Abstract
Intestinal epithelial cells of the neonatal rat and mouse have been shown to express a major histocompatibility complex (MHC) class I-like Fc receptor, or FcRn, which transports IgG in an apical to basolateral direction. Previous studies have suggested the possible expression of this receptor beyond the neonatal period within the liver. Since bile contains high levels of IgG, we sought to determine whether the FcRn was functionally expressed by adult rat hepatocytes. Using primers specific for FcRn, which did not cross hybridize with MHC class I transcripts, FcRn DNA was amplified by reverse transcriptase polymerase chain reaction from RNA of adult rat hepatocytes. This RNA contained functional FcRn transcripts as it encoded a beta 2-microglobulin-associated cell surface protein as determined by immunoprecipitation of biotinylated cell surface proteins with a polyclonal anti-FcRn specific antiserum. Western blotting of hepatocyte canalicular (apical) and sinusoidal (basolateral) plasma membranes with an FcRn-specific monoclonal antibody further confirmed the protein expression and suggested that FcRn was enriched on the canalicular surface membranes. FcRn, on the surface of hepatocytes, was biologically functional as it bound Fc fragments of IgG at pH 6.0 but not 8.0, which is the same pH dependence observed for FcRn in rat neonatal enterocytes. Thus, FcRn is functionally expressed outside of the neonatal period on the canalicular cell surface of adult hepatocytes. This suggests that hepatocyte FcRn may bind luminal IgG, providing a potential functional communication between parenchymal immune cells and bile.
Full text
PDF


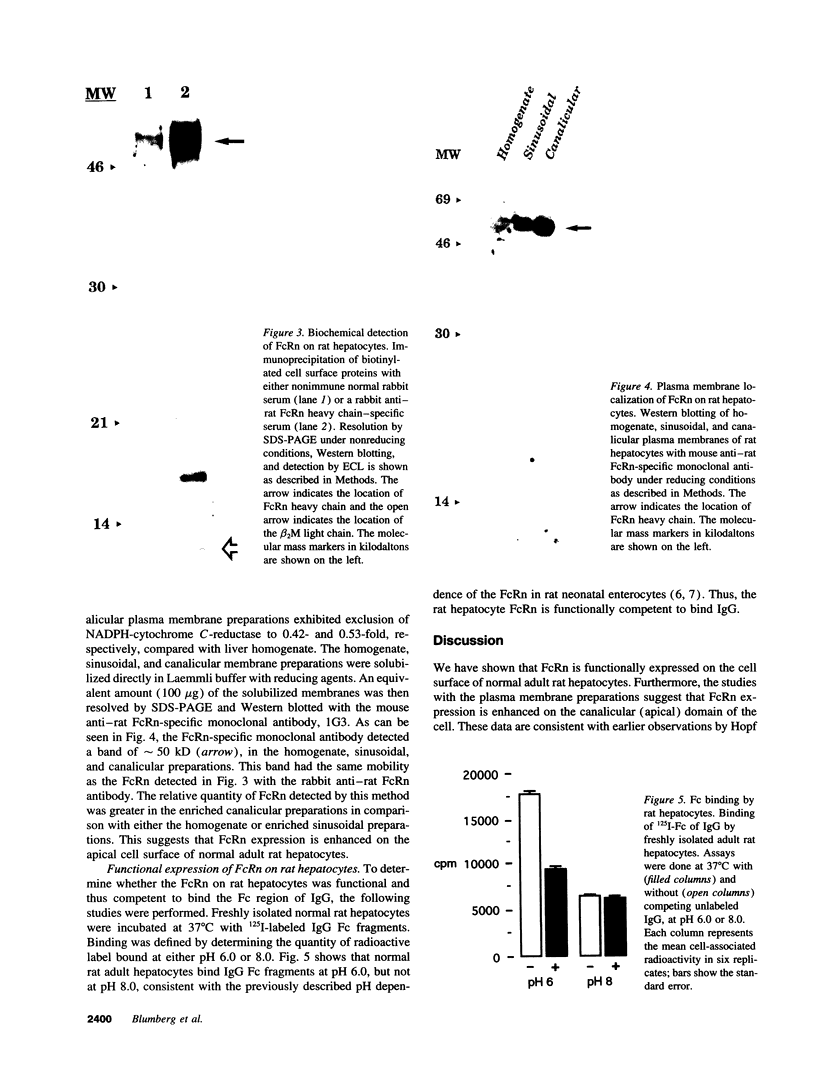
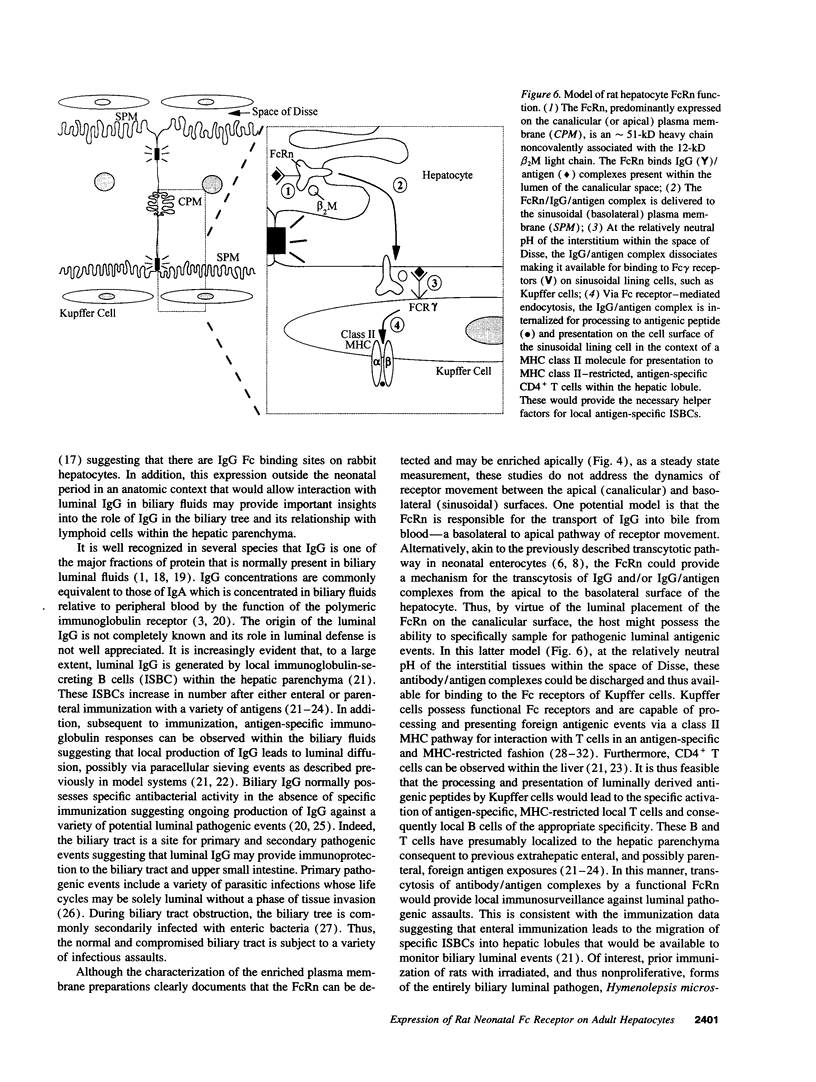

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson D. R., Powers A., Rodewald R. Intestinal absorption of immune complexes by neonatal rats: a route of antigen transfer from mother to young. Science. 1979 Nov 2;206(4418):567–569. doi: 10.1126/science.493961. [DOI] [PubMed] [Google Scholar]
- Ahouse J. J., Hagerman C. L., Mittal P., Gilbert D. J., Copeland N. G., Jenkins N. A., Simister N. E. Mouse MHC class I-like Fc receptor encoded outside the MHC. J Immunol. 1993 Dec 1;151(11):6076–6088. [PubMed] [Google Scholar]
- Blumberg R. S., Ley S., Sancho J., Lonberg N., Lacy E., McDermott F., Schad V., Greenstein J. L., Terhorst C. Structure of the T-cell antigen receptor: evidence for two CD3 epsilon subunits in the T-cell receptor-CD3 complex. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7220–7224. doi: 10.1073/pnas.87.18.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke S., Landau S., Green R., Tseng C. C., Nattakom T., Canchis W., Yang L., Kaiserlian D., Gespach C., Balk S. Rat cluster of differentiation 1 molecule: expression on the surface of intestinal epithelial cells and hepatocytes. Gastroenterology. 1994 May;106(5):1143–1149. doi: 10.1016/0016-5085(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Carter L., Barrington P. J., Cooper G. N., Jackson G. D. Antibody synthesis in the rat liver: an association between antibody-forming cells in the liver and biliary antibodies following intravenous injection of horse erythrocytes. Int Arch Allergy Appl Immunol. 1987;82(2):153–158. doi: 10.1159/000234181. [DOI] [PubMed] [Google Scholar]
- Carter L., Barrington P. J., Jackson G. D. Antibody responses in the liver and bile of rats injected with horse erythrocytes. Immunol Cell Biol. 1989 Apr;67(Pt 2):135–139. doi: 10.1038/icb.1989.19. [DOI] [PubMed] [Google Scholar]
- DVORAK J. A., JONES A. W., KUHLMAN H. H. Studies on the biology of Hymenolepis microstoma (Dujardin, 1845). J Parasitol. 1961 Oct;47:833–838. [PubMed] [Google Scholar]
- Dahlgren U. I., Svanvik J., Svanborg Edén C. Antibodies to Escherichia coli and anti-adhesive activity in paired serum, hepatic and gall bladder bile samples. Scand J Immunol. 1986 Sep;24(3):251–260. doi: 10.1111/j.1365-3083.1986.tb02092.x. [DOI] [PubMed] [Google Scholar]
- Dive C., Heremans J. F. Nature and origin of the proteins of bile. I. A comparative analysis of serum and bile proteins in man. Eur J Clin Invest. 1974 Aug;4(4):235–239. doi: 10.1111/j.1365-2362.1974.tb00398.x. [DOI] [PubMed] [Google Scholar]
- Dive C., Nadalini R. A., Vaerman J. P., Heremans J. F. Origin and nature of the proteins of bile. II. A comparative analysis of serum, hepatic lymph and bile proteins in the dog. Eur J Clin Invest. 1974 Aug;4(4):241–246. doi: 10.1111/j.1365-2362.1974.tb00399.x. [DOI] [PubMed] [Google Scholar]
- Goossens P. L., Jouin H., Marchal G., Milon G. Isolation and flow cytometric analysis of the free lymphomyeloid cells present in murine liver. J Immunol Methods. 1990 Aug 28;132(1):137–144. doi: 10.1016/0022-1759(90)90407-m. [DOI] [PubMed] [Google Scholar]
- Green R. M., Whiting J. F., Rosenbluth A. B., Beier D., Gollan J. L. Interleukin-6 inhibits hepatocyte taurocholate uptake and sodium-potassium-adenosinetriphosphatase activity. Am J Physiol. 1994 Dec;267(6 Pt 1):G1094–G1100. doi: 10.1152/ajpgi.1994.267.6.G1094. [DOI] [PubMed] [Google Scholar]
- Hansen P. G., Hennessy E. J., Blake H., Clancy R. L., Kamath R., Molenaar C., Cripps A. W., Jackson G. D. Appearance of IgG and IgA antibodies in human bile after tetanus toxoid immunization. Clin Exp Immunol. 1989 Aug;77(2):215–220. [PMC free article] [PubMed] [Google Scholar]
- Hansen P. G., Jackson G. D. The occurrence and sources of natural antibody in human bile and serum against the O antigens of two Escherichia coli serotypes. Scand J Immunol. 1990 Nov;32(5):537–544. doi: 10.1111/j.1365-3083.1990.tb03194.x. [DOI] [PubMed] [Google Scholar]
- Hopf U., Meyer zum Büschenfelde K. H., Dierich M. P. Demonstration of binding sites for IgG Fc and the third complement component (C3) on isolated hepatocytes. J Immunol. 1976 Aug;117(2):639–645. [PubMed] [Google Scholar]
- Jakoi E. R., Cambier J., Saslow S. Transepithelial transport of maternal antibody: purification of IgG receptor from newborn rat intestine. J Immunol. 1985 Nov;135(5):3360–3364. [PubMed] [Google Scholar]
- Lautenschlager I. Characteristics of the strongly Ia-positive cells in rat liver. Scand J Immunol. 1984 Oct;20(4):333–338. doi: 10.1111/j.1365-3083.1984.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Magilavy D. B., Fitch F. W., Gajewski T. F. Murine hepatic accessory cells support the proliferation of Th1 but not Th2 helper T lymphocyte clones. J Exp Med. 1989 Sep 1;170(3):985–990. doi: 10.1084/jem.170.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning R. J., Walker P. G., Carter L., Barrington P. J., Jackson G. D. Studies on the origins of biliary immunoglobulins in rats. Gastroenterology. 1984 Jul;87(1):173–179. [PubMed] [Google Scholar]
- Mullock B. M., Shaw L. J., Fitzharris B., Peppard J., Hamilton M. J., Simpson M. T., Hunt T. M., Hinton R. H. Sources of proteins in human bile. Gut. 1985 May;26(5):500–509. doi: 10.1136/gut.26.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richman L. K., Klingenstein R. J., Richman J. A., Strober W., Berzofsky J. A. The murine Kupffer cell. I. Characterization of the cell serving accessory function in antigen-specific T cell proliferation. J Immunol. 1979 Dec;123(6):2602–2609. [PubMed] [Google Scholar]
- Rubinstein D., Roska A. K., Lipsky P. E. Antigen presentation by liver sinusoidal lining cells after antigen exposure in vivo. J Immunol. 1987 Mar 1;138(5):1377–1382. [PubMed] [Google Scholar]
- Scott A. J., Khan G. A. Origin of bacteria in bileduct bile. Lancet. 1967 Oct 14;2(7520):790–792. doi: 10.1016/s0140-6736(67)92231-3. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Mostov K. E. An Fc receptor structurally related to MHC class I antigens. Nature. 1989 Jan 12;337(6203):184–187. doi: 10.1038/337184a0. [DOI] [PubMed] [Google Scholar]
- Simister N. E., Rees A. R. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985 Jul;15(7):733–738. doi: 10.1002/eji.1830150718. [DOI] [PubMed] [Google Scholar]
- Song C. S., Rubin W., Rifkind A. B., Kappas A. Plasma membranes of the rat liver. Isolation and enzymatic characterization of a fraction rich in bile canaliculi. J Cell Biol. 1969 Apr;41(1):124–132. doi: 10.1083/jcb.41.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B. D., Jones A. W. Autoelimination by means of x-rays: distinguishing the crowding factor from others in premunition caused by the mouse bile duct cestode, Hymenolepis microstoma. Exp Parasitol. 1967 Apr;20(2):147–155. doi: 10.1016/0014-4894(67)90033-1. [DOI] [PubMed] [Google Scholar]
- Underdown B. J., Schiff J. M. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- Wu H. Y., Russell M. W. Antibody-secreting cell responses in the mouse liver. Immunology. 1992 Nov;77(3):443–448. [PMC free article] [PubMed] [Google Scholar]
- Zucker S. D., Goessling W., Zeidel M. L., Gollan J. L. Membrane lipid composition and vesicle size modulate bilirubin intermembrane transfer. Evidence for membrane-directed trafficking of bilirubin in the hepatocyte. J Biol Chem. 1994 Jul 29;269(30):19262–19270. [PubMed] [Google Scholar]