Abstract
Background
Tissue injury triggers reparative processes that often involve endothelial progenitor cells (EPC) recruitment. We hypothesized that atherosclerotic renal artery stenosis (ARAS) activates homing signals that would be detectable in both the kidney and endothelial progenitor cells (EPC), and attenuated upon renal repair using selective cell-based therapy.
Methods
Pigs were treated with intra-renal autologous EPC after 6 weeks of ARAS. Four weeks later, expression of homing-related signals in EPC and kidney, single-kidney function, microvascular density, and morphology were compared to untreated ARAS and normal control pigs (n=7 each).
Results
Compared to normal EPC, EPC from ARAS pigs showed increased stromal cell-derived factor (SDF)-1, angiopoietin-1, Tie-2, and ckit expression, but downregulation of erythropoietin and its receptor. The ARAS kidney released the ckit-ligand stem-cell factor (SCF), uric acid, and erythropoietin, and upregulated integrin β2, suggesting activation of corresponding homing signaling. However, angiopoietin-1 and SDF-1/CXCR4 were not elevated. Administration of EPC into the stenotic kidney restored angiogenic activity, improved microvascular density, renal hemodynamics and function, decreased fibrosis and oxidative stress, and attenuated endogenous injury signals.
Conclusion
The ARAS kidney releases specific homing signals corresponding to cognate receptors expressed by EPC. EPC show plasticity for organ-specific recruitment strategies, which are upregulated in early atherosclerosis. EPC are renoprotective as they attenuated renal dysfunction and damage in chronic ARAS, and consequently decreased the injury signals. Importantly, manipulation of homing signals may potentially allow therapeutic opportunities to increase endogenous EPC recruitment.
Keywords: endothelial progenitor cells, renal artery stenosis, homing factors
Introduction
The increasing incidence and prevalence of hypertension, diabetes, obesity, dyslipidemia, and metabolic syndrome is reflected in the increase in the number of patients with cardiovascular disease. Co-existence of these cardiovascular risk factors also amplifies the number of insults that the kidneys are constantly exposed to. By triggering injurious mechanisms such as inflammation, oxidative stress, fibrosis, and ischemia, renal function may progressively deteriorate and advance to irreversible chronic kidney disease.
One of the mechanisms by which cardiovascular risk factors may ultimately impair renal function is by inducing atherosclerosis, the predominant etiology of renal artery stenosis (RAS)1, and thereby compromising blood supply. Even prior to obstruction by atherosclerotic plaques, the atherogenic process can harm renal tissue by promoting functional and structural deterioration of small vessels and the renal parenchyma2, 3. Atherosclerotic renovascular disease is on the rise as a cause of end-stage renal disease, specially in the older population4. Simply restoring blood flow does not regularly restore function. There is a pressing need for improved strategies to arrest or reverse intra-renal injury in this disease.
Endogenous endothelial progenitor cells (EPC) are often mobilized to mediate neovascularization and endothelial replacement that contribute to healing ischemic tissues. The mobilization from bone marrow and subsequent homing of progenitor cells can be regulated by a variety of mediators such as stromal cell-derived factor (SDF)-1, stem cell factor (SCF), erythropoietin (EPO), or angiopoietins 5, 6, which are released by injured tissue to attract the cells and ensure their adherence. In turn, the cells express corresponding cognate receptors such as CXCR4, cKit, EPO-receptors (EPO-R), and Tie, respectively, which allow them to be recognized, recruited, and retained at the injured tissues.
However, the endogenous system may be overwhelmed or dysfunctional, and hence fail to repair the tissues. Therefore, exogenous delivery of EPC collected and expanded in-vitro offers the potential for targeted treatment of conditions such as myocardial7 and hind-limb ischemia8, acute renal injury9, and glomerulonephritis10. We have recently shown the beneficial effects of intra-renal administration of autologous EPC in a porcine model of chronic non-atherosclerotic RAS11. Conceivably, a decrease in tissue damage may resolve the injury signals and homing cues that it releases.
Specific signals that portend chronic ischemic injury and regulate the homing and adherence of endogenous circulating cells into the ischemic kidney, or the ability of successful renal repair to alleviate these signals, have not been elucidated. Therefore, the current study was designed to test the hypotheses that, firstly, renovascular disease activates homing signals detectable in both the ischemic kidney and EPC, and secondly, that these signals are attenuated upon renal repair using selective intra-renal cell-based therapy. For this purpose we utilized a pig model of experimental atherosclerotic RAS (ARAS), which recapitulates many characteristics of early human atherosclerotic renovascular disease3.
Materials and methods
All procedures were approved by Mayo Clinic Institutional Animal Care and Use Committee. Domestic pigs (35–40 kg) were fed with 2% cholesterol diet for six weeks, to induce pre-existing early atherosclerosis. The animals were then anesthetized with 0.5 g of intra-muscular ketamine and xylazine, and maintained with a mixture of ketamine (0.2 mg/kg/min) and xylazine (0.03 mg/kg/min), a local-irritant coil was implanted in the main renal artery to induce RAS, and the high-cholesterol diet continued. Six weeks after induction of RAS, animals were randomized into two groups: one was sham treated (ARAS, n=7) and the other received an intra-renal infusion of autologous EPC (ARAS+EPC, n=7). Additional 7 pigs were used as normal controls. Four weeks later, renal hemodynamics and function were assessed in all pigs by multi-detector computed tomography (MDCT), as previously described11, 12. Mean arterial pressure (MAP) was determined via a carotid artery catheter and the degree of stenosis by renal angiography. Blood samples were collected from a systemic and stenotic renal vein for measurement of creatinine, the EPC homing signal SCF, and the renal injury signal uric acid13.
Three days after completion of in-vivo studies, pigs were euthanized (sodium pentobarbital 100mg/kg, Fort Dodge Laboratories, Fort Dodge, IA). Kidneys were removed and lobes were either shock-frozen in liquid nitrogen and stored at −80°C, preserved in formalin, or prepared for micro-CT to assess renal microvascular (MV) architecture. In-vitro studies were then performed to assess renal inflammation, redox status, and expression of angiogenic and fibrogenic factors. EPC homing signals were examined in both the stenotic kidneys and isolated EPC using immunostaining or Western blotting for SDF-1, angiopoietin-1, EPO and their receptors CXCR4, Tie-2, and EPO-R, as well as the SCF receptor c-Kit, and integrin β2, which mediates the adherence of leukocytes and EPCs to endothelial cell monolayers.
EPC characterization, preparation and delivery
EPC were cultured from mononuclear cells obtained from peripheral blood (100 mL), as described previously11. Briefly, mononuclear cells were isolated from the blood, cultured for 1–3 weeks in endothelial media, and examined for the number of colony forming units (CFU), EPC function, phenotype, and progenitor markers11. Cells were then labeled with a fluorescent membrane dye (CM-DiI) and kept in PBS on ice until delivery (10 ml, 106 cells/ml) into the stenotic renal artery after 6 weeks of ARAS. Retention rate of the injected EPC in the kidney was calculated 4 weeks after administration, as described previously11.
To evaluate homing signals, EPC from normal and ARVD pigs were stained using immunofluorescence with antibodies against SDF-1, CXCR-4, and angiopoietin-1 (all 1:50, Abcam), Tie-2 (1:50, Calbiochem), EPO and EPO-R (1:50, Santa Cruz), c-kit (1:100, Abcam), and integrin β2 (1:50, Thermo Scientific). Surface receptor expression was confirmed by Western blotting, using the same antibodies used for immunofluorescence for CXCR-4, Tie-2, c-kit (all 1:500), and EPO-R (1:200). Immunofluorescence and Western blotting were also repeated on 1-week older EPC (next passage).
Micro-CT
A saline-filled cannula was ligated in a segmental artery perfusing the intact end of the stenotic kidney. Initially saline (containing heparin) was perfused under physiological perfusion pressure, and replaced with infusion (0.8 ml/min) of an intravascular contrast agent (Microfil® MV122, Flow Tech, Carver, MA). Then, a lobe of the contrast-filled tissue was trimmed from the kidney, prepared, and scanned at 0.5° increments and cubic voxels of 18 µm using micro-CT, as previously described14–17.
Renal tissue
Histology and immunohistochemistry: trichrome staining was performed in 5µm paraffin mid-hilar renal cross-sections to assess fibrosis. Immunostaining in 5µm frozen renal cross-sections examine the expression of integrin β2 (1:50, Thermo Scientific, Rockford, IL). Renal oxidative stress was assessed by the in-situ production of superoxide anion detected using dihydroethidium (DHE)18. To investigate the engraftment of EPC in endothelial or tubular structures, double immunofluorescence of the CM-DiI label and CD31 (1:50, AbD Serotec) or cytokeratin (1:100, AbD Serotec), respectively, was performed on frozen slices, as described previously11.
Western blotting: standard blotting protocols were followed, as previously described19, using specific antibodies against vascular endothelial growth factor (VEGF), angiopoietin-1, transforming growth factor (TGF)-β, matrix-metalloproteinase (MMP)-2, tissue-inhibitor of MMP (TIMP)-1, the NAD(P)H oxidase subunit p47phox, the VEGF receptor KDR (Santa Cruz, CA, 1:200 for all), xanthine oxidase (1:10000, non-reducing, no-milk condition, Chemicon International), SDF-1, angiopoietin-1 (1:1000, Abcam), EPO and EPO-R (Santa Cruz, CA, 1:200). GAPDH (1:5000, Covance, Emeryville, CA) served as loading control. Protein expression was determined in each kidney, and the intensities of the protein bands (one per animal) quantified and averaged in each group. For SCF, renal vein plasma proteins were enriched through albumin removal (ProMax, Polysciences, Warrington, PA) following manufacture’s instruction, and then standard Western blotting was performed with Ponceau-S staining as loading control20.
Data analysis
MDCT analysis: Tissue attenuation was sampled in regions of interest selected in MDCT images in the aorta, renal cortex, and medulla. Time- attenuation curves were fitted with extended gamma-variate curve-fits21 and analyzed for renal regional perfusion (ml/minute/g tissue), single-kidney glomerular filtration rate (GFR), and renal blood flow (RBF), using previously-validated methods3, 12, 21–24.
Micro-CT analysis: Images were digitized for reconstruction of 3-D volumes, and analyzed with the Analyze® software package (Biomedical Imaging Resource, Mayo Clinic, Rochester, MN). The cortex was tomographically divided into inner, middle, and outer thirds16, and the spatial density of cortical microvessels (diameters <500µm) calculated in each level14, 15.
Histology: Mid-hilar 5µm cross sections of each kidney (1 per animal) were examined using a computer-aided image-analysis program (MetaMorph®, Meta Imaging). In each slide, trichrome staining or DHE fluorescence was semi-automatically quantified in 15–20 fields by the computer program, expressed as fraction of kidney surface area, and the results from all fields averaged3, 17, 18, 23, 24. For EPC immunofluorescence, 6 fields per sample of 3 pigs from each group were used positive and nuclei area was traced using MetaMorph®, and expressed as ratio of positive/total nuclei.
Statistical Analysis
Results are expressed as mean±SEM. Comparisons within groups were performed using paired student’s t-test, and among groups using ANOVA, with the Bonferroni correction for multiple comparisons, followed by unpaired student’s t-test. Statistical significance was accepted for p≤0.05.
Results
MAP and the angiographic degree of stenosis were similarly greater in ARAS and ARAS+EPC animals compared to normal (Table 1). ARAS pigs showed a significant increase in renal vein levels of uric acid compared to normal controls (1.78±0.1 vs. 1.27±0.1 mg/dL, p<0.01), which was reduced after EPC treatment (1.5±0.1 mg/dL, p=NS vs. Normal, p=0.05 vs. ARAS).
Table 1.
Mean arterial pressure, degree of stenosis, and basal single-kidney hemodynamics and function (mean ± SEM), in normal, atherosclerotic renal artery stenosis (ARAS), and ARAS pigs treated with intra-renal autologous endothelial progenitor cells (ARAS+EPC).
| Parameter | Normal n=7 | ARAS n=7 | ARAS+EPC n=7 | |
|---|---|---|---|---|
| Mean arterial pressure (mmHg) | 102.9±3.5 | 128.7±7.8* | 125.5±8.1* | |
| Degree of stenosis (%) | 0.0±0.0 | 81.3±7.7* | 73.6±6.6* | |
| Renal volume (cc) | Cortex | 130.8±3.8 | 67.8.0±7.3* | 87.9±2.4*† |
| Medulla | 25.3±1.4 | 10.3±1.6* | 8.2±0.6* | |
| Renal blood flow (mL/min) | 567.9±56.1 | 289.6.1±51.7* | 372.9±21.8*† | |
| Perfusion (mL/min/cc) | Cortex | 3.7±0.3 | 3.8±0.5 | 4.3±0.3 |
| Medulla | 3.3±0.7 | 2.2±0.4 | 3.1±0.6 | |
| Glomerular filtration rate (mL/min) | 90.6±9.1 | 50.5±7.6* | 71.5±3.6*† |
p<0.05 vs. Normal
p<0.05 vs. ARAS.
Characterization of the EPC: the number of CFU was similar in normal and ARAS pigs (61.2±5.3 and 58.9±4.1 CFU/cm2, respectively). As we have previously shown11, both CD14 and CD133 were initially expressed in early EPC, but after 21 days the expression of KDR and CD34 increased as CD14 and CD133 diminished in late cells (data not shown), suggesting acquisition of endothelial characteristics.
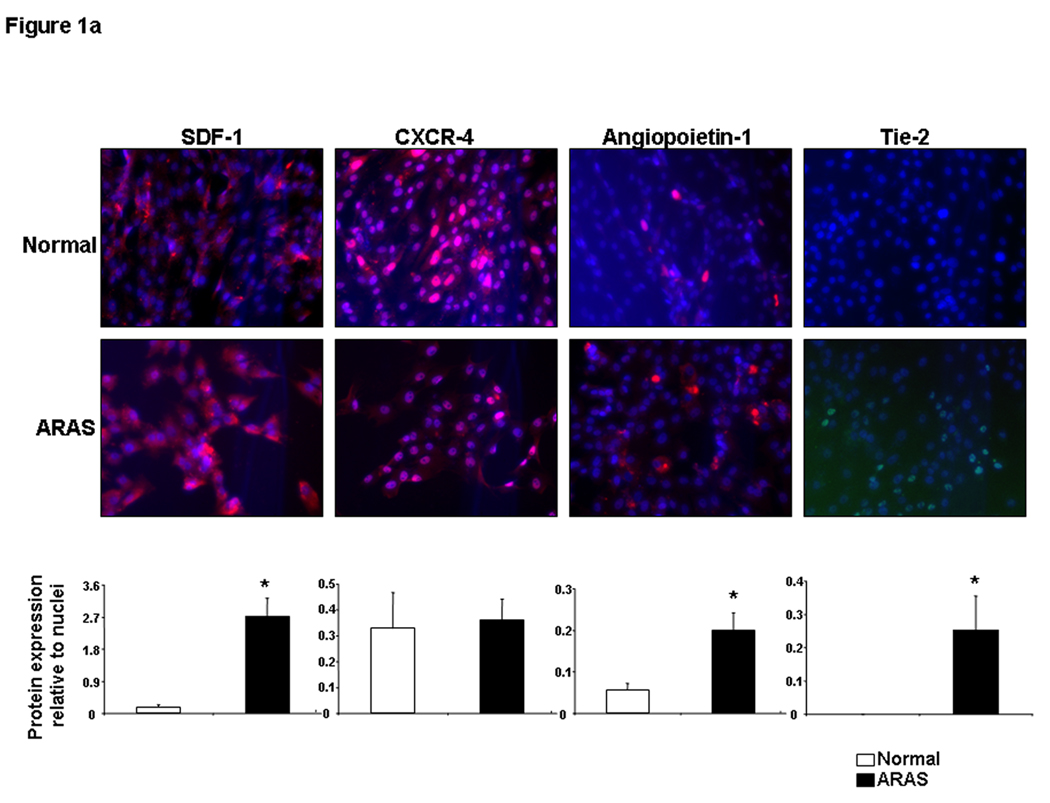
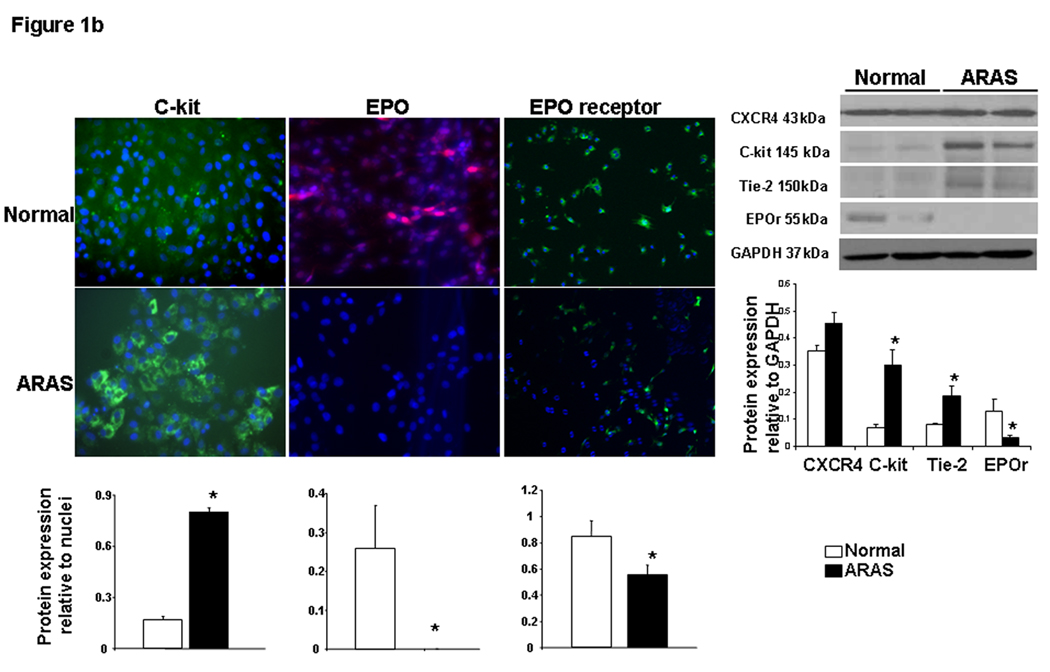
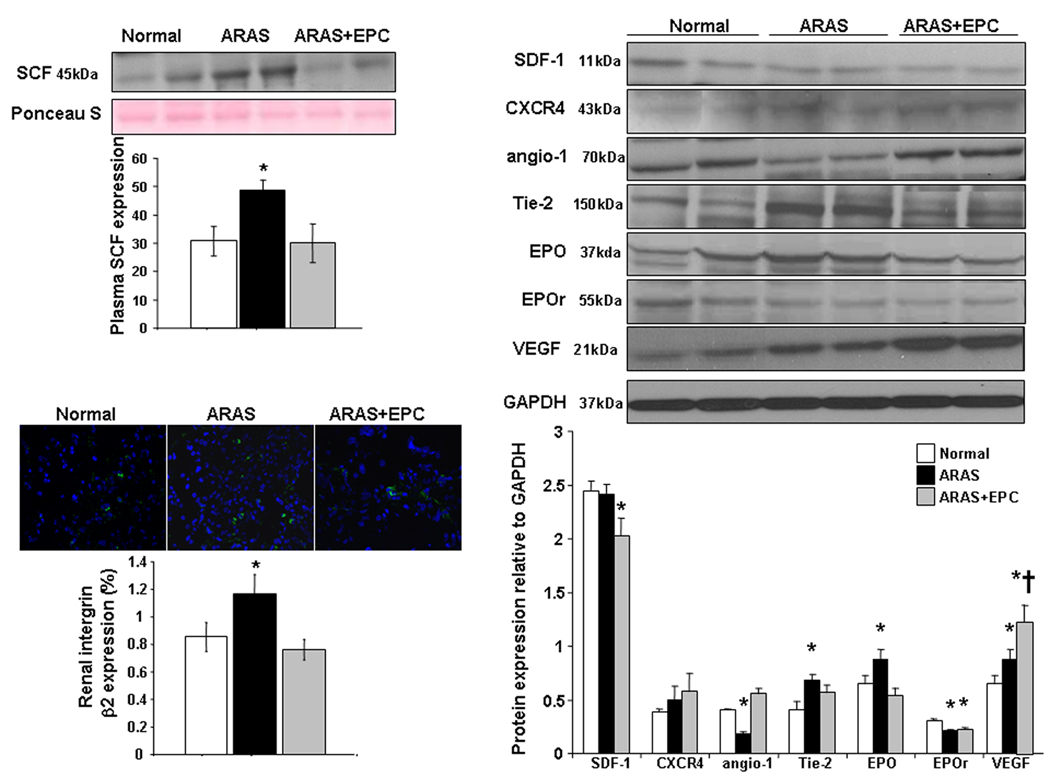
EPC and renal homing and angiogenic signaling: EPC obtained from ARAS animals showed greater expression of SDF-1, angiopoietin-1, its receptor Tie-2, and cKit than EPC obtained from normal pigs (Figure 1a–b), indicating angiogenic and homing activity. However, CXCR-4 remained unchanged, and the expression of EPO and EPO-R in ARAS EPC was blunted (Figure 1a–b). Western blotting confirmed these trends of surface receptor expression (Figure 1b-right). Repeated immunofluorescence and Western blotting showed a similar pattern in next passage EPC (data not shown), suggesting that this expression was sustained under culture conditions. In the kidney ARAS pigs showed a significant increase in renal vein SCF levels (Figure 2-top) and Tie-2, VEGF, integrin β2, and EPO expression, while renal expression of angiopoietin-1 and EPO-R decreased, and SDF-1 and CXCR-4 remained unchanged (Figure 2-right).
Figure 1.
Representative protein expression of stromal cell-derived factor (SDF)-1 and its receptor CXCR4, angiopoietin-1 and its Tie-2 receptor (1a), cKit, erythropoietin (EPO) and its receptor (1b) in progenitor cells obtained from normal and atherosclerotic renal artery stenosis (ARAS) renovascular hypertensive pigs. Surface receptor protein expression was also confirmed using Western blotting. Changes in homing factor expression in progenitor cells obtained from ARAS compared to normal controls suggest modulation of mobilization and homing signaling. *p<0.05 vs. Normal.
Figure 2.
Representative expression and quantification of renal vein stem-cell factor (SCF, top-left), renal tissue SDF-1 and CXCR4, angiopoietin (angio)-1 and Tie-2 receptor, EPO and its receptor, vascular endothelial growth factor (VEGF) (right), and integrin β2 (bottom-left) in normal, atherosclerotic renal artery stenosis (ARAS), and ARAS pigs treated with an intra-renal infusion of autologous endothelial progenitor cells (ARAS+EPC). The ARAS kidney released SCF and increased renal expression of integrin β2 and Tie 2. However, other cell homing mediators such as angiopoietin-1 and SDF-1/CXCR4 were either blunted or unchanged in the stenotic kidney, implying incomplete heralding of an ischemic insult. *p<0.05 vs. Normal, †p<0.05 vs. ARAS.
Effects of EPC delivery in the ARAS kidney: An average of 12.9±5.8% of the injected EPC was detected 4 weeks after delivery in the stenotic kidney. Some of the injected EPC co-expressed CD31 or cytokeratin, and appeared to incorporate into microvessels and tubules, respectively, suggesting acquisition of endothelial and tubular characteristics, as we have recently shown11. EPC delivery into the ARAS kidney further elevated the renal expression of VEGF, improved angiopoietin-1, renal Tie-2, and integrin β2, and decreased renal vein plasma levels of SCF (Figure 2 bottom).
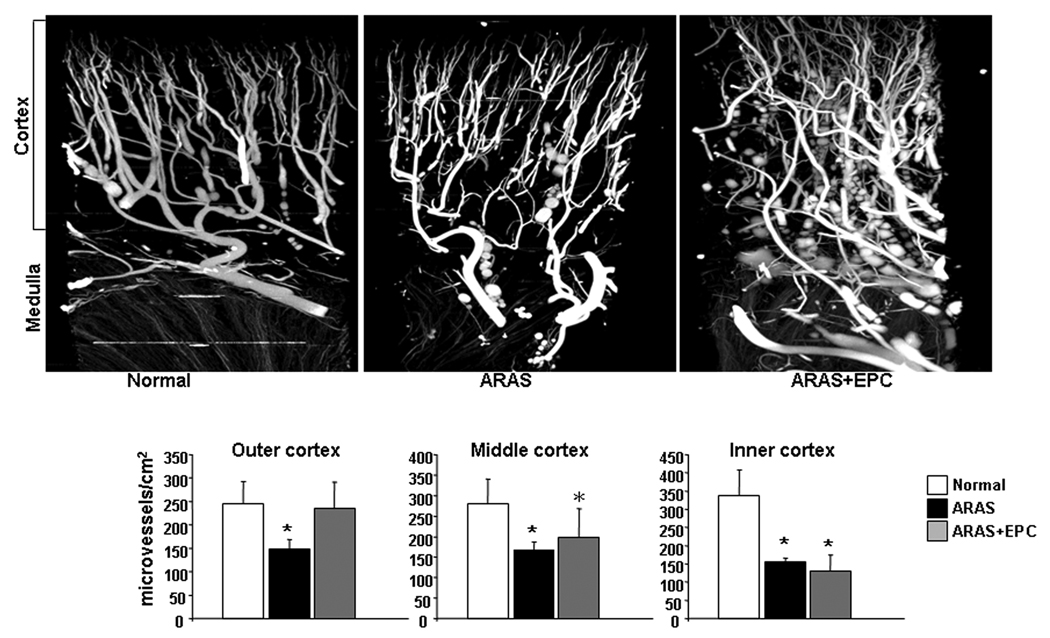
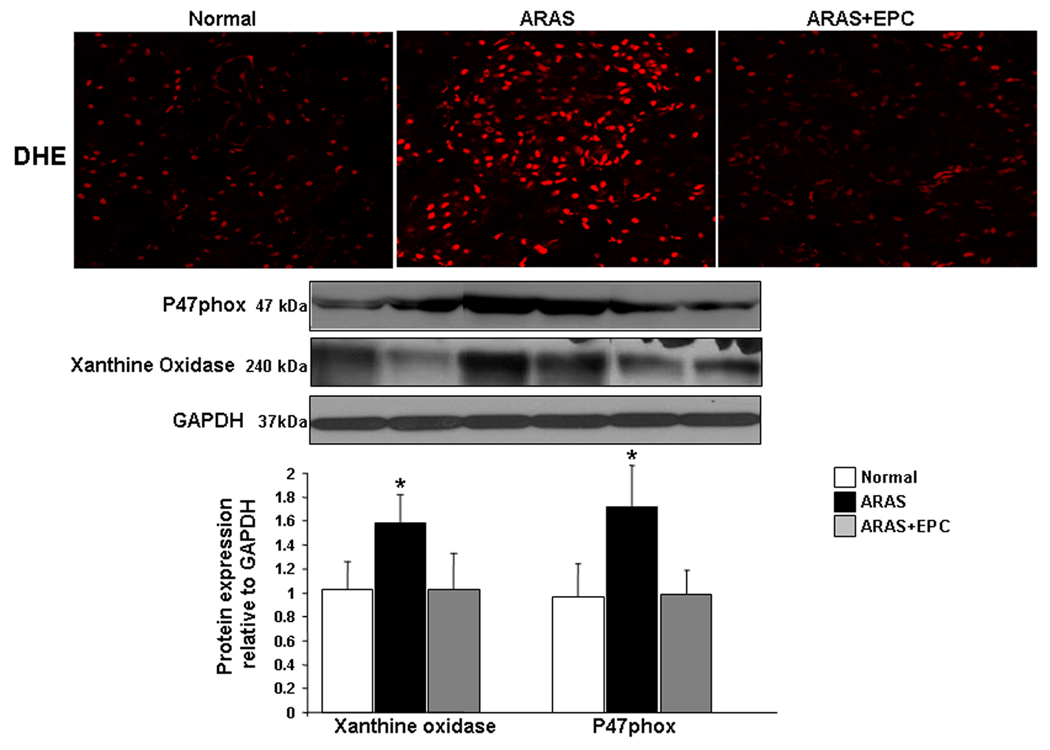
Renal MV 3D architecture and function: MV density was diminished throughout the ARAS renal cortex (inner, middle, and outer). EPC selectively improved MV density in the outer cortex, (Figure 3), while the inner and middle cortical MV density remained attenuated. The increased MV density in ARAS+EPC might have contributed to improve (albeit not normalize) RBF and GFR, which had been decreased in ARAS (Table 1). This improvement was accompanied by downregulated renal expression of NAD(P)H oxidase (p47 subunit), xanthine oxidase, and decreased renal superoxide that were elevated in ARAS, indicating a decrease in oxidative stress by EPC (Figure 4).
Figure 3.
Representative 3D tomographic images of the kidney (top) and quantification (bottom) of renal cortical microvascular density (diameters<500µm) in normal, atherosclerotic renal artery stenosis (ARAS), and ARAS pigs treated with intra-renal autologous endothelial progenitor cells (ARAS+EPC). EPC administration distinctly augmented microvascular density in the stenotic kidney outer cortex. *p<0.05 vs. Normal.
Figure 4.
Top: Representative dihydroethidium (DHE) assay to detect renal superoxide anion (red positive nuclei). Bottom: Representative protein expression and quantification of p47phox and xanthine oxidase in normal, atherosclerotic renal artery stenosis (ARAS), and ARAS kidneys treated with autologous endothelial progenitor cells (ARAS+EPC). EPC administration reduced renal oxidative stress*p<0.05 vs. Normal.
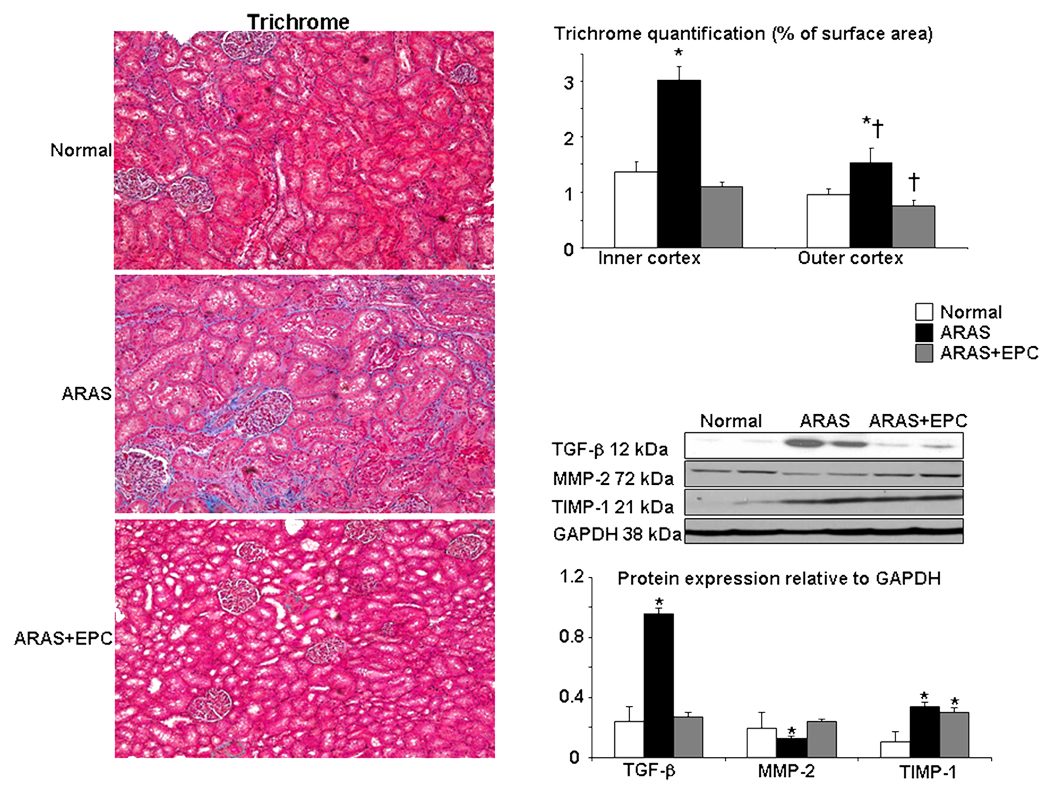
Renal fibrosis and morphology: the ARAS kidney showed renal glomerulosclerosis and tubulo-interstitial and perivascular fibrosis, which was greater in the inner than outer cortex, and accompanied by increased expression of renal TGF-β and TIMP-1, and attenuated MMP-2 (Figure 5). These deleterious changes were largely improved in ARAS+EPC, indicating an overall decrease in renal remodeling, mainly evident in the outer cortex.
Figure 5.
Left: Representative renal trichrome staining (left, ×20) and quantification in the outer and inner renal cortex. Right: protein expression and quantification of transforming growth factor (TGF)-β, matrix-metalloproteinase (MMP)-2 and tissue-inhibitor of metalloproteinase (TIMP)-1 in normal, atherosclerotic renal artery stenosis (ARAS), and ARAS kidneys treated with endothelial progenitor cells (ARAS+EPC). EPC administration decreased renal TGF-β improved MMP-2, and attenuated renal fibrosis. *p<0.05 vs. Normal, † p<0.05 vs. Inner cortex
Discussion
The current study shows that experimental renovascular hypertension modulates systemic EPC homing signaling, and identifies candidate homing/retention signals employed by the ischemic kidney to recruit and retain endogenous circulating EPC in order to stimulate its reparative processes. Autologous EPC expanded in culture expressed a variety of homing signal receptors, some of which corresponded to those expressed in the kidney and likely facilitated their retention and action. Compared to EPC isolated from normal animals, EPC from ARAS showed increased expression of SDF-1, angiopoietin-1, Tie 2, and c-kit, suggesting that early atherosclerosis modulated the homing capability of EPC. On the other hand, while the stenotic kidney activated some corresponding homing signaling, angiopoietin-1 and SDF-1/CXCR4, common mediators of cell homing, were either blunted or unchanged, implying incomplete heralding of an ischemic insult. Notably, delivery of autologous EPC restored renal angiogenic potency, improved cortical MV density, renal hemodynamics and function, decreased fibrosis of the stenotic kidney, and consequently decreased the endogenous injury signals.
Peripheral blood contains stem cell-like and monocytic-like endothelial cell progenitors, which have in vivo homing specificity to injured sites. These circulating cells have the potential to change phenotype when needed to promote vascular or parenchymal repair, and can stimulate neovascularization through paracrine secretion of growth factors, activate resident stem cells, and decrease tissue injury. A number of factors have been proposed to be released from damaged tissues, mobilize endogenous progenitor and stem cells13, and subsequently facilitate their engraftment in the host tissue. The interaction between tissues and circulating cells is mediated by corresponding molecules and cognate receptors released by or expressed in the injured tissue, and reciprocally the EPC.
Of the proposed key mediators of such signaling, SDF-1 and its CXCR4 receptor have been identified as a central signaling axis regulating migration and homing of progenitor cells to ischemic tissues5, 25, 26. A recent report suggests that an ischemic milieu, as characterizes the stenotic kidney, preconditions these cells and improves their migratory and homing capabilities27 partly through stimulation of SDF-1/CXCR428. We found that EPC isolated from ARAS pigs highly expressed SDF-1, while the expression of CXCR4 was similar to EPC from normal pigs. This pattern was relatively stable, as it was sustained during several passages in culture. However, the stenotic kidney did not increase the expression of either SDF-1 or CXCR4 compared to controls, implying a minor (if any) role of the SDF-1/CXCR4 axis in contributing to renal homing of EPC in our model of ARAS. Our observation is supported by recent evidence showing that stem cells can migrate and home into the kidney with acute ischemia/reperfusion injury independent of the SDF-1/CXCR4 axis25.
On the other hand, a number of factors were up-regulated in ARAS. Uric acid, which is overproduced by ischemic tissues and capable of mobilizing EPCs13, as well as SCF, which improves the EPC homing ability29 via its cKit receptor (upregulated in EPC from ARAS), were both increased in the stenotic kidney vein. These were accompanied by increased renal expression of EPO30–32, who attracts and directs circulating endogenous cell progenitors into ischemic tissues, and integrin β2, which promotes the adhesion of cell progenitors and augments neovascularization30, 33. Along the same lines, since angiopoietin-1 is essential to promote endothelial cell survival, vascular branching, and pericyte recruitment34, the increased expression of angiopoietin-1/Tie-2 in EPC from ARAS supports their potential for cell homing and vascular proliferation35. However, the co-expression of both the ligand and receptor in EPC is intriguing and of uncertain significance, yet likely does not diminish the homing potential and may prolong the survival of circulating EPCs36. It is interesting that we observed a dissociation of the upregulated expression of SDF/CXCR4 and angiopoietin-1/Tie-2 in the EPC but not the kidney. This likely reflects the plasticity of EPC to respond to an array of signals released from different injury sites, while in the injured ARAS kidney oxidized lipids37 or increased oxidative stress38 can interfere with EPC function39–41. Indeed, the signals might be tissue- and disease-specific, and thus different in the ARAS kidney from those observed in other conditions, such as the ischemic myocardium42 or hind-limb43, or diabetes44.
Progenitor cells exert their effects not only by lodging in a host tissue, but also by influencing surrounding parenchymal cells in a paracrine and autocrine fashion. We have recently shown in RAS uncomplicated by atherosclerosis that intra-renally infused EPC successfully engraft and incorporate into vascular and tubular structures in the stenotic kidney, improving angiogenic signaling and MV proliferation and decreasing renal damage11. Since atherosclerosis is the main etiology of RAS in humans, in the current study we used a clinically relevant model that mimics early atherosclerotic renovascular disease. This study extends our previous observations3, 19, 37, and demonstrates that renal dysfunction and injury in this model manifests in a decrease in cortical MV density and inadequate angiogenic signaling. Importantly, intra-renal delivery of EPC improved renal expression of most of the angiogenic, mobilizing, and homing factors, likely consequent to partial repair of the ARAS+EPC kidney and decreased need for progenitors. Progenitor cells are rich in vasculogenic factors like VEGF and its mediators, and tissue repair by EPC is partly mediated through increased delivery of VEGF and modulation of angiopoietins in the target tissue45. Angiopoietin-1 is a potent homing6 and angiogenic factor that interacts with VEGF46 to not only promote neovascularization and maturation of new vessels47, 48, but also to increase and extend circulating cell progenitors6. Therefore, the increase in renal expression of the specific angiopoietin receptor Tie-2 may imply improvement in both homing signal for EPC and angiogenic signaling in the ischemic kidney.
We have recently shown that in non-atherosclerotic RAS EPC effectively increased MV proliferation throughout the renal cortex11. Contrarily, generation of new vessels after EPC administration in ARAS was only evident in the small vessels in the outer renal cortex. This diminished effect may reflect preferential lodging of EPC in small vessels or sprouting of microvessels of smaller branching order, which are more abundant in the outer cortex15. The increased vascularity in the outer cortex was accompanied by a decrease (relative also to the inner cortex) in renal fibrosis, implying that the inner cortex may be more vulnerable to insults, possibly due to its spatial and functional relationship with the medulla. It is also possible that marked fibrosis in the inner cortex at the time of EPC delivery impeded neovascularization in this region. Because the number of cells (CFU) in both RAS11 and ARAS was similar to normal pigs, the greater renal functional and structural damage in ARAS3, 19 or lower EPC MV adherence and retention may have reduced their efficacy. In fact, a failure to increase EPC numbers upon injury may have further interfered with renal repair. Nevertheless, decreased cortical fibrosis and MV proliferation in the outer cortex of the ARAS+EPC kidneys was sufficient to improve in the decreased RBF and GFR that characterize ARAS3. This functional recovery likely resulted also from decreased renal oxidative stress and thereby vasoconstriction due to improved function of new or repaired vessels. Furthermore, EPC also distinctly improved the blunted expression of MMP-2 in the ARAS kidney, without affecting the upregulated expression of its inhibitor TIMP-1, and decreased the expression of the pro-fibrotic TGF-β, thereby facilitating matrix turnover. Overall, the increased blood supply and decreased noxious milieu resulted in attenuated renal fibrosis in ARAS+EPC.
The limitations of this study included the early stage of ARAS, because co-morbid conditions, duration, and severity of ischemia may modulate regulation of different mediators. Additional factors may have also contributed to the mobilization, homing, and engraftment of EPC in the ARAS kidney. EPC expanded in culture may not be identical to the endogenous circulating cells, yet may partly represent their function. In addition, the contribution of signals to EPC mobilization and homing, while widely accepted, was not directly demonstrated in this study. Further studies are needed to determine how this expression correlates with the efficacy of EPC-based reparative processes, and whether inadequate renal repair achieved by endogenous EPC is related to incomplete homing signals and progenitor recruitment capacity in ARAS.
In summary, using a clinically relevant model of experimental ARAS, the current study suggests that the stenotic ARAS kidney expresses specific homing factors that correspond to cognate receptors expressed on EPC, suggesting organ-specific mechanisms for EPC recruitment. Furthermore, cultured autologous EPC from ARAS pigs reveal angiogenic capacity and express homing receptors that correspond to a wide array of signals that may permit their response to a variety of signals from injured organs. Indeed, intra-renal delivery of exogenous EPC partly reversed the functional deterioration and damage to the ARAS kidney, which in turn decreased the injury signal it activated. These findings sheds light into the mechanisms by which EPC exert their renoprotective effects and, importantly, show feasibility and potential for cell-based therapy to rescue the kidney in chronic atherosclerotic renovascular disease.
Acknowledgments
Sources of funding: Supported by grant numbers DK-73608, DK-77013, HL-77131, and PO1HL085307 (Project 1, Core A, Core B, and Core C) from the NIH, and by Scientist Development Grant 0830100N from the American Heart Association.
Footnotes
Author contribution summary
Alejandro R. Chade: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript. Xiangyang Zhu: Conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript. James D. Krier: Collection and/or assembly of data, data analysis and interpretation. Kyra L Jordan: Collection and/or assembly of data, data analysis and interpretation. Stephen C. Textor: Data analysis and interpretation, manuscript writing. Joseph P. Grande: Data analysis and interpretation, collection and/or assembly of data. Amir Lerman: Data analysis and interpretation, manuscript writing. Lilach O. Lerman: Conception and design, collection and/or assembly of data, data analysis and interpretation, financial support, manuscript writing, final approval of manuscript.
Disclaimer: None
Disclosure of potential conflicts of interest:
The authors indicate no potential conflicts of interest.
References
- 1.Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431–442. doi: 10.1056/NEJM200102083440607. [DOI] [PubMed] [Google Scholar]
- 2.Chade AR, Mushin OP, Zhu X, et al. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46:772–779. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 3.Chade AR, Rodriguez-Porcel M, Grande JP, et al. Distinct renal injury in early atherosclerosis and renovascular disease. Circulation. 2002;106:1165–1171. doi: 10.1161/01.cir.0000027105.02327.48. [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Collins AJ. End-stage renal disease in the United States: an update from the United States Renal Data System. J Am Soc Nephrol. 2007;18:2644–2648. doi: 10.1681/ASN.2007020220. [DOI] [PubMed] [Google Scholar]
- 5.Mazzinghi B, Ronconi E, Lazzeri E, et al. Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med. 2008;205:479–490. doi: 10.1084/jem.20071903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore MA, Hattori K, Heissig B, et al. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. discussion 45-37. [DOI] [PubMed] [Google Scholar]
- 7.Raval AN. Therapeutic potential of adult progenitor cells in the management of chronic myocardial ischemia. Am J Cardiovasc Drugs. 2008;8:315–326. doi: 10.2165/00129784-200808050-00004. [DOI] [PubMed] [Google Scholar]
- 8.Aicher A, Heeschen C, Sasaki K, et al. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114:2823–2830. doi: 10.1161/CIRCULATIONAHA.106.628623. [DOI] [PubMed] [Google Scholar]
- 9.Patschan D, Krupincza K, Patschan S, et al. Dynamics of mobilization and homing of endothelial progenitor cells after acute renal ischemia: modulation by ischemic preconditioning. Am J Physiol Renal Physiol. 2006;291:F176–F185. doi: 10.1152/ajprenal.00454.2005. [DOI] [PubMed] [Google Scholar]
- 10.Uchimura H, Marumo T, Takase O, et al. Intrarenal injection of bone marrow-derived angiogenic cells reduces endothelial injury and mesangial cell activation in experimental glomerulonephritis. J Am Soc Nephrol. 2005;16:997–1004. doi: 10.1681/ASN.2004050367. [DOI] [PubMed] [Google Scholar]
- 11.Chade AR, Zhu X, Lavi R, et al. Endothelial progenitor cells restore renal function in chronic experimental renovascular disease. Circulation. 2009;119:547–557. doi: 10.1161/CIRCULATIONAHA.108.788653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daghini E, Primak AN, Chade AR, et al. Assessment of renal hemodynamics and function in pigs with 64-section multidetector CT: comparison with electron-beam CT. Radiology. 2007;243:405–412. doi: 10.1148/radiol.2432060655. [DOI] [PubMed] [Google Scholar]
- 13.Patschan D, Patschan S, Gobe GG, et al. Uric acid heralds ischemic tissue injury to mobilize endothelial progenitor cells. J Am Soc Nephrol. 2007;18:1516–1524. doi: 10.1681/ASN.2006070759. [DOI] [PubMed] [Google Scholar]
- 14.Zhu XY, Rodriguez-Porcel M, Bentley MD, et al. Antioxidant intervention attenuates myocardial neovascularization in hypercholesterolemia. Circulation. 2004;109:2109–2115. doi: 10.1161/01.CIR.0000125742.65841.8B. [DOI] [PubMed] [Google Scholar]
- 15.Zhu XY, Chade AR, Rodriguez-Porcel M, et al. Cortical microvascular remodeling in the stenotic kidney: role of increased oxidative stress. Arterioscler Thromb Vasc Biol. 2004;24:1854–1859. doi: 10.1161/01.ATV.0000142443.52606.81. [DOI] [PubMed] [Google Scholar]
- 16.Bentley MD, Rodriguez-Porcel M, Lerman A, et al. Enhanced renal cortical vascularization in experimental hypercholesterolemia. Kidney Int. 2002;61:1056–1063. doi: 10.1046/j.1523-1755.2002.00211.x. [DOI] [PubMed] [Google Scholar]
- 17.Chade AR, Bentley MD, Zhu X, et al. Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol. 2004;15:1816–1825. doi: 10.1097/01.asn.0000130428.85603.6b. [DOI] [PubMed] [Google Scholar]
- 18.Chade AR, Zhu X, Mushin OP, et al. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. Faseb J. 2006;20:1706–1708. doi: 10.1096/fj.05-5680fje. [DOI] [PubMed] [Google Scholar]
- 19.Chade AR, Rodriguez-Porcel M, Grande JP, et al. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:1295–1301. doi: 10.1161/01.ATV.0000077477.40824.52. [DOI] [PubMed] [Google Scholar]
- 20.Boengler K, Gres P, Cabestrero A, et al. Prevention of the ischemia-induced decrease in mitochondrial Tom20 content by ischemic preconditioning. J Mol Cell Cardiol. 2006;41:426–430. doi: 10.1016/j.yjmcc.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 21.Krier JD, Ritman EL, Bajzer Z, et al. Noninvasive measurement of concurrent single-kidney perfusion, glomerular filtration, and tubular function. Am J Physiol Renal Physiol. 2001;281:F630–F638. doi: 10.1152/ajprenal.2001.281.4.F630. [DOI] [PubMed] [Google Scholar]
- 22.Chade AR, Rodriguez-Porcel M, Herrmann J, et al. Beneficial effects of antioxidant vitamins on the stenotic kidney. Hypertension. 2003;42:605–612. doi: 10.1161/01.HYP.0000089880.32275.7C. [DOI] [PubMed] [Google Scholar]
- 23.Chade AR, Best PJ, Rodriguez-Porcel M, et al. Endothelin-1 receptor blockade prevents renal injury in experimental hypercholesterolemia. Kidney Int. 2003;64:962–969. doi: 10.1046/j.1523-1755.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 24.Chade AR, Rodriguez-Porcel M, Herrmann J, et al. Antioxidant intervention blunts renal injury in experimental renovascular disease. J Am Soc Nephrol. 2004;15:958–966. doi: 10.1097/01.asn.0000117774.83396.e9. [DOI] [PubMed] [Google Scholar]
- 25.Stroo I, Stokman G, Teske GJ, et al. Haematopoietic stem cell migration to the ischemic damaged kidney is not altered by manipulating the SDF-1/CXCR4-axis. Nephrol Dial Transplant. 2009;24:2082–2088. doi: 10.1093/ndt/gfp050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wysoczynski M, Reca R, Ratajczak J, et al. Incorporation of CXCR4 into membrane lipid rafts primes homing-related responses of hematopoietic stem/progenitor cells to an SDF-1 gradient. Blood. 2005;105:40–48. doi: 10.1182/blood-2004-04-1430. [DOI] [PubMed] [Google Scholar]
- 27.Rosova I, Dao M, Capoccia B, et al. Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells. 2008;26:2173–2182. doi: 10.1634/stemcells.2007-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang YL, Zhu W, Cheng M, et al. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hart C, Drewel D, Mueller G, et al. Expression and function of homing-essential molecules and enhanced in vivo homing ability of human peripheral blood-derived hematopoietic progenitor cells after stimulation with stem cell factor. Stem Cells. 2004;22:580–589. doi: 10.1634/stemcells.22-4-580. [DOI] [PubMed] [Google Scholar]
- 30.Brunner S, Winogradow J, Huber BC, et al. Erythropoietin administration after myocardial infarction in mice attenuates ischemic cardiomyopathy associated with enhanced homing of bone marrow-derived progenitor cells via the CXCR-4/SDF-1 axis. Faseb J. 2009;23:351–361. doi: 10.1096/fj.08-109462. [DOI] [PubMed] [Google Scholar]
- 31.Heeschen C, Aicher A, Lehmann R, et al. Erythropoietin is a potent physiologic stimulus for endothelial progenitor cell mobilization. Blood. 2003;102:1340–1346. doi: 10.1182/blood-2003-01-0223. [DOI] [PubMed] [Google Scholar]
- 32.Westenbrink BD, Lipsic E, van der Meer P, et al. Erythropoietin improves cardiac function through endothelial progenitor cell and vascular endothelial growth factor mediated neovascularization. Eur Heart J. 2007;28:2018–2027. doi: 10.1093/eurheartj/ehm177. [DOI] [PubMed] [Google Scholar]
- 33.Chavakis E, Aicher A, Heeschen C, et al. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Metheny-Barlow LJ, Li LY. The enigmatic role of angiopoietin-1 in tumor angiogenesis. Cell Res. 2003;13:309–317. doi: 10.1038/sj.cr.7290176. [DOI] [PubMed] [Google Scholar]
- 35.Lemstrom KB, Nykanen AI, Tikkanen JM, et al. Role of angiogenic growth factors in transplant coronary artery disease. Ann Med. 2004;36:184–193. doi: 10.1080/07853890310025243. [DOI] [PubMed] [Google Scholar]
- 36.Tsigkos S, Koutsilieris M, Papapetropoulos A. Angiopoietins in angiogenesis and beyond. Expert Opin Investig Drugs. 2003;12:933–941. doi: 10.1517/13543784.12.6.933. [DOI] [PubMed] [Google Scholar]
- 37.Chade AR, Zhu XY, Grande JP, et al. Simvastatin abates development of renal fibrosis in experimental renovascular disease. J Hypertens. 2008;26:1651–1660. doi: 10.1097/HJH.0b013e328302833a. [DOI] [PubMed] [Google Scholar]
- 38.Thum T, Bauersachs J. Spotlight on endothelial progenitor cell inhibitors: short review. Vasc Med. 2005;10 Suppl 1:S59–S64. doi: 10.1191/1358863x05vm607oa. [DOI] [PubMed] [Google Scholar]
- 39.Salguero G, Akin E, Templin C, et al. Renovascular hypertension by two-kidney one-clip enhances endothelial progenitor cell mobilization in a p47phox-dependent manner. J Hypertens. 2008;26:257–268. doi: 10.1097/HJH.0b013e3282f09f79. [DOI] [PubMed] [Google Scholar]
- 40.Pirro M, Bagaglia F, Paoletti L, et al. Hypercholesterolemia-associated endothelial progenitor cell dysfunction. Ther Adv Cardiovasc Dis. 2008;2:329–339. doi: 10.1177/1753944708094769. [DOI] [PubMed] [Google Scholar]
- 41.Chen JZ, Zhang FR, Tao QM, et al. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond) 2004;107:273–280. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- 42.Kocher AA, Schuster MD, Bonaros N, et al. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol. 2006;40:455–464. doi: 10.1016/j.yjmcc.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Hur J, Yoon CH, Lee CS, et al. Akt is a key modulator of endothelial progenitor cell trafficking in ischemic muscle. Stem Cells. 2007;25:1769–1778. doi: 10.1634/stemcells.2006-0385. [DOI] [PubMed] [Google Scholar]
- 44.Segal MS, Shah R, Afzal A, et al. Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes. 2006;55:102–109. [PubMed] [Google Scholar]
- 45.Awad O, Dedkov EI, Jiao C, et al. Differential healing activities of CD34+ and CD14+ endothelial cell progenitors. Arterioscler Thromb Vasc Biol. 2006;26:758–764. doi: 10.1161/01.ATV.0000203513.29227.6f. [DOI] [PubMed] [Google Scholar]
- 46.Zhu Y, Lee C, Shen F, et al. Angiopoietin-2 facilitates vascular endothelial growth factor-induced angiogenesis in the mature mouse brain. Stroke. 2005;36:1533–1537. doi: 10.1161/01.STR.0000170712.46106.2e. [DOI] [PubMed] [Google Scholar]
- 47.Chen F, Tan Z, Dong CY, et al. Combination of VEGF(165)/Angiopoietin-1 gene and endothelial progenitor cells for therapeutic neovascularization. Eur J Pharmacol. 2007;568:222–230. doi: 10.1016/j.ejphar.2007.04.047. [DOI] [PubMed] [Google Scholar]
- 48.Kim KL, Shin IS, Kim JM, et al. Interaction between Tie receptors modulates angiogenic activity of angiopoietin2 in endothelial progenitor cells. Cardiovasc Res. 2006;72:394–402. doi: 10.1016/j.cardiores.2006.08.002. [DOI] [PubMed] [Google Scholar]