Abstract
Cotreatment with testosterone (T) and 17β-estradiol (E2) is an established regimen for inducing of prostatic intraepithelial neoplasia (PIN) and prostate cancer in rodent models. We previously used the pure antiestrogen ICI 182,780 (ICI) and bromocriptine, a dopamine receptor agonist, to inhibit PIN induction and systemic hyperprolactinemia in Noble rats and found that the carcinogenic action of T+E2 is mediated directly by the effects of E2 on the prostate and/or indirectly via E2-induced hyperprolactinemia. In this study, we delineate the specific action(s) of E2 and prolactin (PRL) in early prostate carcinogenesis by an integrated approach combining global transcription profiling, gene ontology, and gene-network mapping. We identified 2504 differentially expressed genes in the T+E2-treated lateral prostate. The changes in expression of a subset of 1990 genes (∼80%) were blocked upon cotreatment with ICI and bromocriptine, respectively, whereas those of 262 genes (∼10%) were blocked only by treatment with ICI, suggesting that E2-induced pituitary PRL is the primary mediator of the prostatic transcriptional response to the altered hormone milieu. Bioinformatics analyses identified hormone-responsive gene networks involved in immune responses, stromal tissue remodeling, and the ERK pathway. In particular, our data suggest that IL-1β may mediate, at least in part, hormone-induced changes in gene expression during PIN formation. Together, these data highlight the importance of pituitary PRL in estrogen-induced prostate tumorigenesis. The identification of both E2- and pituitary PRL-responsive genes provides a comprehensive resource for future investigations of the complex mechanisms by which changes in the endocrine milieu contribute to prostate carcinogenesis in vivo.
We delineate the direct action of estrogen and its indirect effect via hyperprolactinemia on mediating global transcriptional responses associated with prostate pre-malignancy in Noble rats.
Both androgens and estrogens play important roles in prostate carcinogenesis (1,2,3). However, a cross-sectional study of racial and ethnic variations in circulating sex hormones demonstrated that black men, who have the highest incidence of prostate cancer (PCa) in the United States, also have the highest levels of circulating 17β-estradiol (E2), whereas the levels of circulating testosterone (T) in this group were comparable with white men (4). A phase IIB clinical studies have shown that antiestrogen Toremifene blocks PCa progression in men with high-grade prostatic intraepithelial neoplasia (PIN) (5). Furthermore, a change in intraprostatic hormone profiles toward estrogen predominance was observed in the aged prostate (6). These findings suggest that estrogen is a key determinant of prostate carcinogenesis.
Estrogens have both direct and indirect effects on the prostate. Their direct action is mediated through intraprostatic estrogen receptors. However, their indirect effects can be mediated through induction of hyperprolactinemia (7). It is well established that prolactin (PRL) promotes growth (8) and inflammation (9,10) of the prostate. However, whether this polypeptide is a cause of PCa remains controversial, because elevated circulating PRL has not been related to an increase in PCa risk (11,12). Nevertheless, an increase in prostatic PRL signaling owing to elevated expression of PRL receptor (13) and/or downstream mediator activation via the PRL-Jak2-STAT5 a/b pathway (14,15) could be the culprit in a heightened risk for PCa in the aged prostate.
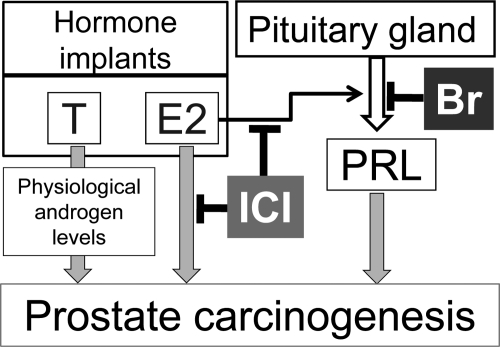
We (16) and others (17,18) have shown, in rodent models, that treatment with T+E2, which causes moderate elevations in E2 and maintains physiological T levels, elicits a malignant transformation in the prostate. Our laboratory has demonstrated that the carcinogenic action of T+E2 in the Noble (NBL) rat prostate is blocked by either the antiestrogen ICI 182,780 (ICI) or bromocriptine (Br), a dopamine receptor agonist that suppresses prolactinemia (19,20). ICI blocks both the local tissue estrogen response and systemic hyperprolactinemia (20), whereas Br inhibits only hyperprolactinemia (Fig. 1).
Figure 1.
A schematic diagram of the actions of T, E2, and estrogen-induced PRL in the NBL rat model of prostate carcinogenesis. ICI antagonizes estrogenic responses in the prostate gland and inhibits the release estrogen-induced PRL. Br only blocks the release of PRL from the pituitary.
In the present study, we used ICI and Br to identify the gene signatures induced by E2 or PRL in the NBL rat model through global transcriptional profiling (Fig. 2). We found that gene networks linked to inflammatory response, oxidative/nitrosative stress, stromal tissue remodeling, and ERK pathways are principally induced by PRL but also identified a set of unique E2-induced genes. Of significant interest was the identification of a PRL-induced IL-1β gene network that may be a mediator of the procarcinogenic effects of sex hormones in the rat prostate. Finally, our data provide strong evidence for the importance of PRL in the development of estrogen-induced PCa in rodent models and begs for confirmation in human studies.
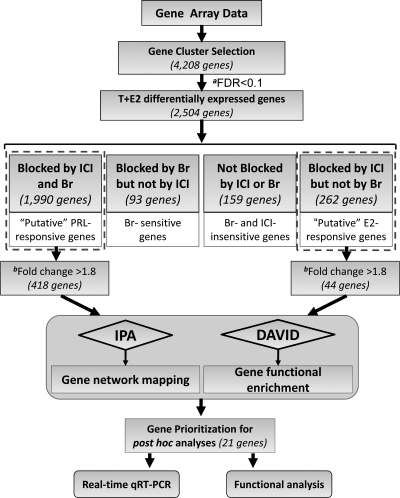
Figure 2.
A schematic diagram of the strategies and approaches used in gene shaving, candidate identification, bioinformatics analysis, and post hoc validation. aFDR, T+E2 vs. control; bFold change, T+E2 vs. control. DAVID, Database for Annotation, Visualization, and Integrated Discovery.
Results
Suppression by Br and ICI of T+E2-induced PIN development and epithelial cell proliferation
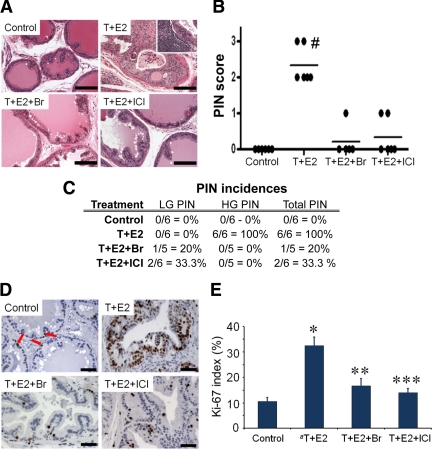
As we showed previously (16,19,20), the T+E2-treated lateral prostates (LPs) developed PIN lesions (PIN incidence, 100%; PIN score, 2.33) (Fig. 3A–C) with considerable inflammatory infiltrates. These changes were effectively blocked by cotreatment with Br (PIN incidence, 20%; PIN score, 0.2) or ICI (PIN incidence, 33.3%; PIN score, 0.33) (Fig. 3, A–C). The histology of LPs of untreated control rats remained normal (PIN incidence, 0%; PIN score, 0) (Fig. 3, A–C). A marked increase in epithelial cell proliferation, as assessed by Ki-67 index, was observed in PIN regions of the T+E2-treated LPs (Fig. 3, D and E). After cotreatment with Br or ICI, the basal epithelial proliferation rates throughout LP tissues remained unchanged as compared with those of untreated control rats (Fig. 3, D and E).
Figure 3.
Blockade of T+E2-induced PIN lesions in LPs with cotreatment with Br or ICI. A, Representative hematoxylin/eosin sections from LPs of the four treatment groups. Inset, Stromal inflammatory infiltrates associated with PIN. Scale bar, 100 μm. B, Mean PIN scores across treatment groups; #, P < 0.01 vs. all other groups. Each data point represents one animal. C, Incidence of PIN lesions across treatment groups. LG PIN, low-grade PIN; HG PIN, high-grade PIN. D, Representative Ki-67 staining of LPs. Red arrow, Ki-67-positive cells in control LP. Scale bar, 50 μm. E, Ki-67 index of LPs across treatment groups (n = 5). a, Data from PIN lesions of T+E2-treated LPs. *, P < 0.001 vs. control; **, P < 0.01 vs. T+E2; ***, P < 0.001 vs. T+E2. Error bar, sem.
Identification of the gene signature specifically associated with E2 or PRL action on the prostate
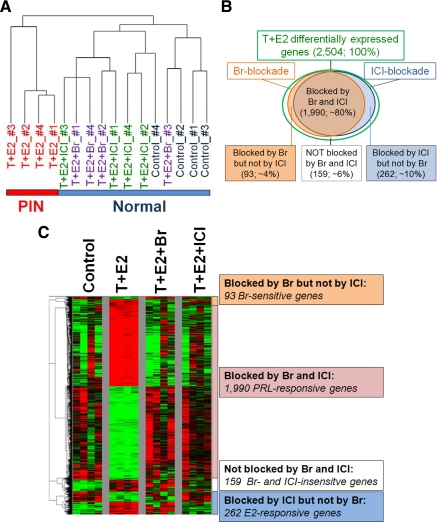
Hierarchical clustering showed that LPs treated with T+E2 formed a cluster distinct from the other three treatment groups (untreated controls, and T+E2+Br-treated and T+E2+ICI-treated LPs) (Fig. 4A), indicating that both Br and ICI effectively abrogated changes in gene expression induced by the T+E2 treatment. Using the strategy of microarray analysis described above (Fig. 2), we identified 2504 genes that were differentially affected by the T+E2 treatment (arbitrarily referred to as 100%). This gene set can be separated into four groups (Fig. 4, B and C, and Supplemental Table 1, A–D, published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org): 1) 1990 overlapping genes that are inhibitable by cotreatment with Br and ICI (putative PRL-responsive gene signature, ∼80%); 2) 262 genes whose changes in expression were inhibited only by ICI (putative E2-responsive gene signature, ∼10%); 3) 93 genes whose changes in expression were blocked only by Br (putative Br-sensitive genes, ∼4%); and 4) 159 genes showing no inhibitory responses to either antagonist (non-estrogen receptor/-PRL modifiable genes, ∼6%).
Figure 4.
Hierarchical clustering analysis of differential gene expression of LPs across treatment groups. A, Dendrogram shows that the clustering (Euclidean distances) separates T+E2-treated LPs harboring PIN lesions from the other three groups (control, T+E2+Br, and T+E2+ICI), which are histologically normal. B, Venn diagram shows T+E2-differentially expressed genes (number and percent) in response to Br and ICI blockade. A circle with a green border represents genes differentially expressed by T+E2 treatment. A circle with a brown border represents genes whose T+E2-induced differential changes are negated by Br. A circle with a blue border denotes genes whose changes are inhibited by ICI. The overlapping region between the brown and blue circles indicates the putative PRL-response genes. The blue region (right) denotes the putative E2-responsive genes. C, Heatmap shows the four signature panels of T+E2-differentially expressed genes in response to Br and ICI blockade. Green represents low expression; red represents high expression.
Selection of putative E2-/PRL-regulated gene candidates for post hoc confirmation
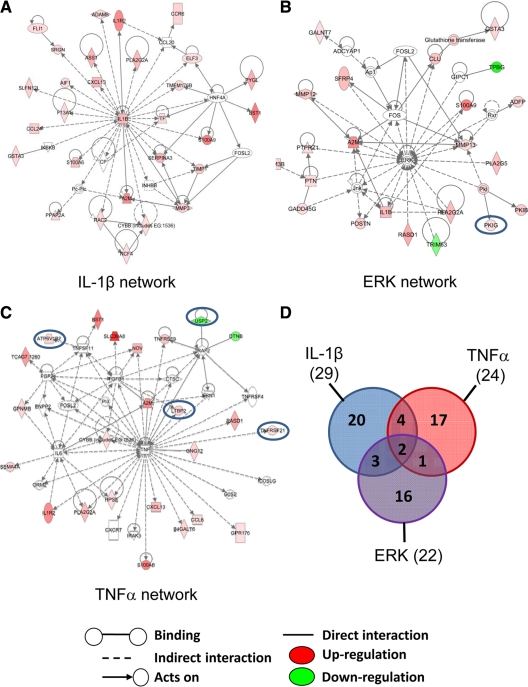
We next examined two gene sets, i.e. the putative PRL-gene signature and putative E2-gene signature, selected those with more than or equal to 1.8-fold changes, and conducted functional enrichment by Database for Annotation, Visualization, and Integrated Discovery and biological network/pathway mapping by Ingenuity Pathway Analysis (IPA). Among the 418 PRL-responsive genes with a T+E2-induced change of more than or equal to 1.8-fold (Supplemental Table 2), four major functional categories, including immune response, taxis, extracellular regions, and pattern binding, were significantly enriched (Table 1). IPA identified the top three networks (Fig. 5, A–C, and Supplemental Table 3, A–C) from which candidates were selected for post hoc analyses: IL-1β network (P value, 2.79 × 10−39), ERK network (P value, 1.27 × 10−25), and TNFα network (P value, 1.14 × 10−18). No significant enrichment of functional categories was observed among the 44 E2-responsive genes with a T+E2-induced change of more than or equal to 1.8-fold (Supplemental Table 4). Interestingly, IPA of this set of E2-responsive genes also revealed the ERK (P value, 3.44 × 10−5) and TNFα (P value, 6.3 × 10−12) networks with modest coverage (one E2 gene in the ERK network and four E2 genes in the TNFα network) (Fig. 5, B and C). There was not much overlapping between the IL-1β, ERK, and TNFα networks (Fig. 5D).
Table 1.
Gene Ontology functions significantly enriched in PRL-responsive genes
| No. of genes | Benjamini P value | |
|---|---|---|
| Immune response | ||
| Immune system process | 72 | 1.50E-17 |
| Immune response | 50 | 1.40E-15 |
| Defense response | 49 | 6.20E-15 |
| Inflammatory response | 38 | 2.10E-13 |
| Regulation of immune system process | 47 | 3.30E-13 |
| Response to wounding | 51 | 2.00E-12 |
| Response to external stimulus | 70 | 4.30E-11 |
| Positive regulation of immune system process | 33 | 5.40E-10 |
| Regulation of immune response | 30 | 5.10E-09 |
| Regulation of response to stimulus | 44 | 6.20E-09 |
| Acute inflammatory response | 21 | 6.80E-09 |
| Response to stimulus | 134 | 2.50E-08 |
| Positive regulation of immune response | 23 | 5.30E-08 |
| Leukocyte migration | 16 | 5.40E-08 |
| Response to stress | 85 | 8.60E-08 |
| Positive regulation of response to stimulus | 27 | 1.70E-06 |
| Response to chemical stimulus | 85 | 3.60E-06 |
| Immune effector process | 18 | 2.40E-05 |
| Activation of immune response | 15 | 3.40E-05 |
| Innate immune response | 14 | 1.20E-04 |
| Adaptive immune response | 13 | 1.90E-04 |
| Complement activation | 9 | 5.30E-04 |
| Activation of plasma proteins involved in acute inflammatory response | 9 | 7.00E-04 |
| Humoral immune response | 10 | 9.20E-04 |
| Taxis | ||
| Taxis | 18 | 1.90E-07 |
| Chemotaxis | 18 | 1.90E-07 |
| Cell chemotaxis | 13 | 5.70E-07 |
| Leukocyte chemotaxis | 12 | 3.20E-06 |
| Chemokine activity | 10 | 2.00E-04 |
| Chemokine receptor binding | 10 | 2.20E-04 |
| Cytokine activity | 15 | 7.30E-04 |
| Extracellular region | ||
| Extracellular region | 100 | 2.10E-19 |
| Extracellular region part | 66 | 4.90E-14 |
| Extracellular space | 50 | 6.10E-11 |
| Extracellular matrix | 29 | 1.70E-07 |
| Proteinaceous extracellular matrix | 25 | 2.70E-06 |
| Pattern binding | ||
| Heparin binding | 16 | 6.70E-06 |
| Carbohydrate binding | 28 | 4.50E-05 |
| Glycosaminoglycan binding | 16 | 1.50E-04 |
| Polysaccharide binding | 16 | 3.20E-04 |
| Pattern binding | 16 | 3.20E-04 |
Figure 5.
Ingenuity pathway analysis shows the most significant three gene networks of T+E2-treated LPs. A, IL-1β network: 29 genes were mapped principally to a network with IL-1β as a central node. B, ERK network: 22 genes were mapped to a network with ERK as a distinct node. C, TNFα network: 24 genes were mapped to a network with TNFα as a prominent node. Blue circles represent “estrogen-responsive” genes in which hormone-inducible changes were negated by antiestrogen ICI but not reversed by PRL blockade with Br. For more details of the IPA network legend, see Supplemental Fig. 1. The intensity of the node color indicates the degree of up- or down-regulation. Genes in uncolored nodes were not identified as differentially expressed in our array experiments and were incorporated into individual networks on the basis of the IPA knowledge database, indicating a relevance to this network. D, Venn diagram shows a partial overlap among the three identified gene networks.
Confirmation of changes in gene expression by real-time quantitative PCR (qPCR)
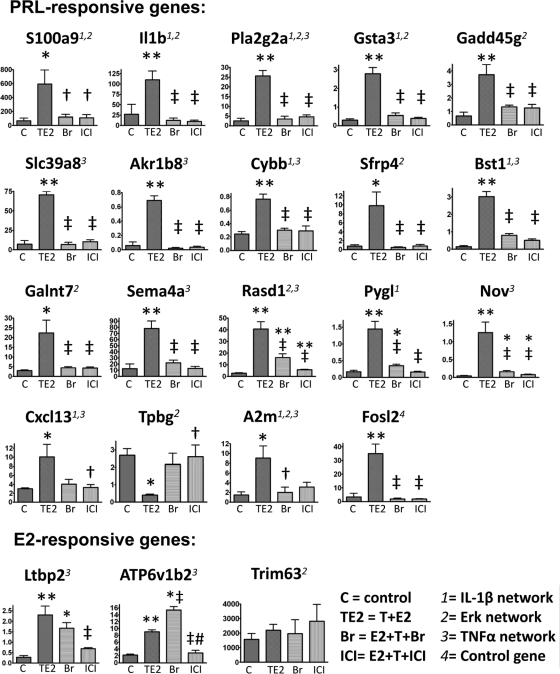
We selected 21 genes from the three gene networks for validation by real-time qPCR (Fig. 6 and Supplemental Table 3, A–C). We confirmed the PRL regulation of 18 genes [chemokine (C-X-C motif) ligand 13 (Cxcl13), S100 calcium binding protein A9 (S100a9), IL1b, phospholipase A2, group IIA (Pla2g2a), glutathione S-transferase α 3 (Gsta3), cytochrome b-245, β polypeptide (Cybb), solute carrier family 39 (zinc transporter), member 8 (Slc39a8), aldo-keto reductase family 1, member B8 (Akr1b8), growth arrest and DNA-damage-inducible 45γ (Gadd45g), secreted frizzled-related protein 4 (Sfrp4), bone marrow stromal cell antigen 1 (Bst1), RAS, dexamethasone-induced 1 (Rasd1), sema domain, Ig domain, transmembrane domain, and short cytoplasmic domain, (semaphorin) 4A (Sema4a), UDP-N-acetyl-α-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7 (Galnt7), phosphorylase, glycogen, liver (Pygl), nephroblastoma overexpressed gene (Nov), trophoblast glycoprotein (Tpbg), and α-2-macroglobulin (A2m)], and E2 regulation of two genes [ATPase, H transporting, lysosomal V1 subunit B2 (ATP6v1b2) and latent transforming growth factor beta binding protein 2 (Ltbp2)]. Fos-like antigen 2 (Fosl2), an internal control for the post hoc study, was previously shown by us to be overexpressed by T+E2 treatment and suppressed by ICI cotreatment (20). We confirmed our earlier observations and also showed that the hormone-induced Fosl2 overexpression was also blocked by Br cotreatment.
Figure 6.
Post hoc real-time qPCR analyses of the PRL- and E2-responsive genes. For PRL-responsive genes, the T+E2-induced differential expression is inhibited by Br (because it blocks PRL release) and ICI (because it blocks the E2-induced hyperprolactinemia). For E2-responsive genes, the T+E2-induced changes in expression are inhibited by ICI (because it blocks estrogenic actions in the prostate gland) but not by Br (because it does not affect the estrogenic signaling pathways). The data were normalized to the levels of Rpl19 (ribosomal protein L19, a housekeeping gene). sem (bars) for four animals in each treatment group; *, P < 0.05 vs. control; **, P < 0.01 vs. control; †, P < 0.05 vs. T+E2; ‡, P < 0.01 vs. T+E2; #, P < 0.01 vs. Br by one-way ANOVA with Tukey post hoc analysis. We previously showed Fosl2 (synonym, Fra2), a control gene, to be a T+E2-induced differentially expressed gene whose change in expression was inhibited by ICI (20). The expression of Trim63 was not induced by T+E2 and was not affected by cotreatment with either Br or ICI.
Increase by exogenous IL-1β of Cxcl13 and S100a9 gene expression in rat prostate epithelial cells
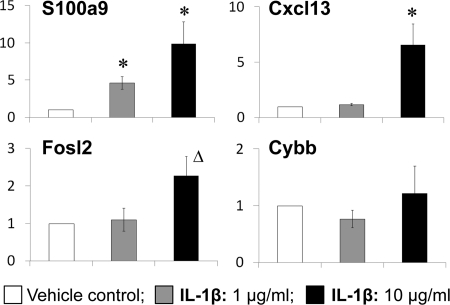
To illustrate that a “master” signaling regulator mediates hormone-induced changes in prostatic gene expression, we treated a normal rat prostate epithelial cell line (NBE-1) with the cytokine IL-1β, the central node of the IL-1β network, and found up-regulation of two of its direct downstream effectors, Cxcl13 and S100a9, but not Fosl2 and Cybb (Fig. 7).
Figure 7.
Effect of IL-1β on rat normal prostate epithelial cells (NBE-1). Relative levels of transcript expression of Cxcl13, s100a9, Cybb, and Fosl2 in response to IL-1β treatment (1 and 10 μg/ml) for 6 h; bars, sem; *, P < 0.05 vs. control; Δ, P = 0.07 vs. control.
Discussion
In the T+E2-induced rat prostate carcinogenesis model, we showed that cotreatment with Br or ICI significantly reduced PIN lesions (19,20), indicating that both E2 and E2-induced hyperprolactinemia are involved in early neoplastic transformation. In this study, we used global transcriptional profiling to uncover gene signatures associated with these two modes of action. First, we found that the majority of changes in gene expression were attributable to E2-induced hyperprolactemia. Using bioinformatics approach, we revealed that the top signaling pathways in the hyperprolactinemia-induced gene signature include IL-1β, ERK, and TNFα networks, and the biological functions demonstrating significant enrichment include immune response, taxis, extracellular region, and pattern binding. We also confirmed the expression pattern of 14 of 15 genes selected from this signature. Furthermore, using a cell-based approach, we validated the linkage of IL-1β, S100a9, and Cxcl13 identified in the IL-1β network. Second, we found that a small group of genes was directly altered by E2. Two of six genes selected from this category were confirmed to be estrogen responsive (Ltbp2 and ATP6v1b2), and the other four were found to be associated with hyperprolactinemia. These data support our hypothesis that both estrogen and PRL are contributors in this prostate carcinogenesis model.
In this study, almost 80% of the transcriptional response to T+E2 treatment was mediated by E2-induced hyperprolactinemia, highlighting the importance of PRL in rat prostate carcinogenesis. Epidemiologic and clinical studies did not show an association between hyperprolactinemia and PCa or aging in men, thus raising doubts that PRL is involved in prostate carcinogenesis. However, recent data from Nevalainen and co-workers (14,15) demonstrated that heightened PRL signaling in the prostate mighty be a key factor in its neoplastic transformation. Their data are supported by findings in a number of animal studies, including our present study. Mice overexpressing PRL ubiquitously or in the prostate developed prostatic hyperplasia but not high-grade PIN and PCa (21,22), whereas knockdown of the PRL receptor blocked PIN and PCa development in a spontaneous PCa mouse model (23). Collectively, these findings emphasize that local, but not circulating, levels of PRL are important for prostate carcinogenesis and suggest the hyperprolactinemia-associated transcriptome we found in our model.
The molecular targets linking PRL to PCa are largely unknown. Here, we identified two gene networks around IL-1β and TNFα nodes associated with PRL actions. IL-1β and TNFα are inflammatory mediators in tumorigenesis (24). These two inflammatory cytokines have been shown to induce protein degradation of NK3 homeobox 1, a prostate tumor suppressor, in human PCa cells (25). Microarray analysis has also identified an IL-1β node in the most significant gene network in human PCa tissues (26). The importance of IL-1β in human PCa was also demonstrated by a recent report showing an association between IL-1β gene polymorphisms and increased PCa aggressiveness in white American men (27).
We identified up-regulation of specific members of the S100 family and a panel of genes related to eicosanoid metabolism in the IL-1β- and TNFα-associated gene networks. S100a8 and S100a9 heterodimerize to act as chemotractants for leukocytes and have been detected in the human prostate, as evident in tumor/PIN epithelium, infiltrating immune cells, and corpora amylacea inclusions (28,29,30). Pla2g2a, phosphatidic acid phosphatase type 2A (Ppap2a), and E74-like factor 3 (Elf3) are key regulators of eicosanoid generation, which is closely linked to inflammatory lipid signaling in tumorigenesis (31,32). Expression of Pla2g2a has been shown to be induced by proinflammatory cytokines IL-1β, IL-6, and TNFα in normal and malignant human prostate cell lines (33), consistent with our observations in the present gene-network analysis. It is noteworthy that our previous study found an increase in cyclooxygenase-2 expression in sex hormone-induced PIN epithelial cells (34). Together, these data suggest that sex hormone-induced dysregulation in eicosanoid metabolism is an early event in prostatic tumorigenesis.
We also found evidence for the overexpression of specific panels of genes related to immune-cell activation [chemokine (C-C motif) ligand 24 (Ccl24), chemokine (C-C motif) ligand 6 (Ccl6), chemokine (C-C motif) receptor 6 (Ccr6), allograft inflammatory factor 1 (Aif1), Bst1, Sema4a, Slc39a8, and serglycin (Srgn)], reactive oxygen/nitrogen species production [Cybb, neutrophil cytosolic factor 4 (Ncf4), ras-related C3 botulinum toxin substrate 2 (Rac2), argininosuccinate synthetase 1 (Ass1), Rasd1, and Elf3] and oxidative/nitrosative stress response (Gsta3 and Akr1b8) in these inflammatory gene networks. These findings are consistent with previous findings, from us and others, reporting prostatic inflammation after exposure to neonatal (10) or adult (9,34,35) estrogens.
Aberrant activation of ERK pathway plays a key role in cell proliferation and malignant transformation (36). Here, we identify a gene network with ERK as the most prominent node, consistent with our previous study showing the protracted mitogenic action of MAPK in the T+E2-induced PIN lesions (37). We showed that regulation of many of the genes in this network was mediated by PRL. Notably, our network mapping analysis suggested a link between several transcripts [IL-1β, S100A9, A2m, pleiotrophin (Ptn), and protein tyrosine phosphatase, receptor-type, Z polypeptide 1 (Ptprz1)] and the MAPK pathway, based on the literature related to prostatic malignancy. In addition to provoking inflammatory responses, IL-1β and S100a9 have also been shown to promote human PCa cell proliferation and migration via an ERK-dependent pathway (38,39). A2m is a plasma protease inhibitor and also a protein binding with cytokines/growth factors. When A2m is cleaved and thus activated by matrix metalloproteinases, it is capable of triggering proliferation and survival of human PCa cells through activation of multiple signaling pathways, including ERK (40). We also found evidence for increased expression of Ptn (also known as heparin affin regulatory peptide) and its cognate receptor, Ptprz1, in the hormone-treated prostate harboring preneoplastic lesions. Although Ptn plays a key role in promoting PCa cell proliferation, migration, and angiogenicity (41,42), little is known about its contribution, if any, to the early development of PCa. Regarding its connection to ERK, Ptn gene expression is mediated by hydrogen peroxide and nitric oxide through ERK activation in human PCa cells. Overall, we propose that this biological network is associated with aberrant ERK signaling in response to the procarcinogenic actions of hormones on the prostate gland.
Remodeling of the stromal microenvironment, mediated by extracellular matrix (ECM)-degrading enzymes and their modulators, has been implicated in early prostate tumorigensis (20,43,44). We identified a group of tissue-remodeling genes in the gene-network mapping and gene-functional enrichment analyses. These genes include enzymes degrading ECM proteins and proteoglycans [matrix metallopeptidase 3 (Mmp3), Mmp12, Mmp13, ADAM metallopeptidase domain 8 (Adam8), and heparanase (Hpse)], protease modulators [tissue inhibitor of metallopeptidase 1 (Timp1), Cxcl13, glycoprotein (transmembrane) nmb (Gpnmb), and Atp6v1b2], ECM structural and associated proteins (Ltbp2 and Nov), cell adhesion/matricellular proteins and proangiogenic factors [periostin, osteoblast specific factor (Postn), dystrobrevin, β (Dtnb), and coagulation factor XIII, A1 subunit (F13a1)]. All genes except Dtnb were unregulated in response to T+E2 treatment. These findings are novel and of particular significance, because many of these genes have not been reported to play a role in early prostatic malignancy (Supplemental Discussion). Our data clearly demonstrate that regulation of the expression of all of the aforementioned tissue-remodeling genes, except Atp6v1b2 and Ltbp2, is mediated by PRL, whereas Atp6v1b2 and Ltbp2 are directly regulated by E2 (which was confirmed by our post hoc analysis). This suggests that E2 has a direct role in regulating a unique set of genes involving stromal remodeling in this rodent model. Of importance is the observation of tissue-remodeling activities in the hyperplastic prostate of Pb-PRL transgenic mice (45). The results reported here, taken together, suggest that concerted actions of PRL and E2 create a favorable stromal microenvironment for the initiation and progression of PCa.
To validate the relevancy of our network analysis, we chose to focus on the IL-1β network and to test whether IL-1β regulates expression of the peripherally connecting genes (S100a9, Cxcl13, Cybb, and Fosl-2) in rat prostate normal epithelial cells in vitro. We showed that exogenous IL-1β significantly elevated the expression of S100a9 and Cxcl13. These data suggest that IL-1β may mediate, at least in part, hormone-induced changes in gene expression during the early phase of neoplastic transformation. Further exploration into other relevant networks is warranted to establish an overall picture of the transcriptional orchestration in prostatic hormonal carcinogenesis.
This study provides not only a view of individual gene networks/pathways but also novel insights into the mechanistic linkage between sex hormones and PCa initiation. The finding that functional PRL receptors are expressed in the human prostate, PIN, and neoplastic lesions (13,46) and evidence that autocrine PRL promotes human PCa growth (15), together with our present work on the NBL rat model, provide a relevant resource for understanding how changes in hormonal milieu contribute to prostate carcinogenesis. Our findings also highlight the importance of PRL in other rodent models for estrogen-induced prostatic malignancy (17,18,47). Our findings may have implications for human prostate tumorigenesis with regard to E2 and the local production of prostatic PRL by an autocrine mechanism or via inflammatory infiltrates, on the complexity of hormonal balance, in this disease.
Materials and Methods
Animals and hormone treatment
The protocol for animal use was approved by the Institutional Animal Care Committee at the University of Cincinnati. Male NBL rats (5–6 wk old) were purchased from Charles River Laboratories (Kingston, NY), kept under standard conditions, and treated as previously reported (16,19,20,34). Animals were randomized into four groups (n = 5 or 6): untreated control, T+E2, T+E2+Br, and T+E2+ICI. At the end of a 16-wk treatment period, animals were killed and their LPs were excised for subsequent analyses.
Histopathology
Formalin-fixed samples were processed for hematoxylin/eosin staining and scored for PIN lesions, as previously described (16,48,49). The incidence and mean PIN score per treatment group were determined and analyzed by the Kruskal-Wallis test followed by the Mann-Whitney U test in the post hoc analysis. To control for multiple testing, we applied a Bonferroni adjustment to adjust the significance level of each comparison by dividing 0.05 by 6, the number of comparisons. P < 0.01 was used to determine significance for comparing treatment means.
Immunohistochemistry
Immunohistochemical analysis of Ki-67 (NCL-Ki67-MM1; Novocastra Laboratories, Newcastle Upon Tyne, UK) was conducted, and the Ki-67 index of epithelial cells was determined, as previously described (49). The data were analyzed by ANOVA and post hoc Bonferroni tests, where P < 0.05 was considered significant.
RNA isolation and microarray hybridization
RNA from LPs of four animals in each group was extracted with the TRIZO reagent as described (Invitrogen, Carlsbad, CA). Rat Genome 230 2.0 Array GeneChips were hybridized with 15 μg of fragmented amplified RNA. The hybridization, staining, and washing were carried out with the Affymetrix GeneChip Hybridization Wash and Stain kit (P/N 900720) per the manufacturer’s protocols. The GeneChips were scanned with Affymetrix GeneChip Scanner 3000 7G with GCOS software and Affymetrix preset settings.
Microarray data analysis
Raw data files (Affymetrix Rat Genome 230 2.0 CEL files) were Robust Multichip Analysis-preprocessed (50) using the Entrez Gene-based custom CDF (version 10) from the Psychiatry/Molecular and Behavioral Neuroscience Institute Microarray Lab at the University of Michigan (51). Minimum Information About a Microarray Experiment-annotated primary and processed microarray data are accessible through the Gene Expression Omnibus database under the accession identifier GSE17819. For more details of clustering and statistical analysis, see Supplemental data.
Identification of E2 and PRL gene signatures
Figure 2 illustrates the strategies and approaches used in gene shaving, candidate identification, bioinformatic analysis, and gene prioritization for post hoc validation. Gene clusters regulated by the direct E2 effect or PRL were identified by visual inspection of the corresponding heatmaps by selecting expression patterns exhibiting differential expression among the four treatment groups. Genes were further filtered by [false discovery rate (FDR), <0.1] over- or underexpression in the T+E2 treatment group. The E2- and PRL-signature genes were identified by their respective responses to ICI and Br cotreatments: 1) genes were considered to be regulated directly by E2 when the changes in expression induced by T+E2 were significantly (FDR, <0.1) blocked by ICI but not by Br; and 2) genes were considered to be affected by an indirect action on E2 via pituitary PRL when T+E2-induced changes in expression were blocked concurrently in the Br and ICI cotreatment groups (FDR, <0.1; in the comparison with the T+E2 and T+E2+hormone blocker groups).
Gene functional enrichment and biological network mapping
Genes regulated by E2 or PRL with a more than 1.8-fold difference between control and T+E2 groups were annotated by the Database for Annotation, Visualization, and Integrated Discovery (National Institute of Allergy and Infectious Diseases, National Institutes of Health) (52) using Gene Ontology database. The top significant biological functions were identified by a cutoff in Benjamini P value of less than 0.001. The PRL- or E2-responsive genes were also analyzed by IPA 7.5 software (Ingenuity, Redwood City, CA). We selected three biological networks with top significance (indicated by P value) for post hoc analysis.
Real-time qRT-PCR
Real-time qRT-PCR was performed as previously described (34). In brief, total RNA was reverse transcribed into cDNA using Superscript III (Invitrogen). The PCR was performed using the SYBR GreenER qPCR SuperMix Universal (Invitrogen). Relative expression was analyzed by the ΔΔCt method (53) or Pfaffl’s model (54) and one-way ANOVA followed by Tukey post hoc analysis, where P < 0.05 was considered statistically significant. Sequences of primers are listed in the Supplemental Table 5.
Prostate cell cultures and IL-1β treatment
A normal prostate epithelial NbE-1 cell line was established from the NBL rat and immortalized as described previously (55). Cell cultures were treated with recombinant rat IL-1β at 1 or 10 μg/ml (R&D Systems, Minneapolis, MN) or PBS as a vehicle control for 6 h. RNA was extracted for real-time RT-PCR quantification of mRNA abundance of Cxcl13, S100a9, Fosl2, and Cybb.
Supplementary Material
Acknowledgments
We thank Mr. Saikumar Karyala and the Genomics and Microarray Laboratory at the University of Cincinnati for their technical expertise in Affymetrix microarray experiments, Dr. Xiang Zhang for help with the IPA analysis, Dr. Shlomo Bakshi, Ms. Hong Xiao, and Ms. Dan Song for their assistance with some of the animal work, and Ms. Dan Song for performing immunohistochemistry. We also thank Dr. Linda Levin for her expertise in the statistical analysis of PIN score.
Footnotes
Present address for C.Y.Y.S.: Department of Clinical Oncology, The University of Hong Kong, Hong Kong.
This work was supported by CA015776, CA112532, ES006096, and ES015584 (to S.-M.H.) and by Ohio Cancer Research Associates-Pilot Grant (to N.N.C.T.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online September 22, 2010
N.N.C.T. and C.Y.Y.S. contributed equally to this work.
Abbreviations: A2m, α-2-Macroglobulin; Br, bromocriptine; Cxcl13, chemokine (C-X-C motif) ligand 13; Cybb, cytochrome b-245, β polypeptide; E2, 17β-estradiol; ECM, extracellular matrix; FDR, false discovery rate; Fosl2, Fos-like antigen 2; ICI, ICI 182,780; IPA, Ingenuity Pathway Analysis; LP, lateral prostate; Ltbp2, latent transforming growth factor beta binding protein 2; NBL, Noble; PCa, prostate cancer; PIN, prostatic intraepithelial neoplasia; Pla2g2a, phospholipase A2, group IIA; PRL, prolactin; Ptn, pleiotrophin; qPCR, quantitative PCR; S100a9, S100 calcium binding protein A9; T, testosterone.
References
- Bosland MC 2000 The role of steroid hormones in prostate carcinogenesis. J Natl Cancer Inst Monogr 39–66 [DOI] [PubMed] [Google Scholar]
- Prins GS, Korach KS 2008 The role of estrogens and estrogen receptors in normal prostate growth and disease. Steroids 73:233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson SJ, Ellem SJ, Risbridger GP 2008 Estrogen-regulated development and differentiation of the prostate. Differentiation 76:660–670 [DOI] [PubMed] [Google Scholar]
- Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, Yager JD, Platz EA 2007 Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. J Clin Endocrinol Metab 92:2519–2525 [DOI] [PubMed] [Google Scholar]
- Price D, Stein B, Sieber P, Tutrone R, Bailen J, Goluboff E, Burzon D, Bostwick D, Steiner M 2006 Toremifene for the prevention of prostate cancer in men with high grade prostatic intraepithelial neoplasia: results of a double-blind, placebo controlled, phase IIB clinical trial. J Urol 176:965–970 [DOI] [PubMed] [Google Scholar]
- Krieg M, Nass R, Tunn S 1993 Effect of aging on endogenous level of 5 alpha-dihydrotestosterone, testosterone, estradiol, and estrone in epithelium and stroma of normal and hyperplastic human prostate. J Clin Endocrinol Metab 77:375–381 [DOI] [PubMed] [Google Scholar]
- Bartke A, Doherty PC, Steger RW, Morgan WW, Amador AG, Herbert DC, Siler-Khodr TM, Smith MS, Klemcke HG, Hymer WC 1984 Effects of estrogen-induced hyperprolactinemia on endocrine and sexual functions in adult male rats. Neuroendocrinology 39:126–135 [DOI] [PubMed] [Google Scholar]
- Costello LC, Franklin RB 1994 Effect of prolactin on the prostate. Prostate 24:162–166 [DOI] [PubMed] [Google Scholar]
- Tangbanluekal L, Robinette CL 1993 Prolactin mediates estradiol-induced inflammation in the lateral prostate of Wistar rats. Endocrinology 132:2407–2416 [DOI] [PubMed] [Google Scholar]
- Gilleran JP, Putz O, DeJong M, DeJong S, Birch L, Pu Y, Huang L, Prins GS 2003 The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology 144:2046–2054 [DOI] [PubMed] [Google Scholar]
- Lissoni P, Bignami A, Frontini L, Manganini V, Dapretto E, Gardani GS, Viganò P, Strada G 2005 Possible involvement of prolactin in endocrine-resistant metastatic prostate cancer. Int J Biol Markers 20:123–125 [PubMed] [Google Scholar]
- Stattin P, Rinaldi S, Stenman UH, Riboli E, Hallmans G, Bergh A, Kaaks R 2001 Plasma prolactin and prostate cancer risk: a prospective study. Int J Cancer 92:463–465 [DOI] [PubMed] [Google Scholar]
- Leav I, Merk FB, Lee KF, Loda M, Mandoki M, McNeal JE, Ho SM 1999 Prolactin receptor expression in the developing human prostate and in hyperplastic, dysplastic, and neoplastic lesions. Am J Pathol 154:863–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagvadorj A, Kirken RA, Leiby B, Karras J, Nevalainen MT 2008 Transcription factor signal transducer and activator of transcription 5 promotes growth of human prostate cancer cells in vivo. Clin Cancer Res 14:1317–1324 [DOI] [PubMed] [Google Scholar]
- Dagvadorj A, Collins S, Jomain JB, Abdulghani J, Karras J, Zellweger T, Li H, Nurmi M, Alanen K, Mirtti T, Visakorpi T, Bubendorf L, Goffin V, Nevalainen MT 2007 Autocrine prolactin promotes prostate cancer cell growth via Janus kinase-2-signal transducer and activator of transcription-5a/b signaling pathway. Endocrinology 148:3089–3101 [DOI] [PubMed] [Google Scholar]
- Leav I, Ho SM, Ofner P, Merk FB, Kwan PW, Damassa D 1988 Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Natl Cancer Inst 80:1045–1053 [DOI] [PubMed] [Google Scholar]
- Ricke WA, McPherson SJ, Bianco JJ, Cunha GR, Wang Y, Risbridger GP 2008 Prostatic hormonal carcinogenesis is mediated by in situ estrogen production and estrogen receptor alpha signaling. FASEB J 22:1512–1520 [DOI] [PubMed] [Google Scholar]
- Wang Y, Hayward SW, Donjacour AA, Young P, Jacks T, Sage J, Dahiya R, Cardiff RD, Day ML, Cunha GR 2000 Sex hormone-induced carcinogenesis in Rb-deficient prostate tissue. Cancer Res 60:6008–6017 [PubMed] [Google Scholar]
- Lane KE, Leav I, Ziar J, Bridges RS, Rand WM, Ho SM 1997 Suppression of testosterone and estradiol-17beta-induced dysplasia in the dorsolateral prostate of Noble rats by bromocriptine. Carcinogenesis 18:1505–1510 [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Tam NN, Joyce JM, Leav I, Ho SM 2002 Gene expression profiling of testosterone and estradiol-17 beta-induced prostatic dysplasia in Noble rats and response to the antiestrogen ICI 182,780. Endocrinology 143:2093–2105 [DOI] [PubMed] [Google Scholar]
- Wennbo H, Kindblom J, Isaksson OG, Törnell J 1997 Transgenic mice overexpressing the prolactin gene develop dramatic enlargement of the prostate gland. Endocrinology 138:4410–4415 [DOI] [PubMed] [Google Scholar]
- Kindblom J, Dillner K, Sahlin L, Robertson F, Ormandy C, Törnell J, Wennbo H 2003 Prostate hyperplasia in a transgenic mouse with prostate-specific expression of prolactin. Endocrinology 144:2269- 2278 [DOI] [PubMed] [Google Scholar]
- Robertson FG, Harris J, Naylor MJ, Oakes SR, Kindblom J, Dillner K, Wennbo H, Törnell J, Kelly PA, Green J, Ormandy CJ 2003 Prostate development and carcinogenesis in prolactin receptor knockout mice. Endocrinology 144:3196–3205 [DOI] [PubMed] [Google Scholar]
- Germano G, Allavena P, Mantovani A 2008 Cytokines as a key component of cancer-related inflammation. Cytokine 43:374–379 [DOI] [PubMed] [Google Scholar]
- Markowski MC, Bowen C, Gelmann EP 2008 Inflammatory cytokines induce phosphorylation and ubiquitination of prostate suppressor protein NKX3.1. Cancer Res 68:6896–6901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savli H, Szendröi A, Romics I, Nagy B 2008 Gene network and canonical pathway analysis in prostate cancer: a microarray study. Exp Mol Med 40:176–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabaleta J, Su LJ, Lin HY, Sierra RA, Hall MC, Sartor AO, Clark PE, Hu JJ, Ochoa AC 2009 Cytokine genetic polymorphisms and prostate cancer aggressiveness. Carcinogenesis 30:1358–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermani A, Hess J, De Servi B, Medunjanin S, Grobholz R, Trojan L, Angel P, Mayer D 2005 Calcium-binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res 11:5146–5152 [DOI] [PubMed] [Google Scholar]
- Yanamandra K, Alexeyev O, Zamotin V, Srivastava V, Shchukarev A, Brorsson AC, Tartaglia GG, Vogl T, Kayed R, Wingsle G, Olsson J, Dobson CM, Bergh A, Elgh F, Morozova-Roche LA 2009 Amyloid formation by the pro-inflammatory S100A8/A9 proteins in the ageing prostate. PLoS One 4:e5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sfanos KS, Wilson BA, De Marzo AM, Isaacs WB 2009 Acute inflammatory proteins constitute the organic matrix of prostatic corpora amylacea and calculi in men with prostate cancer. Proc Natl Acad Sci USA 106:3443–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyanovsky BB, Webb NR 2009 Biology of secretory phospholipase A2. Cardiovasc Drugs Ther 23:61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grkovich A, Dennis EA 2009 Phosphatidic acid phosphohydrolase in the regulation of inflammatory signaling. Adv Enzyme Regul 49:114–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menschikowski M, Hagelgans A, Gussakovsky E, Kostka H, Paley EL, Siegert G 2008 Differential expression of secretory phospholipases A2 in normal and malignant prostate cell lines: regulation by cytokines, cell signaling pathways, and epigenetic mechanisms. Neoplasia 10:279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam NN, Leav I, Ho SM 2007 Sex hormones induce direct epithelial and inflammation-mediated oxidative/nitrosative stress that favors prostatic carcinogenesis in the noble rat. Am J Pathol 171:1334–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernoulli J, Yatkin E, Talvitie EM, Santti R, Streng T 2007 Urodynamic changes in a noble rat model for nonbacterial prostatic inflammation. Prostate 67:888–899 [DOI] [PubMed] [Google Scholar]
- Marshall CJ 1995 Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179–185 [DOI] [PubMed] [Google Scholar]
- Leav I, Galluzzi CM, Ziar J, Stork PJ, Ho SM, Loda M 1996 Mitogen-activated protein kinase and mitogen-activated kinase phosphatase-1 expression in the Noble rat model of sex hormone-induced prostatic dysplasia and carcinoma. Lab Invest 75:361–370 [PubMed] [Google Scholar]
- Hermani A, De Servi B, Medunjanin S, Tessier PA, Mayer D 2006 S100A8 and S100A9 activate MAP kinase and NF-kappaB signaling pathways and trigger translocation of RAGE in human prostate cancer cells. Exp Cell Res 312:184–197 [DOI] [PubMed] [Google Scholar]
- Tsai CY, Lee TS, Kou YR, Wu YL 2009 Glucosamine inhibits IL-1beta-mediated IL-8 production in prostate cancer cells by MAPK attenuation. J Cell Biochem 108:489–498 [DOI] [PubMed] [Google Scholar]
- Misra UK, Deedwania R, Pizzo SV 2006 Activation and cross-talk between Akt, NF-kappaB, and unfolded protein response signaling in 1-LN prostate cancer cells consequent to ligation of cell surface-associated GRP78. J Biol Chem 281:13694–13707 [DOI] [PubMed] [Google Scholar]
- Vacherot F, Caruelle D, Chopin D, Gil-Diez S, Barritault D, Caruelle JP, Courty J 1999 Involvement of heparin affin regulatory peptide in human prostate cancer. Prostate 38:126–136 [DOI] [PubMed] [Google Scholar]
- Hatziapostolou M, Delbe J, Katsoris P, Polytarchou C, Courty J, Papadimitriou E 2005 Heparin affin regulatory peptide is a key player in prostate cancer cell growth and angiogenicity. Prostate 65:151–158 [DOI] [PubMed] [Google Scholar]
- Li SC, Chen GF, Chan PS, Choi HL, Ho SM, Chan FL 2001 Altered expression of extracellular matrix and proteinases in Noble rat prostate gland after long-term treatment with sex steroids. Prostate 49:58–71 [DOI] [PubMed] [Google Scholar]
- Wong YC, Tam NN 2002 Dedifferentiation of stromal smooth muscle as a factor in prostate carcinogenesis. Differentiation 70:633–645 [DOI] [PubMed] [Google Scholar]
- Dillner K, Kindblom J, Flores-Morales A, Shao R, Törnell J, Norstedt G, Wennbo H 2003 Gene expression analysis of prostate hyperplasia in mice overexpressing the prolactin gene specifically in the prostate. Endocrinology 144:4955–4966 [DOI] [PubMed] [Google Scholar]
- Nevalainen MT, Valve EM, Ingleton PM, Nurmi M, Martikainen PM, Harkonen PL 1997 Prolactin and prolactin receptors are expressed and functioning in human prostate. J Clin Invest 99:618–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellem SJ, Wang H, Poutanen M, Risbridger GP 2009 Increased endogenous estrogen synthesis leads to the sequential induction of prostatic inflammation (prostatitis) and prostatic pre-malignancy. Am J Pathol 175:1187–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shappell SB, Thomas GV, Roberts RL, Herbert R, Ittmann MM, Rubin MA, Humphrey PA, Sundberg JP, Rozengurt N, Barrios R, Ward JM, Cardiff RD 2004 Prostate pathology of genetically engineered mice: definitions and classification. The consensus report from the Bar Harbor meeting of the Mouse Models of Human Cancer Consortium Prostate Pathology Committee. Cancer Res 64:2270–2305 [DOI] [PubMed] [Google Scholar]
- Ho SM, Tang WY, Belmonte de Frausto J, Prins GS 2006 Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res 66:5624–5632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP 2003 A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193 [DOI] [PubMed] [Google Scholar]
- Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG, Bunney WE, Myers RM, Speed TP, Akil H, Watson SJ, Meng F 2005 Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic Acids Res 33:e175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT, Lempicki RA 2009 Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57 [DOI] [PubMed] [Google Scholar]
- Winer J, Jung CK, Shackel I, Williams PM 1999 Development and validation of real-time quantitative reverse transcriptase-polymerase chain reaction for monitoring gene expression in cardiac myocytes in vitro. Anal Biochem 270:41–49 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW 2001 A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SM, Chung LW 1989 Interaction between prostatic fibroblast and epithelial cells in culture: role of androgen. Endocrinology 125:2719–2727 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.