Abstract
Development of cardiac fibrosis portends the transition and deterioration from hypertrophy to dilation and heart failure. Here we examined how estrogen blocks this important development. Angiotensin II (AngII) and endothelin-1 induce cardiac hypertrophy and fibrosis in humans. and we find that these agents directly stimulate the transition of the cardiac fibroblast to a myofibroblast. AngII and endothelin-1 stimulated TGFβ1 synthesis in the fibroblast, an inducer of fibrosis that signaled via c-jun kinase to Sma- and Mad-related protein 3 phosphorylation and nuclear translocation in myofibroblasts. As a result, mesenchymal proteins fibronectin and vimentin were produced, as were collagens I and III, the major forms found in fibrotic hearts. 17β-Estradiol (E2) or dipropylnitrile, an estrogen receptor (ER)β agonist, comparably blocked all these events, reversed by estrogen receptor (ER)β small interfering RNA. E2 and dipropylnitrile signaling through cAMP and protein kinase A prevented myofibroblast formation and blocked activation of c-jun kinase and important events of fibrosis. In the hearts of ovariectomized female mice, cardiac hypertrophy and fibrosis were induced by AngII infusion and prevented by E2 administration to wild type but not ERβ knockout rodents. Our results establish the cardiac fibroblast as an important target for hypertrophic/fibrosis-inducing peptides the actions of which were mitigated by E2/ERβ acting in these stromal cells.
Estrogen inhibits fibrosis and collagen synthesis in WT but not ERβ KO mouse hearts and in cardiac fibroblasts through rapid cAMP and PKA generation.
The role of estrogen in preventing heart disease in women has been controversial. Many retrospective studies showed that 17β-estradiol (E2) or other estrogens decreased the incidence of myocardial infarction (MI) by approximately 45% (1,2,3). However, the initial analysis from prospective trials such as the Women’s Health Initiative did not support this conclusion. Rather, the Women’s Health Initiative results indicated no protective effects of estrogen in older women taking hormone replacement therapy (HRT) initiated at a mean of approximately 15 yr after the menopause, and a slight increase in MI or stroke (4,5). Reevaluation of these data for women commencing HRT within 10 yr of the menopause indicated a 36% decrease in the rate of MI, compared with women not taking HRT (6,7). Furthermore, these same women had a strong decrease in the coronary artery calcium score, an indicator of arterial disease (8). Thus, the timing in initiating HRT after the menopause seems to be important in preventing cardiovascular diseases.
Regarding the outcomes of heart and vascular disease, the most common cause of death in humans is cardiac failure. This often occurs after repeated ischemia (compromised coronary artery blood flow) or after poorly controlled hypertension. Hypertension leads to increased arterial vascular resistance, resulting in cardiomyocyte hypertrophy (9). If the underlying cause is not controlled, cardiac hypertrophy progresses to dilation, apoptotic thinning of myocytes, and ultimately heart failure (10). Estrogen may defend against the impetus for these events in several ways, because genetic deletion of estrogen receptor (ER)β results in hypertension developing in both female and male mice (11). Furthermore, estrogen deficiency in hypertensive rats results in endothelial dysfunction and oxidative stress (12).
Estrogen may also directly mitigate the development of cardiac hypertrophy as supported in various animal models (13). Mice deficient for the FKBP12.6 protein have abnormal sarcoplasmic reticulum calcium regulation, due to abnormal control of the cardiomyocyte ryanodine receptor. This leads to profound cardiac hypertrophy and failure in the male mice (14). Interestingly, postnatal female mice do not develop cardiac hypertrophy unless administered tamoxifen, indicating ER protection. Several groups have implicated E2 and ERβ in preventing hypertrophy due to a variety of stimuli in mice (15,16). Jazbutyte et al. (17) showed that administration of an ERβ-specific agonist, 8β-VE2 (Leverkusen, Germany, Bayer Pharmaceutical), to ovariectomized spontaneously hypertensive rats lowers systolic blood pressure and peripheral arterial resistance, and attenuates cardiac hypertrophy. Thus, the overall effects of E2 on the myocardium are probably both indirect and direct.
There is a large literature implicating abnormal activity of the calcium-sensitive phosphatase, calcineurin (PP2B) in cardiac hypertrophy. Calcineurin activity is increased by a variety of hypertrophic stimuli, including angiotensin (18). Calcineurin activation dephosphorylates serine residues of nuclear factor of activated T-lymphocytes transcription factors, leading to their nuclear translocation and subsequent up-regulation of genes that mediate cardiac hypertrophy (19). Calcineurin also activates myocyte-enhancing factor-2, up-regulating hypertrophic genes. We recently published a novel down-regulation of calcineurin activity by estrogen, mediated through stimulation of MCIP1 gene transcription, preventing cardiomyocyte hypertrophy in vitro (20) and in vivo (13). This could account for the ability of E2 to dampen the downstream effects of calcium dysregulation in female mice, a defect that induces cardiac hypertrophy in male mice (14). Regarding human cardiac hypertrophy, hormone replacement lowers vascular resistance and decreases left ventricular hypertrophy in hypertensive, postmenopausal women (21).
A very important event in the progression of cardiac hypertrophy to heart dilation and failure is progressive fibrosis of the heart (22,23). This occurs in both the interstitial and perivascular spaces of the myocardium. Hypertrophic agents such as angiotensin II (AngII) and endothelin 1 (ET-1) are produced in fibroblasts and macrophages, as well as the endothelium. AngII and ET-1 promote fibrosis in part by inducing TGFβ1 production resulting in downstream signaling from TGFβ1 receptors (24). These events occur upon transition of the cardiac fibroblast to a myofibroblast phenotype, the latter synthesizing procollagens I and III (24,25). We previously determined that AngII-induced cardiac hypertrophy was associated with cardiac fibrosis, and E2 inhibited this by unclear mechanisms (13). Recent studies in a chronic model of neurohormonal-induced heart failure also showed reduction of cardiac hypertrophy and fibrosis by estrogen, through undetermined mechanisms (26). We therefore sought to understand the target cell and the cellular and molecular mechanisms by which E2 inhibited this important event in the progression of cardiac disease.
Results
Estrogen and ERβ prevent fibroblast to myofibroblast transition
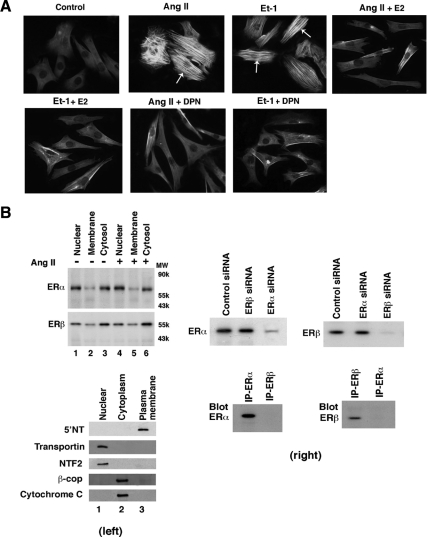
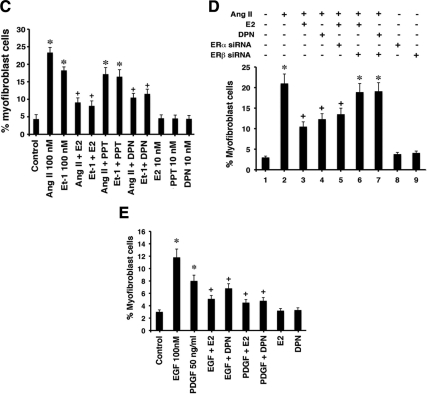
When fibroblasts undergo the transition to myofibroblasts, this bestows the capability of producing collagen and other proteins that cause fibrosis (22,23). Whether estrogen modulates this transition is unknown. We first determined that the well-recognized cardiac hypertrophic factors AngII and ET-1 (27) directly cause the fibroblast-to-myofibroblast transition in many cells, seen as a change in the morphology of fibroblasts to a more rounded, muscle-like phenotype (Fig. 1A). These cells also strongly expressed α-smooth muscle actin (SMA), an important marker of the myofibroblast that is barely expressed in fibroblasts (28). Importantly, 10 nm E2 markedly down-regulated α-SMA expression and caused as much as a 75% decrease in the number of phenotypic myofibroblasts seen from coculture of the sex steroid with AngII or ET-1 (also see Fig. 1C). The ERβ agonist dipropylnitrile (DPN) comparably prevented myofibroblast transition, indicating that E2 probably blocks stimulation of myofibroblast development through ERβ.
Figure 1.
Regulation of the conversion of cardiac fibroblasts to myofibroblasts. A, Hypertrophic factors cause the transition of fibroblasts to myofibroblasts, prevented by E2 or DPN. AngII or ET-1 (100 nm) was incubated with fibroblasts for 24 h. Expression of α-SMA (white arrows) is a marker of myofibroblasts. B, Distribution of ERα and ERβ in cardiac fibroblasts. Left, freshly isolated neonatal rat cardiac fibroblasts were acutely cultured without or with AngII for 24 h, the cells were lysed, and membrane, cytoplasmic, and nuclear fractions were isolated by differential centrifugation for Western blotting, as per Materials and Methods. The study was repeated. Purity of the cell fractions was demonstrated by immunoblotting with antibodies to 5′-NT, an integral plasma membrane protein, transportin and NF T2, nuclear proteins, and β-coatomer protein complex, subunit beta 2 (Golgi) and cytochrome c (mitochondria) cytosolic proteins. Nonspecific IgG antibodies showed no staining, and ER antibodies first preabsorbed with specific ER isoform proteins also produced no ER detection (data not shown). Right, specificity of ER isoform siRNA. Transfection of fibroblasts with either control siRNA or siRNA to ERα or ERβ was performed, and the cells then lysed at 48 h for immunoblotting with either ERα or ERβ antibody. ERα or ERβ antibody does not detect the other isoform: Fibroblasts were lysed, and lysate was immunoprecipitated with each ER isoform antibody and then blotted with each ER isoform antibody. C, Quantification of myofibroblasts in response to AngII or ET-1, significantly prevented by 10 nm E2 or DPN, but not PPT. The bar graph was from three experiments combined. *, P < 0.05 by ANOVA plus Schefe’s test for control vs. AngII, ET-1, or with PPT. +, P < 0.05 for AngII or ET-1 vs. same plus E2 or DPN. D, siRNA knockdown of ERβ reverses the effects of E2 or DPN to prevent myofibroblast formation. Three experiments were combined for bar graph data. *, P < 0.05 for control vs. AngII or AngII + E2 or DPN + ERβsiRNA. +, P < 0.05 for AngII vs. same + E2 or DPN without or with siRNA to ERα. E, EGF- or PDGF-induced myofibroblast formation is inhibited by E2 or DPN. Experimental procedures and data analysis were similar to panel C. IP, Immunoprecipitation.
We then found that fibroblasts (no AngII exposure) and myofibroblasts (incubated with AngII) contain comparable amounts and a similar distribution of ERα and ERβ located in the nucleus, cytoplasm, and plasma membrane (Fig. 1B, left). Interestingly, ERβ was more abundant in extranuclear locations (cytoplasm and plasma membrane fractions) whereas ERα localization was predominantly nuclear. Purity of the cell fractions was shown through immunoblotting the fractions for membrane [5′-neurotropin (NT), nuclear (transportin and NT F2), and cytoplasmic (β-coatomer protein complex, subunit beta 2 and cytochrome c) proteins, and small interfering RNA (siRNA) and antibody specificity is shown also (Fig. 1B, right). Thus, these cells could directly mediate the actions of E2 at either ER isoform present in several cellular pools.
To further investigate this, we compared and quantified the effects of E2, the ERα agonist, propyl-pyrazole-triol (PPT), and DPN, each at 10 nm. AngII and ET-1 caused a near 6-fold increase in myofibroblasts compared with control conditions (Fig. 1C). Only DPN and E2 caused a significant and equivalent inhibition of the fibroblast transition. Further supporting the idea that ERβ mediates these effects of E2, we knocked down ERα or ERβ expression with siRNA for 48 h, as previously described (29). AngII-stimulated transition to myofibroblasts was inhibited equipotently by E2 or DPN; these effects were only reversed by ERβ (but not ERα) siRNA (Fig. 1D).
It has also been reported that epidermal growth factor (EGF) and platelet-derived growth factor (PDGF) contribute to the development of cardiac hypertrophy (30,31), but it is not established that they directly promote fibrosis. Here we found that each hypertrophic factor induced the fibroblast transition to myofibroblast, again blocked by E2 and DPN (Fig. 1E). In summary, important cardiac hypertrophic factors directly stimulate myofibroblast formation, prevented by E2 and ERβ.
Fibrosis-inducing proteins are inhibited by estrogen
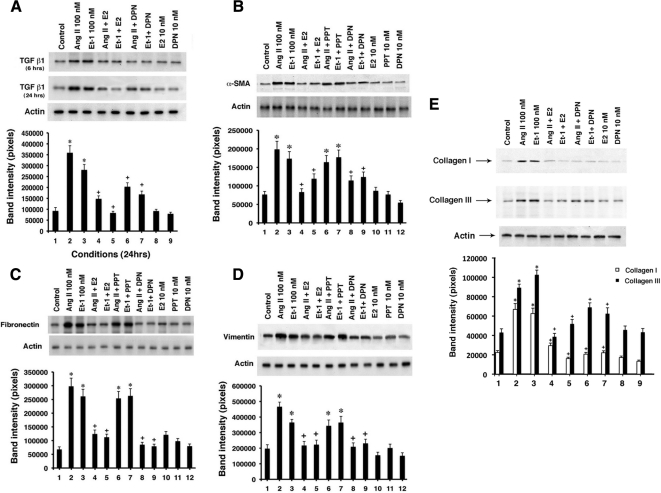
Initially, stimulated fibroblasts produce various proteins that induce the transition to myofibroblasts in an autocrine fashion. The latter cells then produce additional proteins that importantly contribute to fibrosis. We first determined the modulation of the TGFβ1 protein. This growth factor is an important stimulator of fibroblast transition and signals to the production of fibrosis-producing proteins in both autocrine and paracrine fashions (24,25). We found that AngII and ET-1 each significantly stimulated TGFβ1 protein production in cardiac fibroblasts at both 6 and 24 h, significantly inhibited by E2 or DPN (Fig. 2A). We also found that α-SMA, fibronectin, and vimentin proteins each were stimulated by the hypertrophic factors, inhibited by E2 or DPN (and not by PPT) (Fig. 2, B–D). Collagens I and III have been strongly implicated as the major proteins that constitute cardiac fibrosis in disease models (32). AngII or ET-1 induced both collagens, inhibited by E2 or DPN (Fig. 2E). These studies indicate that key proteins implicated in the fibroblast transition and the production of fibrosis are stimulated by hypertrophic factors but inhibited by estrogen acting at ERβ.
Figure 2.
Production of proteins from the myofibroblast that contribute to fibrosis. A, TGFβ1 production in fibroblasts. Cardiac fibroblasts were incubated with 100 nm AngII or ET-1 ± 10 nm E2 or DPN for 6 or 24 h. TGFβ1 was immunoprecipitated from the lysed cells, and separation by SDS-PAGE preceded protein transfer to nylon membranes for immunoblotting. β-Actin protein is shown as a control and loading protein. The bar graph is three experiments combined. *, P < 0.05 for control vs. hypertrophic factors; +, P < 0.05 for hypertrophic proteins vs. same plus E2 or DPN. B–D, α-Smooth muscle actin, fibronectin, and vimentin protein expression, respectively, in fibroblasts. The experiments were similar to panel A. The bar graphs reflect three combined experiments. Data analysis was the same as in Fig. 1C. E, Collagen I and III formation in fibroblasts. Cells were placed in methionine-free media for 1 h, [35S]methionine was then added for 2 h, and cells were incubated with peptides ± steroids for 24 h, at which time new protein synthesis was determined. The bar graph represents data from three experiments. *, P < 0.05 for control vs. AngII or ET-1. +, P < 0.05 for AngII or ET-1 vs. same + E2 or DPN.
Modulation of SMAD (Sma- and Mad-related protein) transcription factors
Signaling to fibrosis has been reported to occur through several pathways directly enacted by TGFβ1 in particular (24) and applicable to AngII and ET-1 actions. Upon stimulation of the fibroblast with hypertrophic agents, TGFβ1 is produced and secreted, binding its Type II receptor (a serine/threonine kinase) that recruits and phosphorylates/activates the Type I TGFβ receptor. As a result, SMAD 2 and 3 proteins are recruited to, then phosphorylated and activated by, the Type I receptor (25,33). Phosphorylation ultimately releases SMAD 2 and 3 proteins from the receptor to sometimes complex with SMAD 4 (cell specific) and translocate to the nucleus. In the nucleus, SMADs 2 and 3 collaborate with other transcription factors to induce gene transcription important to fibrosis.
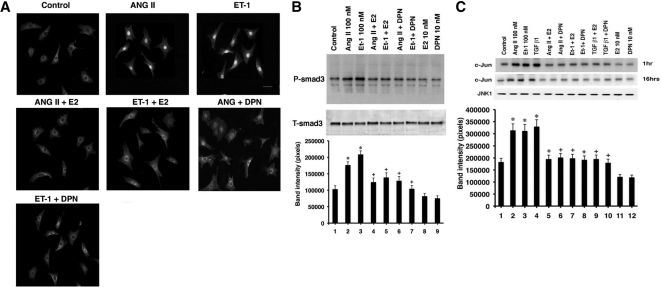
We therefore determined phosphorylation of SMAD 3 and its cellular localization. We used phospho-Ser423/435 antibody directed against the activating phosphorylations of SS (serine-serine) amino acids in the SSXS sequence. AngII and ET-1 stimulated SMAD3 phosphorylation and nuclear localization (Fig. 3A). Both aspects were inhibited by E2 or DPN. To confirm this, we carried out Western blots of serine423/425 phospho-SMAD3 from whole cells exposed to AngII or ET-1 ± E2 or DPN. The more than 2-fold stimulation of p-SMAD by the hypertrophic agents was significantly and comparably prevented by E2 or DPN, implicating ERβ (Fig. 3B).
Figure 3.
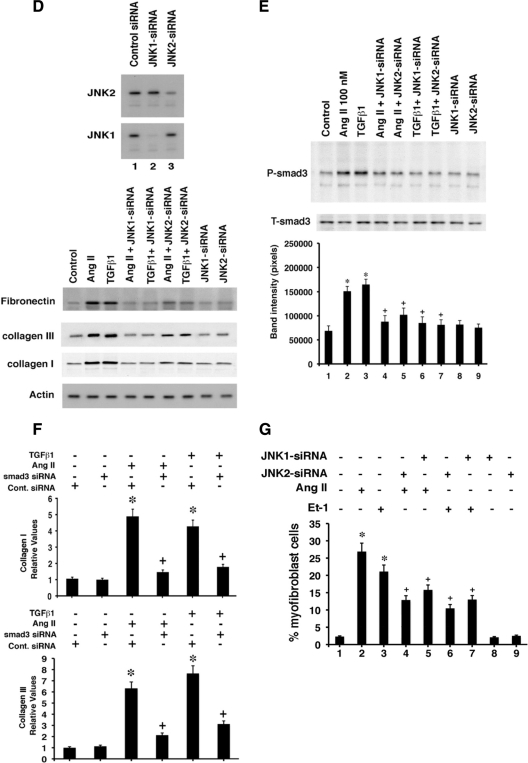
E2 and ERβ prevent signaling to fibrosis. A, Immunofluorescence of SMAD3 activating phosphorylations at ser423/425 as stimulated by AngII and ET-1, resulting in SMAD3 localization to the nucleus of the myofibroblast. E2 or DPN substantially blocked this. A representative study of two is shown. B, Phosphoserine 423/425 SMAD3 immunoblot in fibroblasts exposed to AngII or ET-1 ± E2 or DPN. Cell lysate was immunoprecipitated with antibody to total SMAD 3, and then blotted after SDS-PAGE separation with phospho-SMAD antibody. Total SMAD protein is shown as loading control. The bar graph is three experiments combined. *, P < 0.05 for AngII or ET-1 vs. control. +, P < 0.05 for hypertrophic factors vs. same plus E or DPN. C, JNK activity modulation. Fibroblasts were incubated with hypertrophic agents ± E2 or DPN for 1 or 16 h, and the JNK protein was immunoprecipitated and then used for in vitro kinase activity against exogenous c-jun protein as substrate. Total JNK-1 protein serves as normalization control. The bar graph is three experiments combined. P < 0.05 for AngII, ET-1, or TGF-β vs. control. +, P < 0.05 for hypertrophic factors vs. same plus E or DPN. D (top), siRNA knockdown of JNK1 or JNK2 protein shown by immunoblot at 48 h after transfection. D (bottom), Fibronectin and collagen protein are altered by JNK siRNAs. Cardiac fibroblasts were transfected with control siRNA (control), or JNK1 or JNK2 siRNAs, recovered for 24 h, and then incubated with AngII or TGFβ1 for 24 h. Actin protein serves as loading control. E, JNK signals to SMAD3 phosphorylation. Cells were transfected with JNK1 or JNK2 siRNAs, recovered, and then incubated with TGFβ1 or AngII. Data analysis of three combined studies was carried out as noted in Fig. 1D. F, Quantitative PCR of collagens I and III gene expression in cardiac fibroblasts. Cells were incubated with AngII or TGFβ1 for 24 h, RNA was then extracted, and quantitative PCR was carried out as described in Materials and Methods. In all cells, control or SMAD3 siRNA was transfected 24 h before hypertrophic factor incubation. The study was carried out three times for data analysis. *, P < 0.05 for control siRNA vs. same plus AngII or TGFβ1. +, P < 0.05 for AngII or TGFβ1 + control siRNA vs. AngII or TGFβ1 + SMAD3 siRNA. G, Myofibroblast formation is mediated by JNK. Fibroblasts were incubated with JNK1 or Jnk2 siRNAs, recovered, and then incubated with AngII or ET-1 for 24 h. Myofibroblasts were then counted. Bar graph is from three experiments, mean ± sem. *, P < 0.05 for control vs. AngII or ET-1; +, P < 0.05 for AngII or ET-1 vs. same + JNK siRNA.
Fibrosis-inducing signal transduction is inhibited by E2/ERβ
As a second possible regulatory signal, TGFβ1 stimulates collagen and fibronectin production through a c-jun N-terminal kinase (JNK) pathway (34,35). We first determined that AngII, ET-1, and TGFβ1 each stimulated JNK activity at 1 and 16 h, significantly inhibited by E2 or DPN (Fig. 3C). To determine whether JNK is required for the fibrosis-inducing effects of the hypertrophic agents, we took an RNA interference approach. Specific siRNA to JNK1 or JNK2 each knocked down only the intended target protein at 48 h (Fig. 3D, top). We then determined whether proteins indicative of the fibroblast transition and subsequent production of fibrosis were modulated by the loss of JNK signaling. We found that the JNK 1 and 2 isoforms strongly contributed to the ability of AngII or TGFβ to stimulate fibronectin (indicating myofibroblast transition) or to increase collagens (promoting fibrosis) (Fig. 3D, bottom). This correlated to the proportional ability of each JNK siRNA to knock down the target protein expression.
TGFβ stimulates SMAD nuclear translocation and function on DNA in fibroblasts, in part through JNK, and JNK is therefore upstream to fibroblast protein production (35). To link JNK to SMAD in our model, we determined that AngII or TGFβ-induced SMAD3 phosphorylation in the cardiac fibroblasts was substantially prevented by JNK-1 or JNK-2 siRNA (Fig. 3E). We also investigated the importance of SMAD3 for fibrosis here by examining its role in the modulation of collagens I and III gene(s) transcription as determined by quantitative PCR. AngII and TGFβ1 each stimulated significant increases in the expression of genes encoding both collagens, compared with control, but this was substantially reduced by SMAD3 siRNA (Fig. 3F). JNK also significantly contributed to the ability of AngII and Et-1 to stimulate myofibroblast formation (Fig. 3G). In summary, hypertrophic/fibrosis-inducing peptides significantly stimulated SMAD3 phosphorylation and nuclear localization and myofibroblast formation via JNK signaling, the former inhibited by E2 and DPN. As a result, enhanced collagen production was prevented by the ER ligands.
Rapid signaling by E2/ERβ inhibits fibrosis
How does E2/ERβ signal to inhibit the key features of hypertrophic factor action in fibroblasts? Overexpression and activation of adenylyl cyclase blocks fibroblast to myofibroblast transition (36), suggesting that cAMP generation may play an important role in the actions of E2. Additionally, some evidence suggests that downstream of cAMP, protein kinase A (PKA) may mediate the effects of cAMP (36).
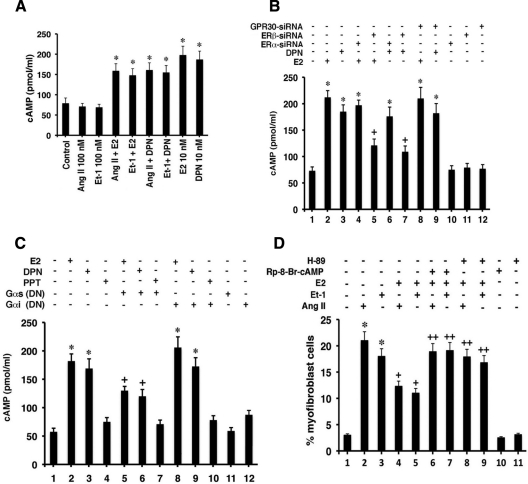
To determine whether this is a potential mechanism, cardiac fibroblasts were incubated with E2 or DPN ± ET-1 or AngII for 5 min and cAMP was measured. As seen in Fig. 4A, both E2 and DPN significantly stimulated cyclic nucleotide production, modestly affected by AngII or ET-1 whereas the latter two proteins had no effect. We also determined that stimulation of cAMP in response to E2 or DPN was unaffected by siRNA to ERα but was significantly compromised by knockdown of ERβ (Fig. 4B). In contrast, no effect of GPR30 knockdown was seen for the ability of E2 or DPN to generate cAMP. Our results are consistent with observations that E2 rapidly stimulates cAMP in other cells (29) and may be a mechanism of E2/ERβ action here. The rapidity of E2 or DPN action suggests that cAMP is generated through membrane-localized ERβ in fibroblasts (Fig. 1B) and is consistent with E2 generating functional and rapid kinase activation in our hypertrophic myocyte models (20).
Figure 4.
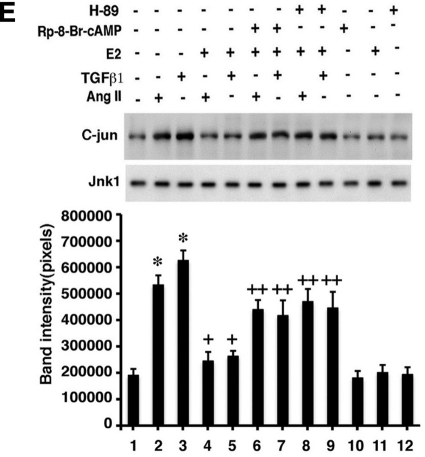
E2 signals to the inhibition of fibrosis. A, E2 or DPN stimulates cAMP generation in cardiac fibroblasts. Fibroblasts were incubated with 10 nm E2 or DPN ± AngII or ET-1, and cAMP was measured after 5-min incubation. Mean ± sem data are from three studies combined with duplicate determinations per condition in each experiment. *, P < 0.05 for control or AngII or ET-1 vs. E2 or DPN or E2/DPN plus hypertrophic factor. B, ERβ, but not ERα, mediates the ability of estrogenic compounds to stimulate cAMP. Fibroblasts were first transfected with siRNA to ERα, ERβ, or GPR30, and the cells were recovered overnight and then incubated with E2 for 5 min. Bar graph is from three experiments; *, P < 0.05 for control vs. E2 or DPN or vs. E2 or DPN with ERα or GPR30 siRNA. +, P < 0.05 for E2 or DPN vs. same + ERβ siRNA. C, Dependence on Gαs coupling to ERβ for cAMP generation by E2. Fibroblasts were transfected with dominant-negative C-terminal minigenes for Gαs and Gαi, and the cells were recovered and then exposed to E2, DPN, or PPT. Bar graph is from three experiments; *, P < 0.05 for control vs. E2 or DPN ± Gαi construct. +, P < 0.05 for E2 or DPN vs. same + Gαs construct. D, Inhibition of cAMP or PKA reverses the E2-induced block of myofibroblast development. Fibroblast to myofibroblast differentiation in response to AngII or ET-1 was inhibited by E2, but the E2 effect was prevented by 5 nm H-89 (PKA inhibitor) or 10 μm Rp-8-Br-cAMP (cAMP inhibitor). Cells were exposed to peptide ± steroid for 24 h, in the absence or presence of cAMP or PKA inhibitor. The bar graph is from three experiments combined. *, P <0/05 for control (lane 1) vs. AngII or ET-1; +, P < 0.05 for AngII or ET-1 vs. same + E2; ++, P < 0.05 for AngII or ET-1 + E2 vs. same + Rp-8-Br-cAMP or H-89. E, cAMP cross talks to inhibit JNK activation. Cells were incubated with TGFβ1 or AngII ± E2 with or without Rp-8-Br-cAMP for 10 min, and then JNK activity was determined as described. The study was done two times and the data were combined.
Adenylyl cyclase is activated upon stimulation of Gαs by many G protein-coupled receptors at the plasma membrane (37). To support the mechanism by which only ERβ generates cAMP, we expressed a dominant-negative (DN) Gαs C-terminal minigene in the fibroblasts (38). As a specificity control, we also expressed a Gαi minigene; Gαi has been shown to have a modest inhibitory effect on cAMP (39), and we previously used these constructs (40). Brief exposure of the cells to E2 or DPN generated increased cyclic nucleotide, and this was significantly although partially prevented by only the Gαs dominant-negative protein (Fig. 4C). PPT did not stimulate cAMP in these cells, and the Gαi DN protein produced an insignificant augmentation of cAMP generation both in basal and E2-treated cells, consistent with the mild inhibitory effect of this small G protein on cAMP generation (39). Thus ERβ is selectively linked to Gαs-mediated cAMP generation.
We then determined that inhibitors of cAMP RP-8-bromo cyclic adenosine monophosphate (RP-8-Br-cAMP) and PKA (H-89) reversed the ability of E2 to inhibit myofibroblast transition (Fig. 4D, lanes 2–9), whereas the inhibitors, by themselves, had no effects. Thus, the cAMP-PKA pathway underlies the effects of E2/ERβ in an important manner.
Might E2/ERβ signaling through cAMP and PKA impact the JNK pathway, potentially indicating a specific cross-target of E2 action? We found that AngII and TGFβ1 stimulation of JNK activity was blocked by E2 but the steroid effect was significantly prevented upon inhibiting cAMP with Rp-8-Br-cAMP and PKA with H-89 (Fig. 4E). Thus, a rapid signaling pathway initiated by liganded ERβ in fibroblasts (likely at the membrane) causes cAMP and PKA augmentation. This signaling blocks AngII and TGFβ1 stimulation of JNK activity that leads to SMAD3 phosphorylation and nuclear localization and fibronectin and collagen production.
In vivo studies
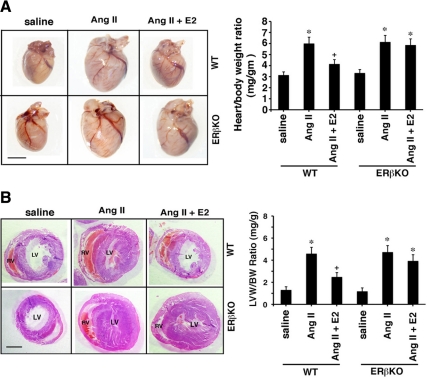
We then investigated E2 effects in WT and ERβ knockout (KO) mice (41) infused with AngII for 21 d to create cardiac hypertrophy and fibrosis (13). E2 pellets produced physiological serum levels in this model, and functional studies as determined by echocardiography were previously reported (13). Previously, we found that AngII-induced cardiac hypertrophy was significantly inhibited by E2 in WT and ERαKO mice but not in ERβKO mice. AngII-induced muscle shortening and enhanced contraction, as seen by echocardiography, were significantly prevented by the sex steroid (13).
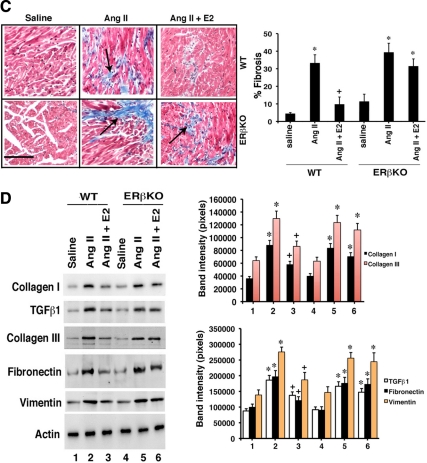
We first corroborated here that AngII induced hypertrophy and E2 prevented this by approximately 60% in WT but not ERβKO mice (Fig. 5A). This was particularly evident for the left ventricle (Fig. 5B). We then assessed fibrosis and found that AngII infusion caused a 7-fold increase in fibrosis relative to saline infusion (control) (Fig. 5C). E2 significantly inhibited fibrosis by more than 80% but only in WT mice (Fig. 5C). Key protein(s) expression in whole ventricle (not just fibroblasts) was determined by immunoblot. All proteins were significantly increased in AngII-infused WT or ERβKO mice, compared with saline-infused (control) mice. E2 significantly inhibited the expression of collagens I and III, TGFβ1, vimentin, and fibronectin in WT but not ERβKO mice (Fig. 5D). These results support the findings from isolated fibroblasts and suggest potential application in vivo.
Figure 5.
In vivo prevention of cardiac fibrosis by E2. A, Hearts are from ovariectomized female WT or ERβKO mice infused with saline or AngII (in saline) ± insertion of E2 pellet for 21 d; representative specimens are shown. The inset bar on the pictures is 2 mm. Heart-body weight ratios reflect cardiac hypertrophy and are calculated from five mice per group. *, P < 0.05 for saline vs. AngII or AngII + E2 in ERβKO mice. +, P < 0.05 for AngII vs. same + E2 in WT mice. B, Left ventricular hypertrophy in the same types and numbers of mice, under the same conditions. The left ventricle of each heart was dissected free and then weighed (LVW), and a ratio was created to body weight (BW). Representative additional transverse sectioned hearts are shown (RV, right ventricle; LV, left ventricle). *, P < 0.05 for saline vs. AngII or AngII + E2 in ERβKO mice. +, P < 0.05 for AngII vs. same + E2 in WT mice. C, Cardiac fibrosis in the left ventricle of ovariectomized female mice. The area of fibrosis was scored as described in Materials and Methods. Bar graph is data from five mice per condition. D, Protein expression in the left ventricles of the same mice exposed to the various experimental conditions. Bar graph data were derived from the densitometry of the protein immunoblots, normalized for total protein in the samples and for β-actin expression.
Discussion
Fibrosis is an important and debilitating development in the progression of cardiac hypertrophy (22,23). This process occurs in both the interstitial and perivascular spaces of the myocardium and is a prime cause of diastolic stiffness, contractility dysfunction, and progression to ventricular wall thinning and heart failure. Here we show that E2 acting at ERβ rapidly signals through cAMP and PKA to directly prevent myofibroblast development and the production of collagen, vimentin, and fibronectin proteins that remodel tissues. We report this also occurs during in vivo hypertrophy induced by AngII where E2 prevents fibrosis in ovariectomized WT but not ERβ KO mice.
The participants involved in fibrosis are multiple and interactive. AngII and ET-1 up-regulate TGFβ1 production in the fibroblast, the latter protein subsequently inducing the phosphorylation of SMAD3 by signaling from the autocrine TGFβ receptor. Phosphorylation of the SMAD transcription factor correlated to nuclear translocation (Fig. 3) where SMAD promoted collagen gene transcription. We implicate Jnk1 and Jnk2 signaling in these critical events, and TGFβ-induced JNK has also been reported to promote SMAD binding to DNA in some cells (34). We importantly find that E2 or DPN blocks the phosphorylation of SMAD3 through stimulating cAMP and PKA production that inhibits JNK activity. cAMP is generated by ERβ specifically coupling to Gαs activation and cAMP, and PKA inhibition of JNK activation provides a novel mechanism for previous observations that cAMP inhibits TGFβ-induced SMAD signaling to collagen production (36,42,43). In addition, we found that cAMP/PKA contributes to E2-inhibition of the fibroblast transition. A cartoon showing these pathways is seen in Fig. 6. EGF and PDGF also have been implicated in hypertrophy and the associated fibroblast transition to myofibroblasts (30,31). We establish here that EGF and PDGF directly act at the fibroblast and provide evidence that E2/ERβ also blocks the myofibroblast-promoting actions of these growth factors.
Figure 6.
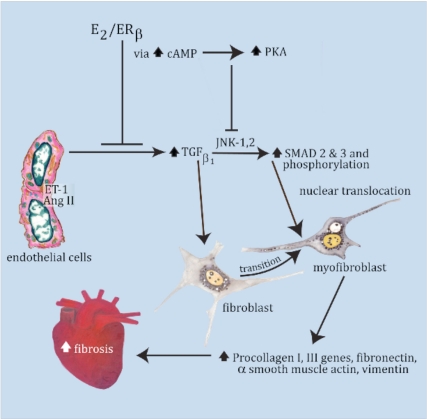
Cartoon showing the pathway of cardiac fibrosis inhibited by E2/ERβ. ET-1 and AngII stimulate TGFβ1 production in fibroblasts, inducing myofibroblast formation, SMAD phosphorylation via JNK activation, and SMAD trafficking to the nucleus. Nuclear SMAD is required for collagen gene up-regulation and enhanced protein production as well as other vital protein(s) production, leading to fibrosis. E2/ERβ inhibits these events via cAMP/PKA signaling to block ET-1- and AngII-induced myofibroblast formation and JNK activation by TGFβ1.
Additional pathways have been proposed for the production of myocardial fibrosis. ET-1 and AngII-induced TGFβ1 stimulates connective tissue growth factor (CTGF). CTGF increases matrix metalloproteinase 2 production via SMAD2 and stimulates fibroblast to myofibroblast conversion (24,44). Additionally, AngII stimulates activation of the small G protein Rho A, leading to Rho kinase activation (45) and myofibril formation in the heart (46). Inhibition of Rho kinase activity or specific deletion of cardiomyocyte Rho kinase type 1 in mice leads to decreased fibrosis in several hypertrophy models (46,47). Rho kinase causes the stimulation of TGFβ1 and CTGF production, implicating Rho as upstream to TGFβ1 but downstream of AngII (45,48). Interestingly, we find that AngII and ET-1 strongly stimulate Rho kinase activity in the fibroblasts, and E2 and DPN significantly inhibit kinase activation (our unpublished data).
Our results implicate a novel function for ERβ. This probably results from the rapid signaling that occurs when E2 binds to the membrane-localized pool of this sex steroid receptor. We demonstrate here the existence of this endogenous subcellular pool in the isolated cardiac fibroblast/myofibroblast and previously showed that it is membrane-localized (and not nuclear-localized) ERβ that is responsible for rapid signaling (49). Also, Gαs and adenylyl cyclase are membrane-associated proteins that interact to stimulate cAMP generation. E2 engaging ERβ rapidly signals to also prevent cardiomyocyte hypertrophy, in vitro and in vivo (13,20). In myocytes, E2/ERβ inhibits AngII-induced protein phosphatase 2B (calcineurin) activity by stimulating transcription of the MCIP1 gene through a phosphatidylinositol-3-kinase mechanism (13,20). Blocking calcineurin activation resulted in the retention of the nuclear factor of activated T-lymphocytes c3 transcription factor in cytoplasm, thereby preventing hypertrophic gene transcription. E2/ERβ also promoted the transcription and resulting autocrine functions of atrial and brain natriuretic peptides in cardiomyocytes to block AngII hypertrophic signaling through ERK MAPK (13,20). In vivo, E2 acted equivalently in WT and ERα KO female mice in these respects but had no effects in ERβ KO mice (13).
These data collectively support the idea that administering a selective ERβ agonist may prevent the development of cardiac hypertrophy (17) and fibrosis (present paper). Such an agonist may also lower blood pressure (11,17). This could be useful, for instance, in hypertensive women whose blood pressure is not well controlled and are therefore at risk for developing cardiac hypertrophy and fibrosis. The advantage of using an ERβ agonist instead of E2 is that the ERβ agonist would avoid the proliferative effects of E2 on the uterus and breast that contribute to hormone-related cancers.
Extending the breadth of our findings here, we propose that the ability of E2/ERβ to prevent fibrosis is not confined to the heart. Supporting this idea, the ERβKO mouse has a prominent phenotype of pulmonary fibrosis (41). In decompensated hypertensive heart disease, pulmonary fibrosis can occur, and perhaps E2/ERβ can prevent this. Furthermore, chronic hepatitis infection (50) or hepatotoxins (51) induce inflammatory changes in the liver resulting in progressive hepatic fibrosis, cirrhosis, and hepatocellular carcinoma. Premenopausal women or female rats are relatively protected from these events due to estrogen action, and some data support a role for ERβ (50). The mechanisms of E2 preventing hepatic or lung fibrosis are not well understood. Investigation of the utility of ERβ agonists might provide mechanistic insight and prevent fibrosis in other organs.
In summary, estrogen acts through ERβ produced in both the cardiomyocyte and cardiac fibroblast to prevent key elements of progressive heart disease, in vitro and in vivo. We believe these studies justify further preclinical and early translational trials using ERβ-specific agonists in women (and perhaps men) at risk for developing progressive cardiac hypertrophy and fibrosis.
Materials and Methods
Reagents
Peptides AngII (Sigma Chemical Co., St. Louis, MO), ET-1 (AnaSpec, Inc., Fremont, CA), EGF, PDGF, and TGFβ1 (R&D Systems, Minneapolis, MN), or steroids E2 (Steraloids Inc., Newport, RI), PPT, and DPN (Tocris Bioscience, Ellisville, MO) were purchased from the designated companies. Antibodies to ERα (C terminus) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) and ERβ (C terminus) (Invitrogen, Carlsbad, CA) were used for immunoblots of subcellular fractions as described (29). Antibodies for immunofluorescent microscopy included α-SMA (Santa Cruz) and phospho-SMAD3 (Abcam Inc., Cambridge, MA). Additional antibodies for immunoblots included TGFβ1 (Abcam), fibronectin (Santa Cruz), and vimentin (Santa Cruz), collagens I and III, JNK 1 and 2, and α-smooth muscle actin (Santa Cruz). siRNAs to JNK1 and JNK2, SMAD 3, GPR30, and ERα and ERβ were from Santa Cruz. Rp-8-Br-cAMP and H89 were from Calbiochem (La Jolla, CA) and ICI 182780 was from Sigma.
In vitro studies
Primary culture of cardiac fibroblasts
Cardiac fibroblast cells were isolated from the hearts of 2-d-old neonatal Sprague Dawley rats (The Jackson Laboratory, Bar Harbor, ME). Briefly, ventricles were removed under sterile conditions, placed in cold sterile Hank’s buffered saline, minced into approximately 2-mm cubes, then treated with 1 mg/ml collagenase (type II; Worthington Biochemical Corp., Lakewood, NJ) under agitation at 37 C for 30 min. The supernatant was discarded, and the tissue pellet was resuspended and agitated in 0.025% trypsin (Life Technologies, Inc., Gaithersburg, MD) in Hank’s buffered saline for 10-min intervals until the tissue was completely dispersed. Cells were then pelleted by centrifugation. Dissociated cells were passed through a 100-μm mesh strainer and preplated for 45 min in DMEM (Sigma) containing 10% heat-inactivated fetal bovine serum (FBS) (Hyclone Laboratories, Logan, UT). This process was repeated twice, and cells were collected after trypsin treatment (to allow preferential attachment of the myocytes, while the fibroblasts are in suspension). The resulting cardiac fibroblasts were pelleted, then resuspended in DMEM containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) and seeded in standard culture dishes. All fibroblasts used in the experiments were at passage 2. Some cells were cultured on poly-d-lysine coated, 35-mm glass-bottom dishes (MatTek Corp., Ashland, MA) for cell localization of proteins by fluorescent microscopy. For functional studies, fibroblasts were cultured in six-well plates and exposed to AngII or ET-1 (100 nm), PDGF (10 nm), EGF (100 nm) ± 10 nm E2, PPT, or DPN. In some experiments, cells were exposed to 10 nm TGFβ1 for 1 or 16 h. siRNAs were transfected and used for experiments after 24–48 h when cells reached approximately 75% cell confluency. Myofibroblasts were identified after the fibroblasts were incubated with AngII or ET-1 (100 nm) ± 10 nm E2 or DPN for 24 h by immunohistochemical staining for α-SMA, and by general morphology. In each experiment, the fibroblast to myofibroblast ratio in each condition was determined by counting 200 cells.
cAMP and JNK kinase determinations
cAMP was measured in fibroblasts using a kit (PerkinElmer, Boston, MA) as previously described (29) after 5 min incubation of the cells with E2 or DPN ± AngII or ET-1. In additional studies, C-terminal Gαs or Gαi plasmids (Cue Biotech, Chicago, IL) (37) that serve as dominant negatives to the endogenous small Gα proteins were transfected into the fibroblasts, the cells recovered overnight and then exposed to E2, PPT, or DPN for 5 min. cAMP was then measured. For JNK activity, cardiac fibroblast cells were cultured and then treated with various experimental conditions for 10 min. Cells were lysed at that time in buffer [50 mm Tris-HCl (pH 7.5), 5 mm EDTA, 100 mm NaCl, 50 mm NaF, 100 μm phenylmethylsulfonyl fluoride, protease inhibitor cocktail, and 0.2% Triton X-100]. Cell lysate from each condition was exposed to Protein A/G agarose to ensure removal of endogenous IgG in a microcentrifuge tube containing 50 μl of the suspended agarose beads, for 30 min at 4 C while rotating. The cell lysate-protein A/G bead complex was centrifuged at maximum speed on a desktop centrifuge for 30 sec at 4 C. Without disturbing the pellet, the supernatant was transferred to a new tube. The precleared supernatants were stored on ice. For the JNK activity assay, 10 μl of JNK antibody were conjugated to 50 ml of protein A/G beads for 2 h at room temperature and washed. Then, 1 ml of precleared whole-cell extract was added to JNK antibody/protein A/G bead complex, and rotated end-over-end overnight at 4 C. Beads were washed once with lysis buffer and twice with HEPES buffer (25 mm HEPES, 10 mm Mg-acetate). To the lysates-antibody-bead complexes, we added 40 μl of a mixture, containing 20 ml of 3× kinase buffer (25 mm HEPES, pH 7.5; 10 mm MgAc, 2 mm dithiothreitol; 40 mm ATP), and 10 ml of H20, to 2 mg c-Jun protein in 9 ml of water, and 1 μl γ-32P-ATP. Each tube was vortexed and incubated at 30 C for 30 min. SDS-PAGE loading buffer (40 μl) was then added, vortexed, boiled for 5 min, and centrifuged at 14,000 rpm for 1 min. Forty microliters per condition sample were loaded onto a 10% gel. After electrophoresis, the gel was fixed, dried, and subjected to autoradiography.
[35S]methionine metabolic labeling of cells
Cells were cultured in 60-mm dishes and were 90% confluent at the time of experiment. To pulse the cells with [35S]methionine, normal culture medium was removed and 2 ml methionine-free DMEM, supplemented with 10% dialyzed FBS, were added into each dish followed by addition of 20 μl (250 μCi) of [35S] methionine/cysteine/well (PerkinElmer). After 2 h of pulsing, 35S-containing medium was removed, and normal culture medium was added back, with or without stimulants for the indicated times. Cells were then lysed with 1 ml lysis buffer. After a complete set of samples was collected, lysates were spun at 12,000 × g for 10 min at 4 C, and clear supernatant was collected. For immunoprecipitation, 10 μl anticollagen I or collagen III antibodies were added to 30 μl protein A/G Plus-agarose (Sigma) and rotated at room temperature for 2 h. After washing four times with lysis buffer, lysates were added to antibody-bead complex and rotated at 4 C overnight. Samples were washed with cold PBS and then were heated at 95 C for 5 min in loading buffer [50 mmol/liter Tris (pH 6.8), 2% sodium dodecyl sulfate, 10% glycerol, 100 mmol/liter dithiothreitol] and separated on a 12% SDS-PAGE gel. Gels were dried and exposed to x-ray film followed by densitometry analysis. Additionally, immunoblots for TGFβ1, α-SMA, vimentin, and fibronectin proteins were determined as previously described (13,20). Briefly, cardiac fibroblasts were exposed to hypertrophic peptides ± steroids for 24 h; the cells were lysed, and individual proteins were immunoprecipitated, reduced, and separated by SDS-PAGE on a gel before transfer to nitrocellulose. Transfer was followed by immunoblotting.
Gene expression by quantitative real-time PCR
Total RNA was extracted using the RNeasy Mini Kit (QIAGEN, Chatsworth, CA) following the manufacturer’s protocol. All the samples were treated with DNAse-free trizol reagent (Ambion, Inc., Austin, TX). RNA purity and concentrations were measured by UV-spectrophotometry (A260 and A280). cDNA was synthesized using approximately 500 ng RNA and Oligo dT primers with the Improm-II reverse transcription system (Promega Corp., Madison, WI). Quantitative real time PCR (qRT-PCR) was used to examine the relative expression of collagen I and collagen III. Expression was normalized using the housekeeper GAPDH gene. Primers were designed using Primer3 (http://frodo.wi.mit.edu/). Blast analysis was used to check specificity [primers: collagen I forward (F) (5′-tgctgccttttctgttcctt-3′); collagen I reverse (R) (5′-aaggtgctgggtagggaagt-3′); collagen IIIF (5′-gtccacgaggtgacaaaggt-3′); collagen IIIR (5′-catcttttccaggaggtcca-3′); GAPDHF (5′-ccacagtccatgccatca-3′); GAPDHR (5′-ggatgaccttgcccacag-3′)]. Primers were designed to have an annealing temperature of 55 C and to amplify regions of 150–200 bp. PCR amplicon sizes were confirmed by agarose gel electrophoresis before qRT-PCR analysis. For qRT-PCR analysis, cDNA was combined in a 50-μl reaction with 25 μl of SYBR GreenER qPCR Supermix (Invitrogen), 1 μl of 10 μm forward/reverse primer stocks, and nuclease-free water. Thermocycling was carried out using the iCycler (Bio-Rad Laboratories, Hercules, CA) with a melting curve temperature of 60 C. Relative mRNA levels were calculated using the Ct method. Data were normalized to the control siRNA condition.
Mice models
All mouse studies were approved by the Animal Use and Research and Development Committees at the Long Beach Veterans Administration Medical Center and the University of California, Irvine. Ovariectomized female wild-type (WT) or ERβ gene-deleted mice were obtained from Dennis Lubahn (University of Missouri) (13). The ERβ mice were originally created by Gustafsson et al. (49), and control mice were WT littermates. Mice were housed in 12-h light, 12-h dark lighting and fed rodent chow devoid of soy or most plant products. AngII (1.1 mg/kg/d) in saline or saline alone-filled osmotic minipumps (Alzet; DURECT Corp., Cupertino, CA.) provided 21-d infusion after sc insertion under inhaled isofluorane anesthesia. In some mice, an E2 pellet (0.1 mg, 21-d release pellets; Innovative Research of America, Sarasota, FL) or placebo pellet was also inserted under the skin. This pellet is well documented to produce physiological levels of E2 in the serum of mice (13,51). At inception and after 21 d, the mice were weighed. At 21 d, the hearts were arrested in diastole by injection of CdCl2 (0.1 mol/liter iv), followed by cervical dislocation. The hearts were removed and weighed, and the ratio of heart to total body weight was determined. The left ventricle was also dissected free and weighed. Estrogen loss or administration did not significantly affect body weight over the 3-wk period of the study, and heart weight was normalized to initial body weight.
Cardiac fibrosis
Hearts and heart sections (left ventricular tissue) were weighed and then processed by fixing in 3.75% paraformaldehyde solution for embedding, or frozen for protein analysis. Paraffin-embedded tissue sections (5 μm) were stained with hematoxylin and eosin or Masson’s trichrome, the latter for the presence of interstitial collagen fiber accumulation indicative of fibrosis. The ratio of interstitial fibrosis to the total left ventricular area was calculated from 10 randomly selected microscopic fields from each of five sections per heart using NIH image J analysis software (n = 5 mice per condition). Frozen tissues were used for relative protein expression, and immunoblots were carried out on protein extracted from the left ventricle of mice from all conditions, following separation by SDS-PAGE and transfer to nitrocellulose, as we described previously (13,20).
Statistics
Data were compared by two-way ANOVA plus Schefe’s test for significant differences between conditions. Statistical significance of difference was at the 0.05 level.
Footnotes
This work was supported by grants from the Research Service of the Department of Veterans Affairs, and National Institutes of Health Grant CA-10036 (to E.R.L.). F.O. is a recipient of a National Biophotonics and Imaging Platform Ireland Career Enhancement and Mobility Fellowship cofunded by Marie Curie Actions.
Disclosure Summary: A.P., M.R., F.O., D.L., and E.R.L. have no conflicts to report. E.R.L. carries out pharmaceutical clinical trials of products developed by Novo-Nordisk and Novartis.
First Published Online September 1, 2010
Abbreviations: AngII, Angiotensin II; CTGF, connective tissue growth factor; DPN, dipropylnitrile; E2, 17β-estradiol; EGF, epidermal growth factor; ER, estrogen receptor; FBS, fetal bovine serum; HRT, hormone replacement therapy; JNK, c-jun N-terminal kinase; KO, knockout; MI, myocardial infarction; NT, neurotropin; PDGF, platelet-derived growth factor; PKA, protein kinase A; PPT, propylpyrazoletriol; qRT-PCR, quantitative real time PCR; RP-8-BR-cAMP, RP-8-bromo cyclic adenosine monophosphate; siRNA, small interfering RNA; SMA, smooth muscle actin; WT, wild type.
References
- Stampfer MJ, Willett WC, Colditz GA, Rosner B, Speizer FE, Hennekens CH 1985 A prospective study of postmenopausal estrogen therapy and coronary heart disease. N Engl J Med 313:1044–1049 [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Manson JE, Rosner B, Speizer FE, Hennekens CH 1991 Postmenopausal estrogen therapy and cardiovascular disease: ten year follow up from the Nurses Health Study. N Engl J Med 325:756–762 [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA 1991 Estrogen replacement and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med 20:47–63 [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J 2002 Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative Randomized Controlled Trial. JAMA 288:321–333 [DOI] [PubMed] [Google Scholar]
- Herrington DM, Reboussin DM, Brosnihan KB, Sharp PC, Shumaker SA, Snyder TE, Furberg CD, Kowalchuk GJ, Stuckey TD, Rogers WJ, Givens DH, Waters D 2000 Effects of estrogen replacement on the progression of coronary-artery atherosclerosis. N Engl J Med 343:522–529 [DOI] [PubMed] [Google Scholar]
- Hernan MA, Alonso A, Logan R, Grodstein F, Michels KB, Willett WC, Manson JE, Robins JM 2008 Observational studies analyzed like randomized experiments and application to postmenopausal hormone therapy and coronary heart disease. Epidemiology 19:766–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Prentice RL, Manson JE, Wu L, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis KL, Stefanick ML 2007 Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA 297:1465–1477 [DOI] [PubMed] [Google Scholar]
- Manson JE, Allison MA, Rossuouw JE, Carr JJ, Langer RD, Hsia J, Kuller LH, Cochrane BB, Hunt JR, Ludlam SE, Pettinger MB, Gass M, Marg olis KL, Nathan L, Ockene JK, Prentice RL, Robbins J, Stefanick ML 2007 Estrogen therapy and coronary-artery calcification. N Engl J Med 356:2591–2602 [DOI] [PubMed] [Google Scholar]
- Tumuklu MM, Erkorkmaz U, Ocal A 2007 The impact of hypertension and hypertension-related left ventricle hypertrophy on right ventricle function. Echocardiography 24:374–384 [DOI] [PubMed] [Google Scholar]
- Subramaniam V, Lip GY 2009 Hypertension to heart failure: a pathophysiological spectrum relating blood pressure, drug treatments, and stroke. Expert Rev Cardiovasc Ther 7:703–713 [DOI] [PubMed] [Google Scholar]
- Zhu Y, Bian Z, Lu P, Karas RH, Bao L, Cox D, Hodgin J, Shaul PW, Thoren P, Smithies O, Gustafsson JA, Mendelsohn ME 2002 Abnormal vascular function and hypertension in mice deficient in estrogen receptor β. Science 295:505–508 [DOI] [PubMed] [Google Scholar]
- Wassmann S, Bäumer AT, Strehlow K, van Eickels M, Grohé C, Ahlbory K, Rösen R, Böhm M, Nickenig G 2001 Endothelial dysfunction and oxidative stress during estrogen deficiency in spontaneously hypertensive rats. Circulation 103:435–441 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Lubahn D, Liu J, Vannan M, Levin ER 2008 Estrogen inhibits cardiac hypertrophy: role of estrogen receptor β to inhibit calcineurin. Endocrinology 149:3361–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, Collier ML, Deng KY, Jeyakumar LH, Magnuson MA, Inagami T, Kotlikoff MI, Fleischer S 2002 Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 416:334–338 [DOI] [PubMed] [Google Scholar]
- Skavdahl M, Steenbergen C, Clark J, Myers P, Demianenko T, Mao L, Rockman HA, Korach KS, Murphy E 2005 Estrogen receptor-β mediates male-female differences in the development of pressure overload hypertrophy. Am J Physiol Heart Circ Physiol 288:H469–H476 [DOI] [PubMed] [Google Scholar]
- Babiker FA, Lips D, Meyer R, Delvaux E, Zandberg P, Janssen B, van Eys G, Grohé C, Doevendans PA 2006 Estrogen receptor β protects the murine heart against left ventricular hypertrophy. Arterioscl Thromb Vasc Biol 26:1524–1530 [DOI] [PubMed] [Google Scholar]
- Jazbutyte V, Arias-Loza PA, Hu K, Widder J, Govindaraj V, von Poser-Klein C, Bauersachs J, Fritzemeier KH, Hegele-Hartung C, Neyses L, Ertl G, Pelzer T 2008 Ligand-dependent activation of ER lowers blood pressure and attenuates cardiac hypertrophy in ovariectomized SHR. Cardiovasc Res 77:774–781 [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN 1998 A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell 93:215–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega RB, Bassel-Duby R, Olson EN 2003 Control of cardiac growth and function by calcineurin signaling. J Biol Chem 278:36981–36984 [DOI] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Aitkenhead M, Levin ER 2005 Estrogen inhibits cardiomyocyte hypertrophy in-vitro: antagonism of calcineurin-related hypertrophy through induction of MCIP1. J Biol Chem 280:26339–26348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light KC, Hinderliter AL, West SG, Grewen KM, Steege JF, Sherwood A, Girdler SS 2001 Hormone replacement improves hemodynamic profile and left ventricular geometry in hypertensive and normotensive postmenopausal women. J Hyperten 19:269–278 [DOI] [PubMed] [Google Scholar]
- Manabe I, Shindo T, Nagai R 2002 Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res 91:1103–1113 [DOI] [PubMed] [Google Scholar]
- Berk BC, Fujiwara K, Lehoux S 2007 ECM remodeling in hypertensive heart disease. J Clin Invest 117:568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J 2007 TGF-β signaling in vascular fibrosis. Cardiovasc Res 74:196–206 [DOI] [PubMed] [Google Scholar]
- Flanders KC 2004 Smad3 as a mediator of the fibrotic response. Int J Exp Pathol 85:47–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thireau J, Aimond F, Poisson D, Zhang B, Bruneval P, Eder V, Richard S, Babuty D 2010 New insights into sexual dimorphism during progression of heart failure and rhythm disorders. Endocrinology 151:1837–1845 [DOI] [PubMed] [Google Scholar]
- Wagenaar LJ, Voors AA, Buikema H, van Gilst WH 2002 Angiotensin receptors in the cardiovascular system. Can J Cardiol 18:1331–1339 [PubMed] [Google Scholar]
- Eyden B 2005 The myofibroblast: a study of normal, reactive, and neoplastic tissues, with an emphasis on ultrastructure. I. Normal and reactive cells. J Submicrosc Cytol Pathol 37:109–204 [PubMed] [Google Scholar]
- Pedram A, Razandi M, Levin ER 2006 Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol 20:1996–2009 [DOI] [PubMed] [Google Scholar]
- Ushikoshi H, Takahashi T, Chen X, Khai NC, Esaki M, Goto K, Takemura G, Maruyama R, Monatoguchi S, Fujiwara T, Nagano S, Yuge K, Kawai T, Murofushi Y, Fujiwara H, Kosai K 2005 Local overexpression of HB-EGF exacerbates remodeling following myocardial infarction by activating noncardiomyocytes. Lab Invest 85:862–873 [DOI] [PubMed] [Google Scholar]
- Raizman JE, Komljenovic J, Chang R, Deng C, Bedosky KM, Rattan SG, Cunnington RH, Freed DH, Dixon IM 2007 The participation of the Na+-Ca2+ exchanger in primary cardiac myofibroblast migration, contraction, and proliferation. J Cell Physiol 213:540–551 [DOI] [PubMed] [Google Scholar]
- González A, López B, Díez J 2004 Fibrosis in hypertensive heart disease: role of the renin-angiotensin-aldosterone system. Med Clin North Am 88:83–97 [DOI] [PubMed] [Google Scholar]
- Towbin JA 2007 Scarring in the heart—a reversible phenomenon? N Engl J Med 1767–1768 [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH 1999 TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J 18:1345–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonska E, Markart P, Zakrzewicz D, Preissner KT, Wygrecka M 2010 TGF-β 1 induces expression of human coagulation factor XII via SMAD3 and JNK signaling in human lung fibroblasts. J Biol Chem 285:11638–11651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA 2005 Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. Proc Natl Acad Sci USA 102:437–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa S, Endoh T, Shibukawa Y, Tsumura M, Ichikawa H, Tazaki M, Furusawa M 2010 Calcitonin gene-related peptide and adrenomedullin-induced facilitation of calcium current by different signal pathways in nucleus tractus solitarius. Brain Res 1327:47–55 [DOI] [PubMed] [Google Scholar]
- Gilchrist A, Li A, Hamm HE 2002 Design and use of C-terminal minigene vectors for studying role of hetero-trimeric G proteins. Methods Enzymol 344:58–69 [DOI] [PubMed] [Google Scholar]
- Willard FS, Low AB, McCudden CR, Siderovski DP 2007 Differential G-α interaction capacities of the GoLoco motifs in Rap GTPase activating proteins. Cell Signal 19:428–438 [DOI] [PubMed] [Google Scholar]
- Razandi M, Pedram A, Park ST, Levin ER 2003 2003 Proximal events in ER signaling from the plasma membrane. J Biol Chem 278:2701–2712 [DOI] [PubMed] [Google Scholar]
- Morani A, Barros RP, Imamov O, Hultenby K, Arner A, Warner M, Gustafsson JA 2006 Lung dysfunction causes systemic hypoxia in estrogen receptor β knockout (ERβ−/−) mice. Proc Natl Acad Sci USA 103:7165–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sun SQ, Hassid A, Ostrom RS 2006 cAMP inhibits transforming growth factor-β-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and smad signaling in cardiac fibroblasts. Mol Pharmacol 70:1992–2003 [DOI] [PubMed] [Google Scholar]
- Huang S, Wettlaufer SH, Hogaboam C, Aronoff DM, Peters-Golden M 2007 Prostaglandin E(2) inhibits collagen expression and proliferation in patient-derived normal lung fibroblasts via E prostanoid 2 receptor and cAMP signaling. Am J Physiol Lung Cell Mol Physiol 292:L405–L413 [DOI] [PubMed] [Google Scholar]
- López B, González A, Diez J 2004 Role of matrix metalloproteinases in hypertension-associated cardiac fibrosis. Curr Opin Nephrol Hypertens 13:197–204 [DOI] [PubMed] [Google Scholar]
- Aoki H, Izumo S, Sadoshima J 1998 Angiotensin II activates RhoA in cardiac myocytes: a critical role of RhoA angiotensin II-induced premyofibril formation. Circ Res 82:666–676 [DOI] [PubMed] [Google Scholar]
- Satoh S, Ueda Y, Koyanagi M, Kadokami T, Sugano M, Yoshikawa Y, Makino N 2003 Chronic inhibition of Rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J Mol Cell Cardiol 35:59–70 [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Oyama N, Wang CY, Noma K, Satoh M, Kim HH, Liao JK 2005 Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploninsufficient mice. Circulation 112:2959– 2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma K, Oyama N, Liao JK 2006 Physiological role of ROCKs in the cardiovascular system. Am J Physiol Cell Physiol 290:C661–C668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedram A, Razandi M, Sainson RCA, Kim JK, Hughes CC, Levin ER 2007 A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem 282:22278–22288 [DOI] [PubMed] [Google Scholar]
- Shimizu I, Kohno N, Tamaki K, Shono M, Huang HW, He JH, Yao DF 2007 Female hepatology: favorable role of estrogen in chronic liver disease with hepatitis B virus infection. World J Gastroenterol 13:4295–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M 2007 Gender disparity in liver cancer due to sex differences in MyDD88-dependent IL-6 production. Science 317:121–124 [DOI] [PubMed] [Google Scholar]