Abstract
Background and Aims
The successful spread of invasive plants in new environments is often linked to multiple introductions and a diverse gene pool that facilitates local adaptation to variable environmental conditions. For clonal plants, however, phenotypic plasticity may be equally important. Here the primary adaptive strategy in three non-native, clonally reproducing macrophytes (Egeria densa, Elodea canadensis and Lagarosiphon major) in New Zealand freshwaters were examined and an attempt was made to link observed differences in plant morphology to local variation in habitat conditions.
Methods
Field populations with a large phenotypic variety were sampled in a range of lakes and streams with different chemical and physical properties. The phenotypic plasticity of the species before and after cultivation was studied in a common garden growth experiment, and the genetic diversity of these same populations was also quantified.
Key Results
For all three species, greater variation in plant characteristics was found before they were grown in standardized conditions. Moreover, field populations displayed remarkably little genetic variation and there was little interaction between habitat conditions and plant morphological characteristics.
Conclusions
The results indicate that at the current stage of spread into New Zealand, the primary adaptive strategy of these three invasive macrophytes is phenotypic plasticity. However, while limited, the possibility that genetic diversity between populations may facilitate ecotypic differentiation in the future cannot be excluded. These results thus indicate that invasive clonal aquatic plants adapt to new introduced areas by phenotypic plasticity. Inorganic carbon, nitrogen and phosphorous were important in controlling plant size of E. canadensis and L. major, but no other relationships between plant characteristics and habitat conditions were apparent. This implies that within-species differences in plant size can be explained by local nutrient conditions. All together this strongly suggests that invasive clonal aquatic plants adapt to a wide range of habitats in introduced areas by phenotypic plasticity rather than local adaptation.
Keywords: Alien weeds, biological invasion, clonal plants, Egeria densa, Elodea canadensis, establishment, genetic diversity, Lagarosiphon major, local adaptation, macrophytes, morphometric characters, phenotypic plasticity
INTRODUCTION
A major question for understanding the ecology of invasive plants is, how do invasive plants adapt to the wide range of habitat conditions? Two different adaptive mechanisms which improve the survival and the dispersal of invasive species are phenotypic plasticity and local adaptation. Phenotypic plasticity is the capacity of a given genotype to express different phenotypes in different environments (Sultan, 2000). If phenotypic plasticity is the primary adaptive mechanism for plants to spread into a range of habitats, plants are able to rapidly change their phenotypic characters and the change is induced by environmental conditions in the habitat. Local adaptation is the capacity of a species to rapidly adapt genetically by virtue of a diverse gene pool (Ward et al., 2008). If local adaptation is the primary adaptive mechanism, differences in plant characters and domination in different habitats among populations are due to local natural selection resulting in local genotypes that have a higher relative fitness in their local habitat than genotypes originating from other habitats (Kawecki and Ebert, 2004).
It is most likely that the number of introductions of a species to a region is important in determining whether phenotypic plasticity or local adaptation is the most important adaptive mechanism for invasive plant species (Parker et al., 2003; Kawecki and Ebert, 2004). If there are multiple introductions, a more diverse gene pool is available and, because of a relative high genetic diversity, species can rapidly respond to selection, and consequently local adaptation is supposed to be the primary adaptive mechanism. Lavergne et al. (2010) showed that novel genotypes arising from new allelic combinations may also undergo genomic re-arrangements, such as genome size reduction, that may have rapid phenotypic and evolutionary implications. Thus, as Parker et al. (2003) and Kawecki and Ebert (2004) point out, factors that favour invasion by local adaptation include high outcrossing rates, low gene flow through dispersal, high number of founders in new populations, and the creation of novel genotypes through gene flow among independent introductions. Alternatively, if only one or few introductions have occurred, a single genotype will dominate and phenotypic plasticity is believed to be the primary adaptive mechanism. Baker (1965) introduced the term ‘general purpose genotype’ to describe colonizing species that grow in a wide range of environmental conditions through phenotypic plasticity. According to Parker et al. (2003) the general purpose genotype facilitates the success of populations founded by a small number of individuals through vegetative reproduction.
For clonal, invasive plants there is generally low genetic variation in the introduced range. Instead, phenotypic plasticity may be the most important adaptive strategy (Parker et al., 2003). For example, of four invasive, clonal and perennial plants related to freshwater, three of them (Egeria densa, Eichornia crassipes and Alternanthera philoxeroides) had relatively low genetic diversity in the introduced area and only one, Phalaris arundinacea, had relatively high genetic diversity in the introduced area compared with native region (Ward et al., 2008). Similarly, Geng et al. (2007) concluded that the primary adaptive strategy of invasive Alternanthera philoxeroides in China is phenotypic plasticity.
The main objective of our study was to determine whether the primary adaptive strategy in three invasive freshwater plants in New Zealand freshwaters is linked to phenotypic plasticity or local adaptation. Phenotypic plasticity of the study species was examined in a common garden growth experiment, to explore whether differences in phenotypic characters among populations would disappear, to test the hypothesis that invasive clonal plants mainly adapt by phenotypic plasticity. Alternatively, if differences among field-collected populations persist in standard conditions, local adaptation would then be implicated as the most important adaptive strategy. Alongside the common garden experiment, genetic variation was also analysed directly within and between populations of each species using AFLPs (amplified fragment length polymorphisms; Vos et al., 1995).
If the primary adaptive strategy of the three invasive species is phenotypic plasticity rather than local adaptation, the factors controlling differences in plant morphology will likely to be a specific subset of the prevailing habitat conditions (Sultan, 2000). Therefore plant material was collected from different populations representing a broad range of habitat types in New Zealand and the relationship between plant morphology and local environmental conditions analysed. This made it possible to test the hypothesis that within-species differences in plant morphology are related to specific local habitat conditions.
MATERIALS AND METHODS
Study species
Three invasive elodeid macrophytes, Egeria densa Planch, Lagarosiphon major (Ridl.) Moss and Elodea canadensis Michx. (Hydrocharitaceae) were used in the study, all of which are common invasive macrophytes in New Zealand lakes and streams (de Winton et al., 2009). According to Chapman (1970) E. canadensis was introduced in 1868 from Tasmania. Lagarosiphon major is native to South Africa and was first reported in New Zealand in 1950 (Howard-Williams and Davies, 1988) and E. densa is native to South America and was first registered in New Zealand in 1946 (de Winton et al., 2009). They are dioecious clonal plants that mainly disperse vegetatively in their natural habitats; even small fragments are able to establish and develop into new macrophyte beds (Howard-Williams, 1993; Riis et al., 2009).
Field samples and chemistry analysis
Sample sites were located on the North Island of New Zealand. Populations showed great phenology variation and were taken from a range of lakes and streams with different chemical and physical properties. Plant populations and habitat conditions were sampled from a total of 14 sites for E. densa, seven sites for E. canadensis and ten sites for L. major (Tables 1 and 2). At each site, macrophytes (five large handfuls) were collected at the depth of maximum biomass within their depth range. A water sample was collected close to the macrophyte bed where plants were collected and two sediment samples were collected among the roots of the macrophyte bed. The samples were kept chilled while returning to the laboratory and were processed within 48 h of collection.
Table 1.
Habitat conditions in sites where plants were collected for genetic analyses and cultivation in common garden conditions to test if the primary adaptative strategy for these invasive clonal aquatic plants was phenotypic plasticity or local adaptation
| Water | Sediment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | pH | Alkalinity (meq l−1) | DIC (mm) | Free CO2 (mm) | NO3-N (mg L−1) | NH4-N (mg L−1) | Tot N (mg L−1) | PO43-P (mg L−1) | Tot P (mg L−1) | Org. matter (% of d. wt) | Tot N (g kg−1 d. wt) | Tot P g (kg−1 d. wt) | Lotic/lentic | Species collected |
| Awakaponga | 6·65 | 0·414 | 0·630 | 0·216 | 1·010 | 0·041 | 1·310 | 0·016 | 0·065 | 15·03 | 4·4 | 0·81 | Lotic | Elo |
| Hamurana spring | 6·56 | 0·538 | 0·882 | 0·344 | 0·721 | 0·003 | 0·751 | 0·072 | 0·078 | 8·32 | 3·1 | 0·39 | Lotic | Lag |
| Kaituna Rv. | 6·71 | 0·342 | 0·498 | 0·156 | 0·626 | 0·090 | 0·914 | 0·025 | 0·060 | 8·51 | 2·0 | 0·45 | Lotic | Ege |
| 8·51 | 2·0 | 0·45 | Lag | |||||||||||
| McLaren | 7·20 | 0·254 | 0·291 | 0·038 | 0·367 | 0·010 | 0·492 | 0·006 | 0·015 | 26·08 | 7·2 | 1·10 | Lentic | Ege |
| 26·08 | 7·2 | 1·10 | Elo | |||||||||||
| Oraka Stm. | 7·12 | 0·490 | 0·576 | 0·086 | 1·770 | 0·008 | 2·000 | 0·057 | 0·078 | 9·54 | 2·2 | 0·50 | Lotic | Elo |
| 11·56 | 3·9 | 0·92 | Lag | |||||||||||
| Pongakawa | 6·90 | 0·538 | 0·695 | 0·157 | 0·011 | 0·011 | 1·500 | 0·020 | 0·129 | 3·66 | 0·5 | 0·24 | Lotic | Elo |
| Lk. Rotorua | 7·63 | 0·144 | 0·151 | 0·008 | 0·001 | 0·004 | 0·403 | 0·001 | 0·032 | 1·24 | 0·3 | 0·07 | Lentic | Elo |
| Rotorua outlet | 6·94 | 0·138 | 0·175 | 0·037 | 0·004 | 0·006 | 0·401 | 0·001 | 0·040 | 14·32 | 7·1 | 1·30 | Lentic | Ege |
| Lk. Tarawera | 7·94 | 2·100 | 2·142 | 0·053 | 0·008 | 0·004 | 0·134 | 0·001 | 0·011 | 6·14 | 2·3 | 2·50 | Lentic | Ege |
| 1·71 | 0·2 | 0·05 | Lag | |||||||||||
| Lk. Tikitapu | 6·95 | 0·056 | 0·071 | 0·015 | 0·001 | 0·004 | 0·166 | 0·001 | 0·002 | 5·50 | 2·1 | 1·70 | Lentic | Lag |
| Waikato Rv. | 6·13 | 0·772 | 2·090 | 1·318 | 0·158 | 0·011 | 0·337 | 0·012 | 0·037 | 3·25 | 0·9 | 3·20 | Lotic | Ege |
Ege, Egeria densa; Elo, Elodea canadensis; Lag, Lagarosiphon major; DIC, dissolved inorganic carbon.
Table 2.
Habitat conditions in sites where additional plants were collected for genetic analyses and morphometric measures
| Water | Sediment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site | pH | Alkalinity (meq L−1) | DIC (mm) | Free CO2 (mm) | NO3-N (mg L−1) | NH4-N (mg L−1 | Tot N (mg L−1 | PO43-P (mg L−1 | Tot P (mg L−1 | Org. Matter (% of d. wt) | Tot. N (g kg−1 d. wt) | Tot. P (g kg−1 d. wt) | Lotic/lentic | Species collected |
| Lk. Hamilton | 7·47 | 0·400 | 0·431 | 0·032 | 0·001 | 0·004 | 0·556 | 0·006 | 0·019 | 10·48 | 2·8 | 0·36 | Lentic | Ege |
| Lk. Nyakakela | 7·27 | 0·450 | 0·506 | 0·056 | 0·001 | 0·047 | 0·380 | 0·001 | 0·019 | 26·37 | 16·3 | 1·10 | Lentic | Ege |
| Lk. Otomangakau | 7·64 | 0·564 | 0·592 | 0·030 | 0·008 | 0·015 | 0·096 | 0·011 | 0·022 | 24·55 | 5·3 | 1·40 | Lentic | Elo, Lag |
| Lk. Parkinsons | 7·02 | 0·714 | 0·871 | 0·157 | 0·027 | 0·016 | 0·565 | 0·009 | 0·022 | 31·66 | 15·9 | 3·60 | Lentic | Ege |
| Lk. Puketi | 7·68 | 0·700 | 0·732 | 0·034 | 0·003 | 0·003 | 0·467 | 0·005 | 0·011 | 48·17 | 17·0 | 1·20 | Lentic | Ege |
| Lk. Rotoaira | 7·88 | 0·774 | 0·794 | 0·023 | 0·001 | 0·004 | 0·071 | 0·004 | 0·009 | 1·59 | <0·2 | 0·15 | Lentic | Lag |
| Lk. Rotoiti | 7·12 | 0·674 | 0·791 | 0·118 | 0·001 | 0·014 | 0·706 | 0·019 | 0·047 | 15·07 | 5·7 | 1·50 | Lentic | Ege |
| Lk. Rotoma | 9·37 | 0·848 | 0·751 | 0·001 | 0·001 | 0·009 | 0·310 | 0·004 | 0·023 | 8·90 | 1·8 | 0·44 | Lentic | Lag |
| Lk. Swan | 7·21 | 0·440 | 0·503 | 0·063 | 0·011 | 0·019 | 0·493 | 0·001 | 0·010 | 4·65 | 0·5 | 0·09 | Lentic | Ege |
| Taupo outlet | 8·60 | 0·728 | 0·715 | 0·004 | 0·001 | 0·003 | 0·078 | 0·002 | 0·007 | 10·45 | 3·8 | 0·43 | Lotic | Ege, Lag |
| Lk. Taupo Sth | 7·52 | 0·850 | 0·907 | 0·059 | 0·004 | 0·021 | 0·121 | 0·011 | 0·015 | 8·10 | 2·6 | 0·44 | Lentic | Elo, Lag |
| Lk. Weavers | 8·79 | 1·062 | 1·029 | 0·004 | 0·001 | 0·004 | 0·297 | 0·002 | 0·010 | 16·96 | 4·6 | 0·37 | Lentic | Ege |
| Lk. Whatihua | 8·79 | 1·314 | 1·273 | 0·005 | 0·002 | 0·018 | 0·552 | 0·004 | 0·012 | 29·57 | 13·7 | 1·50 | Lentic | Ege |
These populations were not grown in common garden. Ege, Egeria densa; Elo, Elodea canadensis; Lag, Lagarosiphon major; DIC, dissolved inorganic carbon.
In the laboratory, the water samples were filtered and analysed for nutrient concentrations using flow injection analysis for NO3−, NH4+ and PO43–. Water pH was measured in the laboratory and alkalinity was determined by end-point titration using 0·02 n HCL. Sediment samples were analysed for organic matter as loss on ignition by drying samples at 104 °C for dry weight (d. wt) and combusting at 400 °C. Total nitrogen in sediment was measured by catalytic combustion at 900 °C on a C/N analyser (CE Instruments). Total phosphorus in sediment was measured by HNO3/HCl digest using ICP_MS (Martin et al., 1994). The sites were separated into lotic (drain, stream or river) and lentic (lake) categories. All data for water and sediment characteristics on the sites are given in Tables 1 and 2.
Common garden experiment
The common garden experiment was set up in a temperature-controlled outdoor tank (1·2 m wide, 2·4 m long and 0·4 m deep water) at the National Institute of Water and Atmospheric Research experimental facility, Hamilton, New Zealand. Detailed physical and operational descriptions of the tanks is given in Burnett et al. (2007). Water was drawn past heating or cooling elements by an electric pump and recirculated into the tank at 1200 L h−1. The water temperature in the tank ranged from 14·5 °C to 24·5 °C during the experimental period, with an average day temperature of 20·5 °C (±2·0 s.d.) and average night temperature of 19·8 °C (±1·9 s.d.). Alkalinity was measured by end-point titration and was 0·6 meq L−1 (±0·01 s.d.) and pH was 7·49 (±0·02 s.d.). Total N was 0·239 mg L−1 with 0·109 mg NO3-N L−1 and 0·002 mg NH4-N L−1. Total P was 0·006 mg L−1 with 0·004 mg PO43-P L−1. Total N and total P were analysed using flow injection analysis. Water chemistry was held constant during the experiment. Sediment used in the pots had organic matter content of 15·6 % d. wt, total N content of 3·9 mg kg−1 (±0·7 s.d.) and total P content of 0·73 mg kg−1 (±0·01 s.d.). Light intensity in the tank was controlled at 50 % of ambient irradiance using mesh screens, i.e. ranging from 300 to 500 µmol photons m−2 s−1.
Plant shoots from five populations of each species were planted in small pots (9 cm diameter and 7 cm deep) containing a mixture of two-thirds garden soil and one-third fine sand. For each species shoots were taken from the five populations so as to include the greatest possible morphological variation within species. In each pot a 17-cm shoot was planted with 5 cm embedded in the sediment and 12 cm above the sediment. No branches were present on the shoots. Six replicates of each population were planted with a total of 90 pots (3 species × 5 populations × 6 replicates). The plants grew in the common garden tank for 7 weeks (20 January to 9 March 2009).
Plant characteristics
Morphometric characters, dry weight and photosynthetic rates in the five plant populations for each species were measured before and after cultivation in standard conditions. Plant characters before transplantation were measured on a separate set of plants (n = 20) than the transplanted plants.
Morphometric characters were measured on 10-cm apical shoots rather than on whole plants, to ensure comparability between tissue grown in the field and new tissue grown in the common garden experiment. For all three species, leaf width and shoot diameter were measured 5 cm from the apex. For E. canadensis and E. densa the number of whorls was also counted on the 10-cm shoot; this was not applicable to L. major which has alternate leaves. The dry weight of the 10 cm shoot (apex d. wt) was then determined by drying at 80 °C for at least 24 h.
Photosynthetic activity was measured under ambient conditions in the tanks on three replicate plants of each population from the collection sites and plants from the common garden experiment. Shoots (4 cm) were incubated in glass-stoppered bottles (volume 30 mL) with ambient water. The water was initially bubbled with N2 to adjust the oxygen concentration to about 80 % air saturation. The bottles were incubated in the tank at 400–600 µmol photons m−2 s−1 for about 30 min, after which oxygen concentrations were measured in the bottles using fibre-optic oxygen microsensors, OXY-4 micro Device and Software Version OXY4v2_11TX (PreSens Precision Sensing GmbH, Germany). Dry weight was subsequently measured for each shoot used in the incubation.
Genetic diversity between populations
Three samples from each population were collected randomly in the field from different macrophyte beds at a distance of about 3 m from each other. As clonal propagation is the main form of reproduction of these species both in New Zealand and in their native ranges, the geographic extension of the sampling area and the number of sampled locations were considered more informative of the genetic diversity present in the northern part of North Island than the number of shoots collected at each location. In total, 21 specimens of E. canadensis collected in seven different locations, 43 specimens of E. densa from 14 locations and 30 specimens of L. major from ten locations were AFLP fingerprinted. This technique detects a high number of DNA fragments, which makes it possible to identify accurately identical genotypes in different populations. A specific study focusing on the genetic diversity pattern of these populations has been carried out by the same authors (Lambertini et al., 2010) and can be referred to for the methodological details and for an extensive presentation of the results.
Data analysis
A two-way ANOVA was conducted to analyse the effect of site (sampling sites) and time (before and after the common garden experiment) on plant characteristics for each plant. Then two one-way ANOVAs were conducted with population and time as independent variables to detect differences in plant characteristics among populations of single species, before and after the common garden experiment. Prior to ANOVA, a Bartlett's test was used to check for homogeneity of variances and data were log-transformed where necessary. Post-hoc differences between treatment means were located by Tukey tests. The change in variability in plant characters between populations was described by calculating: SSpop/SStotal, where SSpop was sum of squares between populations and SStotal was total variability in the one-way ANOVA analyses before and after growth in common garden conditions, respectively. Relationships between plant characters and habitat conditions were analysed by Pearson product moment correlation and regression analyses performed in Statgraphics Centurion XV (StatPoint, Inc., USA).
To examine the overall effect of common garden conditions on phenotypic variation, principal component analyses (PCA) were conducted. Plant characters for plants before they were grown in common garden and plants grown in common garden were used to determine phenotypic similarity before and after growth in standard conditions. Photosynthetic rates were not included in these analyses because there were only three replicates from before and after common garden. Overlay and correlation of plant characteristics with plant scores on the first two axes were performed. All multivariable analyses were performed in PC-Ord (McCune and Mefford, 1999).
The DNA data were analysed to test if the populations were genetically differentiated from each other and to establish if the genetic variation was due to a pool of outcrossing genotypes (precondition for local adaptation) or to other sources. The AFLP chromatograms were scored for presence or absence of polymorphic DNA fragments in the sample set and the resulting binary matrix was analysed with Arlequin ver. 3·11 (Excoffier et al., 2005). The analysis of molecular variance (AMOVA) calculates the percentage of genetic variation which is distributed within and between populations. The resulting fixation index (Fst) is a measure of the extent of genetic differentiation between populations. AMOVA was run based on pairwise differences (number of polymorphic fragments between every pair of samples) and was tested with 1000 permutations. The population comparison test, which calculates pairwise Fst between populations, was tested with 100 permutations. The exact test of population differentiation tests the hypothesis of random distribution of the individuals between pairs of populations and is based on haplotype frequencies. It was calculated with a Markov chain of 100 000 steps and 10 000 dememorization steps.
RESULTS
Common garden experiment
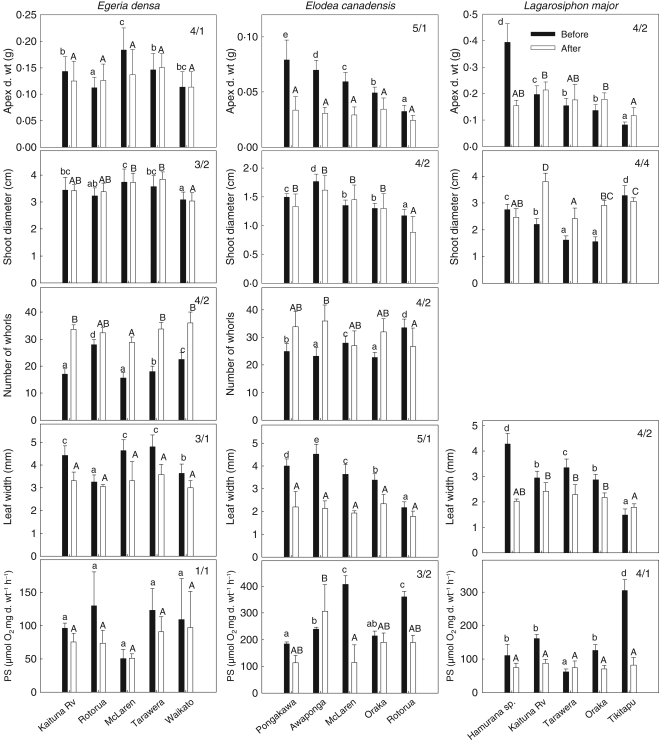
There was generally greater variation in plant characteristics between field populations before growth in standard (common garden) conditions of E. densa, E. canadensis and L. major than afterwards. This observation is supported by the two-way ANOVA showing that 12 out of 14 plant characteristics were not sustained during the common garden growth experiment (Table 3 and Fig. 1). Significantly more distinct population groups (Fig. 1) were also found due to a much larger percentage of variability in the different plant characteristics (Table 4) prior to growth in standard conditions (one-way ANOVA, P < 0·05). No significant variation in photosynthetic rate and shoot diameter in Egeria densa was found before and after growth in standardized growth conditions (Table 3).
Table 3.
F-ratios in a two-way ANOVA to test differences in plant characteristics in five populations of three invasive plant species according to population (collected at five different sites) and time (before and after common garden)
| Apex d. wt (g) | Shoot diameter (cm) | No. of whorls | Leaf width (mm) | PS (μmol g−1 d. wt h−1) | |
|---|---|---|---|---|---|
| Egeria densa | |||||
| Population | 4·25** | 9·75*** | 31·08*** | 18·95*** | 3·82* |
| Time | 7·60** | 0·73 | 497·48*** | 100·84*** | 3·39 |
| Population × time | 3·57** | 0·57 | 18·77*** | 4·85** | 0·53 |
| Elodea canadensis | |||||
| Population | 49·70*** | 1·91 | 37·25*** | 20·66*** | 4·72** |
| Time | 11·03** | 35·07*** | 380·43*** | 178·92*** | 23·09*** |
| Population × time | 5·09*** | 24·31*** | 20·90*** | 11·89*** | 7·05** |
| Lagarosiphon major | |||||
| Population | 58·44*** | 74·93*** | NA | 83·62*** | 14·43*** |
| Time | 8·00** | 177·38*** | NA | 171·59*** | 54·15*** |
| Population × time | 48·20*** | 55·69*** | NA | 45·19*** | 0·18 |
PS, Photosynthetic rates based on dry weights (d. wt); NA, not applicable.
*P < 0·05; **P < 0·01; ***P < 0·001.
Fig. 1.
Variation in morphological characteristics and photosynthetic rate (mean ± s.d.) in plants sampled from invasive New Zealand populations of Egeria densa, Elodea canadensis and Lagarosiphon major, before and after cultivation in common garden conditions. Number of groups before and after transplantation is given by X/Y in each diagram. Letters indicate significant difference between populations before (lowercase letters) and after (uppercase letters) common garden. d. wt, dry weight; PS, photosynthetic rate.
Table 4.
Percentage of variability in plant characteristics between plant populations before and after standard common garden conditions
| % variability before common garden | % variability after common garden | |
|---|---|---|
| Egeria densa | ||
| Apex d. wt | 35·6 | 12·2 |
| Shoot diameter | 26·4 | 51·4 |
| No. of whorls | 82·6 | 51·9 |
| Leaf width | 66·6 | 19·9 |
| Photosynthetic rate | 44·3 | 32·4 |
| Elodea canadensis | ||
| Apex d. wt | 79·2 | 17·4 |
| Shoot diameter | 81·2 | 52·9 |
| Number of whorls | 69·1 | 33·7 |
| Leaf width | 84·2 | 25·6 |
| Photosynthetic rate | 96·8 | 68·9 |
| Lagarosiphon major | ||
| Apex d. wt | 91·0 | 48·4 |
| Shoot diameter | 89·9 | 79·1 |
| Number of whorls | N/A | N/A |
| Leaf width | 90·1 | 46·1 |
| Photosynthetic rate | 95·0 | 16·8 |
Values based on SSpop/SStotal, where SSpop is sum of squares between populations and SStotal is total variability in one-way ANOVA analysis before and after growth in common garden, respectively.
Two-way ANOVA also revealed a high degree of interaction between ‘population’ and ‘time’ within the different species, indicating that changes in plant characteristics before and after the common garden experiment were not necessarily in the same direction (Table 3). For example, E. canadensis plants from Lake Rotorua had most whorls before growth in standard conditions whereas plants from Pongakawa and Awakaponga had most whorls afterwards (Fig. 1); i.e. trait variation is not in a constant direction.
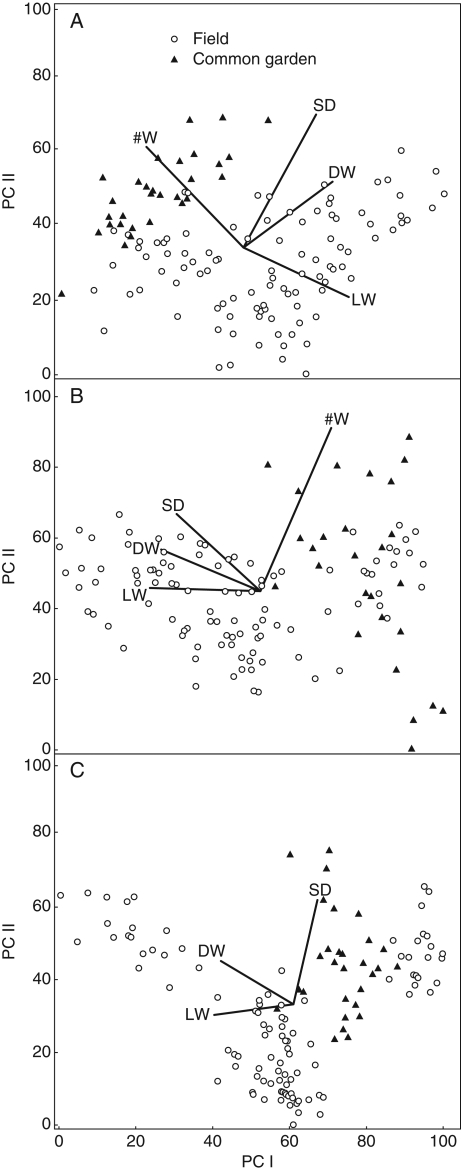
Finally the PCA diagrams (Fig. 2) show that for all three species, plants in the common garden experiment were more similar in terms of plant characteristics than plants collected in the field. Overlay of plant characteristics correlated to species scores showed that those characteristics changing most during the growth experiment were number of whorls and leaf width for E. densa; apex d. wt, leaf width and shoot diameter for E. canadensis; and leaf width and apex d. wt for L. major.
Fig. 2.
Principal component diagram for plant individuals of (A) Egeria densa, (B) Elodea canadensis and (C) Lagarosiphon major based on plant morphological characteristics of plants sampled from invasive New Zealand populations in the field and after common garden, as indicated. Lines indicate correlations of plant traits (LW = leaf width; DW = apex d. wt; SD = shoot diameter and #W = number of whorls). The first and second principal component axes of the diagrams explained 57·6 % and 22·5 % for E. densa; 65·4 % and 19·6 % for E. canadensis; and 60·1 % and 36·2 % for L. major, respectively, of the overall variation of plant characteristics among the individuals.
Genetic variation
A set of very similar genotypes was found in the invasive populations of each species. A total of 102 DNA fragments were scored in E. canadensis, of which 22 were polymorphic (21·6 %). Pairwise differences between genotypes ranged between zero and 11 DNA fragments. Similar levels of polymorphism were detected in E. densa and lower levels in L. major (Table 5). In spite of the different numbers of polymorphic fragments, a widespread genotype was found in almost every location for E. densa and L. major. In E. canadensis, clonal replicates were found only within population distances. In all three species most of the genetic variation was distributed within, rather than between populations (P < 0·01 for E. canadensis and E. densa; P = 0·016 for L. major), indicating that the populations consist of distinct genotypes. However, such genotypes were genetically similar to those of other NZ populations. Population pairwise Fsts (P > 0·05), as well as an exact test of population differentiation (P = 1), indicated that the populations sampled for the common garden experiment were not genetically differentiated from each other.
Table 5.
Genetic similarities between plant samples and populations estimated by the number of polymorphic DNA fragments
| Elodea canadensis | Egeria densa | Lagarosiphon major | |
|---|---|---|---|
| No. populations | 7 | 14 | 10 |
| No. samples | 21 | 38 | 27 |
| No. primers | 3 | 3 | 4 |
| No. of AFLP fragments | 102 | 127 | 142 |
| No. of polymorphic fragments | 22 | 29 | 14 |
| 21·6 % | 22·8 % | 9·8 % | |
| Range of pairwise polymorphic fragments | 0–11 | 0–10 | 0–8 |
| 0–10·8 % | 0–7·9 % | 0–5·6 % |
Number of polymorphic fragments and range of pairwise polymorphic fragments are given as numbers and percentage of the total number of AFLP fragments for each species.
Relationship between plant morphology and habitat conditions
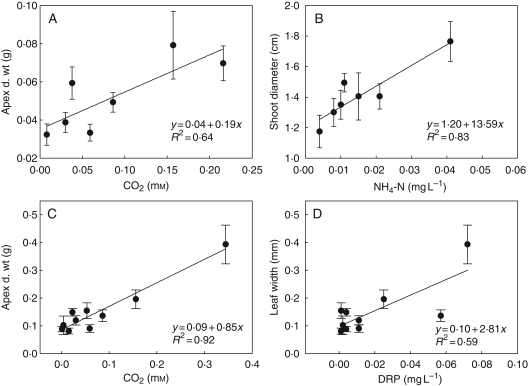
There were few significant relationships between plant morphology and habitat conditions (Table 6). For both E. canadensis and L. major there were positive relationships between apex d. wt and free CO2 concentration (Fig. 3), while for E. canadensis there was also a positive relationship between shoot diameter and inorganic nitrogen of lake water. For L. major, there was a negative quadratic relationship between leaf width and water nitrate content. There was no relationship between E. densa morphology and habitat conditions, and sediment conditions and water movement had no effect on plant morphology for any species.
Table 6.
Pearson correlation coefficient between plant morphometric characteristics as given in Fig. 1 and habitat conditions as given in Tables 1 and 2
| pH | Free CO2 | NH4-N | PO43-P | Total P | |
|---|---|---|---|---|---|
| Elodea canadensis | |||||
| Shoot diameter | –0·75* | 0·88** | 0·91** | ||
| Leaf width | –0·96*** | 0·86* | |||
| Apex d. wt | –0·90** | 0·81* | |||
| Lagarosiphon major | |||||
| Shoot diameter | –0·65* | ||||
| Leaf width | 0·84** | 0·73* | 0·70* | ||
| Apex d. wt | 0·96*** | 0·77** | 0·68* |
Only significant relationships are given. No significant correlations were present for Egeria densa.
*P < 0·05; **P < 0·01; ***P < 0·001.
Fig. 3.
Significant relationships between plant size characteristics of Elodea canadensis (A, B, n = 7 sites) and Lagarosiphon major (C, D, n = 10 sites) and the amount of free CO2 and ammonium on the sites for E. canadensis and free CO2 and dissolved phosphorous (DRP) for L. major. This implies that within-species differences in plant size of E. canadensis and L. major can be explained by local nutrient conditions. Plants were sampled from invasive populations representing a broad range of habitat types in New Zealand. No significant relationships were present for Egeria major.
DISCUSSION
It is concluded that the primary adaptive strategy for invasive E. densa, E. canadensis and L. major populations in the North Island of New Zealand is phenotypic plasticity. No evidence was found in either the common garden experiment or the AFLP analysis that genetic traits caused phenotypic differences in plant characteristics between populations of E. densa, E. canadensis and L. major.
If differences in plant characteristics had been stable before and after the common garden experiment it could have been concluded that different ecotypes of the studied invasive macrophytes are present in New Zealand's freshwater systems. However, the common garden experiment strongly points to phenotypic plasticity as the cause of differences in species traits among populations. The lack of total convergence of the plant characteristics rate among populations in this experiment could be due to the growth period being too short to enable plants to fully change their growth according to the habitat conditions. Even though only biomass grown within the time of the experiment was measured, it is very likely that the plant individuals have inherent characters that do not disappear within short time periods.
AFLP analysis revealed low levels of genetic diversity within and between populations and that the populations selected for the common garden experiment were not genetically differentiated from each other. This appears to confirm that variation in plant characteristics is entirely attributable to phenotypic plasticity. However, a broader study about the genetic variation of these populations in North Island has shown that evolution occurred after introduction to NZ (Lambertini et al., 2010), in spite of the limited genetic variation and apparent absence of sexual reproduction. Consequently, given time, phenotypic plasticity may be replaced or augmented by local adaptation. It is a little more than 100 years since E. canadensis was first observed in New Zealand and considerably less for the other two species. While ecotypic differentiation may take longer, the present study nonetheless suggests that phenotypic plasticity does not exclude local adaptation, even in the case of one single perennial clone.
Overall, the results suggest that at this stage of their invasion into the North Island of New Zealand E. densa, E. canadensis and L. major all consist of a ‘general purpose genotype’ that has colonized a wide range of habitats by virtue of phenotypic plasticity. This colonization is most likely still taking place. In a recent assessment of the status of seven invasive plants in New Zealand, E. densa and L. major have spread into at least 32 and 38 new localities from 2000 to 2008, spanning wide trophic, altitudinal and temperature ranges (de Winton et al., 2009). With such rapid dispersal, it is not surprising that the genetic pool of these species is dominated by common genotypes.
Even though phenotypic plasticity seems to be the main adaptive strategy in these species, the possibility cannot be excluded that local adaptation might be important in other regions. However, very low genetic variation was found in introduced populations of E. densa in North America (Carter and Sytsma, 2001), pointing to phenotypic plasticity as the most likely adaptive strategy in this region. For another invasive Hydrocharitaceae, Elodea nuttallii, morphological variation in populations in norht-east France were also due to phenotypic plasticity and not ecotypic differentiation (di Nino et al., 2007). By contrast, however, Vanderpoorten et al. (2000) found a high level of genetic polymorphism in invasive E. nuttallii in north France and Belgium.
Causes of morphological variation among populations?
To elucidate what controls variation in plant morphology between populations from different habitats, morphological traits were compared with local habitat conditions. The results highlighted that inorganic carbon (as free CO2), inorganic nitrogen and phosphorous are the most important factors in controlling plant size in E. canadensis and L. major. For example, shoot diameter and leaf width decreased with decreasing nitrogen and phosphorus availability for E. canadensis and L. major, respectively, which is unsurprising given that these factors are known to strongly limit plant size and growth (e.g. Sultan, 2000; Useche and Shipley, 2010). Garbey et al. (2003), for instance, found that Ranunculus peltatus in nutrient-poor and nutrient-rich sites adopted small size and long branching shoots, respectively. In the present study, nitrogen limitation may reduce plant size in E. canadensis because CO2 affinity is higher at high tissue nitrogen concentration (Madsen and Baattrup-Pedersen, 1995).
Other studies have shown that some of the habitat conditions included in the present study have a significant effect on plant morphology where habitats with a wider range of conditions are included. For example, Puijalon and Bornette (2006) found that increasing current velocity induces smaller and more compact plants of Berula erecta. In this case, current velocity acts as a stress factor affecting the mass transfer of resources from the water to the plant (Biggs et al., 2003). The effect of current velocity was analysed only on the level of standing or running water, and not within a gradient approach, and no differences were found in morphology. Shading may also influence phenotypic plasticity (Garbey et al., 2003), but the collection sites were not shaded by riparian vegetation, nor was water depth likely to have been important since all sample sites had clear water and all plants were collected from 1·5 to 2·5 m deep.
Conclusions
The primary adaptive strategy for E. densa, E. canadensis and L. major in North Island of New Zealand is phenotypic plasticity rather than local adaptation since greater variation in plant characteristics was found before they were grown in standardized conditions; also only very low levels of genetic diversity were found within and between populations in this study. However, these genetic differences are preconditions for local adaptation and future ecotypic differentiation. These results indicate that invasive clonal aquatic plants adapt to new introduced areas by phenotypic plasticity. Inorganic carbon, nitrogen and phosphorous were important in controlling plant size of E. canadensis and L. major, but no other relationships between plant characteristics and habitat conditions were apparent. This implies that within-species differences in plant size are best explained by local nutrient conditions. All together this strongly suggests that invasive clonal aquatic plants adapt to a wide range of habitats in introduced areas by phenotypic plasticity rather than local adaptation.
ACKNOWLEDGEMENTS
The authors thank The Danish Natural Science Research Council for financial support. We also thank two anonymous referees for valuable comments and suggestions.
LITERATURE CITED
- Baker HG. Characteristics and modes of origin of weeds. In: Baker HG, Stebbins GL, editors. The genetics of colonizing species. New York, NY: Academic Press; 1965. pp. 147–168. [Google Scholar]
- Biggs BJF, Nikora VI, Snelder TH. Linking scales of flow variability to lotic ecosystem structure and function. River Research and Application. 2003;21:283–298. [Google Scholar]
- Burnett DA, Champion PD, Clayton JS, Ogden J. A system for investigation of the temperature responses of emergent aquatic plants. Aquatic Botany. 2007;86:187–190. [Google Scholar]
- Carter MC, Sytsma MD. Comparison of the genetic structure of North and South American populations of a clonal aquatic plant. Biological Invasions. 2001;3:113–118. [Google Scholar]
- Chapman VJ. A history of the lake-weed infestation of the Rotorua lakes and the lakes of the Waikato hydro-electric system. 1970 New Zealand Department of Scientific and Industrial Research, Information Series 78. Government Printer: Wellington. [Google Scholar]
- Excoffier L, Laval G, Schneider S. Arlequin ver. 3·0: an integrated software package for population genetics data analysis. Evolutionary Bioinformatics Online. 2005;1:47–50. [PMC free article] [PubMed] [Google Scholar]
- Garbey C, Thiébaut G, Muller S. Morphological plasticity of a spreading aquatic macrophyte, Ranunculus peltatus, in response to environmental variables. Plant Ecology. 2003;173:125–137. [Google Scholar]
- Geng Y, Pan X, Xu C, et al. Phenotypic plasticity rather than locally adapted ecotypes allows the invasive alligator weed to colonize a wide range of habitats. Biological Invasions. 2007;9:245–256. [Google Scholar]
- Howard-Williams C, Davies J. The invasion of Lake Taupo by the submerged water weed Lagarosiphon major and its impact on the native flora. New Zealand Journal of Ecology. 1988;11:13–19. [Google Scholar]
- Kawecki T.J, Ebert D. Conceptual issues in local adaptation. Ecology Letters. 2004;7:1225–1241. [Google Scholar]
- Lambertini C, Riis T, Olesen B, Clayton JS, Sorrell BK, Brix H. Genetic diversity in three invasive clonal aquatic species in New Zealand. BMC Genetics. 2010;11:52. doi: 10.1186/1471-2156-11-52. doi:10.1186/1471-2156-11-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavergne S, Muenke NJ, Molofsky J. Genome reduction can trigger rapid phenotypic evolution in invasive plants. Annals of Botany. 2010;105:109–116. doi: 10.1093/aob/mcp271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune B, Mefford M J. PC-ORD. Multivariate Analysis of Ecological Data. Version 5.0. 1999 MjM Software, Gleneden Beach, OR, USA. [Google Scholar]
- Madsen T, Baattrup-Pedersen A. Regulation of growth and photosynthesis performance in Elodea canadensis in response to inorganic nitrogen. Functional Ecology. 1995;9:239–247. [Google Scholar]
- Martin TD, Creed JT, Brockhoff CA. Method 200.2, Revision 2.8. 1994 Cincinnati, OH: Environmental Monitoring Systems Laboratory Office of Research and Development, US Environmental Protection Agency. [Google Scholar]
- di Nino F, Thiébaut G, Muller S. Phenology and phenotypic variation of genetically uniform populations of Elodea nuttallii (Planch.) H. St John at sites of different trophic states. Fundamental and Applied Limnology. 2007;168:335–343. [Google Scholar]
- Parker IM, Rodriguez J, Loik ME. An evolutionary approach to understanding the biology of invasions: local adaptation and general-purpose genotypes in the weed Verbascum thapsus. Conservation Biology. 2003;17:59–72. [Google Scholar]
- Puijalon S, Bornette G. Phenotypic plasticity and mechanical stress: biomass partitioning and clonal growth of an aquatic plant species. American Journal of Botany. 2006;93:1090–1099. doi: 10.3732/ajb.93.8.1090. [DOI] [PubMed] [Google Scholar]
- Riis T, Madsen TV, Sennels RSH. Regeneration and growth rates of allofragments in four common stream plants. Aquatic Botany. 2009;90:209–212. [Google Scholar]
- Sultan SE. Phenotypic plasticity for plant development, function and life history. Trends in Plant Science. 2000;5:537–542. doi: 10.1016/s1360-1385(00)01797-0. [DOI] [PubMed] [Google Scholar]
- Useche A, Shipley B. Interspecific correlates of plasticity in relative growth rate following a decrease in nitrogen availability. Annals of Botany. 2010;105:333–339. doi: 10.1093/aob/mcp284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderpoorten A, Lambinon J, Tignon M. Morphological and molecular evidence of the confusion between Elodea callitrichoides and E. nutalii in Belgium and Northern France. Belgian Journal of Botany. 2000;133:41–52. [Google Scholar]
- Vos P, Hogers R, Bleeker M, et al. AFLP: a new technique for DNA fingerprinting. Nucleic Acids Research. 1995;23:4407–4414. doi: 10.1093/nar/23.21.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Gaskin JF, Wilson LM. Ecological genetics of plant invasion: what do we know? Invasive Plant Science and Management. 2008;1:98–109. [Google Scholar]
- de Winton MD, Champion PD, Clayton JS, Wells RDS. Spread and status of seven submerged pest plants in New Zealand lakes. New Zealand Journal of Marine and Freshwater Research. 2009;43:547–561. [Google Scholar]