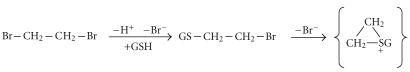Abstract
Glutathione transferase enzymes (GSTs) catalyze reactions in which electrophiles are conjugated to the tripeptide thiol glutathione. While many GST-catalyzed transformations result in the detoxication of xenobiotics, a few substrates, such as dihaloalkanes, undergo bioactivation to reactive intermediates. Many molecular epidemiological studies have tested associations between polymorphisms (especially, deletions) of human GST genes and disease susceptibility or response to therapy. This review presents a discussion of the biochemistry of GSTs, the sources—both genetic and environmental—of interindividual variation in GST activities, and their implications for pharmaco- and toxicogenetics; particular attention is paid to the Theta class GSTs.
1. Introduction: Pharmacogenomics and Personalized Medicine: A Perspective
The Golden Helix Symposium “Pharmacogenomics: paving the path to personalized medicine,” held in Athens in October 2009, brought together scientists and physicians who share the hope and expectation that molecular analysis of human genes affecting pharmacodynamics and pharmacokinetics will soon lead to significant medical advances. Several kinds of improvements can be anticipated. For example, starting drug doses may be tailored to an individual's metabolism, thereby increasing therapeutic efficacy and reducing side effects; individuals for whom a particular drug should be avoided altogether, to avert toxicity or “idiosyncratic” reactions, might be identified by prior genetic screening; and mechanistic insights into the development of particular diseases, drug side effects, or toxicities resulting from environmental exposures might be garnered by analysis of associations with specific genes [1, 2].
Our pursuit of this research agenda should be diligent but also balanced. Despite optimistic predictions, well-publicized in the popular press [3], clinical implementation of genetically guided drug therapy has been slow. Both fundamental and practical obstacles must be overcome before the clinical potential of pharmacogenomics is realized [4–6]. The goal of getting patients “the right drug in the right dose” must be kept in perspective; for many people, the urgent priority is to obtain any access at all to medical care and to authentic prescription drugs [7]. This article presents a review of the human glutathione transferases (GSTs) and their genes, in the context of pharmacogenetics and pharmacogenomics.
Many genetic polymorphisms affecting enzymes of xenobiotic metabolism strongly influence the pharmacokinetics of clinically-important drugs (e.g., warfarin and P450 2C9 [8], 6-mercaptopurine and thiopurine methyltransferase [9], irinotecan and UDP-glucuronosyltransferase 1A1 [10]). To date, there are no such clear cases with respect to GSTs. (The immunosuppressive drug azathioprine may prove to be one instance [11, 12].) This paucity of examples is certainly not due to a lack of genetic polymorphisms: GST polymorphisms are common and some of them have clear phenotypic consequences, as discussed below. Why, then, do GST polymorphisms apparently have less impact on pharmacokinetics? Several factors may be involved. First, GSTs catalyze detoxication of electrophilic compounds by conjugation to glutathione. Candidate drugs which give rise to substantial amounts of electrophilic reactive species at clinically effective doses are likely to be too toxic for use—the exception being cancer chemotherapeutic drugs [13–15], where electrophilic reactivity can be the mechanism of therapeutic action. Second, as discussed below, humans express a large number of different GSTs with overlapping substrate specificities, and the effects of polymorphisms (including gene deletions) affecting one GST may be masked by the activity of others. Third, in some cases where inactivation of a toxic drug metabolite by glutathione is critical for prevention of toxicity, such as the quinoneimine metabolite of acetaminophen, the nonenzymatic reaction may be fast enough that variations in enzyme activity are of little significance [16]. Fourth, genetic polymorphisms probably account for only a small proportion of the large interindividual variation in GST expression and activity [17–19]. Factors such as diet [20, 21], environmental chemical exposures [22], age [23], and gender [24], which remain only poorly understood, may be more important determinants. Nevertheless, our understanding of human GST polymorphisms is still limited, and clinical consequences may simply have gone unnoticed to date.
2. Glutathione Transferase Enzymes
2.1. Overview
Glutathione transferases (GSTs; systematically designated as “RX: glutathione R-transferases”, E.C. 2.5.1.18) are enzymes belonging to two protein superfamilies, the soluble GSTs and the “MAPEG” (Membrane Associated Proteins in Eicosanoid and Glutathione metabolism) proteins [5]. Soluble GSTs are dimers of 25 kDa subunits. Consequently, the homodimeric protein product of the GSTA1 gene, for example, is referred to as GST A1-1. Crystal structures have been determined for many soluble GSTs, often with bound substrates or products. The “canonical fold” of a soluble GST subunit reveals an N-terminal α/β domain forming the GSH-binding site (“G-site”) and a second, α-helical domain forming most of the “H-site” that binds the electrophilic substrate.
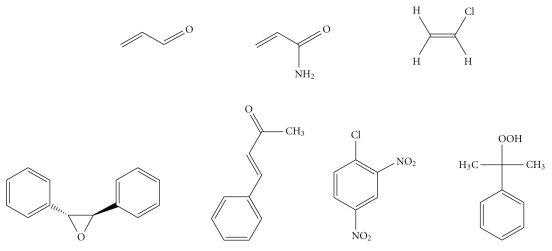
Isaiah Berlin classified philosophers as either “foxes” or “hedgehogs”, based on the classical Greek aphorism “The fox knows many things, but the hedgehog knows one great thing” [25]. Enzymes are commonly regarded as “hedgehogs”: each enzyme only knows how to catalyze one reaction. However, the enzymes of xenobiotic metabolism, such as P450 enzymes and GSTs, are undoubtedly “foxes” and able to catalyze the biotransformation of numerous substrates bearing diverse functional groups. As our understanding of these enzymes has increased, so has the range of known substrates and chemistries. Furthermore, the distinction that was once drawn between enzymes catalyzing xenobiotic metabolism and enzymes catalyzing metabolism of endogenous substrates has largely disappeared. Major chemical classes of GST substrates, such as quinones, epoxides, and hydroperoxides, encompass both exogenous and endogenous compounds. For example, acrolein (see Figure 1), a toxic α,β-unsaturated aldehyde that occurs in cooked foods and tobacco smoke [26–28] and is a metabolite of the cancer chemotherapeutic drug cyclophosphamide [29], is also formed endogenously, by the myeloperoxidase-catalyzed oxidation of threonine [30]; GSTs catalyze the detoxication of hydroperoxides (glutathione peroxidase activity), including both endogenous (e.g., hydrogen peroxide, lipid hydroperoxides) and exogenous (e.g., cumene hydroperoxide) compounds [31, 32].
Figure 1.
Selected electrophilic substrates of GST enzymes. Top row, from left: acrolein; acrylamide; vinyl chloride (bioactivated to the reactive intermediates 2-chloroethylene oxide and chloroacetaldehyde); bottom row, from left: trans-stilbene oxide (TSO); 1-chloro-2,4-dinitrobenzene (CDNB); cumene hydroperoxide.
Overwhelmingly, the metabolic role of GSTs is to detoxify reactive electrophiles by catalyzing their reactions with glutathione, thereby reducing the likelihood of deleterious interactions between such reactive species and essential cellular components, especially proteins and nucleic acids. Many cancer chemotherapeutic agents are electrophilic compounds (or their metabolic precursors) and GST-catalyzed reactions are important pathways for the inactivation and elimination of many such drugs (e.g., 1, 3-bis(2-chloroethyl)-1-nitrosourea [33], cyclophosphamide, melphalan [34], etc.). Drugs from other therapeutic classes may be metabolized to electrophiles that are substrates for GST-catalyzed glutathione conjugation, for example, the phenylpropenal metabolite of the epilepsy drug felbamate [35]. Many other widely used drugs, including acetaminophen, clozapine, and furosemide, are metabolized to glutathione conjugates [36], although the reaction with glutathione is not necessarily dependent on GST catalysis in every case. Glutathione adducts are usually exported from the cell by the action of transporters such as the multidrug resistance protein MRP1 [37] and then processed into mercapturic acids (N-acetylcysteine conjugates) which are excreted in the urine [38] or bile [39]. Despite the important role of glutathione in detoxication, GST-catalyzed conjugations can also, in certain instances, lead to the generation of reactive intermediates. Dihaloalkanes are a notable case [40] and will be mentioned again, later in this article.
2.2. Human GSTs
Human GST enzymes include members of eight classes, assigned on the basis of sequence similarity: Alpha, Mu, Pi, Theta, Kappa, Zeta, Omega, and Sigma. Mammalian GSTs of the Alpha, Mu, and Pi classes bind with high affinity to matrices such as S-hexylglutathione-sepharose or S-hexylglutathione-agarose [41, 42], but GST enzymes from other classes, such as Theta, bind poorly or not at all [43]. (GST-GSH binding affinity is exploited in commercially available systems for expression of recombinant proteins as GST fusions, such as the pGEX vectors.) Because of their relatively high levels of expression and ease of purification, the Alpha, Mu, and Pi class GSTs have been studied more frequently than other classes of human GST enzymes.
The specificities of GST enzymes for the electrophilic substrate overlap considerably. For example, 1-chloro-2, 4-dinitrobenzene (CDNB), commonly used for spectrophotometric GST activity assays, is a substrate for most human GSTs (but not GST T1-1 [44] or GST T2-2 [45]). On the other hand, some substrates are relatively specific for particular GST enzymes, as discussed later.
Further discussion of the biochemistry of GSTs is presented in the monographs “Gluthione Transferases and Gamma-Glutamyl Transpeptidases” [46] and “Toxicology of Glutathione Transferases” [47], and in review articles [48–51].
3. GST Genes
3.1. Overview
Both animal and plant genomes encode large numbers of GST enzymes (and often, multiple pseudogenes with strong sequence similarity to GST genes), for example, 48 GST genes in the nematode Caenorhabditis elegans [52], 26 in the mosquito Aedes aegypti [53], and more than 70 in the Black Cottonwood poplar Populus trichocarpa [54]. The human genome encodes at least 18 expressed GST enzymes, in the eight sequence-similarity classes listed in Section 2.2 [5, 51, 55, 56]. Among these, the Alpha, Mu, Pi, and Theta classes have received most attention with respect to drug metabolism in humans. Some ambiguity persists in the enumeration of human GSTs. For example, the Alpha-class hGSTA5 gene product has been omitted from tabulations of human GSTs, because its expression has never been detected in human cells. However, the coding sequence is intact and an enzymatically active recombinant protein can be expressed; so it is likely that the enzyme is indeed expressed in humans, albeit under conditions that have not yet been discovered [57].
3.2. Human GST Gene Organization: Copy Number Variations
Human GST classes Alpha, Mu, Theta, and Omega all have multiple members, encoded by clusters of paralogous genes on a given chromosome (Figure 2). Deletion polymorphisms of the genes encoding human GST M1-1 and GST T1-1 are common in the human population [58]. A comprehensive review of the significance of these polymorphisms in pharmacology and toxicology was published in 2006 [59]. These deletions presumably arose by homologous recombination events. In both cases, although at least one of the neighbouring GST genes remains intact, individuals homozygous for the null allele show a clear phenotype with respect to glutathione conjugation of specific substrates. Homozygous deletion of the GSTM1 gene eliminates GST activity with respect to conjugation of the characteristic GST M1-1 substrate trans-stilbene oxide (TSO), as measured in lymphocyte homogenates [60–62]; indeed, the phenotypic polymorphism was discovered (with the substrate trans-4-phenyl-3-buten-2-one) before the gene was cloned [63, 64]. Similarly, homozygous deletion of the GSTT1 gene eliminates GST activity with respect to conjugation of the characteristic GST T1-1 substrates methyl bromide (CH3Br) and dichloromethane (CH2Cl2), as measured in erythrocyte homogenates [65, 66]. These biochemical phenotypes have toxicological correlates. For example, genotoxicity of TSO in cultured human lymphocytes (as measured by induction of sister chromatid exchanges) is significantly elevated in GSTM1-null individuals [67], and, as discussed later, clinical toxicity of methyl bromide may be strongly determined by GSTT1 genotype.
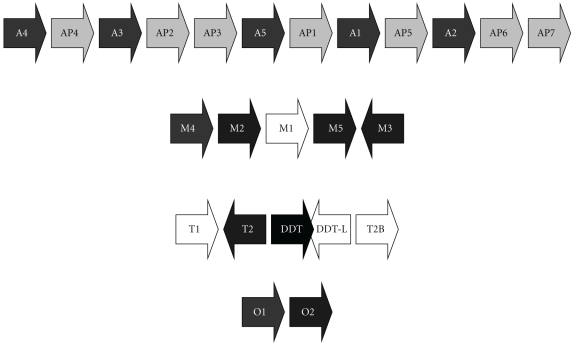
Figure 2.
Organization of selected human GST gene clusters. “P” indicates a pseudogene (e.g., AP3). The GST classes shown are Alpha (chromosome 6p12) [68, 69], Mu (chromosome 1p13) [45, 46], Theta (chromosome 22q11.2) [70, 71], and Omega (chromosome 10q24.3) [72, 73]. (A reverse transcribed pseudogene of the Omega class, found on chromosome 3 [72], is not shown.) GST genes are shown as white text on black background and pseudogenes are shown as black text on a grey background. The direction of each gene is indicated by the arrow. The genes GSTM1, GSTT2B, and GSTT1, each of which is commonly deleted, are shaded white. Genes DDT and DDT-L, in the Theta cluster, encode the enzyme D-dopachrome tautomerase. DDT-L is commonly deleted along with GSTT2B. The figure is not drawn to scale.
Deletion polymorphisms are a specific case of copy-number variation, and the recombination mechanisms that cause deletions can also cause duplications. The extent and significance of gene copy number variation in the human genome has only recently started to become clear, lagging behind the cataloguing of several million human single nucleotide polymorphisms (SNPs) [74]. Human P450 2D6, for instance, provides an important example of copy-number variation—both deletions and duplications—affecting the disposition of drugs, such as the tricyclic antidepressants and tamoxifen [75–77]. The GSTM1 gene is duplicated in some individuals, conferring a +/++ genotype and “ultrarapid” metabolism of TSO [78, 79].
3.3. Molecular Epidemiology of GST Deletion Polymorphisms
The relationships between GST polymorphisms and disease risk have been examined in a vast number of molecular epidemiological studies, beginning around 1990. While these studies are not pharmacogenetic per se, they may be relevant to the more general question of whether human GST polymorphisms influence responses to xenobiotic compounds, since environmental exposures contribute to the risk of specific cancers and other prevalent diseases. A PubMed search using the search string “GST cancer risk” retrieved 599 references (Jan. 2010), and many noncancer diseases have also been studied, for example, Parkinson's disease, Alzheimer's disease, asthma, coronary artery disease, and rheumatoid arthritis (reviewed in [59]). The HuGE Navigator “searchable knowledge base of genetic associations and human genome epidemiology” (http://www.hugenavigator.net/) retrieved 1,027 publications in response to a search for gene symbol GSTT1. Almost all of the molecular epidemiological studies of GSTs have tested the effects of some or all of three genotypes: GSTM1-null, GSTT1-null, and the GSTP1 single-nucleotide polymorphism that results in the coding sequence change Ile105Val [80, 81].
Several circumstances account for the fact that GST polymorphisms have been subject to so many epidemiological analyses. First, the polymorphisms are easily tested. For example, routine PCR analysis can classify individuals as GSTT1 homozygous null versus GSTT1-present and GSTM1 homozygous null versus GSTM1-present; see, for example, [82]. (The limitations of such analysis are discussed later.) Second, homozygous null individuals are common, contributing statistical power to molecular epidemiological analyses. The prevalence of GSTM1 homozygous null individuals in “Caucasian” and Asian populations is about 50%, with a substantially lower frequency among Africans and African Americans. For the GSTT1 homozygous null genotype, the corresponding figures are about 20% for “Caucasian” and African-American populations, but about 50% for Asians [59]. Third, as mentioned earlier, the polymorphisms give rise to detectable phenotypes, in terms of metabolism of some specific substrates. Finally, the acknowledged importance of GSTs in the disposition of toxic compounds and in defense against oxidative stress [83] provides a prima facie justification for testing associations with the risk of cancers and degenerative diseases.
Bolt and Thier have provided an extensive review of the GST molecular epidemiology literature up to about 2005 [59]. Since that time, several new meta-analyses have been published under the auspices of the “HuGE” Human Genome Epidemiology Network, formed in 1998, which facilitates the preparation of “systematic, structured, peer-reviewed synopses of epidemiologic aspects of human genes in relation to specific diseases” [84]. A summary of these meta-analyses is given in Table 1. Overall, it can be seen that GSTT1 or GSTM1 null genotype confers at most a small (less than 50%) increased risk of certain cancers, while many other results are negative (no statistically significant increased risk).
Table 1.
HuGE reviews of GST polymorphisms and disease risk.
| Disease | Alleles | Result | Ref. | Year |
|---|---|---|---|---|
| Bladder ca. | M | null ↑ 1.42 (1.26–1.60) | [85] | 2002 |
| Ovarian ca. | M, P, T | negative | [86] | 2002 |
| Lung ca. | T | Caucasians: negative Asians: null ↑ 1.28 (1.10–1.48) | [87] | 2006 |
| Lung ca. | M | null ↑ 1.22 (1.14–1.30); negative when analysis was limited to the five largest studies | [88] | 2008 |
| Liver ca. | M, T | T null possibly ↑ 1.19 (0.99–1.44); M null ↑ 1.16 (0.89–1.53) | [89] | 2008 |
| Lung ca. | P | (V/V + V/I) versus (I/I) ↑ 1.11 (1.03–1.21) | [90] | 2009 |
| Prostate ca. | M, P, T | M: 1.33 (1.15–1.55); P and T negative | [91] | 2009 |
| Colorectal ca. | P | negative | [92] | 2009 |
| Colorectal ca. | T | null ↑ 1.23 (1.02–1.49) | [93] | 2010 |
| Asthma | M, P, T | negative | [94] | 2010 |
M: GSTM1 null; T: GSTT1 null; P: GSTP1 I105V SNP; ↑: increased risk for individuals with the specified genotype; Results: numbers represent Odds Ratios, with 95% confidence intervals in parentheses; ca.: cancer.
The great majority of the molecular epidemiological investigations published to date suffer from a serious limitation: genotypes were assessed by “yes-no” PCR methods that do not measure gene copy number and therefore cannot distinguish between heterozygous (+/0) and homozygous (+/+ or even ++/+) non-null genotypes. As noted by Minelli et al. [94], “classifying the genotype [only] as “present” or “null” implies a recessive model (one or two copies versus absence of the risk allele), which may not reflect the true underlying genetic model and thus may not provide a valid and accurate estimate of the genetic risk. GSTT1 or GSTM1 copy number variations are correlated with altered enzyme activity, and analysis in a dose-dependent manner would best describe any disease outcome association. ” Analytical methods that assess copy number, such as real-time PCR [95–97], are now available; older and less informative genotyping methods should be abandoned [98].
Several other biases and weaknesses are often found in molecular epidemiological investigations and tend to reduce confidence in published results. (i) Post hoc analysis: data can be recategorized so as to increase statistical significance, by constructing a new hypothesis after the data have already been acquired. For example, in a study of the GSTM1 null polymorphism and ovarian cancer risk [99], very high statistical significance was reported for elevated risk of GSTM1 null genotype with respect to the incidence of combined clear cell and endometrioid pathological subtypes of ovarian cancer, but this combination of subtypes was constructed post hoc, and the biological rationale explaining why these subtypes in particular should be affected by GSTM1 status is not compelling. (ii) Publication bias: studies which find significant associations are more likely to be published than studies which find no association. The recent HuGE meta-analysis of asthma studies [94] noted “clear absence of small studies with negative results, suggesting the presence of publication bias.” (iii) Small sample size: published studies of the GSTT1 and GSTM1 polymorphisms include samples as small as 34 patients with chronic obstructive pulmonary disease [100], 51 liver transplant recipients [101], and 43 schizophrenia patients [102], and such small samples are unlikely to yield reliable data. (iv) Lack of clear biological rationale: is it reasonable to expect that the presence of a non-null GSTT1 gene is associated with, for example, significantly better response to the surgical correction of an enlarged scrotal vein [103]? In contrast, an epidemiological association is more credible when a plausible connection exists between a presumed causative agent and the disease outcome. A strong association between GSTM1 null genotype and hepatocellular carcinoma has been reported in studies in Guangxi, China [104, 105]. The very high incidence of liver cancer in this region is attributed, at least in part, to prevalent aflatoxin contamination of grain, and GST M1-1 catalyzes the detoxication of aflatoxin epoxide [106].
4. Human GST Theta Genes and Enzymes
4.1. Characteristics of Theta Class GSTs
Theta class GSTs are distinct from the Alpha, Mu, and Pi (A-M-P) class enzymes in many respects, including sequence, catalytic activity, and structure [107]. GST Theta genes are evolutionarily very distant from the A-M-P genes and probably diverged from the ancestral A-M-P gene long before the divergence of plants and animals; Theta class GSTs are found in plants, but A-M-P GSTs are absent [108]. The human genome encodes two Theta class GSTs, GST T1-1 and GST T2-2 [70], and possibly a third form, GST T2B-2B (see below). Three Theta class GSTs are encoded on the mouse genome [109].
As noted earlier, Theta class enzymes are distinct from the A-M-P enzymes in failing to bind tightly to glutathione affinity matrices and having little or no activity with the standard GST substrate CDNB [110]. Theta class GSTs have a distinctive (although not unique [111]) activity: catalysis of the conjugation of halo- and dihaloalkanes with glutathione [40], a reaction which can result in the formation of reactive intermediates related to the “sulfur mustards” (S-haloalkanes) [112]. Therefore, Theta class GSTs can catalyze bioactivation [113] as well as detoxication processes, as discussed further below. An intriguing case report [114] suggests that GST T1-1-dependent activation of haloalkanes can be clinically important. Two workers were exposed to a very large inhaled dose of methyl bromide when they entered a sealed mill building being fumigated with the gas, and failed to wear self-contained breathing apparatus. Neurotoxic effects were very severe in one worker but mild in the other. Laboratory investigation showed that the severely affected individual had GST (presumably GST T1-1) activity towards methyl bromide, while the mildly affected individual (presumably a GSTT1 homozygous null) did not. This result, while representing little more than an anecdotal report, is consistent with a determinative role for GST T1-1 in methyl bromide toxicity.
The active sites of Theta class GSTs are also distinctive. In A-M-P class GSTs, a conserved tyrosine residue near the N-terminus forms a hydrogen bond to the thiol sulfur atom of glutathione in the G-site; in Theta class GSTs, a serine residue occupies the corresponding position [48, 115–117]. The G-site of human Theta class GSTs is deeply buried and covered by the C-terminal “tail”, approximately 40 amino acid residues that form a helix-loop-helix extension [116]; this tail also makes the H-site of Theta class enzymes relatively inaccessible [118]. The small size of the H-site is consistent with the selectivity of Theta class GSTs for small xenobiotic substrates, such as dichloromethane [119]. This preference for small xenobiotic substrates is reminiscent of the behaviour of cytochrome P450 2E1, an enzyme which is also notable for an unusually small active site [120], and which catalyzes the oxidation of small substrates such as dimethylnitrosamine [121] and ethanol [122]. Indeed, there may be cases where toxicants are activated by P450 2E1 to reactive species that are subsequently detoxified by GST T1-1-catalyzed glutathione conjugation. Possible examples include vinyl chloride (a plastics monomer and industrial carcinogen) [123, 124], acrylamide [125], and benzene [126, 127].
4.2. Chromosomal Organization of the GSTT Genes
The structure of the human GST Theta region [70, 71] is remarkable (Figure 3). The GST1 and GST2 are oriented head-to-head. GSTT2B was originally referred to as a probable pseudogene, GSTT2P [70], but is now annotated as “glutathione S-transferase theta 2B (gene/pseudogene)” on the NCBI genome database. (Pseudogenes identified as GSTTP1 and GSTTP2 are also annotated on the database but have not been studied in detail.) The GSTT2, DDT, DDT-L, and GSTT2B genes are found within a 61 kb inverted repeat sequence which was recently discovered to be the site of a prevalent (allele frequency approximately 50%) deletion polymorphism that spans the entire GSTT2B gene [71]. Additional characteristics of this previously unknown deletion polymorphism may have important implications for pharmacogenetic studies of the GST Theta genes. Surprisingly, deletion of the GSTT2B gene appears to result in greatly reduced expression of the GSTT2 gene, by an as-yet unknown mechanism; the GSTT2B deletion shows linkage disequilibrium with the much-studied GSTT1 deletion polymorphism, with a very low frequency of alleles carrying deletions of both GSTT1 and GSTT2B [71]. Furthermore, the extent of linkage disequilibrium was very different among three population samples examined: very strong in a northern/western European ancestry sample, strong in a Japanese-Chinese sample, but absent in a Nigerian Yoruba sample [71]. Further investigation of the molecular genetics of human GSTs is very much needed.
Figure 3.
Organization of the human GST Theta gene region. The figure is drawn approximately to scale, based on NCBI Reference Sequence: NC_000022.10, Homo sapiens chromosome 22, GRCh37 primary reference assembly.
4.3. D-Dopachrome Tautomerase
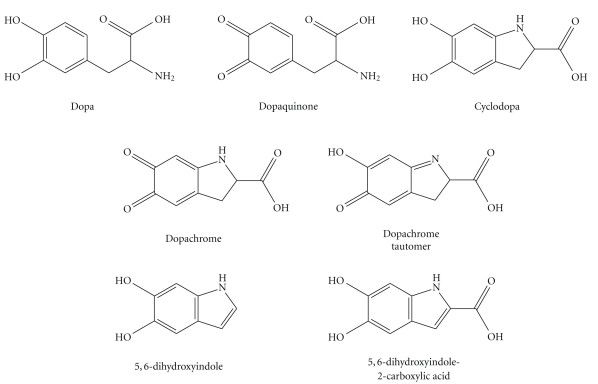
Another intriguing feature of the GST Theta gene region is the presence within the 61 kb inverted repeat of the overlapping, head-to-head DDT (D-dopachrome tautomerase) and DDT-L (D-dopachrome tautomerase-like) coding sequences. As Coggan et al. observed, the proximity of the DDT and GSTT2 genes is “if nothing else, an interesting coincidence.” Does the proximity of these genes have any functional significance? First, we should consider the biochemistry of the DDT gene product, D-dopachrome tautomerase. L-Dopachrome is a tyrosine metabolite required for biosynthesis of the skin pigment melanin [128]. Hydroxylation of tyrosine forms dopa (dihydroxyphenylalanine), which is oxidized to the quinone, dopaquinone (Figure 4); both oxidations are catalyzed by tyrosinase [129]. Dopaquinone can cyclize to give cyclodopa, which undergoes further oxidation to yield dopachrome [130]. In the pathway to eumelanin, L-dopachrome tautomerase (also known as L-dopachrome isomerase) catalyses the isomerization of dopachrome, via a tautomeric form (dopachrome tautomer), to 5,6-dihydroxyindole-2-carboxylic acid [131]. (Alternatively, dopaquinone can react with cysteine to form cysteinyldopa, leading to synthesis of phaeomelanin [130].) All of these metabolites are derived from the natural “L” enantiomer of the amino acid tyrosine. In the course of studies on L-dopachrome tautomerase, researchers used the nonnatural “D” enantiomer of dopachrome as a control; to their surprise, they found that D-dopachrome also underwent tautomerization. Liver (rather than melanin-producing cells) was found to have high D-dopachrome tautomerase activity [132]. D-Dopachrome tautomerase (also known as D-dopachrome decarboxylase) catalyzes the decarboxylation of D-dopachrome to give 5,6-dihydroxyindole [133]. The cytokine macrophage migration inhibitory factor (MIF) [134], another member of the tautomerase superfamily [135], catalyzes the conversion of D-dopachrome into 5,6-dihydroxyindole-2-carboxylic acid [136]. Since D-dopachrome is not found in cells, these enzymes presumably have different endogenous substrates, possibly including phenylpyruvate [137]. The physiological role of D-dopachrome tautomerase remains enigmatic.
Figure 4.
Dopamine and some of its oxidized metabolites relevant to the biosynthesis of melanin.
There are at least some hints of a functional relationship between D-dopachrome tautomerase and GSTs. (i) Dopachrome and related quinones are substrates for GST-catalyzed glutathione conjugation [138, 139]. (ii) The DDT and GSTT2 genes may be coordinately regulated; a recent genomewide analysis identified both genes among a small set of genes that are expressed differentially in a comparison between strains of spontaneously hypertensive rats and the control (Wistar-Kyoto) strain [140]. (iii) A recent proteomic analysis identified D-dopachrome tautomerase as being strongly (more than tenfold) induced in rat liver following exposure to the hepatotoxicant, carbon tetrachloride (CCl4) [141], and GST enzymes may protect against CCl4-induced hepatotoxicity [142]. Further research is needed to clarify the biological role of D-dopachrome tautomerase and its possible interactions with the glutathione/GST system.
4.4. Bioactivation of Mutagens by Theta Class GSTs
As mentioned earlier, GST-catalyzed conjugation of dihaloalkanes gives rise to toxic reactive intermediates. Ethylene dibromide (EDB), a compound that has been used as an antiknock additive in gasoline and as an insecticide, can cause fatal liver, kidney, and cardiac toxicity [143]. EDB is probably the best-characterized example of GST-catalyzed bioactivation [144, 145]. EDB-glutathione conjugation catalyzed by GST T1-1 leads to generation of an electrophilic sulphonium ion that can form covalent adducts with macromolecular targets [146] (Figure 5). It would be very informative to test whether GSTT1 genotype affects the outcome of EDB poisonings in exposed individuals (such as accidental poisonings or suicide attempts).
Figure 5.
Activation of ethylene dibromide to an electrophile by glutathione conjugation.
Mammalian enzymes catalyzing the metabolic activation of xenobiotics can be expressed in bacterial strains for the detection of mutagens [147], as has been demonstrated for aromatic amine N-acetyltransferases [148], P450 enzymes [149], and sulfotransferases [150]. Thier and colleagues demonstrated that rat GST T1-1 (previously known as form 5-5) [151] and human GST T1-1 [152] expressed in Salmonella typhimurium strains catalyze the bioactivation of dihaloalkanes to mutagens that can readily be detected by the Ames test (reversion mutation assay).
4.5. Coding-Sequence SNPs Affecting GST Theta Proteins
With the exception of the GSTP1 SNP mentioned earlier, there have been relatively few studies of coding-sequence SNPs affecting GST proteins. However, the Environmental Genome Project [153] and several recent publications [154–161] have uncovered many new variants in the Mu, Pi, Theta, and Omega GST classes. Characterization of the functional consequences of these variants is required for a thorough understanding of the pharmacogenetics of GSTs [162] and can also provide insight into the structure-activity relationships of the enzyme proteins. The effects of coding-sequence SNPs affecting the human Theta class GSTs are under investigation [163, 164]. A GST expression system based on Escherichia coli strains bearing a lacZ reversion target has been constructed and is being applied in studies of the functional consequences of nonsynonymous SNPs in human GSTT1 [164]. Reported nonsynonymous SNPs in this gene (Entrez SNP database at NCBI; Environmental Genome Project database at www.genome.utah.edu/genesnps/; [160]) encode the protein variants A21T, L30P, D43N, F45C, T65M, R76S, T104P, D141N, V169I, and E173K. In our first study, we expressed the D141N and E173K variants. The D141N variant behaved similarly to the wild-type enzyme, in terms of expression level and specific activities towards a variety of xenobiotic substrates. However, the variant displayed a very much reduced activity for the activation of EDB to a mutagen. Variant E173K, in contrast, was poorly expressed and inactive with most substrates, and the protein appears to be improperly folded, as judged by its altered thermal denaturation profile. Extension of these studies to additional GSTT1 SNPs is in progress.
5. Closing Remarks
A large number of epidemiological studies have tested possible associations between GST polymorphisms, such as the GSTM1 and GSTT1 deletions, with disease risk or therapy outcome. Some meta-analyses have indicated statistically significant but small increases in risk for specific genotypes, while many studies have been negative. However, the genetic analysis used in most of these studies has been limited, especially by the failure to determine between heterozygous and homozygous genotypes (gene dose). GST activity is highly variable among individuals, but genetic factors may account for only a fraction of this variability. Although clear cases of clinically relevant pharmacogenetic consequences and toxicogenetic GST polymorphisms remain very few, greater understanding of the numerous factors affecting GST expression and activity, accompanied by more incisive genetic analysis, may reveal further connections between GST genotypes and individual responses to drugs and toxic compounds.
Acknowledgments
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada. The author wishes to thank Professor Cortland Griswold, University of Guelph, for helpful discussions, and Professor Bengt Mannervik, Uppsala University, for providing valuable comments on the manuscript draft.
Abbreviations
- A-M-P:
Alpha, Mu, and Pi classes of GSTs
- CDNB:
1-chloro-2,4-dinitrobenzene
- GST:
glutathione transferase
- TSO:
trans-stilbene oxide.
References
- 1.Roden DM, Altman RB, Benowitz NL, et al. Pharmacogenomics: challenges and opportunities. Annals of Internal Medicine. 2006;145(10):749–757. doi: 10.7326/0003-4819-145-10-200611210-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desai AA, Innocenti F, Ratain MJ. Pharmacogenomics: road to anticancer therapeutics nirvana? Oncogene. 2003;22(43):6621–6628. doi: 10.1038/sj.onc.1206958. [DOI] [PubMed] [Google Scholar]
- 3.Pollack A. A special drug just for you, at the end of a long pipeline. New York Times. November 2005 [Google Scholar]
- 4.Weinshilboum R, Wang L. Pharmacogenomics: bench to bedside. Nature Reviews Drug Discovery. 2004;3(9):739–748. doi: 10.1038/nrd1497. [DOI] [PubMed] [Google Scholar]
- 5.Josephy PD, Mannervik B. Molecular Toxicology. 2nd edition. New York, NY, USA: Oxford University Press; 2006. [Google Scholar]
- 6.Limdi NA, Veenstra DL. Expectations, validity, and reality in pharmacogenetics. doi: 10.1016/j.jclinepi.2009.09.006. Journal of Clinical Epidemiology. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gautam CS, Utreja A, Singal GL. Spurious and counterfeit drugs: a growing industry in the developing world. Postgraduate Medical Journal. 2009;85(1003):251–256. doi: 10.1136/pgmj.2008.073213. [DOI] [PubMed] [Google Scholar]
- 8.Au N, Rettie AE. Pharmacogenomics of 4-hydroxycoumarin anticoagulants. Drug Metabolism Reviews. 2008;40(2):355–375. doi: 10.1080/03602530801952187. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Weinshilboum R. Thiopurine S-methyltransferase pharmacogenetics: insights, challenges and future directions. Oncogene. 2006;25(11):1629–1638. doi: 10.1038/sj.onc.1209372. [DOI] [PubMed] [Google Scholar]
- 10.Marsh S. Impact of pharmacogenomics on clinical practice in oncology. Molecular Diagnosis and Therapy. 2007;11(2):79–82. doi: 10.1007/BF03256226. [DOI] [PubMed] [Google Scholar]
- 11.Eklund BI, Moberg M, Bergquist J, Mannervik B. Divergent activities of human glutathione transferases in the bioactivation of azathioprine. Molecular Pharmacology. 2006;70(2):747–754. doi: 10.1124/mol.106.025288. [DOI] [PubMed] [Google Scholar]
- 12.Stocco G, Martelossi S, Barabino A, et al. Glutathione-S-transferase genotypes and the adverse effects of azathioprine in young patients with inflammatory bowel disease. Inflammatory Bowel Diseases. 2007;13(1):57–64. doi: 10.1002/ibd.20004. [DOI] [PubMed] [Google Scholar]
- 13.Innocenti F, Ratain MJ. Update on pharmacogenetics in cancer chemotherapy. European Journal of Cancer. 2002;38(5):639–644. doi: 10.1016/s0959-8049(01)00434-8. [DOI] [PubMed] [Google Scholar]
- 14.Townsend DM, Tew KD. Cancer drugs, genetic variation and the glutathione-S-transferase gene family. American Journal of Pharmacogenomics. 2003;3(3):157–172. doi: 10.2165/00129785-200303030-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo H-W, Ali-Osman F. Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Current Opinion in Pharmacology. 2007;7(4):367–374. doi: 10.1016/j.coph.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Coles B, Wilson I, Wardman P, Hinson JA, Nelson SD, Ketterer B. The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Archives of Biochemistry and Biophysics. 1988;264(1):253–260. doi: 10.1016/0003-9861(88)90592-9. [DOI] [PubMed] [Google Scholar]
- 17.Takamatsu Y, Inaba T. Inter-individual variability of human hepatic glutathione S-transferase isozymes assessed by inhibitory capacity. Toxicology. 1994;88(1–3):191–200. doi: 10.1016/0300-483x(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 18.Slone DH, Gallagher EP, Ramsdell HS, et al. Human variability in hepatic glutathione S-transferase-mediated conjugation of aflatoxin B1-epoxide and other substrates. Pharmacogenetics. 1995;5(4):224–233. doi: 10.1097/00008571-199508000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Coles BF, Kadlubar FF. Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs? BioFactors. 2003;17(1–4):115–130. doi: 10.1002/biof.5520170112. [DOI] [PubMed] [Google Scholar]
- 20.Turesky RJ, Richoz J, Constable A, Curtis KD, Dingley KH, Turteltaub KW. The effects of coffee on enzymes involved in metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in rats. Chemico-Biological Interactions. 2003;145(3):251–265. doi: 10.1016/s0009-2797(03)00022-x. [DOI] [PubMed] [Google Scholar]
- 21.Pool-Zobel B, Veeriah S, Bohmer F-D. Modulation of xenobiotic metabolising enzymes by anticarcinogens—focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutation Research. 2005;591(1-2):74–92. doi: 10.1016/j.mrfmmm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Kondraganti SR, Muthiah K, Jiang W, Barrios R, Moorthy B. Effects of 3-methylcholanthrene on gene expression profiling in the rat using cDNA microarray analyses. Chemical Research in Toxicology. 2005;18(11):1634–1641. doi: 10.1021/tx050085n. [DOI] [PubMed] [Google Scholar]
- 23.Leakey JEA, Cunny HC, Bazare J, Jr., et al. Effects of aging and caloric restriction on hepatic drug metabolizing enzymes in the Fischer 344 rat. II: effects on conjugating enzymes. Mechanisms of Ageing and Development. 1989;48(2):157–166. doi: 10.1016/0047-6374(89)90047-x. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell AE, Burns SA, Rudolf JL. Isozyme- and gender-specific induction of glutathione S-transferases by flavonoids. Archives of Toxicology. 2007;81(11):777–784. doi: 10.1007/s00204-007-0210-9. [DOI] [PubMed] [Google Scholar]
- 25.Berlin I. The Hedgehog and the Fox: An Essay on Tolstoy’s View of History. London, UK: Weidenfeld & Nicolson; 1953. [Google Scholar]
- 26.Berhane K, Widersten M, Engstrom A, Kozarich JW, Mannervik B. Detoxication of base propenals and other α,β-unsaturated aldehyde products of radical reactions and lipid peroxidation by human glutathione transferases. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(4):1480–1484. doi: 10.1073/pnas.91.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pal A, Hu X, Zimniak P, Singh SV. Catalytic efficiencies of allelic variants of human glutathione S-transferase Pi in the glutathione conjugation of α,β-unsaturated aldehydes. Cancer Letters. 2000;154(1):39–43. doi: 10.1016/s0304-3835(00)00390-6. [DOI] [PubMed] [Google Scholar]
- 28.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Molecular Nutrition and Food Research. 2008;52(1):7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takamoto S, Sakura N, Namera A, Yashiki M. Monitoring of urinary acrolein concentration in patients receiving cyclophosphamide and ifosphamide. Journal of Chromatography B. 2004;806(1):59–63. doi: 10.1016/j.jchromb.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Anderson MM, Hazen SL, Hsu FF, Heinecke JW. Human neutrophils employ the myeloperoxidase-hydrogen peroxide-chloride system to convert hydroxy-amino acids into glycolaldehyde, 2-hydroxypropanal, and acrolein: a mechanism for the generation of highly reactive α-hydroxy and α,β-unsaturated aldehydes by phagocytes at sites of inflammation. Journal of Clinical Investigation. 1997;99(3):424–432. doi: 10.1172/JCI119176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ketterer B, Meyer DJ. Glutathione transferases: a possible role in the detoxication and repair of DNA and lipid hydroperoxides. Mutation Research. 1989;214(1):33–40. doi: 10.1016/0027-5107(89)90195-4. [DOI] [PubMed] [Google Scholar]
- 32.Blair IA. Endogenous glutathione adducts. Current Drug Metabolism. 2006;7(8):853–872. doi: 10.2174/138920006779010601. [DOI] [PubMed] [Google Scholar]
- 33.Lien S, Larsson A-K, Mannervik B. The polymorphic human glutathione transferase T1-1, the most efficient glutathione transferase in the denitrosation and inactivation of the anticancer drug 1,3-bis(2-chloroethyl)-1-nitrosourea. Biochemical Pharmacology. 2002;63(2):191–197. doi: 10.1016/s0006-2952(01)00846-2. [DOI] [PubMed] [Google Scholar]
- 34.Hall AG, Matheson E, Hickson ID, Foster SA, Hogarth L. Purification of an alpha class glutathione S-transferase from melphalan-resistant Chinese hamster ovary cells and demonstration of its ability to catalyze melphalan-glutathione adduct formation. Cancer Research. 1994;54(13):3369–3372. [PubMed] [Google Scholar]
- 35.Dieckhaus CM, Roller SG, Santos WL, Sofia RD, Macdonald TL. Role of glutathione S-transferases A1-1, M1-1, and P1-1 in the detoxification of 2-phenylpropenal, a reactive felbamate metabolite. Chemical Research in Toxicology. 2001;14(5):511–516. doi: 10.1021/tx000141e. [DOI] [PubMed] [Google Scholar]
- 36.Takakusa H, Masumoto H, Makino C, Okazaki O, Sudo K. Quantitative assessment of reactive metabolite formation using 35S-labeled glutathione. Drug Metabolism and Pharmacokinetics. 2009;24(1):100–107. doi: 10.2133/dmpk.24.100. [DOI] [PubMed] [Google Scholar]
- 37.Cole SPC, Deeley RG. Transport of glutathione and glutathione conjugates by MRP1. Trends in Pharmacological Sciences. 2006;27(8):438–446. doi: 10.1016/j.tips.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 38.Egner PA, Kensler TW, Chen J-G, Gange SJ, Groopman JD, Friesen MD. Quantification of sulforaphane mercapturic acid pathway conjugates in human urine by high-performance liquid chromatography and isotope-dilution tandem mass spectrometry. Chemical Research in Toxicology. 2008;21(10):1991–1996. doi: 10.1021/tx800210k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teichert J, Sohr R, Hennig L, et al. Identification and quantitation of the N-acetyl-L-cysteine S-conjugates of bendamustine and its sulfoxides in human bile after administration of bendamustine hydrochloride. Drug Metabolism and Disposition. 2009;37(2):292–301. doi: 10.1124/dmd.108.022855. [DOI] [PubMed] [Google Scholar]
- 40.Guengerich FP. Activation of dihaloalkanes by thiol-dependent mechanisms. Journal of Biochemistry and Molecular Biology. 2003;36(1):20–27. doi: 10.5483/bmbrep.2003.36.1.020. [DOI] [PubMed] [Google Scholar]
- 41.Guthenberg C, Warholm M, Rane A, Mannervik B. Two distinct forms of glutathione transferase from human foetal liver. Purification and comparison with isoenzymes isolated from adult liver and placenta. Biochemical Journal. 1986;235(3):741–745. doi: 10.1042/bj2350741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mitchell AE, Morin D, Lame MW, Jones AD. Purification, mass spectrometric characterization, and covalent modification of murine glutathione S-transferases. Chemical Research in Toxicology. 1995;8(8):1054–1062. doi: 10.1021/tx00050a009. [DOI] [PubMed] [Google Scholar]
- 43.Jemth P, Stenberg G, Chaga G, Mannervik B. Heterologous expression, purification and characterization of rat class theta glutathione transferase T2-2. Biochemical Journal. 1996;316(1):131–136. doi: 10.1042/bj3160131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jemth P, Mannervik B. Kinetic characterization of recombinant human glutathione transferase T1-1, a polymorphic detoxication enzyme. Archives of Biochemistry and Biophysics. 1997;348(2):247–254. doi: 10.1006/abbi.1997.0357. [DOI] [PubMed] [Google Scholar]
- 45.Tan K-L, Chelvanayagam G, Parker MW, Board PG. Mutagenesis of the active site of the human Theta-class glutathione transferase GSTT2-2: catalysis with different substrates involves different residues. Biochemical Journal. 1996;319(1):315–321. doi: 10.1042/bj3190315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sies H, Packer L, editors. Gluthione Transferases and Gamma-Glutamyl Transpeptidases. New York, NY, USA: Academic Press; 2005. [Google Scholar]
- 47.Awasthi YC, editor. Toxicology of Glutathione Transferases. Boca Raton, Fla, USA: CRC Press; 2006. [Google Scholar]
- 48.Armstrong RN. Structure, catalytic mechanism, and evolution of the glutathione transferases. Chemical Research in Toxicology. 1997;10(1):2–18. doi: 10.1021/tx960072x. [DOI] [PubMed] [Google Scholar]
- 49.Dourado DFAR, Fernandes PA, Ramos MJ. Mammalian cytosolic glutathione transferases. Current Protein and Peptide Science. 2008;9(4):325–337. doi: 10.2174/138920308785132677. [DOI] [PubMed] [Google Scholar]
- 50.Johansson A-S, Mannervik B. Interindividual variability of glutathione transferase expression. In: Pacifici GM, Pelkonen O, editors. Interindividual Variability in Human Drug Metabolism. London, UK: Taylor and Francis; 2001. pp. 460–519. [Google Scholar]
- 51.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annual Review of Pharmacology and Toxicology. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 52.Lindblom TH, Dodd AK. Xenobiotic detoxification in the nematode Caenorhabditis elegans. Journal of Experimental Zoology. Part A. 2006;305(9):720–730. doi: 10.1002/jez.a.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lumjuan N, Stevenson BJ, Prapanthadara L-a, et al. The Aedes aegypti glutathione transferase family. Insect Biochemistry and Molecular Biology. 2007;37(10):1026–1035. doi: 10.1016/j.ibmb.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 54.Lan T, Yang Z-L, Yang X, Liu Y-J, Wang X-R, Zenga Q-Y. Extensive functional diversification of the populus glutathione S-transferase supergene family. Plant Cell. 2009;21(12):3749–3766. doi: 10.1105/tpc.109.070219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nebert DW, Vasiliou V. Analysis of the glutathione S-transferase (GST) gene family. Human Genomics. 2004;1(6):460–464. doi: 10.1186/1479-7364-1-6-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mannervik B, Board PG, Hayes JD, Listowsky I, Pearson WR. Nomenclature for mammalian soluble glutathione transferases. Methods in Enzymology. 2005;401:1–8. doi: 10.1016/S0076-6879(05)01001-3. [DOI] [PubMed] [Google Scholar]
- 57.Singh SP, Zimniak L, Zimniak P. The human hGSTA5 gene encodes an enzymatically active protein. Biochimica et Biophysica Acta. 2010;1800(1):16–22. doi: 10.1016/j.bbagen.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Board PG. Biochemical genetics of glutathione-S-transferase in man. American Journal of Human Genetics. 1981;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- 59.Bolt HM, Thier R. Relevance of the deletion polymorphisms of the glutathione S-transferases GSTT1 and GSTM1 in pharmacology and toxicology. Current Drug Metabolism. 2006;7(6):613–628. doi: 10.2174/138920006778017786. [DOI] [PubMed] [Google Scholar]
- 60.Seidegard J, Vorachek WR, Pero RW, Pearson WR. Hereditary differences in the expression of the human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(19):7293–7297. doi: 10.1073/pnas.85.19.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brockmoller J, Gross D, Kerb R, Drakoulis N, Roots I. Correlation between trans-stilbene oxide-glutathione conjugation activity and the deletion mutation in the glutathione S-transferase class Mu gene detected by polymerase chain reaction. Biochemical Pharmacology. 1992;43(3):647–650. doi: 10.1016/0006-2952(92)90591-6. [DOI] [PubMed] [Google Scholar]
- 62.Brockmoller J, Kerb R, Drakoulis N, Nitz M, Roots I. Genotype and phenotype of glutathione S-transferase class mu isoenzymes mu and psi in lung cancer patients and controls. Cancer Research. 1993;53(5):1004–1011. [PubMed] [Google Scholar]
- 63.Warholm M, Guthenberg C, Mannervik B, von Bahr C, Glaumann H. Identification of a new glutathione S-transferase in human liver. Acta Chemica Scandinavica. Series B. 1980;34(8):607–621. doi: 10.3891/acta.chem.scand.34b-0607. [DOI] [PubMed] [Google Scholar]
- 64.Warholm M, Guthenberg C, Mannervik B, von Bahr C. Purification of a new glutathione S-transferase (transferase mu) from human liver having high activity with benzo[a]pyrene-4,5-oxide. Biochemical and Biophysical Research Communications. 1981;98(2):512–519. doi: 10.1016/0006-291x(81)90870-6. [DOI] [PubMed] [Google Scholar]
- 65.Peter H, Deutschmann S, Reichel C, Hallier E. Metabolism of methyl chloride by human erythrocytes. Archives of Toxicology. 1989;63(5):351–355. doi: 10.1007/BF00303122. [DOI] [PubMed] [Google Scholar]
- 66.Pemble S, Schroeder KR, Spencer SR, et al. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochemical Journal. 1994;300(1):271–276. doi: 10.1042/bj3000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bernardini S, Hirvonen A, Jarventaus H, Norppa H. Trans-stilbene oxide-induced sister chromatid exchange in cultured human lymphocytes: influence of GSTM1 and GSTT1 genotypes. Mutagenesis. 2001;16(3):277–281. doi: 10.1093/mutage/16.3.277. [DOI] [PubMed] [Google Scholar]
- 68.Morel F, Rauch C, Coles B, Le Ferrec E, Guillouzo A. The human glutathione transferase alpha locus: genomic organization of the gene cluster and functional characterization of the genetic polymorphism in the hGSTA1 promoter. Pharmacogenetics. 2002;12(4):277–286. doi: 10.1097/00008571-200206000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Coles BF, Kadlubar FF. Human alpha class glutathione S-transferases: genetic polymorphism, expression, and susceptibility to disease. Methods in Enzymology. 2005;401:9–42. doi: 10.1016/S0076-6879(05)01002-5. [DOI] [PubMed] [Google Scholar]
- 70.Coggan M, Whitbread L, Whittington A, Board P. Structure and organization of the human theta-class glutathione S-transferase and D-dopachrome tautomerase gene complex. Biochemical Journal. 1998;334(3):617–623. doi: 10.1042/bj3340617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhao Y, Marotta M, Eichler EE, Eng C, Tanaka H. Linkage disequilibrium between two high-frequency deletion polymorphisms: implications for association studies involving the glutathione-S transferase (GST) genes. PLoS Genetics. 2009;5(5, article e1000472) doi: 10.1371/journal.pgen.1000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Whitbread AK, Tetlow N, Eyre HJ, Sutherland GR, Board PG. Characterization of the human Omega class glutathione transferase genes and associated polymorphisms. Pharmacogenetics. 2003;13(3):131–144. doi: 10.1097/00008571-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 73.Whitbread AK, Masoumi A, Tetlow N, Schmuck E, Coggan M, Board PG. Characterization of the omega class of glutathione transferases. Methods in Enzymology. 2005;401:78–99. doi: 10.1016/S0076-6879(05)01005-0. [DOI] [PubMed] [Google Scholar]
- 74.Wain LV, Armour JA, Tobin MD. Genomic copy number variation, human health, and disease. The Lancet. 2009;374(9686):340–350. doi: 10.1016/S0140-6736(09)60249-X. [DOI] [PubMed] [Google Scholar]
- 75.Bernard S, Neville KA, Nguyen AT, Flockhart DA. Interethnic differences in genetic polymorphisms of CYP2D6 in the U.S. population: clinical implications. Oncologist. 2006;11(2):126–135. doi: 10.1634/theoncologist.11-2-126. [DOI] [PubMed] [Google Scholar]
- 76.Zhou S-F. Polymorphism of human cytochrome P450 2D6 and its clinical significance—part II. Clinical Pharmacokinetics. 2009;48(12):761–804. doi: 10.2165/11318070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 77.Zhou SF. Polymorphism of human cytochrome P450 2D6 and its clinical significance—part I. Clinical Pharmacokinetics. 2009;48(11):689–723. doi: 10.2165/11318030-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 78.McLellan RA, Oscarson M, Alexandrie A-K, et al. Characterization of a human glutathione S-transferase mu cluster containing a duplicated GSTM1 gene that causes ultrarapid enzyme activity. Molecular Pharmacology. 1997;52(6):958–965. doi: 10.1124/mol.52.6.958. [DOI] [PubMed] [Google Scholar]
- 79.Huang RS, Chen P, Wisel S, et al. Population-specific GSTM1 copy number variation. Human Molecular Genetics. 2009;18(2):366–372. doi: 10.1093/hmg/ddn345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zimniak P, Nanduri B, Pikula S, et al. Naturally occurring human glutathione S-transferase GSTP1-1 isoforms with isoleucine and valine in position 104 differ in enzymic properties. European Journal of Biochemistry. 1994;224(3):893–899. doi: 10.1111/j.1432-1033.1994.00893.x. [DOI] [PubMed] [Google Scholar]
- 81.Johansson A-S, Stenberg G, Widersten M, Mannervik B. Structure-activity relationships and thermal stability of human glutathione transferase P1-1 governed by the H-site residue 105. Journal of Molecular Biology. 1998;278(3):687–698. doi: 10.1006/jmbi.1998.1708. [DOI] [PubMed] [Google Scholar]
- 82.Abdel-Rahman SZ, El-Zein RA, Anwar WA, Au WW. A multiplex PCR procedure for polymorphic analysis of GSTM1 and GSTT1 genes in population studies. Cancer Letters. 1996;107(2):229–233. doi: 10.1016/0304-3835(96)04832-x. [DOI] [PubMed] [Google Scholar]
- 83.Hayes JD, Strange RC. Potential contribution of the glutathione S-transferase supergene family to resistance to oxidative stress. Free Radical Research. 1995;22(3):193–207. doi: 10.3109/10715769509147539. [DOI] [PubMed] [Google Scholar]
- 84.Khoury MJ, Little J. Human genome epidemiologic reviews: the beginning of something HuGE. American Journal of Epidemiology. 2000;151(1):2–3. doi: 10.1093/oxfordjournals.aje.a010117. [DOI] [PubMed] [Google Scholar]
- 85.Engel LS, Taioli E, Pfeiffer R, et al. Pooled analysis and meta-analysis of glutathione S-transferase M1 and bladder cancer: a HuGE review. American Journal of Epidemiology. 2002;156(2):95–109. doi: 10.1093/aje/kwf018. [DOI] [PubMed] [Google Scholar]
- 86.Coughlin SS, Hall IJ. Glutathione S-transferase polymorphisms and risk of ovarian cancer: a HuGE review. Genetics in Medicine. 2002;4(4):250–257. doi: 10.1097/00125817-200207000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Raimondi S, Paracchini V, Autrup H, et al. Meta- and pooled analysis of GSTT1 and lung cancer: a HuGE-GSEC review. American Journal of Epidemiology. 2006;164(11):1027–1042. doi: 10.1093/aje/kwj321. [DOI] [PubMed] [Google Scholar]
- 88.Carlsten C, Sagoo GS, Frodsham AJ, Burke W, Higgins JPT. Glutathione S-transferase M1 (GSTM1) polymorphisms and lung cancer: a literature-based systematic HuGE review and meta-analysis. American Journal of Epidemiology. 2008;167(7):759–774. doi: 10.1093/aje/kwm383. [DOI] [PubMed] [Google Scholar]
- 89.White DL, Li D, Nurgalieva Z, El-Serag HB. Genetic variants of glutathione S-transferase as possible risk factors for hepatocellular carcinoma: a HuGE systematic review and meta-analysis. American Journal of Epidemiology. 2008;167(4):377–389. doi: 10.1093/aje/kwm315. [DOI] [PubMed] [Google Scholar]
- 90.Cote ML, Chen W, Smith DW, et al. Meta- and pooled analysis of GSTP1 polymorphism and lung cancer: a HuGE-GSEC review. American Journal of Epidemiology. 2009;169(7):802–814. doi: 10.1093/aje/kwn417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mo Z, Gao Y, Cao Y, Gao F, Jian L. An updating meta-analysis of the GSTM1, GSTT1, and GSTP1 polymorphisms and prostate cancer: a HuGE review. Prostate. 2009;69(6):662–688. doi: 10.1002/pros.20907. [DOI] [PubMed] [Google Scholar]
- 92.Gao Y, Pan X, Su T, Mo Z, Cao Y, Gao F. Glutathione S-transferase P1 Ile105Val polymorphism and colorectal cancer risk: a meta-analysis and HuGE review. European Journal of Cancer. 2009;45(18):3303–3314. doi: 10.1016/j.ejca.2009.06.029. [DOI] [PubMed] [Google Scholar]
- 93.Liao C, Cao Y, Wu L, Huang J, Gao F. An updating meta-analysis of the glutathione S-transferase T1 polymorphisms and colorectal cancer risk: a HuGE review. International Journal of Colorectal Disease. 2010;25(1):25–37. doi: 10.1007/s00384-009-0805-0. [DOI] [PubMed] [Google Scholar]
- 94.Minelli C, Granell R, Newson R, Rose-Zerilli MJ, Torrent M, Ring SM. Glutathione-S-transferase genes and asthma phenotypes. A Human Genome Epidemiology (HuGE) systematic review and meta-analysis including unpublished data. International Journal of Epidemiology. 2010;39(2):539–562. doi: 10.1093/ije/dyp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roodi N, Dupont WD, Moore JH, Parl FF. Association of homozygous wild-type glutathione S-transferase M1 genotype with increased breast cancer risk. Cancer Research. 2004;64(4):1233–1236. doi: 10.1158/0008-5472.can-03-2861. [DOI] [PubMed] [Google Scholar]
- 96.Girault I, Lidereau R, Bieche I. Trimodal GSTT1 and GSTM1 genotyping assay by real-time PCR. International Journal of Biological Markers. 2005;20(2):81–86. [PubMed] [Google Scholar]
- 97.Timofeeva M, Jäger B, Rosenberger A, et al. A multiplex real-time PCR method for detection of GSTM1 and GSTT1 copy numbers. Clinical Biochemistry. 2009;42(6):500–509. doi: 10.1016/j.clinbiochem.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 98.Parl F. A need for true GSTM1 and GSTT1 genotyping. Cancer Epidemiology Biomarkers and Prevention. 2009;18(10):p. 2793. doi: 10.1158/1055-9965.EPI-09-0556. [DOI] [PubMed] [Google Scholar]
- 99.Baxter SW, Thomas EJ, Campbell IG. GSTM1 null polymorphism and susceptibility to endometriosis and ovarian cancer. Carcinogenesis. 2001;22(1):63–65. doi: 10.1093/carcin/22.1.63. [DOI] [PubMed] [Google Scholar]
- 100.Faramawy MM, Mohammed TO, Hossaini AM, Kashem RA, Abu Rahma RM. Genetic polymorphism of GSTT1 and GSTM1 and susceptibility to chronic obstructive pulmonary disease (COPD) Journal of Critical Care. 2009;24(3):e7–e10. doi: 10.1016/j.jcrc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Azarpira N, Nikeghbalian S, Geramizadeh B, Darai M. Influence of glutathione S-transferase M1 and T1 polymorphisms with acute rejection in Iranian liver transplant recipients. Molecular Biology Reports. 2010;37(1):21–25. doi: 10.1007/s11033-009-9487-5. [DOI] [PubMed] [Google Scholar]
- 102.Bahaoddini A, Farrashbandi H, Saadat M. Genetic polymorphism of glutathione S-transferase T1 (GSTT1) and QT-interval in schizophrenia patients. Journal of Molecular Neuroscience. 2009;38(2):173–177. doi: 10.1007/s12031-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 103.Ichioka K, Nagahama K, Okubo K, Soda T, Ogawa O, Nishiyama H. Genetic polymorphisms in glutathione S-transferase T1 affect the surgical outcome of varicocelectomies in infertile patients. Asian Journal of Andrology. 2009;11(3):333–341. doi: 10.1038/aja.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deng Z-L, Wei Y-P, Ma Y. Polymorphism of glutathione S-transferase mu 1 and theta 1 genes and hepatocellular carcinoma in southern Guangxi, China. World Journal of Gastroenterology. 2005;11(2):272–274. doi: 10.3748/wjg.v11.i2.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Long XD, Ma Y, Wei YP, Deng ZL. The polymorphisms of GSTM1, GSTT1, HYL1*2, and XRCC1, and aflatoxin B1-related hepatocellular carcinoma in Guangxi population, China. Hepatology Research. 2006;36(1):48–55. doi: 10.1016/j.hepres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 106.Johnson WW, Ueng Y-F, Widersten M, et al. Conjugation of highly reactive aflatoxin B1 exo-8,9-epoxide catalyzed by rat and human glutathione transferases: estimation of kinetic parameters. Biochemistry. 1997;36(11):3056–3060. doi: 10.1021/bi962537o. [DOI] [PubMed] [Google Scholar]
- 107.Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochemical Journal. 2001;360(1):1–16. doi: 10.1042/0264-6021:3600001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dixon DP, Hawkins T, Hussey PJ, Edwards R. Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. Journal of Experimental Botany. 2009;60(4):1207–1218. doi: 10.1093/jxb/ern365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Coggan M, Flanagan JU, Parker MW, Vichai V, Pearson WR, Board PG. Identification and characterization of GSTT3, a third murine Theta class glutathione transferase. Biochemical Journal. 2002;366(1):323–332. doi: 10.1042/BJ20011878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meyer DJ, Coles B, Pemble SE, Gilmore KS, Fraser GM, Ketterer B. Theta, a new class of glutathione transferases purified from rat and man. Biochemical Journal. 1991;274(2):409–414. doi: 10.1042/bj2740409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kurtovic S, Shokeer A, Mannervik B. Diverging catalytic capacities and selectivity profiles with haloalkane substrates of chimeric alpha class glutathione transferases. Protein Engineering, Design and Selection. 2008;21(5):329–341. doi: 10.1093/protein/gzn010. [DOI] [PubMed] [Google Scholar]
- 112.Dacre JC, Goldman M. Toxicology and pharmacology of the chemical warfare agent sulfur mustard. Pharmacological Reviews. 1996;48(2):289–326. [PubMed] [Google Scholar]
- 113.Sherratt PJ, Manson MM, Thomson AM, Hissink EA, Neal GE, van Bladeren PJ. Increased bioactivation of dihaloalkanes in rat liver due to induction of class theta glutathione S-transferase T1-1. Biochemical Journal. 1998;335:619–630. doi: 10.1042/bj3350619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garnier R, Rambourg-Schepens M-O, Muller A, Hallier E. Glutathione transferase activity and formation of macromolecular adducts in two cases of acute methyl bromide poisoning. Occupational and Environmental Medicine. 1996;53(3):211–215. doi: 10.1136/oem.53.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tars K, Larsson A-K, Shokeer A, Olin B, Mannervik B, Kleywegt GJ. Structural basis of the suppressed catalytic activity of wild-type human glutathione transferase T1-1 compared to its W234R mutant. Journal of Molecular Biology. 2006;355(1):96–105. doi: 10.1016/j.jmb.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 116.Rossjohn J, McKinstry WJ, Oakley AJ, et al. Human theta class glutathione transferase: the crystal structure reveals a sulfate-binding pocket within a buried active site. Structure. 1998;6(3):309–322. doi: 10.1016/s0969-2126(98)00034-3. [DOI] [PubMed] [Google Scholar]
- 117.Atkinson HJ, Babbitt PC. Glutathione transferases are structural and functional outliers in the thioredoxin fold. Biochemistry. 2009;48(46):11108–11116. doi: 10.1021/bi901180v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shokeer A, Mannervik B. Minor modifications of the C-terminal helix reschedule the favored chemical reactions catalyzed by theta class glutathione transferase T1-1. The Journal of Biological Chemistry. 2010;285(8):5639–5645. doi: 10.1074/jbc.M109.074757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sherratt PJ, Pulford DJ, Harrison DJ, Green T, Hayes JD. Evidence that human class Theta glutathione S-transferase T1-1 can catalyse the activation of dichloromethane, a liver and lung carcinogen in the mouse. Comparison of the tissue distribution of GST T1-1 with that of classes Alpha, Mu and Pi GST in human. Biochemical Journal. 1997;326(3):837–846. doi: 10.1042/bj3260837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Porubsky PR, Meneely KM, Scott EE. Structures of human cytochrome P-450 2E1. Insights into the binding of inhibitors and both small molecular weight and fatty acid substrates. Journal of Biological Chemistry. 2008;283(48):33698–33707. doi: 10.1074/jbc.M805999200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Patten CJ, Ishizaki H, Aoyama T, et al. Catalytic properties of the human cytochrome P450 2E1 produced by cDNA expression in mammalian cells. Archives of Biochemistry and Biophysics. 1992;299(1):163–171. doi: 10.1016/0003-9861(92)90258-x. [DOI] [PubMed] [Google Scholar]
- 122.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radical Biology and Medicine. 2008;44(5):723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dogliotti E. Molecular mechanisms of carcinogenesis by vinyl chloride. Annali dell’Istituto Superiore di Sanita. 2006;42(2):163–169. [PubMed] [Google Scholar]
- 124.Huang C-Y, Huang K-L, Cheng T-J, Wang J-D, Hsieh L-L. The GST T1 and CYP2E1 genotypes are possible factors causing vinyl chloride induced abnormal liver function. Archives of Toxicology. 1997;71(8):482–488. doi: 10.1007/s002040050416. [DOI] [PubMed] [Google Scholar]
- 125.Doroshyenko O, Fuhr U, Kunz D, et al. In vivo role of cytochrome P450 2E1 and glutathione-S-transferase activity for acrylamide toxicokinetics in humans. Cancer Epidemiology Biomarkers and Prevention. 2009;18(2):433–443. doi: 10.1158/1055-9965.EPI-08-0832. [DOI] [PubMed] [Google Scholar]
- 126.Hanioka N, Yamamoto M, Tanaka-Kagawa T, Jinno H, Narimatsu S. Functional characterization of human cytochrome P450 2E1 allelic variants: in vitro metabolism of benzene and toluene by recombinant enzymes expressed in yeast cells. Archives of Toxicology. 2010;84(5):363–371. doi: 10.1007/s00204-009-0504-1. [DOI] [PubMed] [Google Scholar]
- 127.Monks TJ, Butterworth M, Lau SS. The fate of benzene-oxide. Chemico-Biological Interactions. 2010;184(1-2):201–206. doi: 10.1016/j.cbi.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ito S, Wakamatsu K. Chemistry of mixed melanogenesis—pivotal roles of dopaquinone. Photochemistry and Photobiology. 2008;84(3):582–592. doi: 10.1111/j.1751-1097.2007.00238.x. [DOI] [PubMed] [Google Scholar]
- 129.Del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Letters. 1996;381(3):165–168. doi: 10.1016/0014-5793(96)00109-3. [DOI] [PubMed] [Google Scholar]
- 130.Land EJ, Riley PA. Spontaneous redox reactions of dopaquinone and the balance between the eumelanic and phaeomelanic pathways. Pigment Cell Research. 2000;13(4):273–277. doi: 10.1034/j.1600-0749.2000.130409.x. [DOI] [PubMed] [Google Scholar]
- 131.Solano F, Jimenez-Cervantes C, Martinez-Liarte JH, Garcia-Borron JC, Jara JR, Lozano JA. Molecular mechanism for catalysis by a new zinc-enzyme, dopachrome tautomerase. Biochemical Journal. 1996;313(2):447–453. doi: 10.1042/bj3130447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Odh G, Hindemith A, Rosengren A-M, Rosengren E, Rorsman H. Isolation of a new tautomerase monitored by the conversion of D-dopachrome to 5,6-dihydroxyindole. Biochemical and Biophysical Research Communications. 1993;197(2):619–624. doi: 10.1006/bbrc.1993.2524. [DOI] [PubMed] [Google Scholar]
- 133.Nishihira J, Fujinaga M, Kuriyama T, et al. Molecular cloning of human D-dopachrome tautomerase cDNA: N-terminal proline is essential for enzyme activation. Biochemical and Biophysical Research Communications. 1998;243(2):538–544. doi: 10.1006/bbrc.1998.8123. [DOI] [PubMed] [Google Scholar]
- 134.Noels H, Bernhagen J, Weber C. Macrophage migration inhibitory factor: a noncanonical chemokine important in atherosclerosis. Trends in Cardiovascular Medicine. 2009;19(3):76–86. doi: 10.1016/j.tcm.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 135.Poelarends GJ, Veetil VP, Whitman CP. The chemical versatility of the β-α-β fold: catalytic promiscuity and divergent evolution in the tautomerase superfamily. Cellular and Molecular Life Sciences. 2008;65(22):3606–3618. doi: 10.1007/s00018-008-8285-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rosengren E, Bucala R, Aman P, et al. The immunoregulatory mediator macrophage migration inhibitory factor (MIF) catalyzes a tautomerization reaction. Molecular Medicine. 1996;2(1):143–149. [PMC free article] [PubMed] [Google Scholar]
- 137.Orita M, Yamamoto S, Katayama N, Fujita S. Macrophage migration inhibitory factor and the discovery of tautomerase inhibitors. Current Pharmaceutical Design. 2002;8(14):1297–1317. doi: 10.2174/1381612023394674. [DOI] [PubMed] [Google Scholar]
- 138.Dagnino-Subiabre A, Cassels BK, Baez S, Johansson A-S, Mannervik B, Segura-Aguilar J. Glutathione transferase M2-2 catalyzes conjugation of dopamine and dopa o-quinones. Biochemical and Biophysical Research Communications. 2000;274(1):32–36. doi: 10.1006/bbrc.2000.3087. [DOI] [PubMed] [Google Scholar]
- 139.Baez S, Segura-Aguilar J, Widersten M, Johansson A-S, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochemical Journal. 1997;324(1):25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dmitrieva RI, Hinojos CA, Grove ML, et al. Genome-wide identification of allelic expression in hypertensive rats. Circulation. Cardiovascular Genetics. 2009;2(2):106–115. doi: 10.1161/CIRCGENETICS.108.809509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hiyoshi M, Konishi H, Uemura H, et al. D-Dopachrome tautomerase is a candidate for key proteins to protect the rat liver damaged by carbon tetrachloride. Toxicology. 2009;255(1-2):6–14. doi: 10.1016/j.tox.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 142.Hosono-Fukao T, Hosono T, Seki T, Ariga T. Diallyl trisulfide protects rats from carbon tetrachloride-induced liver injury. Journal of Nutrition. 2009;139(12):2252–2256. doi: 10.3945/jn.109.109611. [DOI] [PubMed] [Google Scholar]
- 143.Singh N, Jatav OP, Gupta RK, Tailor MK, Jain R. Outcome of sixty four cases of ethylene dibromide ingestion treated in tertiary care hospital. Journal of Association of Physicians of India. 2007;55:842–845. [PubMed] [Google Scholar]
- 144.Guengerich FP, Peterson LA, Cmarik JL, Koga N, Inskeep PB. Activation of dihaloalkanes by glutathione conjugation and formation of DNA adducts. Environmental Health Perspectives. 1987;76:15–18. doi: 10.1289/ehp.877615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guengerich FP. Activation of alkyl halides by glutathione transferases. Methods in Enzymology. 2005;401:342–353. doi: 10.1016/S0076-6879(05)01021-9. [DOI] [PubMed] [Google Scholar]
- 146.Koga N, Inskeep PB, Harris TM, Guengerich FP. S-[2-(N7-guanyl)ethyl]glutathione, the major DNA adduct formed from 1,2-dibromoethane. Biochemistry. 1986;25(8):2192–2198. doi: 10.1021/bi00356a051. [DOI] [PubMed] [Google Scholar]
- 147.Josephy PD. Genetically-engineered bacteria expressing human enzymes and their use in the study of mutagens and mutagenesis. Toxicology. 2002;181-182:255–260. doi: 10.1016/s0300-483x(02)00292-5. [DOI] [PubMed] [Google Scholar]
- 148.Grant DM, Josephy PD, Lord HL, Morrison LD. Salmonella typhimurium strains expressing human arylamine N-acetyltransferases: metabolism and mutagenic activation of aromatic amines. Cancer Research. 1992;52(14):3961–3964. [PubMed] [Google Scholar]
- 149.Josephy PD, DeBruin LS, Lord HL, et al. Bioactivation of aromatic amines by recombinant human cytochrome P4501A2 expressed in Ames tester strain bacteria: a substitute for activation by mammalian tissue preparations. Cancer Research. 1995;55(4):799–802. [PubMed] [Google Scholar]
- 150.Glatt H, Bartsch I, Christoph S, et al. Sulfotransferase-mediated activation of mutagens studied using heterologous expression systems. Chemico-Biological Interactions. 1998;109(1–3):195–219. doi: 10.1016/s0009-2797(97)00133-6. [DOI] [PubMed] [Google Scholar]
- 151.Thier R, Taylor JB, Pemble SE, et al. Expression of mammalian glutathione S-transferase 5-5 in Salmonella typhimurium TA1535 leads to base-pair mutations upon exposure to dihalomethanes. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(18):8576–8580. doi: 10.1073/pnas.90.18.8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Thier R, Pemble SE, Kramer H, Taylor JB, Guengerich FP, Ketterer B. Human glutathione S-transferase T1-1 enhances mutagenicity of 1,2-dibromoethane, dibromomethane and 1,2,3,4-diepoxybutane in Salmonella typhimurium. Carcinogenesis. 1996;17(1):163–166. doi: 10.1093/carcin/17.1.163. [DOI] [PubMed] [Google Scholar]
- 153.Wilson SH, Olden K. The environmental genome project. Phase I and beyond. Molecular Interventions. 2004;4(3):147–156. doi: 10.1124/mi.4.3.4. [DOI] [PubMed] [Google Scholar]
- 154.Alexandrie A-K, Rannug A, Juronen E, Tasa G, Warholm M. Detection and characterization of a novel functional polymorphism in the GSTT1 gene. Pharmacogenetics. 2002;12(8):613–619. doi: 10.1097/00008571-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 155.Tetlow N, Liu D, Board P. Polymorphism of human alpha class glutathione transferases. Pharmacogenetics. 2001;11(7):609–617. doi: 10.1097/00008571-200110000-00007. [DOI] [PubMed] [Google Scholar]
- 156.Tetlow N, Robinson A, Mantle T, Board P. Polymorphism of human mu class glutathione transferases. Pharmacogenetics. 2004;14(6):359–368. doi: 10.1097/00008571-200406000-00005. [DOI] [PubMed] [Google Scholar]
- 157.Ning B, Wang C, Morel F, et al. Human glutathione S-transferase A2 polymorphisms: variant expression, distribution in prostate cancer cases/controls and a novel form. Pharmacogenetics. 2004;14(1):35–44. doi: 10.1097/00008571-200401000-00004. [DOI] [PubMed] [Google Scholar]
- 158.Tetlow N, Board PG. Functional polymorphism of human glutathione transferase A2. Pharmacogenetics. 2004;14(2):111–116. doi: 10.1097/00008571-200402000-00005. [DOI] [PubMed] [Google Scholar]
- 159.Mukherjee B, Salavaggione OE, Pelleymounter LL, et al. Glutathione S-transferase omega 1 and omega 2 pharmacogenomics. Drug Metabolism and Disposition. 2006;34(7):1237–1246. doi: 10.1124/dmd.106.009613. [DOI] [PubMed] [Google Scholar]
- 160.Moyer AM, Salavaggione OE, Hebbring SJ, et al. Glutathione S-transferase T1 and M1: gene sequence variation and functional genomics. Clinical Cancer Research. 2007;13(23):7207–7216. doi: 10.1158/1078-0432.CCR-07-0635. [DOI] [PubMed] [Google Scholar]
- 161.Moyer AM, Salavaggione OE, Wu T-Y, et al. Glutathione S-transferase P1: gene sequence variation and functional genomic studies. Cancer Research. 2008;68(12):4791–4801. doi: 10.1158/0008-5472.CAN-07-6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Agundez JAG, Ladero JM. Glutathione S-transferase GSTT1 and GSTM1 allozymes: beyond null alleles. Pharmacogenomics. 2008;9(3):359–363. doi: 10.2217/14622416.9.3.359. [DOI] [PubMed] [Google Scholar]
- 163.Josephy PD, Taylor PL, Vervaet G, Mannervik B. Screening and characterization of variant theta-class glutathione transferases catalyzing the activation of ethylene dibromide to a mutagen. Environmental and Molecular Mutagenesis. 2006;47(9):657–665. doi: 10.1002/em.20252. [DOI] [PubMed] [Google Scholar]
- 164.Josephy PD, Kent M, Mannervik B. Single-nucleotide polymorphic variants of human glutathione transferase T1-1 differ in stability and functional properties. Archives of Biochemistry and Biophysics. 2009;490(1):24–29. doi: 10.1016/j.abb.2009.07.025. [DOI] [PubMed] [Google Scholar]