Abstract
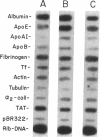
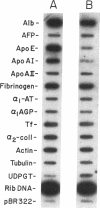
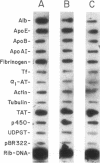
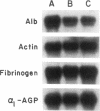
A novel feedback regulatory mechanism operating on transcription of the albumin gene is described in the rat. In 1946, it was proposed that circulating colloids, including serum albumin, may affect the synthesis and/or secretion of albumin in the liver. The molecular basis for this proposed regulation has now been investigated by adding oncotically active macromolecules to the circulation of normal or genetically albumin-deficient Nagase analbuminemic rats (NAR) and analyzing the hepatic expression of genes, including albumin after 24 h. The transcription rate of the albumin gene was higher in NAR than in normal rats and was dramatically reduced by raising serum albumin to 1.6 g/dl. Intravenous infusion of albumin into normal rats also decreased transcriptional activity of the albumin gene by 50-60%, and this decrease correlated with changes in serum colloid osmotic pressure after albumin infusion. Inhibition of albumin gene transcription was also observed upon intravenous infusion of other protein or nonprotein macromolecules, such as gamma-globulin and dextran. This down-regulation appears to control the steady-state level of albumin mRNA in the liver. Aside from a concomitant decrease in apo E gene transcription after albumin or macromolecule infusion, there was no change in the transcription rate of other genes, including those exhibiting liver-preferred or -specific expression (e.g., tyrosine amino-transferase, cytochrome P-450, alpha 1-antitrypsin, apolipoproteins A-I and B, and transferrin) or general cellular expression (e.g., alpha-tubulin, pro alpha 2 collagen, and beta-actin). Feedback regulation of albumin gene expression by serum colloids may serve as a specific homeostatic mechanism to maintain the steady-state level of total protein in the circulation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alper C. A. Plasma protein measurements as a diagnostic aid. N Engl J Med. 1974 Aug 8;291(6):287–290. doi: 10.1056/NEJM197408082910606. [DOI] [PubMed] [Google Scholar]
- BJORNEBOE M., SCHWARTZ M. Investigations concerning the changes in serum proteins during immunization; the cause of hypoalbuminemia with high gamma globulin values. J Exp Med. 1959 Aug 1;110(2):259–270. doi: 10.1084/jem.110.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUER R. W. Liver circulation and function. Physiol Rev. 1963 Jan;43:115–213. doi: 10.1152/physrev.1963.43.1.115. [DOI] [PubMed] [Google Scholar]
- Baumann H. Hepatic acute phase reaction in vivo and in vitro. In Vitro Cell Dev Biol. 1989 Feb;25(2):115–126. doi: 10.1007/BF02626167. [DOI] [PubMed] [Google Scholar]
- Baumhueter S., Mendel D. B., Conley P. B., Kuo C. J., Turk C., Graves M. K., Edwards C. A., Courtois G., Crabtree G. R. HNF-1 shares three sequence motifs with the POU domain proteins and is identical to LF-B1 and APF. Genes Dev. 1990 Mar;4(3):372–379. doi: 10.1101/gad.4.3.372. [DOI] [PubMed] [Google Scholar]
- Castell J. V., Andus T., Kunz D., Heinrich P. C. Interleukin-6. The major regulator of acute-phase protein synthesis in man and rat. Ann N Y Acad Sci. 1989;557:87–101. [PubMed] [Google Scholar]
- Chojkier M., Brenner D. A., Leffert H. L. Vasopressin inhibits type-I collagen and albumin gene expression in primary cultures of adult rat hepatocytes. J Biol Chem. 1989 Jun 5;264(16):9583–9591. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Clayton D. F., Darnell J. E., Jr Changes in liver-specific compared to common gene transcription during primary culture of mouse hepatocytes. Mol Cell Biol. 1983 Sep;3(9):1552–1561. doi: 10.1128/mcb.3.9.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dich J., Hansen S. E., Thieden H. I. Effect of albumin concentration and colloid osmotic pressure on albumin synthesis in the perfused rat liver. Acta Physiol Scand. 1973 Nov;89(3):352–358. doi: 10.1111/j.1748-1716.1973.tb05530.x. [DOI] [PubMed] [Google Scholar]
- Doumas B. T., Watson W. A., Biggs H. G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971 Jan;31(1):87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Goetz K. L. Physiology and pathophysiology of atrial peptides. Am J Physiol. 1988 Jan;254(1 Pt 1):E1–15. doi: 10.1152/ajpendo.1988.254.1.E1. [DOI] [PubMed] [Google Scholar]
- Gorski K., Carneiro M., Schibler U. Tissue-specific in vitro transcription from the mouse albumin promoter. Cell. 1986 Dec 5;47(5):767–776. doi: 10.1016/0092-8674(86)90519-2. [DOI] [PubMed] [Google Scholar]
- Heard J. M., Herbomel P., Ott M. O., Mottura-Rollier A., Weiss M., Yaniv M. Determinants of rat albumin promoter tissue specificity analyzed by an improved transient expression system. Mol Cell Biol. 1987 Jul;7(7):2425–2434. doi: 10.1128/mcb.7.7.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R. S., Boczko E. M., Darnell J. E., Jr, Babiss L. E. The mouse albumin enhancer contains a negative regulatory element that interacts with a novel DNA-binding protein. Mol Cell Biol. 1990 Aug;10(8):3896–3905. doi: 10.1128/mcb.10.8.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman A. The in vitro effect of colloid osmotic pressure on albumin biosynthesis in normal rat liver. Rev Invest Clin. 1973 Oct-Dec;25(4):321–330. [PubMed] [Google Scholar]
- Hüssinger D., Lang F., Bauers K., Gerok W. Control of hepatic nitrogen metabolism and glutathione release by cell volume regulatory mechanisms. Eur J Biochem. 1990 Nov 13;193(3):891–898. doi: 10.1111/j.1432-1033.1990.tb19414.x. [DOI] [PubMed] [Google Scholar]
- Januszewicz P., Gutkowska J., De Lean A., Thibault G., Garcia R., Genest J., Cantin M. Synthetic atrial natriuretic factor induces release (possibly receptor-mediated) of vasopressin from rat posterior pituitary. Proc Soc Exp Biol Med. 1985 Feb;178(2):321–325. doi: 10.3181/00379727-178-2-rc4. [DOI] [PubMed] [Google Scholar]
- Joles J. A., Willekes-Koolschijn N., Braam B., Kortlandt W., Koomans H. A., Dorhout Mees E. J. Colloid osmotic pressure in young analbuminemic rats. Am J Physiol. 1989 Jul;257(1 Pt 2):F23–F28. doi: 10.1152/ajprenal.1989.257.1.F23. [DOI] [PubMed] [Google Scholar]
- Katz J., Bonorris G., Okuyama S., Sellers A. L. Albumin synthesis in perfused liver of normal and nephrotic rats. Am J Physiol. 1967 Jun;212(6):1255–1260. doi: 10.1152/ajplegacy.1967.212.6.1255. [DOI] [PubMed] [Google Scholar]
- LAURENT T. C. THE INTERACTION BETWEEN POLYSACCHARIDES AND OTHER MACROMOLECULES. 5. THE SOLUBILITY OF PROTEINS IN THE PRESENCE OF DEXTRAN. Biochem J. 1963 Nov;89:253–257. doi: 10.1042/bj0890253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang T. J., Grieninger G. Direct effect of insulin on the synthesis of specific plasma proteins: biphasic response of hepatocytes cultured in serum- and hormone-free medium. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6972–6976. doi: 10.1073/pnas.78.11.6972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtsteiner S., Schibler U. A glycosylated liver-specific transcription factor stimulates transcription of the albumin gene. Cell. 1989 Jun 30;57(7):1179–1187. doi: 10.1016/0092-8674(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Liu J. K., DiPersio C. M., Zaret K. S. Extracellular signals that regulate liver transcription factors during hepatic differentiation in vitro. Mol Cell Biol. 1991 Feb;11(2):773–784. doi: 10.1128/mcb.11.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER L. L., BLY C. G., WATSON M. L., BALE W. F. The dominant role of the liver in plasma protein synthesis; a direct study of the isolated perfused rat liver with the aid of lysine-epsilon-C14. J Exp Med. 1951 Nov;94(5):431–453. doi: 10.1084/jem.94.5.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire P., Wuarin J., Schibler U. The role of cis-acting promoter elements in tissue-specific albumin gene expression. Science. 1989 Apr 21;244(4902):343–346. doi: 10.1126/science.2711183. [DOI] [PubMed] [Google Scholar]
- Matsui H., Yazawa H., Suzuki N., Hosoya T. Effects of glucocorticoid and cycloheximide on the activity and amount of RNA polymerase I in nuclei of rat liver. Biochem J. 1986 May 1;235(3):699–705. doi: 10.1042/bj2350699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagase S., Shimamune K., Shumiya S. Albumin-deficient rat mutant. Science. 1979 Aug 10;205(4406):590–591. doi: 10.1126/science.451621. [DOI] [PubMed] [Google Scholar]
- OGSTON A. G., PHELPS C. F. The partition of solutes between buffer solutions and solutions containing hyaluronic acid. Biochem J. 1961 Apr;78:827–833. doi: 10.1042/bj0780827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oratz M., Rothschild M. A., Schreiber S. S., Burks A., Mongelli J., Matarese B. The role of the urea cycle and polyamines in albumin synthesis. Hepatology. 1983 Jul-Aug;3(4):567–571. doi: 10.1002/hep.1840030415. [DOI] [PubMed] [Google Scholar]
- PETERS T., Jr, ANFINSEN C. B. Net production of serum albumin by liver slices. J Biol Chem. 1950 Oct;186(2):805–813. [PubMed] [Google Scholar]
- Panduro A., Shalaby F., Shafritz D. A. Changing patterns of transcriptional and post-transcriptional control of liver-specific gene expression during rat development. Genes Dev. 1987 Dec;1(10):1172–1182. doi: 10.1101/gad.1.10.1172. [DOI] [PubMed] [Google Scholar]
- Panduro A., Shalaby F., Weiner F. R., Biempica L., Zern M. A., Shafritz D. A. Transcriptional switch from albumin to alpha-fetoprotein and changes in transcription of other genes during carbon tetrachloride induced liver regeneration. Biochemistry. 1986 Mar 25;25(6):1414–1420. doi: 10.1021/bi00354a034. [DOI] [PubMed] [Google Scholar]
- Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987 May;1(3):268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- ROE J. H. The determination of dextran in blood and urine with anthrone reagent. J Biol Chem. 1954 Jun;208(2):889–896. [PubMed] [Google Scholar]
- ROTHSCHILD M. A., ORATZ M., WIMER E., SCHREIBER S. S. Studies on albumin synthesis: the effects of dextran and cortisone on albumin metabolism in rabbits studied with albumin-I-131. J Clin Invest. 1961 Mar;40:545–554. doi: 10.1172/JCI104282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Evans C. D., Schreiber S. S. Role hepatic interstitial albumin in regulating albumin synthesis. Am J Physiol. 1966 Jan;210(1):57–68. doi: 10.1152/ajplegacy.1966.210.1.57. [DOI] [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Mongelli J., Schreiber S. S. Albumin metabolism in rabbits during gamma globulin infusions. J Lab Clin Med. 1965 Nov;66(5):733–740. [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Mongelli J., Schreiber S. S. Effect of albumin concentration on albumin synthesis in the perfused liver. Am J Physiol. 1969 May;216(5):1127–1130. doi: 10.1152/ajplegacy.1969.216.5.1127. [DOI] [PubMed] [Google Scholar]
- Rothschild M. A., Oratz M., Schreiber S. S. Serum albumin. Hepatology. 1988 Mar-Apr;8(2):385–401. doi: 10.1002/hep.1840080234. [DOI] [PubMed] [Google Scholar]
- Schreiber G., Tsykin A., Aldred A. R., Thomas T., Fung W. P., Dickson P. W., Cole T., Birch H., De Jong F. A., Milland J. The acute phase response in the rodent. Ann N Y Acad Sci. 1989;557:61–86. doi: 10.1111/j.1749-6632.1989.tb24000.x. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Yap S. H., Strair R. K. Regulation of albumin synthesis in rat liver. Mol Biol Rep. 1979 May 31;5(1-2):71–78. doi: 10.1007/BF00777491. [DOI] [PubMed] [Google Scholar]
- Tracht M. E., Tallal L., Tracht D. G. Intrinsic hepatic control of psma albumin concentration. Life Sci. 1967 Dec 15;6(24):2621–2628. doi: 10.1016/0024-3205(67)90112-9. [DOI] [PubMed] [Google Scholar]
- Trowell O. A. The experimental production of watery vacuolation of the liver. J Physiol. 1946 Dec 6;105(3):268–297. [PMC free article] [PubMed] [Google Scholar]
- WALDENSTROM J. Abnormal proteins in myeloma. Adv Intern Med. 1952;5:398–440. [PubMed] [Google Scholar]
- Weisberg H. F. Osmotic pressure of the serum proteins. Ann Clin Lab Sci. 1978 Mar-Apr;8(2):155–164. [PubMed] [Google Scholar]