Abstract
Background and Aims
To date, current research involving pollen viability has been evaluated in a relatively low number of orchid species. In the present study, we focused on five related Mediterranean orchid genera (Anacamptis, Orchis, Dactylorhiza, Ophrys and Serapias) that are characterized by different types of deceptive pollination.
Methods
The in vitro germination ability of increasingly aged pollinaria of eight food-, seven sexually and two shelter-deceptive species was evaluated. Pollination experiments on two food-, one sexually and one shelter-deceptive species were also performed and the percentage of embryonate seeds derived from the increasingly aged pollinaria was checked.
Key Results
All of the examined species showed long-term viabilities (=50 % pollen tube growth) that ranged from 8 to 35 d. Species with the same deceptive pollination strategies exhibited the same pollen viability trends. Interestingly, pollen viabilities of species groups with different deception types have shown significant differences, with sexually and shelter- deceptive species exhibiting a shorter life span than food-deceptive species.
Conclusions
This study confirms the prolonged germination and fertilization capacities of orchid pollinaria, and to our knowledge is the first report demonstrating a clear relationship between pollen viability and pollination system. It is proposed that this relationship is attributed to the different types of reproductive barriers, pre- or post-zygotic, that characterixe Ophrys and Serapias and the food-deceptive species, respectively.
Keywords: Anacamptis, Dactylorhiza, fruit set, Ophrys, Orchis, pollen tube, pollination strategy, seed, Serapias
INTRODUCTION
Following in the footsteps of Mendel (1865) and Darwin (1862), plant biologists have largely used controlled pollination experiments to study the reproductive biology (Barret and Charlesworth, 1991), ecology (Desrochers and Rieseberg, 1998) and evolution (Bradshaw and Schemske, 2003) of angiosperms. In this context, the issue of pollen viability has been investigated in terms of its contribution to incompatibility and fertility studies or crop improvement and breeding projects (Stone et al., 1995, and references therein). By means of in vitro and in vivo germination tests, the life span of pollen grains has been reported to range from a few minutes to several days (Pacini et al., 1997; Dafni and Firmage, 2000, and reference therein). Additionally, it has been documented that internal, morphological and environmental factors all play a role in determining the duration of pollen viability, with dehydration and/or ultraviolet light producing the most severely damaging effects (Dafni and Firmage, 2000). The orchid family is considered to have the most protected pollen grains because they are surrounded by pollenkitt or elastoviscin (Schill and Wolter, 1986; Pacini, 1997) and commonly packaged into dispersal units (Pacini and Hesse, 2002).
Therefore, it is not surprising that among the angiosperms that have been investigated thus far, the orchid species have displayed the most long-term pollen viabilities (i.e. 50 d in Dactylorhiza spp.; Neiland and Wilcock, 1995). However, there are currently a high number of orchid species in existence, of which relatively few taxa have been investigated, and few studies have closely examined a systematically or ecologically homogeneous orchid group.
The Euro-Mediterranean sub-tribe of Orchidinae is a monophyletic group encompassing several genera (Bateman et al., 2003), most of which have a food- or sexually deceptive pollination strategy (van der Pijl and Dodson, 1966; Dressler, 1981; Ackerman, 1986). The sexually deceptive pollination is typical of the genus Ophrys, whose flowers mimic the female of its own pollinator in shape and scent (Kullenberg, 1961; Schiestl et al., 1999). Food-deceptive species, such as the widespread genera Orchis, Anacamptis and Dactylorhiza, display large, showy flowers resembling those of rewarding species but that lack the energetic rewards (Dafni and Bernhardt, 1989). Another widespread, deceptive Mediterranean orchid is the genus Serapias, characterized by a different, less-understood pollination strategy usually defined as shelter deception, since pollinators visit its flowers to rest or sleep (Gumprecht, 1977; Dafni et al., 1981).
The wide occurrence of deceptive pollination among orchids seems to be a true enigma because it has been proven that orchids have a low reproductive fitness due to pollinator limitation (Zimmerman and Aide, 1989). Deceptive species are exposed to a further decrease in fruit formation rates because lured pollinators tend to fly away and may learn to avoid these deceptive plants (Jersáková et al., 2006). Thus, high pollen viabilities may be suitable with regards to the rarity of pollinator visits and length of time necessary for transfer to conspecific stigma (Proctor, 1998).
Limited information is available regarding the effects of protracted pollinia adhesion to the insect body on the production of fruit and seed sets (Luyt and Johnson, 2001; Luangsuwalai et al., 2008) and on the relationships between pollen viability and different pollination systems (Pacini and Hesse, 2002)
In this study, we proposed to expand upon current information concerning the pollen viability of widespread Euro-Mediterranean orchids characterized by different types of pollination strategies. In vitro germination tests and controlled pollination experiments were performed to evaluate the duration of pollen viability and the ability of increasingly aged pollen to produce fruits and seeds containing embryos. We also aimed to evaluate the relationships between pollen viability and pollination strategies of selected Orchidinae.
MATERIALS AND METHODS
Plant materials
Seventeen species of the genera Anacamptis, Dactylorhiza, Ophrys, Orchis and Serapias, all belonging to the sub-tribe Orchidinae, were selected, taking into account their pollination strategies (van der Cingel, 1995, and references therein). Eight food-deceptive species from three different genera (three Anacamptis spp., four Orchis spp. and Dactylorhiza sambucina), seven sexually deceptive species all belonging to the genus Ophrys, and two Serapias species characterized by shelter-deceptive pollination strategies were selected (Table 1).
Table 1.
Sampling locations, pollination types and predominant pollinators of the studied orchid species
| Species | Sampling location* | Pollination syndrome | Predominant pollinators† |
|---|---|---|---|
| Anacamptis morio | Mangone | Food deception | Female bees |
| Anacamptis papilionacea | Cassano | Food deception | Eucera males |
| Anacamptis pyramidalis | Acquaformosa | Food deception | Butterflies |
| Dactylorhiza sambucina | Sila | Food deception | Bumblebees |
| Ophrys apifera | Cassano | Sexual deception | Eucera males |
| Ophrys bertolonii | Cassano | Sexual deception | Andrena males |
| Ophrys bombyliflora | Cassano | Sexual deception | Eucera males |
| Ophrys fusca | Acquaformosa | Sexual deception | Andrena males |
| Ophrys incubacea | Cassano | Sexual deception | Andrena males |
| Ophrys lutea | Cassano | Sexual deception | Andrena males |
| Ophrys tenthredinifera | Cassano | Sexual deception | Eucera males |
| Orchis anthropophora | Cassano | Food deception | Beetles |
| Orchis italica | Cassano | Food deception | Female bees |
| Orchis mascula | Sila | Food deception | Female bees |
| Orchis provincialis | Rogliano | Food deception | Female bees |
| Serapias cordigera | Piano Monello | Sleeping holes | Eucera males |
| Serapias vomeracea | Mangone | Sleeping holes | Eucera males |
*All locations are in Italy
†From Van der Cingel (1995); Felicioli et al. (1998); Cozzolino et al. (2005).
In vitro pollen germinability
To test the duration of pollen germinability, in spring 2009 plants with flowers at the onset of anthesis for each selected species were collected and their pollinia were removed using toothpicks. The toothpicks carrying the pollinia were kept in an open box at approximate field conditions. Squashed pollinia were placed in a 20 % sucrose solution in 96-well test plates and incubated at 18 °C for 24 h, then stained with lactophenol cotton blue, and the presence of pollen tubes was visualized and recorded using an optical microscope set at ×100 magnification. A pollen grain was considered to have germinated when the pollen tube was at least as long as the diameter of the pollen grain (Stanley and Liskens, 1974). Tests began using fresh pollinia and proceeded with stored pollinia, which were checked every day for the first week, every 3 d for the following 3 weeks and every 5 d up to the 50th day of the experiment. All of the tests were performed using ten pollinia of each aged class for each species.
Since pollen viability decreases progressively over time (Kearns and Inouye, 1993), the common practice of defining ‘pollen viability duration’ as the time period in which >50 % of the pollen grains still produce pollen tubes was followed (Kumar et al., 1995). The time at which pollen grains still possessed 10 % germinability was also checked.
Fruit and seed set
To test the ability of aged pollen to produce fruits and seeds with embryos, four species were selected: two food- (Anacamptis papilionacea and Orchis italica), one sexually (Ophrys lutea) and one shelter-deceptive species (Serapias vomeracea). It was decided to examine these four taxa alone because of the potential problems involved excavating, potting and transferring plants of 17 species to the laboratory every 3 d (approx. 1700 plants).
To carry out hand-pollination experiments, a second stock of pollinia from plants in the field containing flowers at anthesis was removed using toothpicks, and they were stored in an open box left at approximate field conditions. Every 3 d, we excavated, potted and transferred those individuals that had about 50 % unopened flowers from each selected species to the laboratory, so that we could choose flowers with specific and comparable stigma receptivity. The first round of hand-pollinations was accomplished with fresh pollinia, while the stored pollinia were used every day in the first week, every 2 d in the third and fourth weeks and then every 4–5 d up to the 30th day.
A total of 400 intraspecific hand-pollinations were performed, including ten flowers from various plants for each species with a pollinarium of each aged class.
Fruit production was signified by the development of swollen ovaries, and the percentage of fruit set was calculated as the ratio of swollen ovaries to the number of treated flowers. Ripe fruits were stored in silica gel to prevent their degradation, and the percentage of seeds containing embryos was determind by counting at least 1000 seeds removed from the centre of the capsule using an optical microscope set at ×100 magnification. Seeds were categorized as viable or non-viable according to the presence or absence of viable embryos.
Data analysis
The effects of pollen age on germinability were evaluated using a two-way analysis of variance (ANOVA) with taxa and pollination strategy as fixed factors and pollen age as a covariate factor using the SPSS software package (SPSS v. 13·0 for Windows Chicago, IL, USA). In particular, we compared ‘pollen viability duration’ (sensu Kumar) and ‘10 % pollen viability’ among the three species grouped by their different pollination strategies. In addition, we performed replicate one-way ANOVAs to test the effects of pollen age on germinability between the different pollination strategies.
Moreover, bivariate analyses were performed using the SAS package (SAS Institute, Inc., 1988) to evaluate the significance of the correlation between pollen germinability and pollen age among species with the same pollination type.
To test for significant differences in abilities of aged pollen to produce fruits and seeds with embryos, a two-way ANOVA was performed to partition the variation among the four examined species and a replicate one-way ANOVA was performed to evaluate effects between pairs of species.
RESULTS
In vitro pollen germinability
Tests of in vitro germination showed that pollen of all examined orchids remained fully viable (100 % of pollen tube growth) for up to 5 d, while more aged pollinia revealed different trends of pollen viability decline (Figs 1–3). To uncover potential relationships between pollen viability duration and deceptive pollination types, we statistically compared the values obtained for the food-, sexually and shelter-deceptive species. Evidence was found of highly significant differences in pollen germinability with respect to pollen age among species (F16,254 = 6·278, P < 0·001) and among pollination strategies (F2,268 = 27·279, P < 0·001). In particular, the replicate one-way ANOVAs showed highly significant differences in the effects of pollen age with respect to pollen germinability, in the food- vs. the sexually (F1,238 = 22·521, P < 0·001) and shelter- (F1,157 = 31·324, P < 0·001) deceptive species, while a marginally significant difference occurred between the Ophrys (sexually) and Serapias (shelter) species (F1,238 = 22·521, P < 0·05).
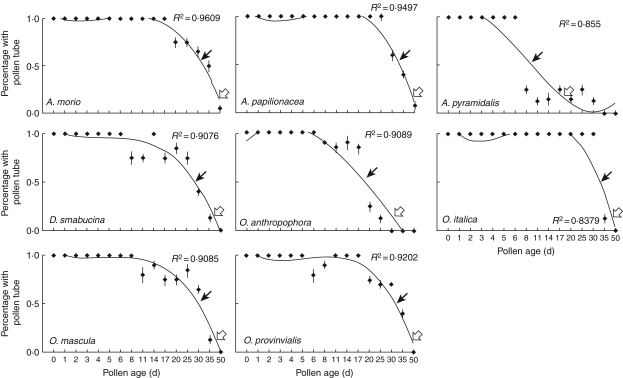
Fig. 1.
Relationships between pollen germinability and pollen age for food-deceptive orchids. Black and white arrows indicate 50 and 10 % pollen germinability, respectively.
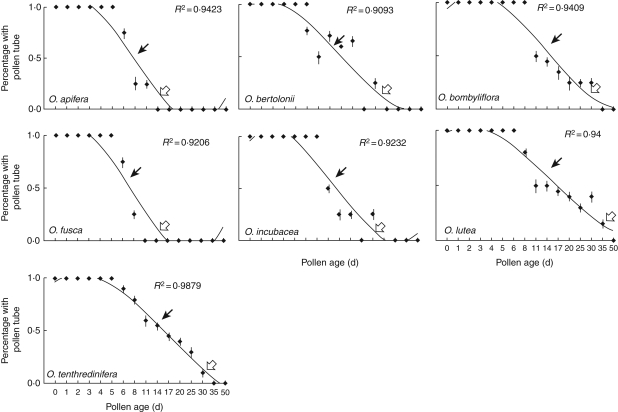
Fig. 2.
Relationships between pollen germinability and pollen age for sexually deceptive orchids. Black and white arrows indicate 50 and 10 % pollen germinability, respectively.
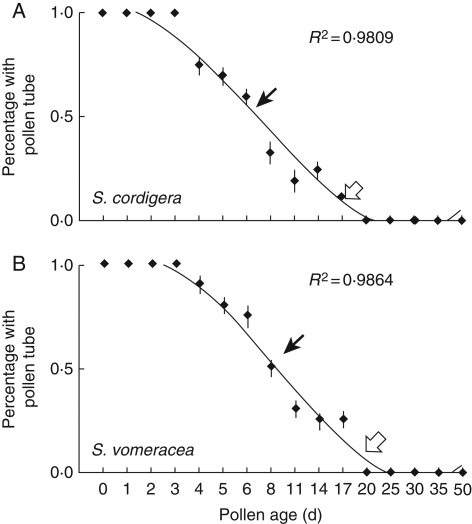
Fig. 3.
Relationships between pollen germinability and pollen age for shelter-deceptive orchids. Black and white arrows indicate 50 and 10 % pollen germinability, respectively.
On the other hand, there was a positive correlation among pollen germinability trends observed in those species with similar pollination strategies (food deceptive rs = 0·82, P < 0·001; sexually deceptive rs = 0·92, P < 0·001; shelter deceptive rs = 0·95, P < 0·001).
The duration of pollen viability (50 % of pollinia) was 8 d in A. pyramidalis (Fig. 1) and both Serapias species (Fig. 3), 8–14 d in all of the Ophrys species (Fig. 2), 20–30 d in all of the Orchis species and in D. sambucina (Fig. 1), and 30–35 d in the remaining two Anacamptis species (Fig. 1). Overall, these data indicate that the examined Orchidinae show a pollen life span in line with values previously reported for the orchid species.
Under the pollen viability threshold of 50 %, a 10 % viability was detectable at time points ranging from 35 to 50 d (mean value 40 d) in food-deceptive species (Fig. 1), 17–20 d (mean value 18·5 d) in Serapias species (Fig. 3) and 11–35 d (mean value 23 d) in sexually deceptive orchids (Fig. 2). Interestingly, there were highly significant differences in ‘pollen viability duration’ (F2,268 = 24·199, P < 0·001) and ‘10 % pollen viability’ (F2,268 = 22·865, P < 0·001) among pollination strategies. Regardless of the time required, total viability loss is marked by a sudden or progressive decrease.
Fruit production and viable seed
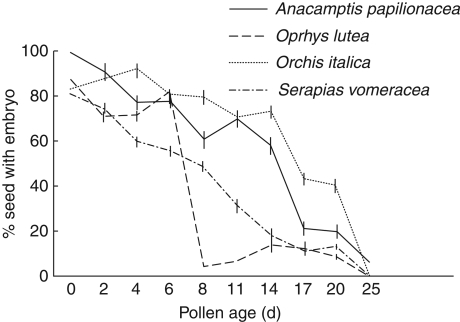
All of the manual crosses performed on plants of A. papilionacea, O. italica and S. vomeracea using fresh and older pollen triggered the development of fruits, while the hand-pollination performed in O. lutea with pollen older than 20 d did not produce fruits.
The presence of viable embryos was ascertained for each produced fruit. There was a significantly different pattern of embryo viability (F3,368 = 25·165, P < 0·001) between the four examined species. Anacamptis papilionacea and O. italica showed a high percentage of seeds containing embryos even in manual crosses with older pollen (Fig. 4), while the percentage of seeds with embryos in O. lutea and S. vomeracea decreased quickly in the manual crosses performed with pollen of 8 and 11 d old, respectively (Fig. 4). ANOVA showed highly significant differences in the effects of pollen age with respect to the percentage of seeds containig embryos between the food- (A. papilionacea and O. italica) and the sexually and shelter-deceptive species (F1,368 = 24·732, P < 0·001), while pollen age did not have an effect on the number of embryonated seeds produced in relation to A. papilionacea and O. italica (F1,198 = 0·499, P = 0·72) and to O. lutea and S. vomeracea (F1,197 = 0·660, P = 0·61).
Fig. 4.
Relationships between percentage of seeds with embryos and pollen age for Anacamptis papilionacea and Orchis italica (food-deceptive species), Ophrys lutea (sexually deceptive species) and Serapias vomeracea (shelter-deceptive species).
DISCUSSION
Experiments performed in the 1990s revealed that some orchid species possess the highest pollen viability durations, a trait that was regarded as a perfect fit with respect to their peculiar reproductive biology and pollination ecology (Neiland and Wilcock, 1995; Proctor, 1998). This led to the general assumption that orchid pollinia would remain viable for relatively long time periods, a prediction that has been confirmed by several studies, none of which specifically addressed the pollen longevity issue (Johnson and Edwards, 2000; Murren, 2002).
The aim of our study was to examine the pollen viability duration of several members of the deceptive Euro-Mediterranean orchid guild, which is particularly associated with infrequent pollination visits and long-distance pollen dispersal because most of its members have evolved a deceptive pollination system. Our results unequivocally demonstrate that pollen grains of all selected species maintain a prolonged capacity for in vitro germination, fertilization and embryonate seed development. In fact, pollen grains from all of the examined species even displayed 100 % germination rates 5 d after their dislodgement from the original flowers. After this period, pollen viability duration sensu Kumar (50 % pollen tube growth) varied depending on the species, and ranged from 8 to 35 d, while some species still retained 10 % pollen tube formation after 40–50 d (Figs 1–3). Similarly, we assessed the prolonged ability to stimulate fruit formation and embryonate seeds (Fig. 4).
Proctor (1998) studied pollen that was aged for a maximum of 8 d and he found both high in vitro germination rates and embryonate seed production in three deceptive North America orchids, although one of them, namely Cypripedium calceolum, releases pollen as monads. Neiland and Wilcock (1995) found that pollen of three Scottish species (Gymnadenia conopsea, Dactylorhiza maculata and D. purpurella) was still able to germinate after a storage period of 37–51 d, but did not check their seed set capacity. Thus, our work is the first, to our knowledge, that has documented prolonged in vitro germination and seed formation, confirming the assumption that orchid pollen grains within pollinia are able to survive for a long period of time.
Even more interestingly, we have demonstrated the existence of a relationship between pollen viability duration and the type of deceptive pollination utilized by our selected species. In fact, the food-deceptive group showed significantly higher values of pollen viability than both the sexually and shelter-deceptive groups, while these two latter groups showed very marginally significant differences. This finding has been validated by the existence of a higher percentage of embryonate seeds as produced in the two food-deceptive species (A. papilionacea and O. italica) than in O. lutea and S. vomeracea.
Our study is the first to have found a significant relationship between the pollen viability duration and pollination system. However, this issue has received little attention, probably because pollen viability is seen as greatly variable even at the intraspecific level, and it is strictly affected by environmental factors (Ottaviano and Mulcahy, 1989). Pacini et al. (1997) examined pollen viability in relation to pollination in three anemophilous and three entomophilous species, but failed to find a direct correlation; rather, in both groups they observed variable short- and long-term pollen grain variability. In the current study, many species of strictly related genera that prefer similar or the same habitats were examined, while the six angiosperm species examined by Pacini et al. (1997) belonged to different, unrelated genera.
Thus, we have developed some interesting inferences about the deceptive, Mediterranean orchid guild. Species of this group usually grow in sympatry, bloom in the same period, share the same pollinator faunas and, as a result, frequently undergo hybrid and backcross mating (van der Cingel, 1995). Recent studies have pointed out that food-deceptive species maintain their genetic identity by means of post-zygotic barriers (Scopece et al., 2007), while both Ophrys and Serapias species have effective pre-pollination barriers (Scopece et al., 2007; Bellusci et al., 2010). In consideration of this, we argue that a shortened pollen viability duration, like that exhibited in this study by sexually and shelter-deceptive species, could limit the number of interspecific mates. Similarly, species with active post-zygotic barriers, like all of the food-deceptive species, may have more viable pollen because they may eliminate genetic contamination. This hypothesis is sustained by recent reports of first-generation hybrids in admixed populations of food-deceptive species (Moccia et al., 2007) and of introgression among sympatric species, including both Ophrys (Soliva and Widmer, 2003) and Serapias (Bellusci et al., 2010). Interestingly, the genera Serapias and Ophrys have proven to be closely phylogenetically related (Bateman et al., 2003) and are able to emit comparable floral bouquets (Schiestl and Cozzolino, 2008) that may be part of their reproductive pre-pollination barriers.
In conclusion, this study has shown that prolonged germination and fertilization capacities of pollinaria are typical traits of the Mediterranean deceptive orchid guild. This demonstrates further evidence that the high pollen viability and longevity of orchids is an adaptive response for developing efficient pollination strategies to overcome the long travelling distance and low likelihood of pollinaria removal (Dafni and Firmage, 2000). These authors have also predicted, from an evolutionary ecology viewpoint, the relevance of potentially complex interactions between pollen longevity, pollination chances and breeding systems. Our paper supports this prediction by demonstrating a clear relationship between pollen viability and pollination systems that in turn could be linked to the different types of reproductive barriers.
LITERATURE CITED
- Ackerman JD. Mechanisms and evolution of food deceptive pollination systems in orchids. Lindleyana. 1986;1:108–113. [Google Scholar]
- Barret SCH, Charlesworth D. Effect of a change in the level of inbreeding on the genetic load. Nature. 1991;352:522–524. doi: 10.1038/352522a0. [DOI] [PubMed] [Google Scholar]
- Bateman RM, Hollingsworth PM, Preston J, Yi-Bo L, Pridgeon AM, Chase MW. Molecular phylogenetics and evolution of Orchidinae and selected Habenariinae (Orchidaceae) Botanical Journal of the Linnean Society. 2003;142:1–40. [Google Scholar]
- Bellusci F, Pellegrino G, Palermo AM, Musacchio A. Crossing barriers between the unrewarding Mediterranean orchids Serapias vomeracea and Serapias cordigera. Plant Species Biology. 2010;25:68–76. [Google Scholar]
- Bradshaw HD, Schemske DW. Allele substitution at a flower colour locus produces a pollinator shift in monkeyflowers. Nature. 2003;426:176–178. doi: 10.1038/nature02106. [DOI] [PubMed] [Google Scholar]
- Dafni A, Bernhardt B. Pollination of terrestrial orchids of Australia and the Mediterranean: systematical, ecological and evolutionary implications. Evolutionary Biology. 1989;24:193–252. [Google Scholar]
- Dafni A, Firmage D. Pollen viability and longevity: practical, ecological and evolutionary implications. In: Dafni A, Pacini E, Hesse M, editors. Pollen and pollination. Berlin: Springer; 2000. pp. 113–132. [Google Scholar]
- Dafni A, Ivri Y, Brantjes NBM. Pollination of Serapias vomeracea Briq. (Orch.) by imitation of holes for sleeping solitary male bees (Hym.) Acta Botanica Neerlendiana. 1981;30:69–73. [Google Scholar]
- Darwin CR. On the two forms, or dimorphic condition, in the species of Primula, and on their remarkable sexual relations [Read 21 November 1861] Journal of Proceedings of the Linnean Society. 1862;6:77–96. [Google Scholar]
- Desrochers AM, Rieseberg LH. Mentor effects in wild species of Helianthus (Asteraceae) American Journal of Botany. 1998;85:770–775. [PubMed] [Google Scholar]
- Dressler R. Orchids – natural history and classification. Cambridge, MA: Harvard University Press; 1981. [Google Scholar]
- Gumprecht R. Seltsame bestäubungsvorgänge bei Orchideen. Die Orchidee. 1977;28:1–23. [Google Scholar]
- Jersáková J, Johnson SD, Kindlmann P. Mechanisms and evolution of deceptive pollination in orchids. Biological Reviews. 2006;81:219–235. doi: 10.1017/S1464793105006986. [DOI] [PubMed] [Google Scholar]
- Johnson SD, Edwards TJ. The structure and function of orchid pollinaria. Plant Systematics and Evolution. 2000;222:243–269. [Google Scholar]
- Kearns CA, Inouye DW. Techniques for pollination biologists. Colorado: Niwot University Press of Colorado; 1993. [Google Scholar]
- Kullenberg B. Studies in Ophrys pollination. Zoologiska Bidrag fran Uppsala. 1961;34:1–340. [Google Scholar]
- Kumar A, Chowdhury RK, Dahiya OS. Pollen viability and stigma receptivity in relation to meteorological parameters in pearl millet. Seed Science and Technology. 1995;23:147–156. [Google Scholar]
- Luangsuwalai K, Ketsa S, Wisutiamonkul A, van Doorn WG. Lack of visible post-pollination effects in pollen grains of two Dendrobium cultivars: relationship with pollinia ACC, pollen germination, and pollen tube growth. Functional Plant Biology. 2008;35:152–158. doi: 10.1071/FP07245. [DOI] [PubMed] [Google Scholar]
- Luyt R, Johnson SD. Hawkmoth pollination of the African epiphytic orchid Mystacidium venosum, with special reference to flower and pollen longevity. Plant Systematics and Evolution. 2001;228:49–62. [Google Scholar]
- Mendel G. Versuche über pflanzen-hybriden. Verhandlungen des Naturforschenden Vereines Brünn. 1866;4:3–47. [Google Scholar]
- Moccia MD, Widemer A, Cozzolino S. The strength of reproductive isolation in two hybridizing food-deceptive orchid species. Molecular Ecology. 2007;16:2855–2866. doi: 10.1111/j.1365-294X.2007.03240.x. [DOI] [PubMed] [Google Scholar]
- Murren CJ. Effects of habitat fragmentation on pollination: pollinators, pollinia viability and reproductive success. Journal of Ecology. 2002;90:100–107. [Google Scholar]
- Neiland MRM, Wilcock CC. Maximisation of reproductive success by European Orchidaceae under conditions of infrequent pollination. Protoplasma. 1995;187:39–48. [Google Scholar]
- Ottaviano E, Mulcahy DL. Genetics of angiosperm pollen. Advances in Genetics. 1997;26:1–64. [Google Scholar]
- Pacini E. Tapetum character states: analytical keys for tapetum types and activity. Canadian Journal of Botany. 1997;75:1448–1459. [Google Scholar]
- Pacini E, Hesse M. Types of pollen dispersal units in orchids, and their consequences for germination and fertilization. Annals of Botany. 2002;89:653–664. doi: 10.1093/aob/mcf138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacini E, Franchi GG, Lisci M, Nepi M. Pollen viability related to type of pollination in six angiosperm species. Annals of Botany. 1997;80:83–87. [Google Scholar]
- Proctor HC. Effect of pollen age on fruit set, fruit weight, and seed set in three orchid species. Canadian Journal of Botany. 1998;76:420–427. [Google Scholar]
- Schiestl FP, Cozzolino S. Evolution of sexual mimicry in the orchid subtribe orchidinae: the role of preadaptations in the attraction of male bees as pollinators. BMC Evolutionary Biology. 2008;8:27. doi: 10.1186/1471-2148-8-27. doi:10·1186/1471-2148-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl FP, Ayasse M, Paulus HF, et al. Orchid pollination by sexual swindle. Nature. 1999;399:421–422. [Google Scholar]
- Schill R, Wolter M. On the presence of elastoviscin in all the subfamilies of the Orchidaceae and the homology to pollenkitt. Nordic Journal of Botany. 1986;6:321–324. [Google Scholar]
- Scopece G, Musacchio A, Widmer A, Cozzolino S. Patterns of reproductive isolation in Mediterranean orchids. Evolution. 2007;61:2623–2642. doi: 10.1111/j.1558-5646.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- Soliva M, Widmer A. Gene flow across species boundaries in sympatric, sexually deceptive Ophrys (Orchidaceae) species. Evolution. 2003;57:2252–2261. doi: 10.1111/j.0014-3820.2003.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Stanley RG, Linskens HF. Pollen: biology, biochemistry and management. New York: Springer; 1974. [Google Scholar]
- Stone JL, Thomson JD, Denta-Acosta SJ. Assessment of pollen viability in hand-pollination experiments: a review. American Journal of Botany. 1995;82:1186–1197. [Google Scholar]
- van der Cingel NA. An atlas of orchid pollination. Rotterdam: Balkema; 1995. [Google Scholar]
- Van der Pijl L, Dodson CH. Orchid flowers: their pollination and evolution. Coral Gables, Forida: University of Miami Press; 1966. [Google Scholar]
- Zimmerman M, Aide TM. Patterns of fruit production in a Neotropical orchid: pollinator vs. resource limitation. American Journal of Botany. 1989;76:67–73. [Google Scholar]