Abstract
Background and Aims
The rate of photosynthesis in paddy rice often decreases at noon on sunny days because of water stress, even under submerged conditions. Maintenance of higher rates of photosynthesis during the day might improve both yield and dry matter production in paddy rice. A high-yielding indica variety, ‘Habataki’, maintains a high rate of leaf photosynthesis during the daytime because of the higher hydraulic conductance from roots to leaves than in the standard japonica variety ‘Sasanishiki’. This research was conducted to characterize the trait responsible for the higher hydraulic conductance in ‘Habataki’ and identified a chromosome region for the high hydraulic conductance.
Methods
Hydraulic conductance to passive water transport and to osmotic water transport was determined for plants under intense transpiration and for plants without transpiration, respectively. The varietal difference in hydraulic conductance was examined with respect to root surface area and hydraulic conductivity (hydraulic conductance per root surface area, Lp). To identify the chromosome region responsible for higher hydraulic conductance, chromosome segment substitution lines (CSSLs) derived from a cross between ‘Sasanishiki’ and ‘Habataki’ were used.
Key Results
The significantly higher hydraulic conductance resulted from the larger root surface area not from Lp in ‘Habataki’. A chromosome region associated with the elevated hydraulic conductance was detected between RM3916 and RM2431 on the long arm of chromosome 4. The CSSL, in which this region was substituted with the ‘Habataki’ chromosome segment in the ‘Sasanishiki’ background, had a larger root mass than ‘Sasanishiki’.
Conclusions
The trait for increasing plant hydraulic conductance and, therefore, maintaining the higher rate of leaf photosynthesis under the conditions of intense transpiration in ‘Habataki’ was identified, and it was estimated that there is at least one chromosome region for the trait located on chromosome 4.
Keywords: Chromosome segment substitution lines, diffusive conductance, hydraulic conductance, photosynthetic rate, quantitative trait locus, rice, Oryza sativa, root hydraulic conductivity
INTRODUCTION
More than 90 % of the world's rice is produced in Asia, where rice (Oryza sativa) is the dominant staple food. As the size of the Asian population increases rapidly, increased rice production is now essential. With only minor possibilities for expansion of areas under rice cultivation, increases in yield are necessary to meet the demand for rice in Asia (International Rice Research Institute, 2008). In Japan, while sufficient rice is produced to meet local demand for human consumption as boiled rice, increases in yield might be effective in reducing production costs.
Yield involves both total dry matter production and harvest index. The harvest index is the cumulative result of the interactions of numerous processes, and modern varieties of rice are characterized by high values for this index (Evans, 1993; Mann, 1999). Indeed, the harvest index is considered to be close to the maximum possible for modern, high-yielding varieties and, thus, increases in dry matter production might be the key to increases in rice yields (Mann, 1999).
Dry matter production of crops is determined by the efficiency with which light is intercepted by leaves and by the efficiency of utilization of the energy of the intercepted light for carbon fixation (Long et al., 2006). The rate of leaf photosynthesis influences dry matter production during the whole growth period. The maximum photosynthetic rate, or the capacity of photosynthesis, of a single leaf is measured at full leaf expansion under conditions of saturating light, ambient concentration of CO2, optimum temperature and low vapour pressure deficit (Murata, 1961; Hayami, 1982). The rate of photosynthesis decreases with senescence (Jiang et al., 1988) and, also, at midday and during afternoon as a result of abiotic stresses, such as water stress (Ishihara and Saito, 1987; Hirasawa et al., 1989). Improvements in traits associated with dry matter production, by improved cultivation methods or by breeding, might allow dry matter production and, eventually, yield to be increased.
The rate of photosynthesis of paddy rice, grown under submerged conditions, often decreases at midday in fine weather despite the availability of adequate water around the roots. Hydraulic conductance affects significantly the maintenance of the rate of leaf photosynthesis under the conditions of high light intensity and high vapour pressure deficit (Hirasawa and Ishihara, 1992; Hirasawa et al., 1992b). However, there are varietal differences in the maintenance. For example, the high-yielding japonica-indica variety ‘Akenohoshi’ maintains higher rates of photosynthesis from midday into the afternoon on fine days than the japonica variety ‘Nipponbare’ (Jiang et al., 1988). This feature of ‘Akenohoshi’ is attributable to its hydraulic conductance which is higher than that of ‘Nipponbare’ (Jiang et al., 1988). Hydraulic conductance in whole rice plants is determined mainly by the hydraulic conductance of the roots (Hirasawa et al., 1992a). Since the axial resistance of roots is very small compared with the radial resistance, root hydraulic conductance can be considered to be derived from two components, namely, total root surface area and root hydraulic conductivity (Lpr), which is the conductance per root surface area (Steudle and Peterson, 1998; Hirasawa, 2001). The development of root systems exhibits significant varietal differences (Nagai and Hirota, 1959; Yoshida et al., 1982; Morita et al., 1995). Plants with larger amounts of roots have higher hydraulic conductance (Jiang et al., 1988; Hirasawa et al., 1992b). On the other hand, no significant varietal difference in Lpr was observed when two varieties contrasting in drought resistance were compared (Miyamoto et al., 2001).
Recently, various DNA markers have been developed and used for the genetic analysis of rice, whose entire genome has been sequenced (Sasaki, 2003; International Rice Genome Sequencing Project, 2005). Such DNA markers can be used to identify quantitative trait loci (QTLs) for useful traits and can be expected to improve selection efficiency. Various QTLs responsible for a variety of traits have been identified (Yano, 2001; Yamamoto et al., 2009), and chromosome segment substitution lines (CSSLs) have been developed recently for the identification of QTLs (Yano, 2001; Yamamoto et al., 2009). CSSLs are mapping populations, usually consisting of approx. 40 lines, with each line having a chromosome segment from one variety on the genetic background of another variety. The genotypes of the lines were confirmed by using 100–200 uniformly distributed DNA markers. Substituted chromosome segments overlap with other segments and cover whole rice chromosomes (Ebitani et al., 2005; Ando et al., 2008; Rice Genome Resource Center, 2010). A population of CSSLs is much smaller than other primary mapping populations, such as an F2 population and a population of recombinant inbred lines and thus, phenotypic analysis is simplified considerably (Ebitani et al., 2005; Ishikawa et al., 2005). In addition, because each CSSL carries only one chromosome segment, CSSLs allow detection of QTLs with only minor effects by comparison, in terms of phenotypic effects, between alleles at one or a few QTLs (Takeuchi et al., 2007). Once a QTL for a particular trait has been identified, CSSLs can be used as near isogenic lines themselves or as starting materials for development of near isogenic lines (Ebitani et al., 2005).
The indica variety ‘Habataki’ has large numbers of spikelets, with high yield, as compared with common japonica varieties in Japan (Kobayashi et al., 1990). ‘Habataki’ showed the higher rate of the maximum photosynthesis after the booting stage through the late ripening stage than the commercial variety ‘Sasanishiki’ (Asanuma et al., 2008a). This was one of the causes of the higher dry matter production after the booting stage and the higher grain yield in ‘Habataki’. It has also been shown previously that ‘Habataki’ maintained a higher rate of photosynthesis during the midday hours in a preliminary measurement. In the present study, the differences in the rate of leaf photosynthesis, on a sunny day, and the hydraulic conductance between ‘Habataki’ and ‘Sasanishiki’ were confirmed, and the trait accounting for higher hydraulic conductance in ‘Habataki’ was characterized by comparing it with ‘Sasanishiki’. Then, a QTL for hydraulic conductance and the trait responsible for it we identified using CSSLs derived from a cross between ‘Sasanishiki’ and ‘Habataki’.
MATERIALS AND METHODS
Plant materials
Rice plants (Oryza sativa) japonica variety ‘Sasanishiki’, indica variety ‘Habataki’, and plants of 39 CSSLs with chromosome segments of ‘Habataki’ on the genetic background of ‘Sasanishiki’ were used in this study. Details of the development of the CSSLs have been described previously by Ando et al. (2008).
Cultivation
Rice plants were grown in the paddy field of the University Farm (35°41′N, 139°29′E) in alluvial soil (clay loam) from the Tama River in 2005, 2006 and 2007. Details of cultivation were basically similar in each of the three years. Seedlings at the fourth-leaf stage were transplanted at a rate of 22·2 hills m−2 (spacing, 30 cm × 15 cm) with one plant per hill. As a basal dressing, manure was applied at a rate of approx. 2 t per 10 a, and chemical fertilizer was applied at a rate of 5·0, 13·6 and 7·2 kg per 10 a for N, P2O5 and K2O, respectively. One-third of the total N was applied as nitrogen sulfate, one-third as elution-controlled urea, LP-50 (Chisso Asahi Fertilizer, Tokyo, Japan), and one-third as LPS-100 (Chisso Asahi Fertilizer). No topdressing was applied. The experiments were designed with three randomly arranged replicates in each case.
Rice plants were also grown in 3-L and 12-L pots filled with a mixture of paddy soil and Kanto diluvial soil (1 : 1, v/v) in a growth chamber (Koito Manufacturing Co. Ltd, Tokyo, Japan) and outdoors, respectively. In the growth chamber, day/night temperature was 28 °C/23 °C; relative humidity, 60 %/80 %; photoperiod, 12 h and photosynthetic photon flux density (PPFD) at the top of the canopy, approx. 1000 µmol m−2 s−1. Basal fertilizer was applied at a rate of 0·5, 0·5 and 0·5 g per pot for N, P2O5 and K2O, respectively, and no additional fertilizer was applied to 3-L pots. Basal fertilizer was applied at a rate of 1·0, 1·0 and 1·0 g per pot for N, P2O5 and K2O, respectively, and additional fertilizer (N) was applied to 12-L pots at a rate of 0·75 g per pot at the booting stage.
Diurnal changes in photosynthetic rates, diffusive conductance and intercellular CO2 concentration of plants grown in the paddy field
The leaf photosynthetic rate and the diffusive conductance of the flag leaf on a main stem were measured simultaneously in the paddy field on cloudless days at the heading-to-ripening stage with a gas-exchange apparatus (LI-6200; LI-COR, Lincoln, NE, USA). Measurements were started at an air CO2 concentration of about 370 µL L−1 in the assimilation chamber. Measurements, for 8 s, were repeated three times and mean values were taken as the measured values. The leaves were exposed to natural light at a PPFD of >1200 µmol m−2 s−1. The intercellular CO2 concentration (Ci) was calculated according to von Caemmerer and Farquhar (1981).
Hydraulic conductance to osmotic water transport in plants grown in the paddy field
Regarding exudation from the cut surface at the base of stems as a result of osmotic water uptake, hydraulic conductance to osmotic water transport (Cos) can be calculated from the following equation (Fiscus, 1975; Miyamoto et al., 2001):
| (1) |
where E is the exudation rate per stem, σ is the root reflection coefficient for solutes in the xylem, Ψso is the osmotic potential of the soil solution immediately outside the roots, and Ψxs is the osmotic potential of the xylem sap. The osmotic potential of the flooded water on the paddy field (–0·012 MPa on average) was used as Ψso and 0·4 was used as the value of σ (Miyamoto et al., 2001). Exudates from the cut surface of stems, 15 cm above the ground, were collected by putting absorbent cotton on the cut surface. The absorbent cotton was covered with a plastic bag to prevent loss of water by evaporation, and the bag was insulated from incident solar radiation with aluminium foil. Exudates were collected in the morning, for approx. 3 h, from 0830 h. The collected exudates were quickly frozen at –80 °C in a freezer and stored frozen until measurements of osmotic potential were made. The osmotic potentials of exudates and the flooded water were measured with a freezing-point osmometer (OM802; Vogel, Giessen, Germany).
Hydraulic conductance to passive water transport in plants grown in the paddy field
The hydraulic conductance to passive transport of water from the soil through the roots to the flag leaf (Cpa) was calculated from the following equation (Hirasawa and Ishihara, 1991):
| (2) |
where T is the transpiration rate per leaf area at steady state, Ψs is the water potential of the soil immediately outside the root, and Ψl is the water potential of a single leaf. Since rice plants were grown under submerged conditions, Ψs could be regarded here as 0 when compared with Ψl. The transpiration rate of a single intact leaf was measured in an air-sealed acrylic assimilation chamber (Tsunoda, 1974) under natural sunlight. Air, with the dewpoint controlled to 10 (±0·1) °C, was pumped into the chamber at a rate of 6·67 × 10−5 m3 s−1. The humidity of the air that was pumped into and out of the chamber was measured with a dewpoint hygrometer (model 660; EG&G Inc., Waltham, MA, USA). When the transpiration rate reached a constant value, the water potential of a leaf was measured with a pressure chamber (model 3005; Soil Moisture Equipment Inc., Santa Barbara, CA, USA) as described by Hirasawa and Ishihara (1991). Transpiration rate was calculated per leaf area.
Hydraulic conductivity of plants grown in pots
Hydraulic conductance per root surface area, referred to here as the hydraulic conductivity of a plant (Lp) was measured for plants grown in pots. Lp was calculated as follows:
| (3) |
where Uw is the water-uptake rate of the whole plant, A is the root surface area and Ψl is the leaf water potential. Measurements were made in an environment-controlled chamber (air temperature, 28 °C; relative humidity, 58–62 %; and PPFD at the top leaves, approx. 1000 µmol m−2 s−1). The water-uptake rate was determined by rate of weight loss of the pot after a steady state had been reached. To prevent evaporation from the surface of the pot, the top of the pot was covered with polystyrene foam and oily clay was used to seal the gap between the foam and the stem. After measurements of the water-uptake rate, the leaf water potential of the uppermost three leaves was measured with a pressure chamber. The mean of the water potential of the three leaves was used to calculate Lp. After roots had been washed gently with water, the root surface area (A) was measured with an image analyser (Win-Rhizo REG V 2004 b; Regent Inc., Quebec, Canada).
Leaf areas and root dry weights of plants grown in pots
For plants grown in pots, the leaf area of detached leaf blades was measured with an area meter (AAM-9; Hayashi Denko Inc., Tokyo, Japan). Soil in pots was gently washed away with tap water. Plants were separated into roots, leaf sheaths plus stems, and panicles. All plant organs were dried for several days at 80 °C in a ventilated oven.
RESULTS
Diurnal changes in photosynthetic rates and hydraulic conductance from roots to leaves
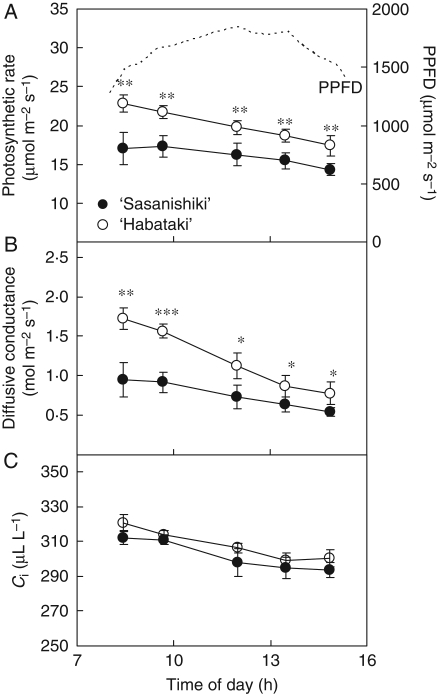
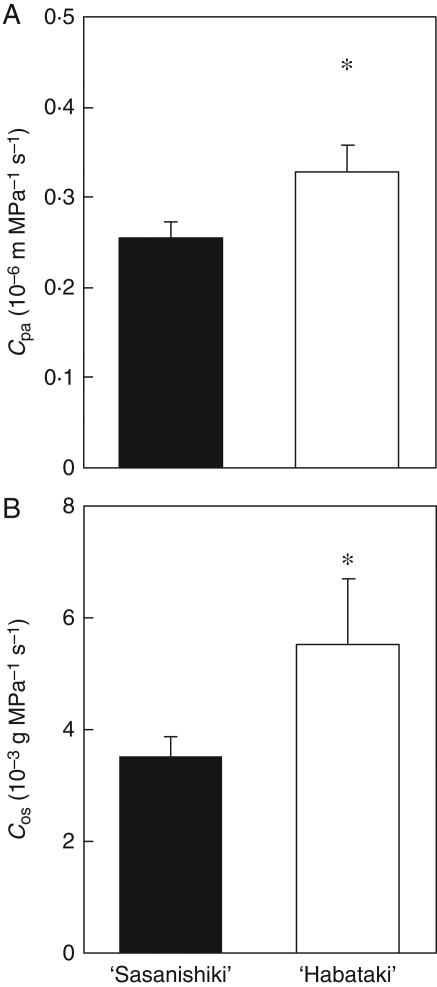
Diurnal changes in photosynthetic rate, diffusive conductance and Ci were measured on a cloudless day at the ripening stage (Fig. 1). Compared with ‘Sasanishiki’, ‘Habataki’ had a higher photosynthetic rate in the morning and maintained a higher rate of photosynthesis during intense transpiration in the afternoon (Fig. 1A). Despite the large decrease in diffusive conductance in ‘Habataki’, as compared with ‘Sasanishiki’, diffusive conductance remained significantly higher in ‘Habataki’ than in ‘Sasanishiki’ in the afternoon under intense-transpiration conditions (Fig. 1B). Ci remained higher in ‘Habataki’ than in ‘Sasanishiki’ during the day, although the differences were not statistically significant (Fig. 1C). This indicates that the higher diffusive conductance of ‘Habataki’ than of ‘Sasanishiki’ contributed to its higher photosynthetic rate in the afternoon, as well as in the morning, through increased CO2 uptake from air to the leaf. The higher photosynthetic rate and diffusive conductance in ‘Habataki’ from morning to afternoon was observed from the heading through the ripening stage (data not shown). The hydraulic conductance to passive water transport from roots to the leaf (Cpa) was significantly higher in ‘Habataki’ than in ‘Sasanishiki’ (Fig. 2A). The hydraulic conductance to osmotic water transport from roots to the base of a stem (Cos) was higher in ‘Habataki’ than in ‘Sasanishiki’ as well as Cpa (Fig. 2B). And it was assumed that the higher root hydraulic conductance in ‘Habataki’ is responsible for its higher hydraulic conductance in whole plants between these two varieties. The higher hydraulic conductance in ‘Habataki’ was observed through the ripening stage (data not shown). From these results, it was confirmed that hydraulic conductance is responsible for the maintenance of leaf photosynthesis from midday to the afternoon.
Fig. 1.
(A) Diurnal changes in photosynthetic rate, (B) diffusive conductance and (C) intercellular CO2 concentration (Ci) of flag leaves of ‘Sasanishiki’ and ‘Habataki’ (as indicated) grown in the paddy field at the ripening stage. The upper dotted line represents the photosynthetic photon flux density (PPFD). Data are means ± s.d. (n = 4). *, ** and ***, values significantly different at the 0·05, 0·01 and 0·001 levels, respectively (t-test).
Fig. 2.
(A) Comparison between the hydraulic conductance to passive water transport through roots to the flag leaf (Cpa) and (B) the hydraulic conductance to osmotic water transport through roots to the base of a stem (Cos) of ‘Sasanishiki’ and ‘Habataki’ grown in the paddy field at the ripening stage. Cpa and Cos were measured between 0900 h and 1500 h and between 0830 h and 1200 h, respectively. Data are means and standard deviation (n = 3). *, values significantly different at the 0·05 level as compared with ‘Sasanishiki’ (t-test).
Characteristics of root and shoot growth and hydraulic conductivity
The growth of roots and shoot and hydraulic conductivity (Lp) in ‘Sasanishiki’ and ‘Habataki’ was examined using plants grown in 3-L pots at the full-heading stage. Root length and root surface area were 55 % and 67 % larger, respectively, in ‘Habataki’ than in ‘Sasanishiki’ (Table 1). Since there was no varietal difference in terms of leaf area, both ratios of root length to leaf area and of root surface area to leaf area were also higher in ‘Habataki’ than those in ‘Sasanishiki’.
Table 1.
Comparison of leaf area, root length and root surface area of a plant, respectively, between ‘Sasanishiki’ and ‘Habataki’ grown in 3-L pots at the full heading stage
| Leaf area (m2) | Root length (km) | Root surface area (m2) | Root length/leaf area (m cm−2) | Root surface area/leaf area (m2 m−2) | Root surface area/root dry weight (m2 g−1) | |
|---|---|---|---|---|---|---|
| ‘Sasanishiki’ | 0·126 ± 0·007 | 1·20 ± 0·09 | 0·94 ± 0·09 | 0·95 ± 0·06 | 7·47 ± 0·74 | 0·24 ± 0·02 |
| ‘Habataki’ | 0·120 ± 0·014 | 1·86 ± 0·32 | 1·57 ± 0·31 | 1·56 ± 0·22 | 13·11 ± 1·99 | 0·22 ± 0·01 |
| n.s. | ** | ** | *** | *** | n.s. |
Data are means ± s.d. (n = 4 or 5). ** and ***, Values significantly different at the 0·01 and 0·001 levels, respectively; n.s., non-significantly different (t-test).
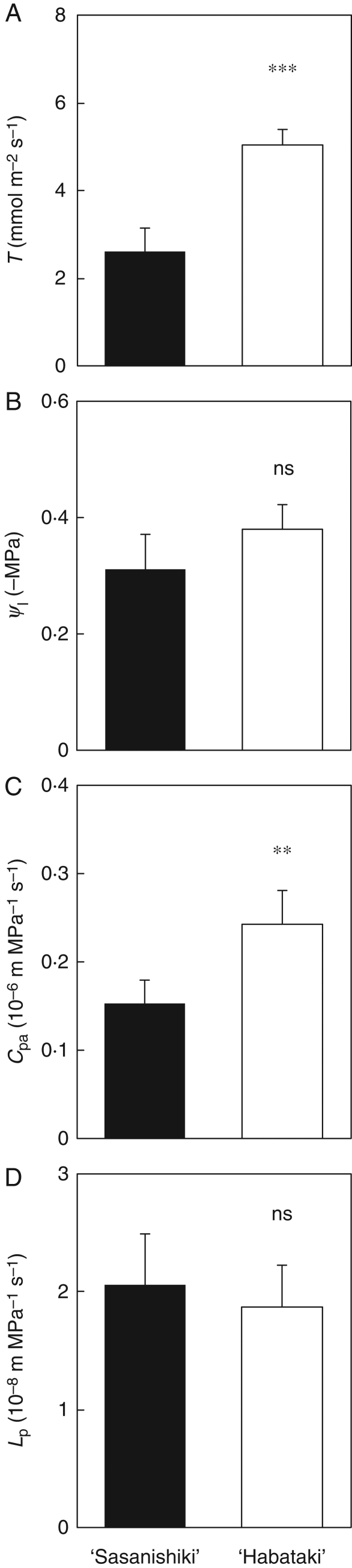
The water-uptake rate per plant (Uw) and water potential of uppermost three leaves were measured in a growth chamber at an air vapour pressure deficit of approx. 1·5 kPa, an air temperature of 28 °C and at a light intensity of approx. 1000 µmol m−2 s−1 at the flag leaves. Transpiration rate per leaf area was significantly higher in ‘Habataki’ than in ‘Sasanishiki’, but Ψl not significantly different in the two varieties (Fig. 3A, B). Hydraulic conductance from roots to leaves in ‘Habataki’ was significantly higher than that in ‘Sasanishiki’ grown in pots, as it was in the field (Fig. 3C). However, there was no difference in Lp between the two varieties (Fig. 3D). These results suggested that higher hydraulic conductance in ‘Habataki’ might be caused by increased effectiveness of the root mass.
Fig. 3.
(A) Comparison between the transpiration rate per leaf area (T), (B) the leaf water potential (Ψl), (C) hydraulic conductance to passive water transport through roots to leaves (Cpa) and (D) hydraulic conductivity of a plant (Lp), respectively, of ‘Sasanishiki’ and ‘Habataki’ grown in 3-L pots at the full-heading stage. Data are means and s.d. (n = 4 or 5). Ψl is given as the mean value of measurements from the uppermost three leaves, measured in a growth chamber at an air vapour pressure deficit of approx. 1·5 kPa, an air temperature of 28 °C and a light intensity at the flag leaves of 1000 µmol m−2 s−1. ** and ***, values significantly different at the 0·01 and 0·001 levels, respectively, as compared with ‘Sasanishiki’ (t-test); ns, not significantly different.
Comparison of hydraulic conductance between ‘Sasanishiki’ and CSSLs
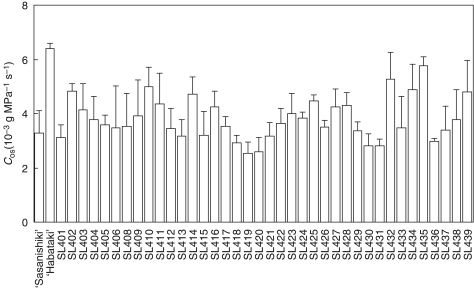
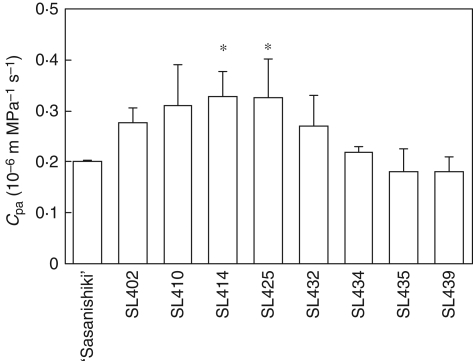
To detect QTLs controlling hydraulic conductance, hydraulic conductance was compared with osmotic water transport (Cos) in CSSLs with that in ‘Sasanishiki’ 2 weeks after heading in the paddy field (Fig. 4). The Cos of ‘Habataki’ (6·4 ± 0·2 × 10−3 g MPa−1 s−1) was far larger than that of ‘Sasanishiki’ (3·3 ± 0·8 × 10−3 g MPa−1 s−1). There was considerable variation in Cos among CSSLs, with values ranging from 2·5 × 10−3 g MPa−1 s−1 to 5·8 × 10−3 g MPa−1 s−1 on average. Eight lines (SL402, SL410, SL414, SL425, SL432, SL434, SL435 and SL439) with a relatively large Cos were selected and they were used for measurements of Cpa for comparisons of hydraulic conductance to passive water transport in the paddy field in 2006 and 2008.
Fig. 4.
Hydraulic conductance to osmotic water transport though roots to the base of a stem (Cos) in CSSLs and parental varieties 2 weeks after heading in the paddy field in 2005. Data are means and s.d. (n = 3).
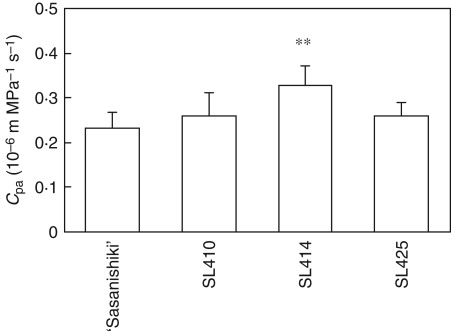
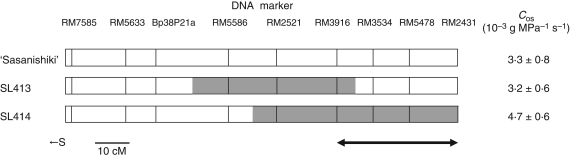
Among eight of CSSLs, two lines (SL414 and SL425) had significantly higher Cpa than ‘Sasanishiki’ in 2006 (Fig. 5). The Cpa of SL410 tended to be higher but the difference was not statistically significant. To confirm these results, values of Cpa of the three lines SL410, SL414 and SL425 were compared with Cpa of ‘Sasanishiki’ in 2008 (Fig. 6). Only SL414 had a significantly higher Cpa than ‘Sasanishiki’. The Cpa of SL410 and that of SL425 tended to be higher than that of ‘Sasanishiki’ but the difference was not statistically significant. These results indicated that at least one or multiple QTLs for hydraulic conductance might be located on the segment in chromosome 4 substituted in SL414. However, SL413, also carrying a chromosome segment of ‘Habataki’ on chromosome 4, had lower hydraulic conductance than SL414 (Figs 4 and 7). These observations suggest that the region, containing QTLs associated with hydraulic conductance, can be narrowed to the region between RM3916 and RM2431, from a comparison of the lengths of the substituted segment between SL413 and SL414 (Fig. 7).
Fig. 5.
Hydraulic conductance to passive water transport though roots to the flag leaf (Cpa) in ten CSSLs and ‘Sasanishiki’ 2–3 weeks after heading in the paddy field in 2006. Data are means and s.d. (n = 3). *, values significantly different at the 0·05 level as compared with ‘Sasanishiki’ (Dunnett's test).
Fig. 6.
Hydraulic conductance to passive water transport though roots to the flag leaf (Cpa) in three CSSLs and ‘Sasanishiki’ 2–3 weeks after heading in the paddy field in 2008. Data are means and standard deviation (n = 6). **, Values significantly different at the 0·01 level as compared with ‘Sasanishiki’ (Dunnett's test).
Fig. 7.
Substitution mapping of elevated hydraulic conductance on chromosome 4. The values of Cos were taken from Fig. 4. White and grey boxes indicate ‘Sasanishiki’-homozygous and ‘Habataki’-homozygous regions, respectively. ←S indicates the side of the short arm; ↔ the putative region associated with elevated hydraulic conductance.
Root and shoot growth characteristics of SL414
To analyse the function of the QTL region, the root and shoot growth characteristics of SL414 were compared with those of pot-grown ‘Sasanishiki’ and ‘Habataki’ in 2007 (Table 2). There were no significant differences in shoot weights and leaf areas among them. However, the dry weight of SL414 roots was almost 1·5 times larger than that of ‘Sasanishiki’ and ratios of root weight to shoot weight, of root weight to leaf weight and of root weight to leaf area of SL414 were also significantly larger than those of ‘Sasanishiki’. The same relationships were observed in 2006 (data not shown). These findings indicate that this region on chromosome 4 is associated with an increase in the absolute amount of roots. There was not much difference in terms of root surface area per root dry weight between ‘Sasanishiki’ and ‘Habataki’ (Table 1), indicating that the root surface area of SL414 was larger than that of ‘Sasanishiki’, and that the higher hydraulic conductance of SL414 was caused by the larger root surface area.
Table 2.
Leaf area and dry weights of separated organs of plants grown in 12-L pots and examined at the ripening stage in 2007
| Shoot wt. (g hill−1) | Leaf area (m2 hill−1) | Leaf wt. (g hill−1) | Root wt. (g hill−1) | Total wt. (g hill−1) | Root wt./shoot wt. (g g−1) | Root wt./leaf wt. (g g−1) | Root wt./leaf area (g m−2) | |
|---|---|---|---|---|---|---|---|---|
| ‘Sasanishiki’ | 45·9 ± 2·8a | 0·184 ± 0·015a | 8·3 ± 0·6a | 4·5 ± 0·2c | 50·4 ± 2·9b | 0·098 ± 0·005c | 0·54 ± 0·05c | 24·5 ± 2·1c |
| ‘Habataki’ | 49·7 ± 4·8a | 0·176 ± 0·023a | 8·4 ± 1·1a | 9·2 ± 0·2a | 58·9 ± 5·0a | 0·186 ± 0·014a | 1·10 ± 0·12a | 52·7 ± 5·8a |
| SL414 | 46·6 ± 3·4a | 0·170 ± 0·013a | 7·0 ± 0·5a | 6·5 ± 0·4b | 53·1 ± 3·8ab | 0·140 ± 0·001b | 0·93 ± 0·01b | 38·4 ± 0·9b |
Data are means ± s.d. (n = 3 or 4). Plants were harvested 2 weeks after heading. Mean values followed by different letters are significantly different at the 5 % level (Fisher's least significant difference).
DISCUSSION
The extent of the maintenance of the rate of photosynthesis during daytime on sunny days is one of the most important characteristics of a single leaf photosynthesis. ‘Habataki’ could maintain a higher rate of leaf photosynthesis by keeping a higher diffusive conductance in the ripening stage than ‘Sasanishiki’ in this research (Fig. 1). Hydraulic conductance influences the maintenance of a high photosynthetic rate and diffusive conductance at midday, as noted in the Introduction (Jiang et al., 1988; Hirasawa and Ishihara, 1992). The higher rate of photosynthesis and diffusive conductance in ‘Habataki’ was supported by its higher hydraulic conductance (Fig. 2A).
Since stem and leaf hydraulic conductance is relatively large and usually does not change under a wide range of environmental conditions and at different stages of growth, the hydraulic conductance of the whole plant is determined by hydraulic conductance of its roots (Boyer, 1971; Hirasawa and Ishihara, 1992; Hirasawa et al., 1992a; Steudle and Peterson, 1998; Ehlert et al., 2009). Therefore, we postulated that differences in Cpa would be reflected by differences in root hydraulic conductance. In fact, the Cos, a measure of hydraulic conductance from roots to the base of a stem, of ‘Habataki’ was also higher than that of ‘Sasanishiki’ (Figs 2B and 4). Water moves from the root surface to the xylem vessels and moves up stems towards the leaves. The axial hydraulic conductance of roots is much larger than the radial conductance (Frensch and Steudle, 1989) and, thus, varietal differences in root hydraulic conductance are, apparently, determined by the differences between root surface area and between root hydraulic conductance per root surface area referred to as root hydraulic conductivity (Lpr). It might be possible to use Lp, calculated in this research, to compare Lpr because of the reasons mentioned above. ‘Habataki’ had a better-developed root system and larger root surface area than ‘Sasanishiki’ (Table 1). Because there was no difference in Lp between ‘Sasanishiki’ and ‘Habataki’ (Fig. 3D), the high hydraulic conductance in ‘Habataki’ might be due mainly to its larger root mass than that of ‘Sasanishiki’. Miyamoto et al. (2001) found, similarly, no difference in Lpr between the rice varieties Azucena and IR64, even though the former showed higher drought tolerance than the latter.
Varietal differences in hydraulic conductance were investigated in several previous studies (Jiang et al., 1988; Asanuma et al., 2008b), which demonstrated that genetic factors control hydraulic conductance. However, as far as is known, there have been no reports of the genetic analysis of hydraulic conductance to date. In the present study, a chromosome region, which is associated with elevated hydraulic conductance, was detected between RM 3916 and RM2431 on chromosome 4 (Figs 4–7). Currently work is in progress to determine the precise location of the QTL on chromosome 4. Further analysis of near-isogenic lines may lead to an increased understanding of the physiological function of the QTL and allow us to identify genes. It was also shown that SL414, in which the region substituted with ‘Habataki’ chromosome segment in the ‘Sasanishiki’ background, had a larger root mass than ‘Sasanishiki’. The rice root system consists of seminal and nodal roots with numerous lateral roots and these roots also have root hairs (Morita and Nemoto, 1995). The differences among the characteristics of development of root systems among Sasanisiki, ‘Habataki’ and CSSLs are an important issue that needs to be investigated.
Multiple QTLs for root morphological traits have been detected on the long arm of chromosome 4 around the region identified in the present study. Qu et al. (2008) located QTLs which had relatively large effects for maximum root length and for root flesh weight at the grain-filling stage around RM3534 on chromosome 4 using recombinant inbred lines derived from rice varieties IRAT109 and Tuefu. A common QTL for root mass might exist on the chromosome region identified, although the mapping populations were different. Moreover, QTLs for root thickness (Champoux et al., 1995; Zheng et al., 2000; Zhang et al., 2001; Price et al., 2002; Kamoshita et al., 2002; Qu et al., 2008) and deep root (Kamoshita et al., 2002) have also been detected around RM 5478. The connections between these results and the increased root mass in the present study remain to be investigated.
The root mass of SL414 was smaller than that of ‘Habataki’, suggesting that additional QTLs might be involved in the difference in root mass between ‘Sasanishiki’ and ‘Habataki’. In this study, relatively high levels of Cpa (not statistical significant) were observed in SL410 and SL425. Segments on chromosome 3 and chromosome 8 were substituted in SL410 and SL425, respectively. Further analysis will be required to validate the existence of QTLs in those chromosomal regions. Identification of QTLs for hydraulic conductance and pyramiding of alleles increasing hydraulic conductance in the ‘Sasanishiki’ genetic background will contribute to improving the water-uptake capacity of ‘Sasanishiki’.
In conclusion, it was found that the higher hydraulic conductance in ‘Habataki’, as a trait for maintaining a higher rate of leaf photosynthesis during a sunny day, was caused by its larger root surface area, not by the larger hydraulic conductivity of the roots, and a chromosome region was detected between RM3916 and RM2431 on chromosome 4, which is associated with elevated hydraulic conductance by increasing the effectiveness of the root surface area.
ACKNOWLEDGEMENTS
This work was supported in part by Grants-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (no. 19380009) and from the Ministry of Agriculture, Forestry and Fisheries, Japan (Green Technology Project; DM-U1119 and Genomics for Agricultural Innovation; QTL-1002).
LITERATURE CITED
- Ando T, Yamamoto T, Shimizu T, et al. Genetic dissection and pyramiding of quantitative traits for panicle architecture by using chromosomal segment substitution lines in rice. Theoretical and Applied Genetics. 2008;116:881–890. doi: 10.1007/s00122-008-0722-6. [DOI] [PubMed] [Google Scholar]
- Asanuma S, Nito N, Ookawa T, Hirasawa T. Yield, dry matter production and ecophysiological characteristics of rice cultivar, Habataki compared with cv. Sasanishiki. Japanese Journal of Crop Science. 2008a;77:474–480. [Google Scholar]
- Asanuma S, Ookawa T, Hirasawa T. Rapid determination of root resistance to water transport: the resistance to water transport in the osmotic water absorption of rice plants. Abstracts of 5th International Crop Science Congress. 2008b 13–18 April 2008 in Jeju, Korea: 9. [Google Scholar]
- Boyer JS. Resistances to water transport in soybean, bean, and sunflower. Crop Science. 1971;11:403–407. [Google Scholar]
- von Caemmerer S, Farquhar GD. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta. 1981;153:376–387. doi: 10.1007/BF00384257. [DOI] [PubMed] [Google Scholar]
- Champoux MC, Wang G, Sarkarung S, et al. Locating genes associated with root morphology and drought avoidance in rice via linkage to molecular markers. Theoretical and Applied Genetics. 1995;90:969–981. doi: 10.1007/BF00222910. [DOI] [PubMed] [Google Scholar]
- Ebitani T, Takeuchi Y, Nonoue Y, Yamamoto T, Takeuchi K, Yano M. Construction and evaluation of chromosome segment substitution lines carrying overlapping chromosome segments of indica rice cultivar Kasalath in a genetic background of japonica elite cultivar Koshihikari. Breeding Science. 2005;55:65–73. [Google Scholar]
- Ehlert C, Maurel C, Tardieu F, Simonneau T. Aquaporin-mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiology. 2009;150:1093–1104. doi: 10.1104/pp.108.131458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT. Crop evolution, adaptation and yield. Cambridge: Cambridge University Press; 1993. [Google Scholar]
- Fiscus EL. The interaction between osmotic- and pressure-induced water flow in plant roots. Plant Physiology. 1975;55:917–922. doi: 10.1104/pp.55.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frensch J, Steudle E. Axial and radial hydraulic resistance to roots of maize (Zea mays L.) Plant Physiology. 1989;91:719–726. doi: 10.1104/pp.91.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayami K. Studies on the physiological and ecological characteristics of high yielding rice variety with high fertilizer response. 1. The effect of nitrogen supply on the photosynthetic characteristics of high yielding rice variety with high fertilizer response. Bulletin of the Tohoku National Agricultural Experiment Station. 1982;67:43–75. [Google Scholar]
- Hirasawa T. Regulation of water status and water transport in plants. Japanese Journal of Crop Science. 2001;70:477–488. [Google Scholar]
- Hirasawa T, Ishihara K. On resistance to water transport in crop plants for estimating water uptake ability under intense transpiration. Japanese Journal of Crop Science. 1991;60:174–183. [Google Scholar]
- Hirasawa T, Ishihara K. The relationship between resistance to water transport and the midday depression of photosynthetic rate in rice plants. In: Murata N, editor. Research in photosynthesis. IV. Dordrecht: Kluwer; 1992. pp. 283–286. [Google Scholar]
- Hirasawa T, Iida Y, Ishihara K. Dominant factors in reduction of photosynthetic rate affected by air humidity and leaf water potential in rice plants. Japanese Journal of Crop Science. 1989;58:383–389. [Google Scholar]
- Hirasawa T, Gotou T, Ishihara K. On resistance to water transport from roots to the leaves at the different positions on a stem in rice plants. Japanese Journal of Crop Science. 1992a;61:153–158. [Google Scholar]
- Hirasawa T, Tsuchida M, Ishihara K. Relationship between resistance to water transport and exudation rate and the effect of the resistance on the midday depression of stomatal aperture in rice plants. Japanese Journal of Crop Science. 1992b;61:145–152. [Google Scholar]
- International Rice Genome Sequencing Project. The map-based sequence of the rice genome. Nature. 2005;436:793–800. doi: 10.1038/nature03895. [DOI] [PubMed] [Google Scholar]
- International Rice Research Institute. The rice crisis: what needs to be done? 2008 http://beta.irri.org/solutions/images/the_rice_crisis.pdf. (17 December 2009) [Google Scholar]
- Ishihara K, Saito K. Diurnal courses of photosynthesis, transpiration, and diffusive conductance in the single-leaf of the rice plants grown in the paddy field under submerged condition. Japanese Journal of Crop Science. 1987;56:8–17. [Google Scholar]
- Ishikawa S, Ae N, Yano M. Chromosomal regions with quantitative trait loci controlling cadmium concentration in brown rice (Oryza sativa) New Phytologist. 2005;168:345–350. doi: 10.1111/j.1469-8137.2005.01516.x. [DOI] [PubMed] [Google Scholar]
- Jiang CZ, Hirasawa T, Ishihara K. Physiological and ecological characteristics of high yielding varieties in rice plants. II. Leaf photosynthetic rates. Japanese Journal of Crop Science. 1988;57:139–145. [Google Scholar]
- Kamoshita A, Zhang J, Siopongco J, Sarkarung S, Nguyen HT, Wade LJ. Effects of phenotyping environment on identification of quantitative trait loci for rice root morphology under anaerobic conditions. Crop Science. 2002;42:255–265. doi: 10.2135/cropsci2002.2550. [DOI] [PubMed] [Google Scholar]
- Kobayashi A, Koga Y, Uchiyamada H, et al. Breeding a new rice variety ‘Habataki. Bulletin of the Hokuriku National Agricultural Experiment Station. 1990;32:65–84. [Google Scholar]
- Long SP, Zhu X-G, Naidu SL, Ort DR. Can improvement in photosynthesis increase crop yield? Plant, Cell & Environment. 2006;29:315–330. doi: 10.1111/j.1365-3040.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- Mann CC. Crop scientists seek a new revolution. Science. 1999;283:310–314. [Google Scholar]
- Miyamoto N, Steudle E, Hirasawa T, Lafitte R. Hydraulic conductivity of rice roots. Journal of Experimental Botany. 2001;52:1835–1846. doi: 10.1093/jexbot/52.362.1835. [DOI] [PubMed] [Google Scholar]
- Morita S, Nemoto K. Morphology and anatomy of rice roots with special reference to coordination in organo- and histogenesis. In: Baluska F, Ciamporova M, Gasparikova O, Barlow PPW, editors. Structure and function of roots. Dordrecht: Kluwer; 1995. pp. 75–86. [Google Scholar]
- Morita S, Yamada S, Abe J. Analysis on root system morphology in rice with reference to varietal differences at ripening stage. Japanese Journal of Crop Science. 1995;64:58–65. [Google Scholar]
- Murata Y. Studies on the photosynthesis of rice plants and cultural significance. Bulletin of the National Institute of Agricultural Sciences D. 1961;9:1–169. [Google Scholar]
- Nagai Y, Hirota H. Cultivated rice varieties viewed from root characters. I. Some ecological characters of rice plants continuously grown in nursery. II. Root diameter, rooting and others. Proceedings of the Crop Science Society of Japan. 1959;27:217–220. [Google Scholar]
- Price AH, Steele KA, Moore BJ, Jones RGW. Upland rice grown in soil-filled chambers and exposed to contrasting water-deficit regimes. II. Mapping QTL for root morphology and distribution. Field Crops Research. 2002;76:25–43. [Google Scholar]
- Qu Y, Mu P, Zhang H, et al. Mapping QTLs of root morphological traits at different growth stages in rice. Genetica. 2008;133:187–200. doi: 10.1007/s10709-007-9199-5. [DOI] [PubMed] [Google Scholar]
- Rice Genome Resource Center. DNA/Seed Stocks. 2010 http://www.rgrc.dna.affrc.go.jp/stock.html. (accessed 24 May 2010) [Google Scholar]
- Sasaki T. Rice genome analysis: understanding the genetic secrets of the rice plant. Breeding Science. 2003;53:281–289. [Google Scholar]
- Steudle E, Peterson CA. How does water get through roots? Journal of Experimental Botany. 1998;49:775–788. [Google Scholar]
- Takeuchi Y, Nonoue Y, Ebitani T, et al. QTL detection for eating quality including glossiness, stickiness, taste and hardness of cooked rice. Breeding Science. 2007;57:231–242. [Google Scholar]
- Tsunoda S. Methods for measuring photosynthesis. In: Matsuo T, editor. Handbook of plant breeding. Tokyo: Yokendo; 1974. pp. 170–174. [Google Scholar]
- Yamamoto T, Yonemaru J, Yano M. Towards the understanding of complex traits in rice: substantially or superficially? DNA Research. 2009;16:141–154. doi: 10.1093/dnares/dsp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M. Genetic and molecular dissection of naturally occurring variation. Current Opinion in Plant Biology. 2001;4:130–135. doi: 10.1016/s1369-5266(00)00148-5. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Bhattacharjee DP, Cabuslay GS. Relationship between plant type and root growth in rice. Soil Science and Plant Nutrition. 1982;28:473–482. [Google Scholar]
- Zhang J, Zheng HG, Aarti A, et al. Locating genomic regions associated with components of drought resistance in rice: comparative mapping within and across species. Theoretical and Applied Genetics. 2001;103:19–29. [Google Scholar]
- Zheng HG, Babu RC, Pathan MS, et al. Quantitative trait loci for root-penetration ability and root thickness in rice: comparison of genetic backgrounds. Genome. 2000;43:53–61. [PubMed] [Google Scholar]