Abstract
Background and Aims
Predicting the response of plant communities to variation in resources and disturbance is still a challenge, because findings depend on how ecological gradients are characterized and how grassland functional composition is assessed. Focusing on leaf dry matter content (LDMC), the efficacy of different methods for evaluating the best response of plant communities to either environmental or disturbance change is examined.
Methods
Data were collected on 69 grasslands located at four sites in the Pyrenees and Massif Central. N-Ellenberg indices and plant nutrient content (Ni) were compared to assess fertility, and either LDMC (meas) measured or calculated from a trait database for which traits were measured under the same environmental conditions (db). Management regime (MR) was characterized in terms of categories (grazing, cutting) and plant height.
Key Results
LDMCdb was positively correlated to LDMCmeas, but depended significantly on site temperature. N-Ellenberg and Ni were significantly correlated, and there was a significant effect of MR and temperature. LDMC responded to fertility, MR and temperature. Replacing MR by plant height in an REML analysis reduced the uncertainty of the LDMC prediction. LDMC was correlated to plant height at community level, whereas the correlation was weak at species level. Differences in LDMC between plant communities under any of the management regimes were significantly correlated to the standing herbage mass.
Conclusion
The N-Ellenberg index is a better indicator of fertility than Ni which is short-term and environment-dependent. LDMC taken from a database allows plant trait variation due to species abundance (excluding variation due to trait plasticity in response to management) to be captured. So the former is better suited for assessing agricultural services that mainly depend on plant phenology and tissue composition. LDMC responded to defoliation regime in addition to fertility because plant height is roughly correlated with LDMC at plant community level.
Keywords: Cutting, N-Ellenberg indices, fertility, grazing, leaf dry matter content, meadow, nitrogen, pasture, plant functional trait, plant height
INTRODUCTION
The need to predict a plant community's response to changes in land management has prompted a search for key community functional parameters, or traits (Violle et al., 2007) that take account of (a) the capacity of a species to exploit resource-rich or -poor environments, (b) its capacity for competitive dominance and (c) its response to disturbance (Wilson et al., 1999). A few key plant functional parameters have been identified (Diaz and Cabido, 1997) for characterizing a community. This allows response traits to be analysed, which are those whose values change in response to factors applied to the community, and effect traits that act on the processes of the ecosystem (productivity and nutrient cycling among others; Lavorel and Garnier, 2002). For managed grasslands, it has been shown previously that such an approach is useful for assessing the agronomic outputs they provide, namely the herbage growth pattern over a given season and forage quality (Ansquer et al., 2009a; Duru et al., 2010). In such managed grasslands, measurement of plant traits has shown that leaf dry matter content (LDMC) performed better than specific leaf area and plant height for assessing these properties (Ansquer et al., 2009a).
Predicting the response of plant communities to variation in resources and disturbance is still a challenge, particularly because findings depend on how ecological gradients are characterized and how grassland functional composition is assessed. Focusing on LDMC, it is intended to compare methods for assessing the impact of different disturbances and nutrient conditions upon functional characteristics of grassland communities. It is known that there is a fundamental trade-off between a set of plant traits that allow rapid acquisition of resources (‘acquisitive’ plant type having low LDMC) and those that permit conservation of resources within well-protected tissues (‘conservative’ plant type having a high LDMC) (Wilson et al., 1999, Diaz et al., 2004). It was found that LDMC values from databases do allow for a consideration of disturbance because species composition will change and this will change the relative weighting of the contributed trait (Martin et al., 2008). Regarding the response of LDMC to management regime, it was assumed that in managed grasslands there is a trade-off between this plant trait and the individual's capacity for competitive dominance, at least for dominant species within a plant community. Plant or canopy height (Gaudet and Keddy, 1988; Hodgson et al., 1999) or specific shoot height (Weiher et al., 1999), are considered to be the best functional parameters to indicate the plant's capacity for competitive dominance, because they express an ability to capture light (Hartvigsen and McNaughton, 1995; Vesk et al., 2004).
In this paper, some methodological issues generated by the study of LDMC response to management practices and environmental factors are analysed. They are related to how grassland management is characterized and how LDMC is calculated at plant community level. The first issue is for the characterization of fertility and management regime that should be suitably characterized because the response of LDMC probably depends on the indicators chosen for assessing them. However, the ideal way to estimate fertility has not yet been established (Martin et al., 2008). There is no consensus whether to use soil or plant methods. Hence, the ability was compared of two integrated plant indicators to encapsulate the effects of natural soil fertility and fertilization rate (applied mineral or organic fertilizer). For management regime, it was assumed that managed grasslands experience periodic disturbance (partial or almost complete loss of the living material) which has three dimensions (timing, frequency and intensity; Grubb, 1998). A rough descriptor to take account of these three components is sought. The second issue is for the assessment of LDMC. Previous studies have shown that there is a consistent ranking for plant traits between field and controlled conditions (Poozesh et al., 2007; Mokany and Ash, 2008). However, the expression of the LDMC of a given plant community from field measurements or from a trait database gave very different values due to plant trait plasticity (Mokany and Ash, 2008). Plant traits taken from a database have the advantage of being easier to use in an applied situation because they avoid tedious measurements. However, it is not known which expression of LDMC performs better for explaining plant community response to management regime.
Using several grassland datasets to avoid biogeographic peculiarities, it was examined whether there were discrepancies between the two ways of estimating LDMC (database or field measurement) and fertility (N-Ellenberg indices versus method based on plant nutrient content) and, if so, how to explain them by taking into account management and environmental variables. Then it is analysed whether measuring plant architecture instead of qualitative assessment of management regime performed better for predicting the plant community composition. Finally, the relationship between LDMC and architectural traits is analysed at both species and plant community levels. In the last part, we discuss how much effort should be put into the collection of traits to sufficiently evaluate the impact of management treatments across plant communities.
MATERIALS AND METHODS
Description of the studied regions
Sixty-nine grassland fields in four regions under different management regimes were chosen (Table 1). In the Pyrenees, 18 grasslands representing field diversity in terms of management regime (three types) and fertility (two levels) were selected within four farms. There were three management regimes: (1) meadows where the first cut was made after flowering; (2) pastures that are only grazed; and (3) meadows that are mown after late-spring grazing. The two fertility levels, assessed empirically from organic fertilizer management and qualitative observations on botanical composition in the previous year, are denoted + and –. Each combination of fertility × defoliation regime was considered as a treatment. There were three replicates per treatment. This dataset was used for analysis done at plant level. The three other grassland datasets were located in the south of the Massif Central and consisted of three sites. Eight farms per site with contrasting management intensity (average stocking rate) were chosen, and then grassland fields differing in their defoliation regimes (cutting versus grazing) were selected. For these three sites, there is a total of 15 meadows for which the first cut was made at around flowering time, 27 pastures that are only grazed, and nine meadows that are mown after early spring grazing.
Table 1.
Description of the four sites in the two regions
| Pyrenees | Massif Central | |||
|---|---|---|---|---|
| Site | Ercé | Aubrac | Cantal | Margeride |
| Latitude/longitude | 42 °50′/1 °17' | 44 °41'/2 °51' | 45 °13'/2 °20' | 44 °48'/3 °17' |
| Annual temperature (°C)* | 11·8 (0·4) | 10·1 (0·6) | 9·7 (0·7) | 8 (0·4) |
| Annual rainfall (mm)* | 1014 (258) | 1284 (133) | 1634 (176) | 882 (102) |
| Year for botanical survey | 2004 | 2005 | 2005 | 2005 |
| Range of altitude of the data collection (m. a.s.l.) | 650–800 | 750–1050 | 600–905 | 865–1060 |
| Size of the sample area at site level | 2 × 1 km | 60 × 10 km | 40 × 40 km | 40 × 40 km |
| No. of grassland fields | 18 | 15 | 18 | 18 |
| Soil developed on | Schist | Basalt | Basalt | Granite |
| Method for floristic survey | Frequency-rank | BOTANAL | BOTANAL | BOTANAL |
| Characterization of management regime | Dates of defoliation and mode of utilization (cutting vs. grazing); rate of herbage utilization | Dates of defoliation and mode of utilization (cutting vs. grazing) | ||
| Method for characterizing plant architecture | Measurement of plant height | Measurement of sward height | ||
| N-Ellenberg (mean and standard error); range (x–x) | 5·6 (0·9); 3·7–6·8 | 5·5 (0·8); 4·4–6·7 | 5·4 (0·7); 4·2–6·1 | 4·7 (0·8); 3·8–6·1 |
| Ni (mean and standard error) | 0·69 (0·12); 0·42–0·88 | 0·65 (0·19); 0·40–0·96 | 0·58 (0·09); 0·47–0·75 | 0·68 (0·11); 0·51–0·89 |
* Climatic data are averages over the last 30 years: mean (s.e.).
LDMC calculation
LDMC was calculated in two ways: (1) from plant measurements within the 69 fields or (2) using a plant trait database. Then, the community weighted mean trait values (labelled w.), which weight the trait values from each species based on their abundance, were calculated (Garnier et al., 2004; Vile et al., 2006).
In Ercé, plant species composition was measured by randomly sampling forage at peak growth (Table 1). Biomass samples were collected using electric shears, then each species was sorted and weighed. In Aubrac, Cantal and Margeride plant species composition was evaluated following the BOTANAL method (Tothill et al., 1992) which consists of visual estimates of species ranking and biomass using a ranking method. Both methods allow robust and similar estimates of weighted plant traits (Lavorel et al., 2008), as verified previously (Fallour et al., 2008). The studied grasslands were usually dominated by grasses: from 47 to 82 % of the total standing biomass (Ercé), and from 76 to 84 % for other sites (Table S1 in Supplementary data, available online). LDMC measurements were made during spring growth on 15 leaves of each grass species, using the protocol proposed by Garnier et al. (2001) and Cornelissen et al. (2003), i.e. on the youngest fully expanded leaves of sampled tillers which have to be healthy and grown under full natural radiation (no shaded leaves). Leaves were rehydrated by immersing them into demineralized water for at least 8 h in cold (4 °C) and dark conditions. After measurements of their fresh weight and area, leaves were dried at 60 °C for 48 h.
LDMC measurements and calculations were made only on grass species for two reasons. It has been shown previously that grass and rosette species coexisting within a plant community have a similar herbage growth pattern (Duru et al., 2010) whereas there were large and consistent differences in LDMC between these two life forms (Viegas et al., 2005; Ansquer et al., 2009b). Usually it was the dominant functional group in managed grasslands. This avoids bias in the evaluation of agronomic characteristics of all the vegetation (Duru et al., 2010).
The measured weighted LDMC (LDMCw.meas.) was compared, and then weighted LDMC were calculated from a database (LDMCw.db.) where the values were collected in a common garden experiment at Auzeville, France, a region close to the study sites (Table S1 in Supplementary data). In this common garden, plants were fertilized so that there was no nutrient limitation to plant growth (Al Haj Khaled et al., 2005). This database was preferred to a bigger and more complete one such as LEDA (Kleyer et al., 2008) because trait values are environment-dependent, traits measured in the same environment are sought. It is a condition for LDMC to capture plant features such as tissue composition and plant phenology that are key characteristics for evaluating agronomic services and are somewhat dependent on the environment (Duru et al., 2010). Significant correlations between the measured values of LDMC were found in the four locations and the Auzeville database (Table S1 in Supplementary data). The database LDMC values will only vary with environmental conditions when these conditions are significant enough to change species composition, whereas measured LDMC values include measures of phenotypic plasticity.
Characterization of fertility
For quantifying fertility, there is no consensus about a single method to take account of the main nutrient sources (fertilizer, soil fertility, etc.) Thus, two methods frequently used for this purpose were compared. The first is based on the Ellenberg index database (Ellenberg et al., 1992) which characterizes species habitat preference with regard to nutrient availability on a scale of 1–9. Recording species abundance makes it possible to calculate an Ellenberg abundance-weighted mean nutrient index across the plant community (N-Ellenberg). Species not available in the Ellenberg database were excluded from the calculation. The advantage of the Ellenberg index is that it includes an integration of plant species' behaviour over many years (Schaffers and Sykora, 2000). Furthermore the N-Ellenberg index for nitrogen is also well correlated with P availability in the topsoil (Ersten et al., 1998), and may therefore be regarded as an indicator of the overall grassland nutrient status. The second method, the plant nutrient index (Ni), is an agronomic one based on the nitrogen nutrition index (Lemaire and Gastal, 1997) representing the extent of nitrogen limitation experienced by the plant in achieving the potential growth permitted by local weather conditions. Lemaire and Gastal (1997) introduced the concept of critical N concentration [%Nc = 4·8 (DM)−0·32] (DM being the standing herbage dry matter in tonnes per hectare). It represents the inverse relationship between N concentration in herbaceous vegetation and DM accumulation called the N dilution curve. The ratio between the actual and critical N concentration is the NNI. The phosphorus index (NPI) was computed as a function of P and N contents (Duru and Ducrocq, 1997). To compare the plant communities for their nutrient status, a global nutrient index (Ni) was calculated from the values of these two indices according to Duru and Ducrocq (1997). An Ni value of 1 means that herbage growth is not limited by nutrients. Thus, for a given nutrient availability, Ni is higher when the time elapsed between two defoliations increases and when weather conditions (temperature, rainfall) are better for plant growth, because there is more N ‘dilution’.
Assuming that N-Ellenberg is an index of ecosystem productivity (Hill and Carey, 1997), and that the Ni is a measurement of the ability of the soil and fertilizer to provide the amount of nutrients needed to produce the amount of growth permitted by the weather and management regime, it can be expected that N-Ellenberg is positively correlated to the plant N index and any factor that increases plant growth rate. In spring, the average daily temperature can be considered as a rough indicator of the effect of weather on plant growth. On the other hand, the management regime (early grazing + cutting versus cutting) has a big effect on plant growth because spring grazing removes apices in such a way that herbage growth rate is about twice as high for meadows as it is for pastures (Magda et al., 2003).
Characterization of management regime
In a previous inter-site study, Garnier et al. (2007) suggested that there are similarities in responses of grassland functional traits to management in spite of inter-site differences in specific management type (grazing, cutting, mixed use). The methodology used by Garnier et al. (2007) was extended in two ways. First, considering that competition for light occurs mainly when leaf area index is >3 (Simon and Lemaire, 1987), it was assumed that it is appropriate to rank defoliation regimes by the expected maximum standing herbage mass. In this way, grazed meadows for which grazing stops before the threshold of 500 degree-days (starting on 1 February) were regarded as meadows (M) while those grazed between 600 and 900 degree-days were regarded as a specific defoliation regime, i.e. grazed meadows (GM) (Magda et al., 2003; M. Duru, unpubl. res.). Thus, three defoliation regimes were considered and initially described as categorical variables denoted 1 (P, pastures), 2 (GM) and 3 (M, meadows), according to the expected maximum standing herbage mass. Secondly, to characterize them quantitatively, an index of the percentage removal of biomass (Pakeman et al., 2008), i.e. an adaptation of a method based on sward height (Hodgson et al., 2005) was calculated. To this end, herbage mass was measured before and after each cut or grazing period made by the farmer at the Pyrenees site. Measurements were made on a 0·25 m × 0·75 m quadrat, with three replicates per field. Herbage mass measurements were made at different places for each growing cycle. Rate of herbage utilization or difference in herbage mass before and after cutting or grazing were calculated for the first two growth cycles. Results and analyses for characterizing treatments in this way are given in Table S2 in Supplementary data.
Other measurements were made which clearly characterized the effect of management regimes on plant functional composition, considering the plant architecture. The ratio of sward dry matter mass (DM) to sward height (g DM m−2 per metre of plant height) was preferred to simple height measurements because it reduces bias resulting from differences in stage of plant development between sites. Plant height was assessed in two ways: (1) for grassland dataset 1, it was measured for 80 % of species (20 plants per species) with a metre rule, after which a weighted plant height was calculated as for LDMC.w.meas; for grassland datasets 2, 3 and 4, 60 measurements for each grassland field were made at the leafy stage of canopy growth using a sward stick (Duru and Bossuet, 1992).
Data analysis
To analyse the relationships between plant features at plant community level, management and environmental descriptors, two steps were taken: (1) regression analyses were made for examining relationships between single variables, comparing both methods for assessing LDMC and nutrient availability, then introducing management (cutting versus grazing) and environmental (temperature) variables using stepwise regression; (2) two statistical analyses were made to examine the response of LDMC to management regimes (assessed as categories). For the first step, sites were treated as a variable in the regression analysis through its average daily temperature (Table 1). For the second step, a linear mixed model was chosen to take account of both fixed and random effects of explanatory variables. Management regime and resource availability (N-Ellenberg, Ni) were treated as fixed effects while site was modelled as a random effect. This random term is described by a specific variance parameter, different from the error variance. For such mixed models, the restricted maximum likelihood (REML) estimate method is most appropriate as the estimates of variance parameters is unbiased, unlike maximum likelihood estimates. First the response of LDMC to management regime and resource availability indicators was analysed, then the management regime was replaced by plant architecture.
RESULTS
Comparison of methods for assessing LDMC, fertility and for taking account of management regimes
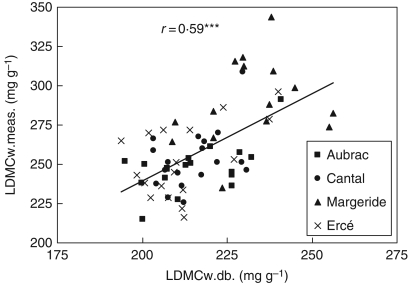
First, methods for assessing fertility and LDMC were compared, then it was examined whether taking account of management and environmental variables can explain possible discrepancies among methods. It was found that N-Ellenberg and Ni were significantly correlated (r = 0·53; P < 0·05), and that there was a significant effect of management regime (P < 0·05) and temperature (P < 0·01). On the other hand, LDMCw.meas. was positively correlated to LDMCw.db. (P < 0·001; Fig. 1), and there was a significant negative effect of temperature (P < 0·01) and management regime (P < 0·05), r = 0·66; P < 0·01. In other words, high temperature as well as cutting (as opposed to grazing) decreased the calculated LDMC; the measured LDMC being considered as the control method, i.e. the variable to explain.
Fig. 1.
Relationship between measured and calculated values from a database for leaf dry matter content (LDMC) for the four sites; each point represents individual means. *** P < 0·001.
Secondly, it was examined whether LDMC responded to management regime assessed as categories (cutting versus grazing), and whether architectural plant traits performed better. It was found that LDMCw.db. was significantly correlated with management regime (r = – 0·33, P = 0·006) (as found previously for other datasets) and the same was true for LDMCw.meas. (r = – 0·27; P = 0·03). Furthermore, it was found that plant architecture was significantly correlated with LDMCw.meas. (r = 0·23; P = 0·05), meaning that a similar response to management and environmental variables can be expected.
Considering all variables together, it was found that whichever method was used for computing LDMC, there was always a significant effect of N-Ellenberg (the first variable selected during the stepwise calculation) and temperature (the second variable selected) (Table 2). Ni and elevation were not selected, and management regime was selected only for LDMCw.db. On the other hand, to replace the site effect by temperature, REML analyses were done for both expressions of LDMC with the two indicators of resource availability (N-Ellenberg and Ni) treating the site as a random factor. Management regimes were compared directly, treating defoliation methods as category variables, and indirectly through architectural plant traits (Table 3). Both management type and resource availability reduced LDMCw. (P < 0·001; Table 3). Grazed and/or nutrient-poor grasslands showed higher LDMCw. values compared with cut and/or nutrient-rich grasslands. Of the two methods for calculating LDMC, the one using the database produced the least uncertainty in the LDMCw. prediction (sum of σ site and σ residual term; Table 3). On the other hand, for estimating fertility, Ni was not significant, or less significant than N-Ellenberg. Considering plant architectural traits instead of management regimes did not change the order of the F-values for the different variables, but reduced the uncertainty of the LDMCw. prediction.
Table 2.
Regression analysis between leaf dry matter content (measured versus taken from a database) and environmental or management variables
| Variables | LDMCw.meas. | LDMCw.db. |
|---|---|---|
| Environmental variable | ||
| Temperature | 222* | 222 |
| Management variables | ||
| N-Ellenberg | 501 | 351 |
| Plant nutrient index | n.s. | n.s. |
| Management regime | n.s. | 113 |
| r2 | 0·55 | 0·50 |
Superscript numbers indicate the order of addition during the stepwise selection variables.
* Marginal effects (percentage variance explained) for variables selected in the stepwise model (P < 0·05).
Table 3.
REML analysis of management regime (MR), resource availability (N-Ellenberg and plant nutrient index: Ni) on LDMCw.meas. and LDMCw.db.
| LDMCw.meas. | LDMCw.db. | ||||
|---|---|---|---|---|---|
| REML | Variable | F(NDF, DDF) | P-value | F(NDF, DDF) | P-value |
| With LUT | N-Ellenberg | F(1,62) = 29·5 | <0·001 (–) | F(1,62) = 24·2 | <0·001 (–) |
| Ni | F(1,62) = 12·9 | <0·001 (–) | F(1,62) = 0·02 | 0·88 | |
| MR | F(1,62) = 8·9 | 0·003 (–) | F(1,62) = 10·8 | <0·001 (–) | |
| σ Site | 145 | 21·1 | |||
| σ Residual term | 48 | 19·5 | |||
| With architectural trait | N-Ellenberg | F(1,62) = 37·6 | <0·001 (–) | F(1,62) = 37·1 | <0·001 (–) |
| Ni | F(1,62) = 13·5 | <0·001 (–) | F(1,62) = 0·01 | 0·9 | |
| Plant architecture | F(1,62) = 5·9 | 0·015 (+) | F(1,62) = 14·3 | <0·001 (–) | |
| σ Site | 135 | 15 | |||
| σ Residual term | 46 | 17·2 |
LUT, land use type.
Standard errors due to the site random term and to the residual term are quantified.
F(NDF, DDF) = F-test with degrees of freedom in the numerator (NDF) and the denominator (DDF). When the effect is significant (P < 0·05), the direction of change of the variable in the column title, when the variables of the left column are increasing, is given in parenthesis.
In short, moving from regression analysis to REML analysis showed that the same variables, N-Ellenberg and management regime (qualitative assessment), were selected for calculated LDMCw., whereas only N-Ellenberg (regression) or N-Ellenberg, management regime, Ni (REML) were selected for measured LDMCw.
Analysing how management regime affects LDMC (Ercé site)
Relationship between leaf and plant architectural traits at species and community levels
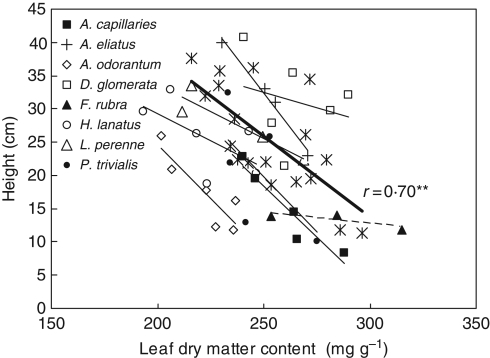
Eight grass species, for which LDMC and height measurements were made for at least four of the eight treatments, were selected. An inverse relationship between LDMC and measured plant height (Fig. 2) was always observed but to a lesser extent for red fescue. However, there is much variation in the intercept (visual assessment) corresponding to a 2-fold height difference for a given LDMC value. In spite of such architectural plant species differences, there was a significant correlation (r = 0·70; P < 0·01) at plant community level when species data were weighted by their abundance. Considering species separately gave a poor correlation (r = 0·26; n = 43; P < 0·1). This means that dominant species smoothed out the largest species differences in plant architecture. On the other hand, there is a significant correlation between LDMCw.meas. and plant architecture and N-Ellenberg (as in Table 3 for all datasets), r = 0·53 (P < 0·05), supporting the relationship between plant architecture and LDMC.
Fig. 2.
Relationship of plant height to leaf dry matter content (LDMC) measured for some grass species and weighted value at plant community level (n = 18). At species level, each value corresponds to an average value per treatment (see column 1 in Table S2 in Supplementary data, available online). The thin lines relate to the individual species, as indicated in the key on the graph; the thick line and asterisks relate to weighted values considering all grass species. ** P < 0·01.
Herbage utilization and plant competition
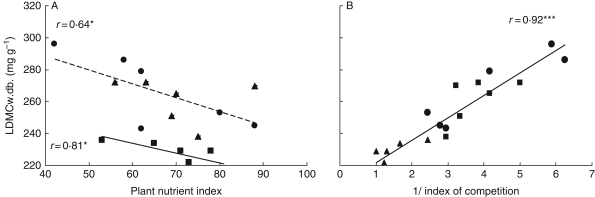
It was shown above that expressing LDMCw.meas. according to nutrient availability (N plant index as well as Ellenberg N indices) gave poor correlations (r = 0·71, P < 0·001 and r = 0·49, P < 0·05, respectively, whereas taking account of the management regime (meadows versus grazed meadows and pastures) gave better correlations for both indices (r = 0·84, P < 0·001). In this way, expressing LDMCw.meas. according to Ni shows that there were two distinctly significant relationships – one for meadows and the other for grazed meadows and pastures (Fig. 3A). Hence, the value of characterizing the defoliation regime was examined more precisely than previously, considering firstly the standing herbage mass before defoliation and the proportion of herbage removed, then an index of plant competition for taking into account both driving factors.
Fig. 3.
Relationship between LDMCw.meas. (mg g −1) and (A) plant nutrient index (dashed fitted line is for pastures and grazed meadows; continuous fitted line is for meadows) and (B) the index of plant competition−1 [DMmax/DMi; DMmax being the highest observed standing herbage mass and DMi being the standing herbage mass observed on each of the 18 plots of the first dataset for the three management regimes: meadows (squares), meadows grazed in spring (triangles) and pastures (circles)]. * P < 0·05, *** P < 0·001.
LDMCw.meas. was best correlated to the rate of herbage utilization for the first growing cycle (r = 0·93; P < 0·001). The correlation was lowest for the growth cycle with the biggest difference in herbage mass before and after cutting or grazing (r = 0·60; P < 0·01) or rate of herbage utilization (r = 0·57; P < 0·05).
Secondly, to take into account the effect of defoliation and nutrient availability, LDMCw.meas. was expressed according to the plant competition index (ratio of maximum standing herbage observed in the experiment to standing herbage mass observed in a given plot) (Fig. 3B). The greater the herbage yield, whether due to nutrient availability, time of regrowth or plant physiological state (vegetative versus reproductive), the lower was LDMCw.meas. Indeed, the competition index expressed well the effects of the different management practices or environmental conditions.
DISCUSSION
Do measured or database LDMCs have the same value for characterizing functional composition of grassland communities?
The two expressions of LDMCw. were significantly correlated which follows the findings of other studies (Lavorel et al., 2008), although the absolute values differed (Fig. 1). In fact, LDMC values calculated from databases depend on environmental variables only through changes in species abundance or presence/absence. Conversely, measured LDMC also integrates the species' plasticity for LDMC in response to these environmental or management factors (Mokany and Ash, 2008).
For evaluating trait plasticity in response to temperature, the results were compared with the literature. Based on data for the four sites and at Auzeville (Table S1 in Supplementary data,), it was found that an increase in temperature of 1 °C lead to a decrease in LDMC by 8 mg g−1 (r = 0·96; P < 0·01) – Roche et al. (2004) found 12 mg g−1 in a Mediterranean climate. To limit bias due to differences in management, a subset of field grasslands submitted to the same management intensity (meadows having the highest Ni, i.e. > 0·5; n = 5 per site) was selected and compared for two sites differing in temperature (Aubrac, 10·1 °C; Margeride, 8 °C). It was found that average LDMC differences between the two sites were 34 and 48 mg g−1 for LDMC.db. and LDMC.meas., respectively. The difference in LDMC between the two methods (14 mg g−1) can be attributed to the plant trait plasticity in response to temperature, which is of similar magnitude to the expected value (2·1 °C × 8 or 12 mg g−1 = 16·8 or 25 mg g−1). It is deduced that the direct effect of temperature on LDMC through trait plasticity contributes about one-third of the whole effect. This explains why the standard errors for the site and the residual term were lower for LDMCw.db. than for LDMCw.meas. (Table 3). Given the present objectives, LDMC.db. was better than LDMC.meas. for analysing the response of management, especially when comparing sites with different climates.
Are methods based on plant nutrient content or botanical composition equally valuable for assessing fertility and predicting functional composition of grassland communities?
Previous results have suggested that the N-Ellenberg index is a good indicator of productivity and nutrient availability because it encapsulates the effect of mineral fertility and climate (Schaffers and Sykora, 2000; Wamelink et al., 2002). Based on a multi-site study, the present findings confirm that the N-Ellenberg index calculated at plant community level is an indicator of plant productivity resulting from nutrient availability (soil fertility and fertilizer management), environment factors (temperature) and management regime. Conversely, the Ni assesses the level of nutrient deficiency or sufficiency experienced by the plant community for a given weather and defoliation regime (Lemaire and Gastal, 1997).
As expected, LDMCw., obtained by either method, decreased significantly when fertility increased. This is in agreement with much of the data in the literature (Weiher et al., 1999; Lavorel et al., 2005). However, only the N-Ellenberg index was significant for characterizing fertility when considering a set of management regimes and growing conditions together. On the other hand, consistent correlations were found between LDMCw. and Ni when considering defoliation regime and only one geographical site at a time (Ercé dataset), in agreement with the expected relationship (first section of Results). This result is in agreement with the findings of Martin et al. (2008) on a larger database used for studying relationships between LDMCw.db. and fertility evaluated with the same indices. They found significant relationships with both indices, but the correlation was better using N-Ellenberg than Ni.
Thus, although based on good science, the Ni is less successful than an empirical index when working in situations where weather as well as disturbance varied in addition to fertility. N-Ellenberg indices weighted at plant community level seem to be an absolute measurement of fertility independent of environmental conditions, while the Ni is short-term and environment-dependent. It only represents the extent of nutrient limitation experienced by the plant in achieving the potential growth permitted by local weather conditions. Thus it is rather an indicator of plant community disturbance than an absolute indicator of nutrient availability.
Is assessment of management regime in terms of categories enough for representing their effect on plant functional composition?
Most studies analysing the effect of grazing intensity on plant traits do not state whether the nutrient availability was the same for all treatments (Kruess and Tscharntke, 2002), leaving doubt about the origin of the differences in results. On the other hand, Vesk and Westoby (2001) considered that our ability to predict vegetation change under grazing is limited. They indicated that it may be mainly due to the difficulty in characterizing grazing, which may not be uniform in intensity and frequency (Fynn et al., 2005). Thus it is difficult to define the treatment in which the greatest disturbance occurs: large and infrequent variation in standing herbage mass or frequent variation, with each defoliation removing only a little herbage mass. On the other hand, comparison with data from the literature is difficult because sometimes differences in grazing intensity correspond to differences of a few centimetres in sward height in continuous grazing (Pakeman, 2004); other studies consist of a comparison between intensively and extensively grazed (Louault et al., 2005) or abandoned plant communities (Köhler et al., 2001; Peco et al., 2006) that correspond to large differences in herbage mass removed. By considering both cut meadows and grazed pastures, larger differences in standing herbage mass (sward height) were produced than would be the case if working on just grazed plant communities. This experimental design allows an analysis of how a plant trait known to respond to fertility also responds to disturbance, i.e. defoliation regime in the present study.
Results obtained from a larger database showing that LDMCw. depended on the management regime in addition to fertility (Martin et al., 2008) were confirmed. However, having shown that architectural traits described the effect of defoliation well (see REML analysis in Table 3), it was found that defoliation regimes for which competition for light was greatest (the most upright herbage mass before cutting/grazing and/or least afterwards, as for meadows) have the lowest LDMCw. This new result is consistent with the fact that these plant communities are composed of fast-growing species (Werger et al., 2002) which can compete very successfully for light (Weiher et al., 1999). At an early stage of regrowth, they are composed of tall individual plant species of low LDMC (Ercé dataset) or swards having low mass : height ratio (Aubrac, Cantal and Margeride datasets) – two features indicating competitive dominance.
In the Ercé dataset, contrasting defoliation regimes had >4 t DM ha−1 (meadows) and <2 t DM ha−1 (pastures) removed at a time (for the same plant nutrient status), considerably modifying competition for light. Thus, in meadows, only species which can grow tall can compete. More precisely, the relationship found between LDMC and plant architectural traits for characterizing the management regime is an emergent property clearly observable at plant community level but weak at species level (Fig. 2). Weighting plant traits by species abundance smoothed out such differences observed at species level. When a grassland is cut instead of grazed (leading to the possibility of accumulating tall standing herbage), plants need to grow fast to survive, and this is associated with high plant trait values for specific leaf area and plant height (Kühner and Kleyer, 2008). Conversely, fast-growing species with a low LDMC lose their competitive advantage and slow-growing species with a high LDMC are encouraged when herbivores consume the vegetation, whatever the fertility, and not only on infertile soil as found previously (Grime, 1977; Fraser and Grime, 1999). This explains why Louault et al. (2005) found that highest-use plots (four grazings and one cut per year in comparison to only one or four grazings) were dominated by one type that is weakly competitive for light and has a lower mature plant height.
The present framework could be extended to a wider range of defoliation regimes than those studied in this paper. Increased grazing intensity (usually continuous grazing from 8 to 4 cm) was accompanied by an increase in the proportion of species with a higher light requirement and a lower minimum height (Pakeman, 2004). When standing herbage mass was potentially high (in the reproductive phase), species with high LDMCw. were not able to challenge those with low LDMCw., because the latter were mostly taller (or sward density was lower per gram of shoot biomass), enabling them to monopolize light (Schippers et al., 1999). Tall species replaced short species when heavy grazing ceased because the latter are not able to compete for light, even though there is plasticity in above-ground biomass partitioning (Werger et al., 2002). Finally, in managed grasslands, considering the plant architectural characteristics instead of management regimes only slightly improves the prediction of LDMCw.
CONCLUSIONS
Based on a multi-site analysis, it was found that, for the objectives, LDMC taken from a database is a better option than LDMC measurement in the field because the former allows plant trait variation to be captured which is related only to species abundance, avoiding the plant trait plasticity in response to management. It was also less time-consuming. It was found also that at this scale it is necessary to take account of environmental characteristics (e.g. temperature) of the grassland fields, in addition to management practices. Nevertheless, the phenotypic plasticity of LDMC could be important to consider for assessing how different ecosystems respond to changes in management, especially for understanding the rate at which changes in species functional composition occurred.
For assessing fertility, the method based upon plant nutrient content is less successful than N-Ellenberg indices. The former is short-term and environment-dependent while the latter appears as an absolute measure of fertility independent of environmental conditions. About management regimes, the results show that LDMC responded to a defoliation regime in addition to fertility, because in managed grasslands plant architectural traits (plant height or derived plant traits) are roughly correlated with LDMC at plant community level. Thus management practices which favour plant species with a low LDMCw., i.e. an acquisitive resource strategy, allow them to compete better for light, whether due to high fertility level, a long period of regrowth or a reproductive stage that increased the standing herbage mass. However, considering the plant architectural characteristics instead of qualitative assessment of management regimes only slightly improves the prediction.
SUPPLEMENTARY DATA
Supplementary Material
ACKNOWLEDGEMENTS
This work was funded by the EU project VISTA (Vulnerability of Ecosystem Services to Land Use Change in Traditional Agricultural Landscapes) (Contract no. EVK2-2001-15 000356), and the PSDR ‘Climfourel’ project (INRA and Midi Pyrenees region). We thank two anonymous reviewers for their very valuable insight.
LITERATURE CITED
- Al Haj Khaled R. L'évaluation des caractéristiques agronomiques d'espèces par leurs traits de vie comme étape préalable au diagnostic des communautés à flore complexe. INPL, Nancy: France; 2005. PhD Thesis. [Google Scholar]
- Al Haj Khaled R, Duru M, Theau J P, Plantureux S, Cruz P. Variation of leaf traits through seasons and N-availability levels and its consequences for ranking grassland species. Journal of Vegetation Science. 2005;16:391–398. [Google Scholar]
- Ansquer P, Duru M, Theau JP, Cruz P. Functional traits as indicators of fodder provision over a short time scale in species-rich grasslands. Annals of Botany. 2009a;103:117–126. doi: 10.1093/aob/mcn215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansquer P, Duru M, Theau JP, Cruz P. Convergence in plant traits between species within grassland communities simplifies their monitoring. Ecological Indicators. 2009b;9:1020–1029. [Google Scholar]
- Cornelissen JHC, Lavorel S, Garnier E, et al. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany. 2003;51:335–380. [Google Scholar]
- Diaz S, Cabido M. Plant functional types and ecosystem functions in relation to global change. Journal of Vegetation Science. 1997;8:463–474. [Google Scholar]
- Diaz S, Hodgson JG, Thompson K, et al. The plant traits that drive ecosystems: evidence from three continents. Journal of Vegetation Science. 2004;15:295–304. [Google Scholar]
- Duru M, Bossuet L. Estimation de la masse d'herbe par le ‘sward-stick’: premiers résultats. Fourrages. 1992;131:283–300. [Google Scholar]
- Duru M, Ducrocq H. A nitrogen and phosphorus herbage nutrient index as a tool for assessing the effect of N and P supply on the dry matter yield of permanent pastures. Nutrient Cycling in Agroecosystems. 1997;47:59–69. [Google Scholar]
- Duru M, Cruz P, Theau JP. A simplified method for characterizing agronomic services provided by species-rich grasslands. Crops and Pasture Science. 2010;61:420–433. [Google Scholar]
- Ellenberg H, Weber HE, Düll R, Wirth V, Werner W, Paulissen D. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica. 1992;18:1–258. [Google Scholar]
- Ersten ACD, Alkemade JRM, Wassen MJ. Calibrating Ellenberg indicator values for moisture, acidity, nutrient availability and salinity in the Netherlands. Plant Ecology. 1998;135:113–124. [Google Scholar]
- Fallour D, Theau JP, Corler K, et al. A simplified method to determine the abundance of grass functional groups in natural grasslands. Uppsala, Sweeden: EGF; 2008. pp. 93–95. [Google Scholar]
- Fraser LH, Grime JP. Interacting effects of herbivory and fertility on a synthesized plant community. Journal of Ecology. 1999;87:514–525. [Google Scholar]
- Fynn RWS, Morris CD, Edwards TJ. Effect of burning and mowing on grass and forb diversity in a long-term grassland experiment. Applied Vegetation Science. 2005;7:1–10. [Google Scholar]
- Garnier E, Shipley B, Roumet C, Laurent G. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology. 2001;15:688–695. [Google Scholar]
- Garnier E, Cortez J, Billes G, et al. Plant functional markers capture ecosystem properties during secondary succession. Ecology. 2004;85:2630–2637. [Google Scholar]
- Garnier E, Lavorel S, Ansquer P, et al. Assessing the effects of land-use change on plant traits, communities and ecosystem functioning in grasslands, a standardized methodology and lessons from an application to 11 European sites. Annals of Botany. 2007;99:967–985. doi: 10.1093/aob/mcl215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet CL, Keddy PA. A comparative approach to predicting competitive ability from plant traits. Nature. 1988;334:242–243. [Google Scholar]
- Grime JP. Evidence for the existence of three primary strategies in plants and its relevance to ecological and evolutionary theory. American Naturalist. 1977;111:1169–1194. [Google Scholar]
- Grubb PJ. A reassessment of the strategies of plants which cope with shortages of resources. Perspectives in Plant Ecology, Evolution and Systematics. 1998;1:3–31. [Google Scholar]
- Hartvigsen G, McNaughton SJ. Tradeoff between height and relative growth rate in a dominant grass from the Serengeti ecosystem. Oecologia. 1995;102:273–276. doi: 10.1007/BF00329793. [DOI] [PubMed] [Google Scholar]
- Hill MO, Carey PD. Prediction of yield of the Rothamsted park grass by Ellenberg indicator values. Journal of Vegetation Science. 1997;8:579–586. [Google Scholar]
- Hodgson JG, Wilson PJ, Hunt R, Grime JP, Thompson K. Allocating C-S-R plant functional types, a soft approach to a hard problem. Oikos. 1999;85:282–294. [Google Scholar]
- Hodgson JG, Montserrat-Marti G, Cerabolini B, et al. A functional method for classifying European grasslands for use in joint ecological and economic studies. Basic and Applied Ecology. 2005;6:119–131. [Google Scholar]
- Kleyer M, Bekker RM, Knevel IC, et al. The LEDA Traitbase: a database of life-history traits of the Northwest European flora. Journal of Ecology. 2008;96:1266–1274. [Google Scholar]
- Köhler B, Ryser P, Güsewell S, Gigon A. Nutrient availability and limitation in traditionally mown and in abandoned limestone grasslands: a bioassay experiment. Plant and Soil. 2001;230:323–332. [Google Scholar]
- Kruess A, Tscharntke T. Contrasting responses of plant and insect diversity to variation in grazing intensity. Biological Conservation. 2002;106:293–302. [Google Scholar]
- Kühner A, Kleyer M. A parsimonious combination of functional traits predicting plant response to disturbance and soil fertility. Journal of Vegetation Science. 2008;19:681–692. [Google Scholar]
- Lavorel S, Garnier E. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology. 2002;16:545–556. [Google Scholar]
- Lavorel S, Diaz S, Cornelissen JH, et al. In: Plant functional types, are we getting any closer to the Holy Grail? Canadell J, Pitelka LF, Pataki D, editors. Springer-Verlag; 2005. [Google Scholar]
- Lavorel S, Grigulis K, McIntyre S, et al. Assessing functional diversity in the field – methodology matters! Functional Ecology. 2008;22:134–147. [Google Scholar]
- Lemaire G, Gastal F. N uptake and distribution in plant canopies. In: Lemaire G, editor. Diagnosis of the nitrogen status in the crops. Berlin: Springer Verlag; 1997. pp. 3–44. [Google Scholar]
- Louault F, Pillar VD, Garnier E, Soussana JF. Plant traits and functional types in response to reduced disturbance in a semi-natural grassland. Journal of Vegetation Science. 2005;16:151–160. [Google Scholar]
- Magda D, Theau JP, Duru M, Coleno FC. Hay-meadows production and weed dynamics as influenced by management. Journal of Range Management. 2003;56:127–133. [Google Scholar]
- Martin G, Cruz P, Theau JP, et al. A multi-site study to classify semi-natural grassland types. Agriculture, Ecosystems and Environment. 2008;129:508–515. [Google Scholar]
- Mokany K, Ash J. Are traits measured on pot grown plants representative of those in natural communities? Journal of Vegetation Science. 2008;19:119–126. [Google Scholar]
- Pakeman RJ. Consistency of plant species and trait responses to grazing along a productivity gradient: a multi-site analysis. Journal of Ecology. 2004;92:893–905. [Google Scholar]
- Pakeman RJ, Reid CL, Lennon JJ, Kent M. Possible interactions between environmental factors in determining species optima. Journal of Vegetation Science. 2008;19:201–208. [Google Scholar]
- Peco B, Sanchez AM, Azcarate FM. Abandonment in grazing systems: consequences for vegetation and soil. Agriculture, Ecosystems and Environment. 2006;113:284–294. [Google Scholar]
- Poozesh V, Cruz P, Choler P, Bertoni G. Relationship between the Al resistance of grasses and their adaptation to an infertile habitat. Annals of Botany. 2007;97:947–954. doi: 10.1093/aob/mcm046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche P, Diaz-Burlinson N, Gachet S. Congruency analysis of species ranking based on leaf traits: which traits are the more reliable? Plant Ecology. 2004;174:37–48. [Google Scholar]
- Schaffers AP, Sykora KV. Reliability of Ellenberg indicator values for moisture, nitrogen and soil reaction: a comparison with field measurements. Journal of Vegetation Science. 2000;11:225–244. [Google Scholar]
- Schippers P, Snoeijing I, Kropff MJ. Competition under high and low nutrient levels among three grassland species occupying different positions in a successional sequence. New Phytologist. 1999;143:547–559. doi: 10.1046/j.1469-8137.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- Simon JC, Lemaire G. Tillering and leaf area index in grass in the vegetative phase. Grass and Forage Science. 1987;42:373–380. [Google Scholar]
- Tothill JC, Hargreaves JNG, Jones RM, McDonald CK. BOTANAL – a comprehensive sampling and computing procedure for estimating pasture yield and composition. 1. Field sampling. Tropical Agronomy Technical Memorandum. 1992;78:1–24. [Google Scholar]
- Vesk PA, Westoby M. Predicting plant species' responses to grazing. Journal of Applied Ecology. 2001;38:897–909. [Google Scholar]
- Vesk PA, Leisham MR, Westoby M. Simple traits do not predict grazing response in Australian dry shrublands and woodlands. Journal of Applied Ecology. 2004;41:22–31. [Google Scholar]
- Viégas J, Cruz P, Theau JP, et al. Variation of LDMC and SLA relationship between growth forms in natural grasslands. In: O'Mara FP, Wilkins RJ, ‘t Mannetje L, et al., editors. XX International Grassland Congress: offered papers. Dublin, Ireland: Wageningen Academic Publishers; 2005. p. 866. [Google Scholar]
- Vile D, Shipley B, Garnier E. Ecosystem productivity can be predicted from potential relative growth rate and species abundance. Ecological Letters. 2006;9:1061–1067. doi: 10.1111/j.1461-0248.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- Violle C, Navas ML, Vile D, et al. Let the concept of trait be functional! Oikos. 2007;116:882–892. [Google Scholar]
- Wamelink GWW, Joosten V, Dobben van HF, Berendse F. Validity of ellenerg indicator value judged from physico-chemical field measurements. Journal of Vegetation Science. 2002;13:269–278. [Google Scholar]
- Weiher E, Van der Werf A, Thompson K, Roderick M, Garnier E, Eriksson O. Challenging Theophrastus: a common core list of plant traits for functional ecology. Journal of Vegetation Science. 1999;10:609–620. [Google Scholar]
- Werger MJA, Hirose T, During HJ, et al. Light partitioning among species and species replacement in early successional grasslands. Journal of Vegetation Science. 2002;13:615–626. [Google Scholar]
- Wilson PJ, Thompson K, Hodgson JG. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist. 1999;143:155–162. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.