earlier or later if acute cellular rejection is a significant component of graft dysfunction.
Orthoclone OKT3 is also an effective agent when cyclosponne sparing is indicated. We have had success using OKT3 in place of cyclosporine during the first 2 weeks after transplantation in patients unable to tolerate cyclosporins, usually because of nephrotoxicity, or rarely because of severe hypertension or CNS toxicity.
As is true of other efficacious immunosuppressive agents, Orthoclone OKT3 is associated with a high incidence of opportunistic infection, especially with cytomegalovirus, herpes virus, and Pneumocystis carinii. The high infection rate we have experienced may in pan reflect our policy of continuing with cyclosporine therapy in most patients treated for acute cellular rejection with Orthoclone OKT3. Perhaps it is safer arid equally efficacious to reduce or discontinue cyclosporine therapy during the initial phase of OKT3 therapy and return to therapeutic treatment with cyclosporine during the last several days of OKT3 administration. We have not seen a high rate of rebound rejection after OKT3 in patients who are at therapeutic levels of cyclosporine on completion of OKT3 therapy.
During the past 18 months we have retreated patients with OKT3 for subsequent steroid-resistant acute rejection episodes with success provided the patients have not developed antimurine antibodies after their first course of therapy. Except in the one case cited above, serious adverse reactions with retreatment have not been a significant problem. It is our impression that OKT3 can be effectively reused in many patients and that the drug should not be withheld when indicated to save it for possible use at some later and indefinite time.
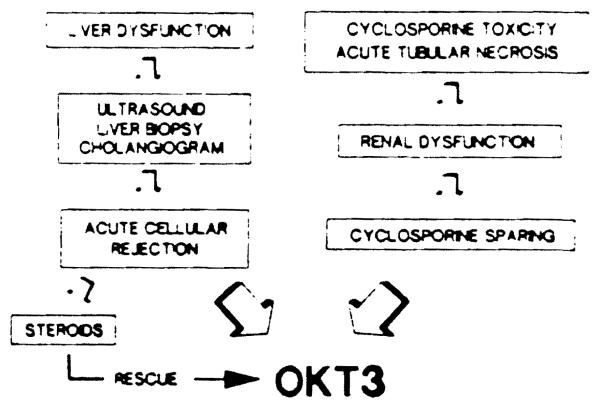
Our current protocol for use of Orthoclone OKT3 is summarized in Fig 2. Orthoclone OKT3 may also have a role as a prophylactic agent in patients with a history of high immunorcactivity, such as patients undergoing retransplantation for rejection of a previous graft. Further study of this application of Orthoclone OKT3 is needed.
Fig 2.
Current protocol for use of Orthoclone OKT3 in the management of liver transplant recipients. Prophylactic use of OKT3 in high-risk patients needs also to be considered.
Acknowledgments
Supported by Research Project Grant No. AM-29961 from the National Institutes of Health. Bethesda. MD. LM. is the recipient of a Centennial Fellowship from the Medical Research Council of Canada.
Footnotes
Consensus Conference on Monoclonal Antibodies in Transplantation sponsored by the National Kidney Foundation, Scottsdale, AZ. June 26-27, 1987.
REFERENCES
- 1.Gordon RD, Shaw BW, Jr, Iwatsuki S, et al. Indications for liver transplantation in the cyclosporine era. Surg Clin North Am. 1986;66:541–556. doi: 10.1016/s0039-6109(16)43939-3. [DOI] [PubMed] [Google Scholar]
- 2.Shaw BW, Jr, Gordon RD, Iwatsuki S, et al. Hepatic re-transplantation. Transplant Proc. 1985;17:264–271. [PMC free article] [PubMed] [Google Scholar]
- 3.Starzl TE, Marchioro TL, Porter KA, et al. The use of heterologous antilymphoid agents in canine renal and liver homotransplantation and in human renal homotransplantation. Surg Gynecol Obstet. 1967;124:301–318. [PMC free article] [PubMed] [Google Scholar]
- 4.Cosimi AB, Colvin R, Burton R, et al. Use of monoclonal antibodies to T-cell subsets for immunological monitoring and treatment in recipients of renal allografts. N Engl J Med. 1981;305:308–314. doi: 10.1056/NEJM198108063050603. [DOI] [PubMed] [Google Scholar]
- 5.Cosimi AB, Burton R, Colvin R, et al. Treatment of acute allograft rejection with OKT3 monoclonal antibody. Transplantation. 1981;32:535–539. doi: 10.1097/00007890-198112000-00018. [DOI] [PubMed] [Google Scholar]
- 6.Fung JJ, Demetris AJ, Porter KA, et al. Use of OKT3 with cyclosporine and steroids for reversal of acute kidney and liver allograft rejection. Nephron. 1987;96:19–33. doi: 10.1159/000184431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon RD, Starzl TE, Fung JJ. Monoclonal antibody therapy with cyclosporine and steroids in nonmatched cadaveric renal transplants. Nephron. 1987;96:56–59. doi: 10.1159/000184436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Esquivel CO, Fung JJ, Markus B, et al. OKT3 in the reversal of acute hepatic allograft rejection. Transplant Proc. 1987;19:2378–2382. [PMC free article] [PubMed] [Google Scholar]