It is an established fact that class II molecules play a crucial role in the initial recognition of foreign antigens.1,2 Exogenous antigen peptide and these class II molecule α-, β-chain complexes are recognized by the T-cell receptor. On the other hand, in cytoplasm, the invariant chain, which is a nonpolymorphic peptide, binds the class II molecules and prevents the binding of endogenous peptides.3 In addition, it has been reported that an invariant chain is expressed on the cell surface in the human4 and mouse,5 but is not reported in the rat. The distribution of the invariant chain on the cell surface is very similar to that of class II molecules, but the invariant chain was observed without class II molecules in certain cells. The differential expression of class II and invariant chain with interferon-γ treatment in the mouse was reported.6 Therefore, we developed a unique monoclonal antibody (MAb), L21-6, recognizing an invariant chain on the cell surface in the rats and studied the tissue and strain distributions.
MATERIALS AND METHODS
Animals
All inbred strains of rats used in this study. LEW (RT1l), BN (RT1n), AC1 (RT1avl), F344 (RT1lvl), WWFF(RT1u), BUF (RT1b), and DA (RT1avl), were purchased from Harlan Sprague-Dawley Inc (Indianapolis, Ind) as were the BALB/c mice.
MAb
BALB/c mice were immunized four times intraperitoneally (IP) with 1 × 107 LEW spleen cells at 10-day intervals. Four days after the last immunization, approximately 1 × 108 mouse spleen cells were fused with 4 × 107 P-3 mouse myeloma cells by PEG-4000, in accordance with the method as described previously.7
The supernatants were screened by indirect immunofluorescence for antibody activity against LEW and BN spleen cells.
The positive hybrids were cloned by limiting dilution. In further studies, we used one of these hybridomas, designated as L21-6.
Detection of Cell Surface Antigen by FACScan
The cells were stained with L21-6 by indirect immunofluorescence. Analysis was performed after the cell viability (more than 95%) was tested by trypan blue dye exclusion.
Immunohistochemical Staining
Cryostat sections of brain, lung, heart, thymus, liver, spleen, pancreas, kidney, skin, small intestine, lymph node, and aorta from LEW rats were obtained. These sections were stained by the ABC method.7 L21-6 or MRC-OX6 was used as first antibody.
Inhibition of Mixed Lymphocyte Reaction
Lymphocytes from mesenteric lymph nodes were used. Responder cells (1 × 105/well) were cocultured with 2 × 105/well 20-Gy-irradiated stimulator cells in the presence of L21-6 in round-bottomed 96-well plates for 5 days. The cells were pulsed with 3H-thymidine, and harvested 18 hours later. Results were obtained from triplicate cultures.
Molecular Determination by SDS-PAGE
The procedures for immunoprecipitation and surface iodination used in this study have been described elsewhere.8 The LEW spleen cells were labeled with 125I by use of lactoperoxidase. SDS-PAGE was performed in 12.5% polyacrylamide gel under the reducing condition.
RESULTS
Reactivity of L21-6 to rat strains (LEW, ACI, DA, F344, WF, and BUF) was studied using spleen cells and positive staining patterns were obtained in all instances. The SDS-PAGE analysis provided a band at 32 kd (Fig 1). MAb L21-6 was capable of 95.2% inhibition of MLR of LEW against BN. The expression of MAb L21-6-defined antigen in normal LEW rat tissues was examined. Staining patterns are summarized in Table 1.
Fig 1.
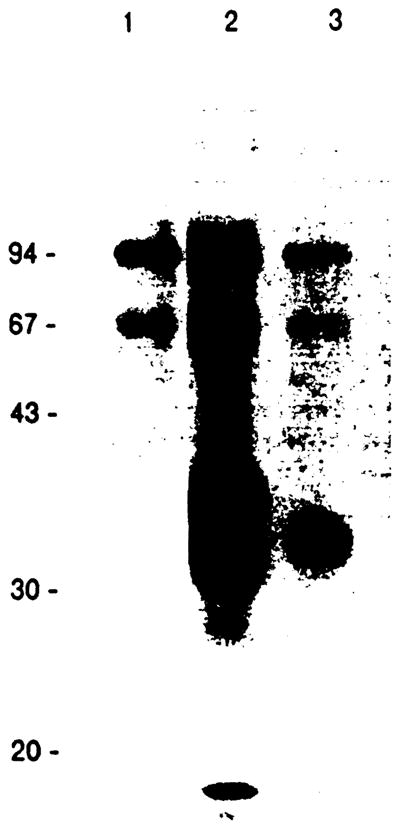
SDS-PAGE analysis of immunoprecipitates made with control (lane 1), MRC OX-6 (lane 2) or L21-6 MAb (lane 3) and LEW spleen cell lysate. Immune complexes were applied to 12.5% polyacrylamide gel under the reducing condition.
Table 1.
Tissue Distribution of Class II Antigen Recognized by L21-6 in Normal LEW Rat Tissues
| Tissue | Reaction With L21-6 |
|---|---|
| Central nervous system | |
| Brain | − |
| Gastrointestinal system | |
| Ileum* | + |
| Liver | |
| Hepatocyte | − |
| Biliary epithelium | − |
| Sinusoidal lining cell | − |
| Artery, vein | − |
| Interstitial dendritic cell | + |
| Respiratory and cardiovascular system | |
| Lung† | |
| Bronchial epithelium | − |
| Alveolar epithelium | − |
| Heart | |
| Endocardium | − |
| Epicardium | − |
| Myocardium | − |
| Aorta | − |
| Interstitial dendritic cell | + |
| Urogenital system | |
| Kidney | |
| Glomeruli | − |
| Tubules | − |
| Interstitial dendritic cell | + |
| Miscellaneous | |
| Pancreas | |
| Exocrine portion | − |
| Endocrine portion | − |
| Skin | |
| Langerhans cell | + |
| Interstitial dendritic cell | + |
| Lymphoid tissue | |
| Lymph node | + |
| Thymus‡ | |
| Medulla | + |
| Cortex | + |
| Spleen | |
| White pulp | + |
| Red pulp | + |
| Bone marrow§ | + |
| Peripheral blood lymphocyte| | + |
(−) = no staining; (+) = positive staining.
Lamina propria was stained diffusely. Peyer’s patch showed the same staining pattern as lymph nodes.
Alveolar macrophages were stained positively.
Cortex of thymus was weakly staining.
Approximately, 10% of bone marrow cells were positive using indirect immunofluorescence.
Approximately, 20% of PBLs were positive using indirect immunofluorescence.
DISCUSSION
Our study demonstrated: (1) the MAb L21-6 recognized the determinant on the cell surface component of invariant chain because the band at 32 kd, which was different from an α- and β-chain of class II molecules, was detected by SDS-PAGE using cell-surface iodinated material; and (2) tissue staining patterns of L21-6 were quite similar to those stained by antiinvariant chain antibodies reported earlier,6,9 but L21-6-defined determinant on the invariant chain was not expressed in BN rats. It was reported that the invariant chain, which was located between the α- and β-chain in the slab gel, was coprecipitated with rat class II molecules recognized by HOK 7 (anti-class II) MAb using soluble glycoprotein fraction from WKAH rat spleen cells.10 However, anti-rat invariant chain MAb was not reported. To our knowledge this is the first report of an anti-rat invariant chain MAb. It is unclear whether or not the invariant chain is expressed on the cell surface. Wraight et al noted that the possible explanation for the varying conclusion of the previous study on invariant chain cell surface expression was the previous absence of antibodies directed against C-terminal portions of the invariant chain, because the invariant chain is inserted into the membrane with its N-terminal on the cytoplasmic side.4 L21-6 is probably specific for the determinant on the C-terminal side (extra-cytoplasmic domain) of the invariant chain. Our result of SDS-PAGE also suggested that the cell surface invariant chain may be not associated with the class II α- and β-chain as is the case in the cytoplasm. L21-6 was capable of 95.2% inhibition of MLR of LEW against BN suggesting the possibility that the invariant chain interacts with antigen presentation as an adhesion molecule. In addition, why the BN rat does not express the invariant chain must be studied. Two anti–class II antibodies were reported to express similar specificity on strain distribution. The alloantibody, RT1B.9, which was raised by skin grafting of BN.ILV1 (F344) onto BN, reacted with all haplotypes except RT1n.10 The antibody HIS 19, which was produced by immunization with lymphocytes isolated from AO (RT1u) Peyer’s patch, reacted with all haplotypes except RT1n.11
The polymorphism of the class II antigen might influence the expression of L21-6-defined antigen. Thus, L21-6 is useful to study the function of the invariant chain on the cell surface.
References
- 1.Schwartz RH. Annu Rev Immunol. 1985;3:237. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- 2.Townsend A, Bodmer H. Annu Rev Immunol. 1989;7:601. doi: 10.1146/annurev.iy.07.040189.003125. [DOI] [PubMed] [Google Scholar]
- 3.Roche PA, Cresswell P. Nature. 1990;345:615. doi: 10.1038/345615a0. [DOI] [PubMed] [Google Scholar]
- 4.Wraight CJ, Endert PV, Moller P, et al. J Biol Chem. 1990;265:5787. [PubMed] [Google Scholar]
- 5.Koch N, Koch S, Hammerling GJ. Nature. 1982;299:664. doi: 10.1038/299644a0. [DOI] [PubMed] [Google Scholar]
- 6.Momburg F, Koch N, Moller P, et al. J Immunol. 1986;136:940. [PubMed] [Google Scholar]
- 7.Yagihashi A, Sato N, Torigoe T, et al. Cancer Res. 1988;48:2798. [PubMed] [Google Scholar]
- 8.Epstein AL, Marder RJ, Winter JN, et al. J Immunol. 1978;133:1028. [PubMed] [Google Scholar]
- 9.Ikeda H, Matsuno Y, Tsuchimoto S, et al. Transplant Proc. 1985;17:18202. [Google Scholar]
- 10.Wettstein PJ. Immunogenetics. 1981;14:541. doi: 10.1007/BF00350126. [DOI] [PubMed] [Google Scholar]
- 11.Stet RJM, Rozing J, Majoor GD, et al. Transplant Proc. 1985;17:1829. [Google Scholar]


