Abstract
Background
Timothy is a long-day grass species well adapted for cultivation in northern latitudes. It produces elongating tillers not only in spring growth but also later in summer. As the quantity and quality of harvested biomass is dictated by canopy architecture and the proportion of stem-forming flowering tillers, the regulation of flowering is of great interest in forage grass production.
Methods
Canopy architecture, stem morphology and freezing tolerance of vernalized timothy were investigated in greenhouse and field experiments. The molecular control of development was examined by analysing the relationship between apex development and expression of timothy homologues of the floral inducer VRN1 and repressor VRN2.
Key Results
True stem formation and lignification of the sclerenchyma ring occur in both vernalized and regrowing stems irrespective of the developmental stage of the apex. The stems had, however, divergent morphology. Vernalization enhanced flowering, and the expression of the VRN1 homologue was elevated when the apex had passed into the reproductive stage. High VRN1 homologue expression was not associated with reduction in freezing tolerance and the expression coincided with increased levels of the floral repressor VRN2 homologue. Field experiments supported the observed linkage between the upregulation of the VRN1 homologue and the transition to the reproductive stage in vernalized tillers. The upregulation of putative VRN1 or VRN2 genes was restricted to vernalized tillers in the spring yield and, thus, not detected in non-vernalized tillers of the second yield; so-called regrowth.
Conclusions
The formation of a lignified sclerenchyma ring that efficiently reduces the digestibility of the stem was not related to apex development but rather to a requirement for mechanical support. The observed good freezing tolerance of reproductive timothy tillers could be one important adaptation mechanism ensuring high yields in northern conditions. Both VRN1 and VRN2 homologues required a vernalization signal for expression so the development of yield-forming tillers in regrowth was regulated independently of the studied genes.
Keywords: Apex development, canopy structure, elongation, flowering, freezing tolerance, lignification, Phleum pratense, regrowth, sclerenchyma ring, spring growth, timothy, vernalization, VRN1, VRN2
INTRODUCTION
The canopy architecture of grasses is controlled genetically, although it is sensitive to environmental stimuli (Doust, 2007). In perennial grasses and winter cereals, vernalization has a great impact on the canopy architecture during spring growth, since the inhibition of flowering is released in vernalized shoots and the canopy consists of elongating and flowering tillers. Some species, such as Lolium perenne and Festuca arundinacea, require vernalization for the formation of true stems and flowering tillers (Heide, 1994). The digestibility of grasses, and especially that of stems, declines during maturation (Akin, 1989). Accumulation of lignin and the formation of a highly lignified sclerenchyma ring are thought to be responsible for the reduced microbial degradation and digestibility of stem cell walls (Grabber et al., 1992; Wilson and Hatfield, 1997; Chen et al., 2002). The transition of the apex from the vegetative to the generative stage is considered to be necessary for the formation and gradual accumulation of true stem biomass (Bélanger and McQueen, 1997), so there is a negative correlation between the quality and quantity of grass yield. The spring growth of vernalized grass tillers and the development of the yield and its quality can be predicted accurately by the accumulation of temperature sum in Nordic conditions (Rinne et al., 2001). Forage grasses are harvested several times during the year, and the growth and quality development of non-vernalized tillers of second and thirds yields – so-called regrowth – cannot be predicted accurately, leading to challenges in optimizing harvesting times (Kuoppala et al., 2008). The yield of regrowth tends to be quantitatively important but lower than that of spring growth, and consists of living and senesced leaves and tillers with vegetative structures (Pakarinen et al., 2008). The formation of flowering stems in regrowth is inhibited, most probably due to the lack of vernalization and to the presence of signals of shortening daylength.
Timothy (Phleum pratense) is well adapted for cultivation in Nordic conditions, since it has good overwintering capacity and the harvested forage grass is palatable to livestock. Compared with other cultivated forage grass species such as L. perenne, it has a superior freezing tolerance (Moriayma et al., 1995). The flowering does not require vernalization, but it is induced under long-day conditions (Heide, 1982). Knievel and Smith (1970) showed, however, that vernalization does affect the canopy structure in timothy, and the non-vernalized summer tillers produce more secondary tillers than the vernalized winter tillers. The release of flowering during vernalization and expression of floral inducers have also been connected with decreased freezing tolerance in winter cereals (Mahfoozi et al., 2001; Danyluk et al., 2003). Thus, the fulfilment of the vernalization requirement may increase the risk of winter deaths of tillers shifted to a reproductive stage. In grasses, such deaths of vernalized tillers may have an effect on the canopy structure and number of flowering tillers in spring growth. However, the compensation capacity of grasses to replace lost tillers is high, which may explain the lack of reports on the relationship between vernalization and freezing tolerance in forage grasses.
The amount of flowering and stem-forming tillers is important for the quantity and quality of forage biomass. The floral transition and formation of stem-forming tillers is induced by signals from both environmental and endogenous (or autonomous) pathways. According to current understanding, the floral inductive signal is perceived in vegetative tissues such as leaves, and then transported to the shoot apical meristem (Colasanti and Coneva, 2009). Flowering is known to be induced by several pathways such as vernalization, photoperiod, gibberellic acid (GA) and an autonomous pathway (He and Amasino, 2005). The autonomous and GA pathways respond to endogenous factors such as plant age, leaf number and energy status, whereas the photoperiod and vernalization pathways respond to environmental factors. In both dicots and monocots, vernalization promotes flowering by repressing the transcription of controlling gene(s) with simultaneous upregulation of flowering inducer gene(s) (Greenup et al., 2009). The molecular control of vernalization has been studied in L. perenne and L. temulentum among the forage grasses, and orthologues of the floral inducers VRN1 (VERNALIZATION 1) and VRN3 (VERNALIZATION 3) have been identified (Gocal et al., 2001; Andersen et al., 2006; Ciannamea et al., 2006; Studer et al., 2009). Moreover, floral repressors LpMADS10 (L. perenne SHORT VEGETATIVE PHASE like gene) (Ciannamea et al., 2006) and LpTFL1 (L. perenne TERMINAL FLOWER 1) (Jensen et al., 2001), and other genes, LpCO (L. perenne CONSTANS) (Martin et al., 2004) and LpLIR1 (L. perenne LIGHT INDUCED RICE) (Ciannamea et al., 2007), related to the vernalization and daylength response of L. perenne, have been cloned from subtractive libraries. The identification of genes controlling flowering and manipulation of their expression can have great potential in attempts to increase the quantity as well as the quality of economically important forage grasses. Therefore, delayed or suppressed flowering has been one of the targets in breeding forage grasses for improved digestibility (Jensen et al., 2004).
The objective of this study was to reveal the physiological and molecular mechanisms of the vernalization response in timothy. Vernalization promotes both stem elongation and flowering in forage grasses and, therefore, the relationships of stem elongation and apex development to stem morphology and lignin localization was also studied. A homology-based identification of vernalization genes was used to design primers for VRN1 and VRN2 homologues in timothy, and the expression was examined under controlled vernalization and daylength conditions in greenhouse as well as in field experiments. While having an important role for yield formation in second and third harvests, the molecular control of development in regrowing tillers of grasses has not been previously studied. This study provides the first insights into how the apex development is related to stem lignification and how the two vernalization genes VRN1 and VRN2 are regulated in spring growth and regrowing tillers of timothy.
MATERIALS AND METHODS
Plant material, growth conditions and determination of apex development
For controlled vernalization conditions, timothy plants (Phleum pratense L. ‘Iki’) were grown in a greenhouse at 20 °C under a 16 h day for 2 weeks prior to the transfer to vernalization conditions. The light intensity was 200–500 µmol m−2 s−1 and natural daylight was supplemented with 400 W high-pressure sodium lamps (Lucalox, LU 400/HO/T/40 NG, Hungary). The plants were grown in 5 L pots containing fertilized and limed peat (Kekkilä B2, Finland).
The timothy plants (‘Tammisto II’) grown in field conditions were located at the MTT Agrifood Research, Maaninka Research Station, Finland (63°10′N, 27°18′E). The experimental field was established in 2005 in three replicates of experimental plots of 12 m2 using barley as a cover crop, which was harvested after heading. During three consecutive years, the experimental field was fertilized for primary growth with 90, 13·5 and 22·5 kg ha−1 and for regrowth with 90, 0 and 31·5 kg ha−1 of N, P and K, respectively, and cut twice per growing season according to the typical cultivation practice of the area. The samples for anatomical and RNA analysis were harvested during the development of the sward in the second and third harvesting years, namely seasons 2007 and 2008.
The developmental stage of the shoot apex of tillers was recorded using a scale adapted from the 11-stage scale developed for L. perenne by Sweet et al. (1991). In this determination, the shoot apices at stages A1 (fully vegetative) and A2 (apex vegetative but elongated) were considered as vegetative, at stages A3 (apex elongated with visible leaf and spikelet primordia) and A4 (double ridge stage) as being at the transition from the vegetative to reproductive stage, and of stages A5 (development of the apex beyond the double ridge stage) and further as reproductive shoot apex. In addition to the original scale, three further stages of development were defined: at stage A12, lemmas were more extended and had reached the height of the floret initials; at stage A13, glumes were well above the floret initials, which were more hidden by the lemmas; and at stage A14, some hairy structures could be seen on the external surface of the glumes. The apex height was measured from ground level to the base of the apex. The dissection of the apex was done if needed. The total length was measured from ground level to the top of the plants as the plant was stretched to its full length.
Vernalization treatments and freezing tests
Timothy plants grown in a greenhouse were subjected to vernalization treatment at 6 °C/4 °C (day/night), 8 h daylength in a growth chamber (Weiss Technik, Germany) for 0, 2, 10, 18 or 20 weeks. The pots were arranged in a completely randomized design with three replications in the growth chamber, and rotated once a week in order to minimize the effects of possible temperature or light difference within the chamber. The development of the shoot apex was followed during vernalization and the developmental stage was recorded. For RNA samples, the leaves and apices of ten plants were harvested immediately after the indicated vernalization time points and after 7–28 d of growth in the greenhouse.
The development of freezing tolerance during vernalization was evaluated by regrowth tests. After vernalization, plants were carefully removed from the soil, and the roots were rinsed with water. The plants had one to two tillers which was not dependent on the length of the vernalization period. Plants were placed in a controlled-temperature cooling bath (–1 °C) in covered culture tubes, four plants per tube, with a piece of wet tissue paper in the bottom. Extracellular ice was initiated by adding a small piece of ice to each tube, and the temperature was held at –1 °C for 1 h, then lowered at 2·5 °C h−1 to the pre-determined minimum temperature (–15 to –25 °C). Samples were removed from the cooling bath at 2·5 °C intervals and thawed at 4 °C overnight. Meanwhile, the control samples were kept at 4 °C. After thawing, the plants were planted in soil in the greenhouse and the regrowth was evaluated weekly until the development of new tillers had ceased (5–6 weeks). Four plants per replication were subjected to each test temperature. The development of plants was monitored after vernalization treatments and freezing tests by calculating the number of leaves, stems and inflorescences weekly.
Anatomical studies
Tillers for anatomical studies were collected from the field experiment during spring growth and regrowth in 2007. At each collection time, 12–20 random tillers containing true stems were cut at the base. Tillers were kept in 4 °C, and total height, ligule and apex height, leaf and node number and the developmental stage of the shoot apex were all determined. Tillers were separated by the developmental stage of the apex into classes A1, A2, A3, A4 and A5 or further.
Pieces of true stem were fixed immediately after collection and stored at room temperature in formalin–acetic acid–alcohol (FAA) before sample preparation. After measurement of ligule height, the internode located halfway up the true stem was used for anatomical studies. Stem samples were collected from the middle part of the selected internodes. Simultaneously samples from two or three of the youngest fully developed leaves were collected in RNAlater solution (Applied Biosystems, USA) and kept at 4 °C until frozen at –70 °C before analysis. The stem samples were dehydrated in a graded ethanol series and embedded in paraffin at 60 ± 2 °C. Cross-sections of 8–12 µm were cut with a microtome (Leica RM2125, McBain Instruments). Paraffin was removed with Histo-Clear (National Diagnostic, UK), and samples were rehydrated in a graded ethanol series. Sections were stained with conventional safranin–alcian blue stain for lignin and polysaccharides (1 % safranin in 50 % ethanol and 0·1 % alcian blue in 3 % acetic acid), and finally mounted in Histomount (National Diagnostic, UK). Three replicate stems of each apex developmental stage were prepared and examined, and photographed with an Olympus DP 50 digital camera connected to a Leitz Laborluxs light microscope or a Leica MZFLIII stereomicroscope (Nilomark).
Identification of putative VRN1 and VRN2 orthologues
For the amplification of two putative vernalization-responsive gene orthologues in timothy, the flowering inducer VRN1 and the flowering repressor VRN2, primers were designed against the corresponding VRN1 genes in L. perenne MADS1 (AY198326), L. temulentum MADS1 (AF035378) as well as T. aestivum TaVRT1 (AY280870) and corresponding VRN2 (TCCT) genes in Triticum monococcum (AY485969) and Hordeum vulgare (AY485977, AY485978, AY687931). The primers for VRN1, LpMADS1_F341_b (forward 5′-GAGCGGTATGAGCGCTACTC-3′) and LpMADS1_R450_b (reverse 5′-GTCTCAACCTTCGCCTTCAG-3′), amplified a 1103 bp genomic DNA fragment that was verified by sequencing as a VRN1 homologue in timothy. The primers for VRN2, VRN2F (forward 5′-GGTGATGAGGTATAGGGAGAAGAG-3′) and VRN2R (reverse 5′-GAACCATCCGAGGTGAAGTT-3′), amplified a 192 bp genomic DNA fragment that showed sequence homology to the T. monococcum VRN2 gene. The PCR (50 µL) contained 0·5 U of Taq polymerase (DyNAzyme II, Finnzymes, Finland), 1× primer buffer, 200 nmol of each primer, 200 nmol of dNTPs and 100 ng of total genomic DNA (‘Iki’). PCR was perfomed (Eppendorf Nethaler- Hinz, Hamburg, Germany) in the following conditions; 5 min at 95 °C, followed by 35 cycles of 30 s at 94 °C, 45 s at 53 °C and 90 s at 72 °C. The final extension step of 30 s at 72 °C was included. The PCR fragments were excised from a 1 % TAE agarose gel and purified using the QIAquick gel extraction kit (QIAGEN, Hilden, Germany), and cloned immediately into the pCR2·1-TOPO or pCRII-TOPO cloning vectors (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. Cloned fragments were sequenced (Institute of Biotechnology, Finland) and the sequence identity searches for VRN1 and VRN2 genes were performed at the NCBI (http://www.ncbi.nlm.nih.gov) using the basic local alignment search tool (BLAST). Species-specific primers for VRN1 (forward 5′-GAGCGGTATGAGCGCTACTC-3′, reverse 5′-TTCAGATTCGGTTGAAATGAGA-3′) and VRN2 (forward 5′- AAGCTTATGCCCAGTTGAGG-3′, reverse 5′-GGGATCGTAGGACGATTGTG-3′) were designed for quantitative real-time PCR analysis to produce a 60–100 bp fragment, and qPCR product sizes were verified in agarose gels.
RNA extraction, cDNA synthesis and quantitative real-time PCR analysis
RNA samples were collected from leaves and apices of 15 plants under controlled vernalization conditions. The samples were pooled and three replicates were used for PCR analysis. The leaves of 3–4 field-grown tillers of the indicated apex stage were collected and used as replicates in PCR analysis. The RNA was extracted according to the manufacturer's instructions (RNAeasy Plant Mini, Qiagen, Hilden, Germany). The first-strand cDNA synthesis was performed in nuclease-free microcentrifuge tubes containing 100 ng of oligo(dT)18 primer, 1 µg of total RNA, 1 µL of 10 mm dNTP Mix and sterile RNase-free water to 13 µL. The mixture was heated to 65 °C for 5 min and then chilled on ice. After a brief centrifugation, 4 µL of 5× First-Strand buffer (Invitrogen, Karlsruhe, Germany), 1 µL of dithiothreitol (DTT; 0·1 m) and 1 µL (200 U) of SuperScript III RT (Invitrogen, Karlsruhe, Germany) were added and the mixture was heated for 5 min at 25 °C, for 45 min at 50 °C and for 15 min at 70 °C. The mixture was diluted with water to 95 µL, 5 µL of which was used per real-time PCR.
Quantitative real-time PCR analysis was carried out in an ABI7000 (Applied Biosystems, Foster City CA, USA) in a 20 µL reaction containing 10·4 µL of SYBR Green master mix (Finnzymes Oy, Espoo, Finland), 0·5 µm forward primer, 0·5 µm reverse primer, 5 µL of cDNA template and 2·6 µL of MQ-water. Actin (forward 5′-ACTGGGACGACATGGAGAAG-3′, reverse 5′-CTGTTAGCCTTGGGGTTCAG-3′) and 18S (forward 5′-TCTTGCTATAAAACAGGCTCGT-3′, reverse 5′-TCTGGGTCTGGAACCAGTAA-3′) genes were tested as endogenous controls. All primers were tested in a decreasing series of cDNA template concentration (100, 50, 20, 10, 5 and 1 %). The efficiency (E) values of the VRN1, VRN2 and actin primers were 2·0, 2·1 and 1·9, respectively. For the calculations of relative gene expression, actin was chosen since its expression was constant between the treatments. The expression level units were calculated for each candidate gene and time points with the Q-gene software tool (Muller et al., 2002).
RESULTS
Interaction of the developmental stage of the apex with stem morphology
Under field conditions, a higher proportion of tillers are reproductive in spring growth than in summer or autumn regrowth. When both the main and side tillers were taken into account, the percentage of flowering tillers in primary growth was 30–40 % whereas the percentage in regrowth was only 1–2 % of all tillers. In this study, it became clear that the transition of the apex to the reproductive stage is not necessary for the initiation of stem elongation, and thus elongating tillers, in which the apical meristem is vegetative, can be found from both primary growth and regrowth (Table 1). In spring growth, the stem elongation occurs with increasing temperatures and lengthening photoperiods. Prior to this stage, all nodes are developed but they are still packed tightly together. As the internodes elongate, the nodes become visible. In field conditions in 2007, vegetative shoot apices were clearly above the ground level and true stems had been formed: the stem height of elongating tillers at the vegetative or transition stages (A1–A4) in spring growth ranged from 320 to 426 mm and in regrowth from 95 to 270 mm (Table 1). There were 2–4 visible nodes above the basal node in the elongating tillers of spring growth and 1–3 visible nodes in the regrowth (data not shown), and the lengths of the internodes were shorter in the regrowth than in the spring growth (Table 1). Elongating tillers with a reproductive apex (A12) in spring were considerably taller than those in regrowth. Although the height of the studied stems varied significantly between different apex classes, the height of the stem was not determined by the developmental stage of the apex.
Table 1.
The developmental stage of the apex and measured lengths of internodes, total height, height of the highest ligule and apex height, i.e. true stem length, in elongating vegetative tillers used for anatomical studies in spring growth (SG) and regrowth (RG) in 2007.
| Internode length (mm) |
Total height (mm) |
Ligule height (mm) |
Apex height (mm) |
|||||
|---|---|---|---|---|---|---|---|---|
| Apex | SG | RG | SG | RG | SG | RG | SG | RG |
| A1 | 101 | 22 | 652 | 237 | 479 | 104 | 426 | 48 |
| A2 | 106 | 53 | 573 | 528 | 381 | 222 | 367 | 165 |
| A3 | 90 | 65 | 570 | 584 | 402 | 337 | 347 | 270 |
| A4 | 92 | 25 | 562 | 545 | 375 | 328 | 320 | 95 |
| A5 | 69 | – | 416 | – | 190 | – | 95 | – |
| A10 | 68 | 73 | 454 | 572 | 226 | 319 | 122 | 223 |
| A12 | 28 | 68 | 756 | 493 | 437 | 296 | 93 | 209 |
| A14 | 120 | – | 710 | – | 513 | – | 477 | – |
| n total | 111 | 62 | 111 | 62 | 111 | 62 | 109 | 62 |
| P-value | – | – | <0·0001*** | 0·015* | <0·0001*** | 0·030* | <0·0001*** | 0·026* |
* P < 0·05; *** P < 0·001.
–, no observations.
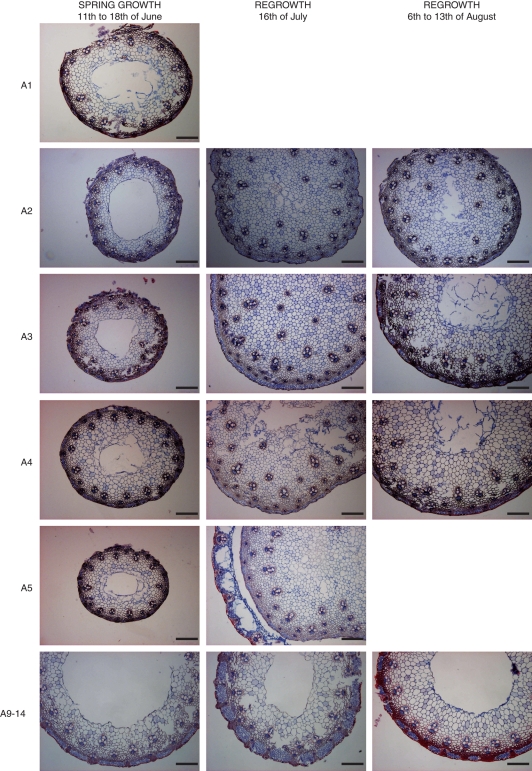
During booting in spring, the internodes of vernalized tillers became hollow at an earlier developmental stage than internodes of regrowing tillers in July and August (Fig. 1). In spring growth, all analysed stem samples at all developmental stages of the apex already had hollow internodes. In contrast, most of the stem samples harvested in the autumn had solid internodes, although some hollow internodes were also observed. The total height of elongating tillers in spring and regrowth was similar from apex stage A2 onward, but the internodes in regrowing stems were shorter and this may explain the observed differences in stem morphology (Table 1, Fig. 1). A lignified schlenchyma ring formed at early stages of apex development, especially in the vernalized tillers during spring growth (Fig. 1). The formation seemed not to be associated with the developmental stage of the apex, but was rather related to stem height during spring growth (Fig. 1, Table 1) and sampling time during regrowth (Fig. 1, July vs. August). Although the anatomical analysis does not allow precise quantification of lignin content, the sclerenchyma ring in regrowing tillers was apparently less lignified in July than in August.
Fig. 1.
The morphology of stems in spring growth (11–18 June) and regrowth (16 July and 6–13 August) at different developmental stages of the apex. A1 to A2 refer to the vegetative stage, A3 to A4 to the transition stage and A5 and more to reproductive stages of apexes. Stem samples of three individual tillers of the indicated apex stage were used for anatomical studies; n = 3 replicate stem sections per apex stage and sampling time. Scale bars = 200 µm in each photomicrograph.
Vernalization, freezing tolerance and canopy architecture
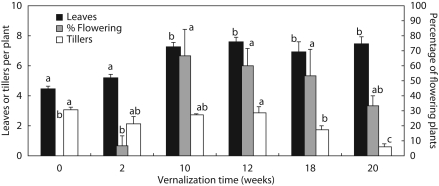
The transition of apices to the reproductive stage was first observed in plants vernalized for 10 weeks. The percentage of flowering plants was also the highest (67 %) in plants vernalized for 10 weeks (Fig. 2) and it decreased gradually from 60 to 33 % in plants vernalized for 12–20 weeks. The decrease was not, however, statistically significant. After 12 weeks of vernalization, the number of developing tillers started to decrease, and after 20 weeks of vernalization, the canopy was thin, consisting of only a few lateral tillers, some with inflorescences. The leaf number increased until 10 weeks of vernalization and then was unaffected by prolonged vernalization time.
Fig. 2.
Vernalization time and canopy structure in timothy. The number of leaves, the number of tillers and the percentage of flowering plants (as indicated) were determined 30–45 d after vernalization, when the development of new tillers had ceased. The seedlings were vernalized at 6 °C/4 ° C (day/night) for 0, 2, 10, 18 or 20 weeks and transferred to the greenhouse for growth monitoring for 5–6 weeks. The transition of the apical meristem from the vegetative to the reproductive stage occurred in seedlings vernalized for ≥10 weeks. Data show the means ± s.e. of a total of 15 seedlings. Bars indicating the leaf or tiller number or percentage of flowering stems above which there is a common letter do not differ significantly at P ≤ 0·05 with Tukey test.
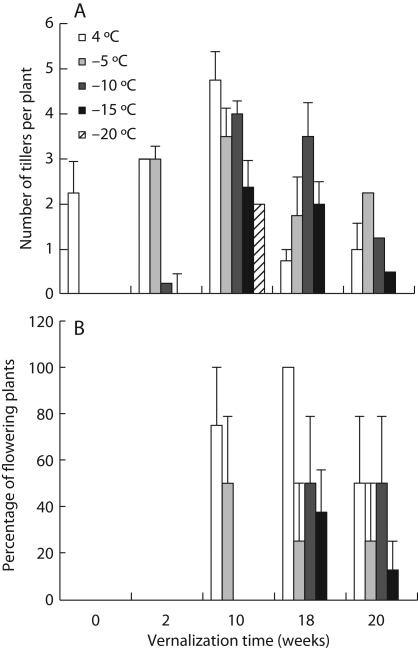
The plants vernalized for 10 weeks had the best freezing tolerance as they could recover from freezing down to –20 °C (Fig. 3A). Prolonged vernalization reduced the number of developing tillers gradually, so that at each tested freezing temperature there were fewer tillers than in plants vernalized for 10 weeks. The reduced number of tillers per plant was observed simultaneously with a higher percentage of flowering plants in plants vernalized for 18–20 weeks after freezing (Fig. 3B). The freezing test procedure seemed to recover the flowering induction in plants vernalized for 18–20 weeks, since the percentage of flowering plants was 50–100 % after freezing tests in plants vernalized for 18–20 weeks (Fig. 3B) but only 33–53 % if the plants were directly transferred to the greenhouse (Fig. 2).
Fig. 3.
The impact of vernalization time on the freezing tolerance of timothy and canopy structure was determined in terms of (A) the number of developing lateral tillers and (B) the percentage of flowering plants after freezing. The plants were vernalized at 6 °C/4 °C (day/night) for 0, 2, 10, 18 or 20 weeks and exposed to controlled freezing conditions. Data show the means ± s.e. of 9–12 plants.
Expression of putative timothy VRN1 and VRN2 orthologues
PCR primers, designed to amplify putative VRN1 and VRN2 orthologues in timothy, produced fragment of 1103 and 192 bp, respectively. The first 75 bp of the putative genomic VRN1 sequence had a high homology (83 %) with the second exon of the VRN1 gene of T. monococcum (EU875079·2, 2012–2090), and with L. perenne (96 %) (AY198326, 361–468) and L. temulentum (96 %) MADS1 (AF035387, 338–445) VRN1 genes (Fig. 4A). The position of the T. monococcum intron at 2091–3225 and, thus, the potential start site of an intron at 66 bp also in the 1103 bp genomic sequence in timothy, led to primer design on a sequence with high homology with VRN1 genes in other species. The VRN2 candidate had 82 % homology to H. vulgare and T. monococcum VRN2 genes, also named ZCCT-Hb (DQ492696, 1853–2042) and ZCCT1 (AY485969·1, 542–731), respectively (Fig. 4B).
Fig. 4.
Sequence alignment of VRN1 and VRN2 candidate genes in timothy (Phleum pratense, P.p.) with corresponding orthologues in T. monococcum (T.m., EU875079·2), L. perenne MADS1 (L.p., AY198326) and L. temulentum MADS1 (L.t., AF035387) for VRN1, and T. monococcum (T.m. AY485969) and H. vulgare (H.v DQ492696) for VRN2. Primer sequences used for qPCR analysis are underlined.
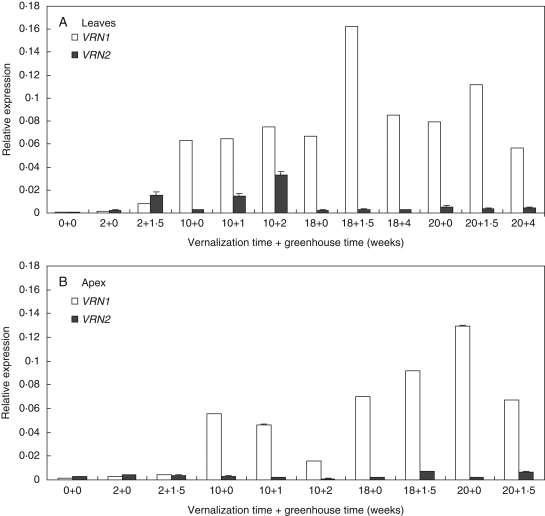
The expression of the timothy VRN1 candidate gene was upregulated in leaves and apex by vernalization (Fig. 5A, B). Significantly increased expression of VRN1 was first detected in the leaves of plants vernalized for 10 weeks and not in non-vernalized controls, and the level of expression remained high during prolonged vernalization. In the apex, VRN1 transcripts were also first detected after 10 weeks of vernalization but higher transcript accumulation was found as the vernalization time increased. VRN1 transcripts accumulated under short days during vernalization (10 + 0, 18 + 0, 20 + 0), and the transfer to long days in the greenhouse did not have a consistent effect on trancript levels. The VRN2 candidate transcript accumulated in long days after vernalization in leaves of plants vernalized for 2–10 weeks (2 + 1·5, 10 + 1, 10 + 2), but not in those vernalized for longer. During vernalization, altered expression of VRN2 was not observed in the apex. The VRN1 expression in the leaves of plants vernalized for 10 weeks coincided with the observed transition of apical meristem from the vegetative to the reproductive phase, as well as with the highest percentage of flowering plants.
Fig. 5.
Quantitative real-time PCR expression analysis of putative VRN1 and VRN2 orthologues, as compared with Actin transcript levels, in (A) leaves and (B) apex of timothy plants during vernalization for 0, 2, 10, 18 and 20 weeks in a growth chamber and after 1–4 weeks of growth in a greenhouse. Data are the means ± s.e of three replicate samples.
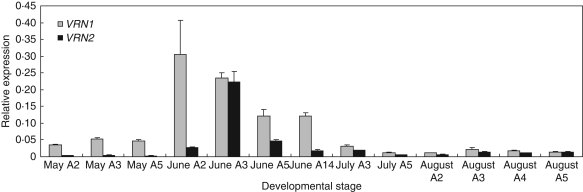
Results from the field experiments supported the observations obtained from controlled vernalization conditions. The expression of the VRN1 candidate gene was elevated in elongating tillers during spring growth, and the upregulation coincided with the transition of the shoot apex from the vegetative (June A2–A3) to the reproductive stage (June A5) (Fig. 6). In the regrowing sward in July and August, elevated transcript levels of VRN1 were not detected in elongating tillers at the same apex developmental stage (July A3, August A3). The highest level of VRN2 expression was observed at the transition from the vegetative to the reproductive stage of the apex (June A3) and coincided with slightly reduced levels of VRN1 expression.
Fig. 6.
Relative amounts of putative VRN1 and VRN2 orthologue transcripts as compared with Actin transcript levels in the leaves of field-grown timothy plants at different developmental stages. Samples were harvested from vernalized plants of spring growth during June and regrowth during July–August. The leaves of individual plants were classified according to the developmental stage of the apex. Data are the mean ± s.e. of 3–4 plants. The transition from vegetative to reproductive stage occurs at apex stage A3–A4.
DISCUSSION
The quantity and quality of harvested forage biomass are defined by the canopy structure of the grass sward. The spring growth is mainly regulated by vernalization and photoperiod, leading to a high number of flowering tillers and gradually decreasing digestibility of the yield, whereas the regrowth is probably affected by photoperiod and plant endogenous signals. A significant reduction in the digestibility of developing forage yield is thought to be the result of extensive lignification of flowering stems (Akin, 1989; Grabber, 2005), and the transition of the apex from vegetative to reproductive is often thought to be critical for the digestibility of the forage biomass. Our observations with timothy under field conditions proved that the differentiation of the stem apex to the reproductive stage is not needed for the formation of true stem in this species, and a lignified sclerenchyma ring, which reduces the digestibility of the true stem (Wilson and Hatfield, 1997), can already be found in vegetative tillers. In F. arundinacea, a lignified sclerenchyma ring was observed in elongating tiller stems when the second node was palpable, and a significant increase in cell wall lignin content as well as accumulation of S-lignin monomers was observed later, when tillers were reproductive (Chen et al., 2002), although the developmental stage of the apex was not analysed in those studies. It seems that in timothy, the lignification process may be related to stem elongation and to the requirement for mechanical support of the stem rather than to the developmental stage of the apex. The sclerenchyma cell walls increase in thickness during maturation, leading to reduced lumen volume (Wilson and Hatfield, 1997). Such thick-walled sclerenchyma cells were observed only in regrowing stems in late summer (stage A9). This indicates that the development of the apex may have been arrested during regrowth, due to environmental and endogenous signals inhibiting flower induction, but the stem internodes had continued maturing processes without further development of the apex. Thus, the developmental stage of the apex does not necessarily correlate with the maturation stage of the stem. In timothy, arrested or backwards development of the apex was reported by Heide (1994), who found that flowering induction is not necessarily unidirectional but in some conditions a reproductive apex can revert back to a vegetative, leaf-forming apex.
The morphology of regrowing tillers differed notably from that of vernalized tillers; hollow stems were seldom observed in regrowing tillers but were already seen at the apex developmental stage A1 in vernalized tillers. The observed difference in morphology could not be explained by total height or by ligule height of the stems. Rather, the regrowing tillers also had shorter internodes and the height of the apex was lower, especially at early developmental stages of the apex. The maturation of the vernalized tillers seemed to be relatively synchronized, leading to high number of flowering tillers with hollow stems and most probably high cell wall content in the spring harvest. Taken together, the results show that the yield-forming stems in spring and regrowth have different morphology and the regulatory processes for stem formation and flowering are probably different. Our results support the observations by Knievel and Smith (1970) who also reported that non-vernalized and vernalized timothy tillers can have the same height. In their experiments vernalization did not increase the number of inflorescences but rather enhanced the rate of development. Those field experiments were, however, conducted during spring when the photoperiod is not repressing the transition to the reproductive stage as is probably the case in the regrowing tillers in this study.
Vernalization time seemed to have an optimum, after which longer times under vernalization conditions did not increase, but rather decreased, the number of flowering stems. Langer (1955) made similar observations on the reduction of the number of ears in timothy plants as the vernalization time of seeds was extended from 4 to 8–10 weeks. The induction of flowering in forage grass species that require double induction, e.g. both low temperature and long-day treatment, is shown to be sensitive to temperature and daylength conditions after low temperature treatment (Heide, 1994). Vernalization causes chromatin modifications that allow flowering induction to proceed (reviewed in Greenup et al., 2009). In this study, the formation of flowering stems after long vernalization was more intensive in plants which had been transferred to freezing test conditions for 1 d prior to planting in the greenhouse. The result indicates that the freezing test treatment after prolonged vernalization may have released some epigenetic regulation of flowering. Alternatively, the temperature and light conditions during freezing treatment were more optimal than greenhouse conditions for flowering induction in those plants.
In cereals, the winter or spring growth habit is defined by the vernalization requirement. Perennial grass species are harvested several times during the growing season, and the vernalization requirement for stem elongation and flowering most probably affect the yield and canopy structure of the first yield but not the second and third yields during summer. The molecular control of flowering in regrowing tillers of grasses has not been studied previously, although they have an important role for yield formation in the second and third yields. There is great variation in the vernalization requirement between species of forage grasses (Heide, 1994). Some species have an obligatory vernalization requirement (L. perenne, Festuca spp.) whereas in others vernalization enhances flowering as shown in this study on timothy. The spring growth of forage grasses is probably mainly regulated via vernalization and photoperiod signals, whereas the endogenous pathway may have a significant role in developmental processes during summer. However, in several temperate grasses (Bromus inermis, F. pratensis, Dactylis glomerata and L. perenne), it has been shown that flowering induction can be transferred from vernalized tillers to their offspring, probably by molecular messenger exchange through vascular tissue at the base of the tiller (Havstad et al., 2004). In Arabidopsis and rice, FT protein and its orthologue Hd3a have been shown to act as long-distance flowering signals (Jaegger and Wigge, 2007). Orthologues of FT have been identifed in cereals and Lolium (VRN3) (Yan et al., 2006), and it is likely that the function is conserved across grasses. Thus, orthologues of FT and VRN3 may have a role in transferring signals from vernalized timothy tillers to their offspring in regrowing swards.
In this study the induced expression of VRN1 coincided with the transition of the apical meristem in vernalized tillers to the reproductive stage in both greenhouse and field conditions. VRN1 was highly induced in apex stages A2 and A3 in the spring growth and the expression of VRN2 was also high at stage A3, after which both transcripts decreased at stages A5 and A14. In field conditions, the expression of both VRN1 and VRN2 peaked in June when the daylength was the longest, 20 h. Also, the relative expression level was higher in field conditions compared with plants grown in the greenhouse. These observations indicate that the expression level of VRN1 and VRN2 was dependent on daylength and light intensity. The studied genes were also active only in vernalized tillers, which may indicate a low-temperature requirement of VRN1 induction in timothy similar to that which has been reported in barley (Trevaskis et al., 2006; Sasani et al., 2009). An increased level of VRN1 expression and transition from the vegetative to the reproductive stage is associated with lowered freezing tolerance in winter cereals, and in spring-habit genotypes VRN1 was constitutively upregulated (Danyluk et al., 2003). Elevated VRN1 expression did not seem to decrease the freezing tolerance of timothy, indicating that its reproductive tillers are not very sensitive to freezing. This may be one of the crop's adaptation mechanisms ensuring high yields in northern conditions.
In cereals, vernalization releases VRN1 expression and induces flowering through repression of VRN2 and induction of VRN3 by long days (Hemming et al., 2008). VRN2 expression is repressed at low temperatures and it integrates the vernalization and photoperiod pathways in winter cereals. VRN2 is not strictly a vernalization gene but it is also thought to repress floral induction under long-day conditions during summer to avoid flowering just prior to winter (Dubcovsky et al., 2006; Trevaskis et al., 2007). Although rice does not require vernalization for flowering, it has a gene closely related to VRN2, Ghd1, that represses flowering under non-inductive long days (Xue et al., 2008). In contrast to cereals, elevated levels of the VRN2 transcript were absent in regrowing timothy tillers. The hexaploid nature of timothy and the possibility for allelic variation in the VRN2 gene may have affected the results and, thus, definite conclusions on the role of VRN2 in regrowing timothy tillers cannot be drawn yet. A full understanding of how the development of elongating and flowering stems is regulated in vernalized and non-vernalized timothy tillers also requires detailed studies on the expression of other vernalization genes, such as VRN3, and regulatory genes of the photoperiod, GA and autonomous pathways.
ACKNOWLEDGEMENTS
We thank Mrs Lilia Sarelainen and Aira Vainiola for excellent assistance in the anatomical studies of timothy stem sections. This work was supported by the Ministery of Agriculture and Forestry and Kylvösiemensäätiö, and these contributors are greatfully acknowledged.
LITERATURE CITED
- Akin DE. Histological and physical factors affecting digestibility of forages. Agronomy Journal. 1989;81:17–25. [Google Scholar]
- Andersen JR, Jensen LB, Asp T, Lubberstedt T. Vernalization response in perennial ryegrass (Lolium perenne L.) involves orthologues of diploid wheat (Triticum monococcum) VRN1 and rice (Oryza sativa) Hd1. Plant Molecular Biology. 2006;60:481–494. doi: 10.1007/s11103-005-4815-1. [DOI] [PubMed] [Google Scholar]
- Bélanger G, McQueen RE. Leaf and stem nutritive value of timothy cultivars differing in maturity. Canadian Journal of Plant Science. 1997;77:237–245. [Google Scholar]
- Chen L, Auh C, Chan F, Cheng X, Aljoe H, Dixon RA, Wang Z. Lignin deposition and associated changes in anatomy, enzyme activity, gene expression and ruminal degradability in stems of tall fescue at different developmental stages. Journal of Agricultural and Food Chemistry. 2002;50:5558–5565. doi: 10.1021/jf020516x. [DOI] [PubMed] [Google Scholar]
- Ciannamea S, Kaufmann K, Frau M, et al. Protein interactions of MADS box transcription factors involved in flowering in Lolium perenne. Journal of Experimental Botany. 2006;57:3419–3431. doi: 10.1093/jxb/erl144. [DOI] [PubMed] [Google Scholar]
- Ciannamea S, Jensen CS, Agerskov H, et al. A new member of the LIR gene family from perennial ryegrass is cold-responsive, and promotes vegetative growth in Arabidopsis. Plant Science. 2007;172:221–227. [Google Scholar]
- Colesanti J, Coneva V. Mechanisms of floral induction in grasses: something borrowed, something new. Plant Physiology. 2009;149:56–62. doi: 10.1104/pp.108.130500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danyluk J, Kane NA, Breton G, Limin AE, Fowler B, Sarhan F. TaVRT1, a putitative transcription factor associated with vegetative to reproductive transition in cereals. Plant Physiology. 2003;132:1849–1860. doi: 10.1104/pp.103.023523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobcovsky J, Loukoianov A, Fu D, Valerik M, Sanchez A, Yan L. Effect of photoperiod on the regulation of wheat vernalization genes VRN1 and VRN2. Plant Molecular Biology. 2006;60:469–480. doi: 10.1007/s11103-005-4814-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doust AN. Grass architecture: genetic and environmental control of branching. Current opinion in Plant Biology. 2007;10:21–25. doi: 10.1016/j.pbi.2006.11.015. [DOI] [PubMed] [Google Scholar]
- Gocal GFW, King RW, Blundell CA, Schwartz OM, Andersen CH, Weigel D. Evolution of floral meristem identity genes. Analysis of Lolium temulentum genes related to APETALA1 and LEAFY of Arabidopsis. Plant Physiology. 2001;125:1788–1801. doi: 10.1104/pp.125.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabber JH. How lignin composition, structure, and cross-linking affect degradability. A review of cell wall model studies. Crop Science. 2005;45:820–831. [Google Scholar]
- Grabber JH, Jung GA, Abrams S, Howard DB. Digestion kinetics of parenchyma and sclerenchyma cell walls isolated from orchardgrass and switchgrass. Crop Science. 1992;32:806–810. [Google Scholar]
- Greenup A, Peacock WJ, Dennis ES, Trevaskis B. The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Annals of Botany. 2009;103:1165–1172. doi: 10.1093/aob/mcp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havstad LT, Aamlid TS, Heide OM, Junttila O. Transfer of flower induction stimuli on non-exposed tillers in a selection of temperate grasses. Acta Agriculturae Scandinavia Section B, Soil and Plant Science. 2004;54:23–30. [Google Scholar]
- He YH, Amasino RM. Role of chromatin modification in flowering-time control. Trends in Plant Science. 2005;10:30–57. doi: 10.1016/j.tplants.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Heide OM. Effects of photoperiod and temperature on growth and flowering in Norwegian and British timothy cultivars (Phleum pratense L.) Acta Agriculturae Scandinavia Section B, Soil and Plant Science. 1982;32:241–252. [Google Scholar]
- Heide OM. Control of flowering and reproduction in temperate grasses. New Phytologist. 1994;128:347–362. doi: 10.1111/j.1469-8137.1994.tb04019.x. [DOI] [PubMed] [Google Scholar]
- Hemming MN, Peacock WJ, Dennis ES, Trevaskis B. Low-temperature and daylength cues are integrated to regulate FLOWERING LOCUS T in barley. Plant Physiology. 2008;147:355–366. doi: 10.1104/pp.108.116418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaegger KE, Wigge PA. FT protein act as a long-range signal in Arabidopsis. Current Biology. 2007;17:1050–1054. doi: 10.1016/j.cub.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Jensen CS, Salchert K, Nielsen KK. A TERMINAL FLOWER1-like gene from perennial ryegrass involved in the floral transition and axillary meristem identity. Plant Physiology. 2001;125:1517–1528. doi: 10.1104/pp.125.3.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CS, Salchert K, Gao C, Andersen CH, Didion T, Nielsen KK. Floral inhibition of red fescue (Festuca rubra L.) through expression of a heterologous flowering repressor from Lolium. Molecular Breeding. 2004;13:37–48. [Google Scholar]
- Knievel DP, Smith D. Yields and chemical composition of timothy (Phleum pratense L.) plants derived from summer and winter tillers. Crop Science. 1970;10:270–273. [Google Scholar]
- Kuoppala K, Rinne M, Nousiainen J, Huhtanen P. The effect of cutting time of grass silage in primary growth and regrowth and the interactions between silage quality and concentrate level on milk production of dairy cows. Livestock Science. 2008;116:171–182. [Google Scholar]
- Langer RHM. Ear formation in timothy grass (Phleum pratense) following vernalization and short-day treatments. Nature. 1955;176:263. [Google Scholar]
- Mahfoozi S, Limin AE, Fowler DB. Influence of vernalization and photoperiod responses on cold hardiness in winter cereals. Crop Science. 2001;41:116–1011. [Google Scholar]
- Martin J, Stodgaard M, Andersen CH, Nielsen KK. Photoperiodic regulation of flowering in perennial ryegrass involving a CONSTANS-like homologue. Plant Molecular Biology. 2004;56:159–169. doi: 10.1007/s11103-004-2647-z. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Abe J, Yoshida M, Tsurumi Y, Nakayama S. Seasonal changes in freezing tolerance, moisture content and dry weight of three temperate grasses. Grassland Science. 1995;41:21–25. [Google Scholar]
- Muller PY, Janovjak H, Miserez AR, Doppie Z. Processing of gene expression data generated by quantitative real-time RT-PCR. BioTechniques. 2002;32:1372–1379. [PubMed] [Google Scholar]
- Pakarinen K, Virkajärvi P, Seppänen M, Rinne M. Effect of different tiller types on the accumulation and digestibility of the herbage mass of timothy (Phleum pratense L.) Grassland Science in Europe. 2008;13:495–497. [Google Scholar]
- Rinne M, Nousiainen J, Mattila I, Nikander H, Huhtanen P. Proceedings of the 19th International Grassland Congress. Brazil: São Pedro, São Paulo; 2001. Digestibility estimates based on a grass growth model are distributed via internet to Finnish farmers; pp. 1072–1073. [Google Scholar]
- Sasani S, Hemming MN, Oliver SN, et al. The influence of vernalization and daylength on expression of flowering-time genes in the shoot apex and leaves of barley (Hordeum vulgare) Journal of Experimental Botany. 2009;60:2169–2178. doi: 10.1093/jxb/erp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet N, Wiltshire JJJ, Baker CK. A new descriptive scale for early reproductive development in Lolium perenne L. Grass Forage Science. 1991;46:201–206. [Google Scholar]
- Studer B, Jensen LB, Fiil A, Asp T. ‘Blind’ mapping of genic DNA sequence polymorphism in Lolium perenne L. by high resolution melting curve analysis. Molecular Breeding. 2009;24:191–199. [Google Scholar]
- Traveskis B, Hemming MN, Peacock WJ, Dennis ES. HvVRN2 responds to daylength, whereas HvVRN1 is regulated by vernalization and developmental status. Plant Physiology. 2006;140:1397–1405. doi: 10.1104/pp.105.073486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevaskis B, Hemming MN, Dennis ES, Peacock WJ. The molecular basis of vernalization-induced flowering in cereals. Trends in Plant Science. 2007;12:352–357. doi: 10.1016/j.tplants.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Wilson JR, Hatfield RD. Structural and chemical changes in cell wall types during stem development: consequences for fibre degradation by rumen microflora. Australian Journal of Agricultural Research. 1997;48:165–180. [Google Scholar]
- Xue W, Xing Y, Weng X, et al. Natural variation in Ghd1 is an important regulator of heading date and yield potential in rice. Nature Genetics. 2008;40:761–767. doi: 10.1038/ng.143. [DOI] [PubMed] [Google Scholar]
- Yan L, Fu D, Li C, et al. The wheat and barley vernalization gene VRN3 is an orthologue of FT. Proceedings of the National Academy of Sciences, USA. 2006;103:19581–19586. doi: 10.1073/pnas.0607142103. [DOI] [PMC free article] [PubMed] [Google Scholar]