Abstract
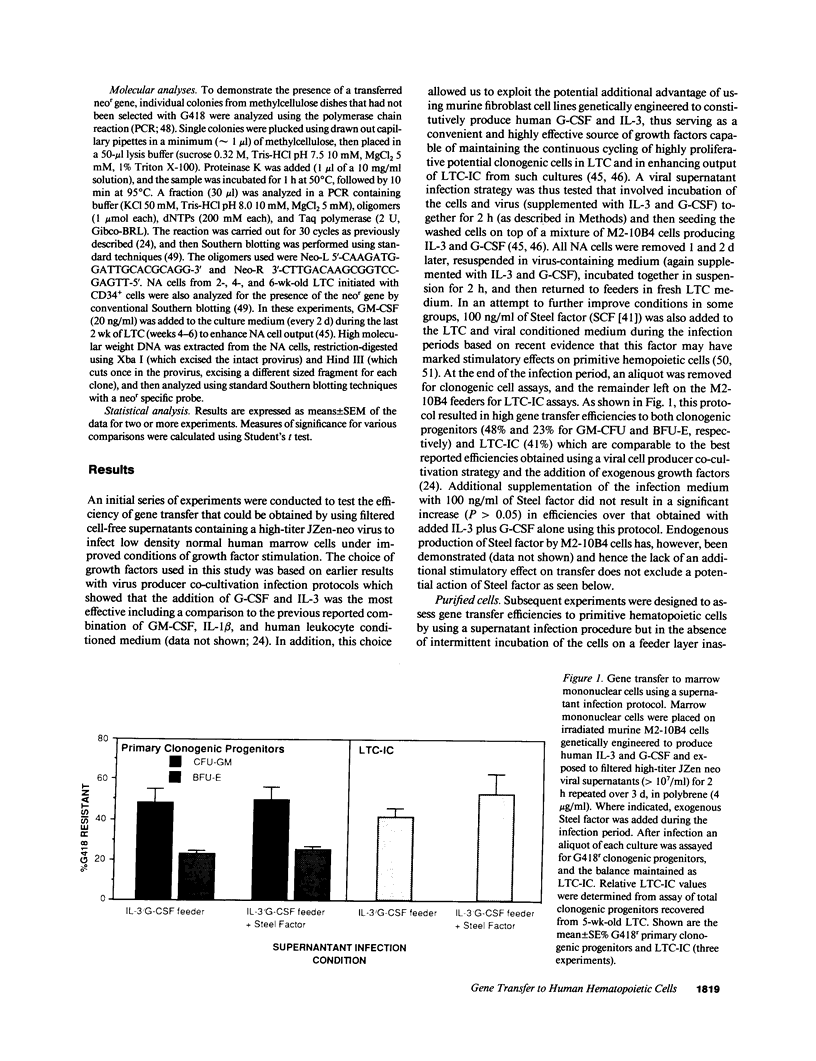
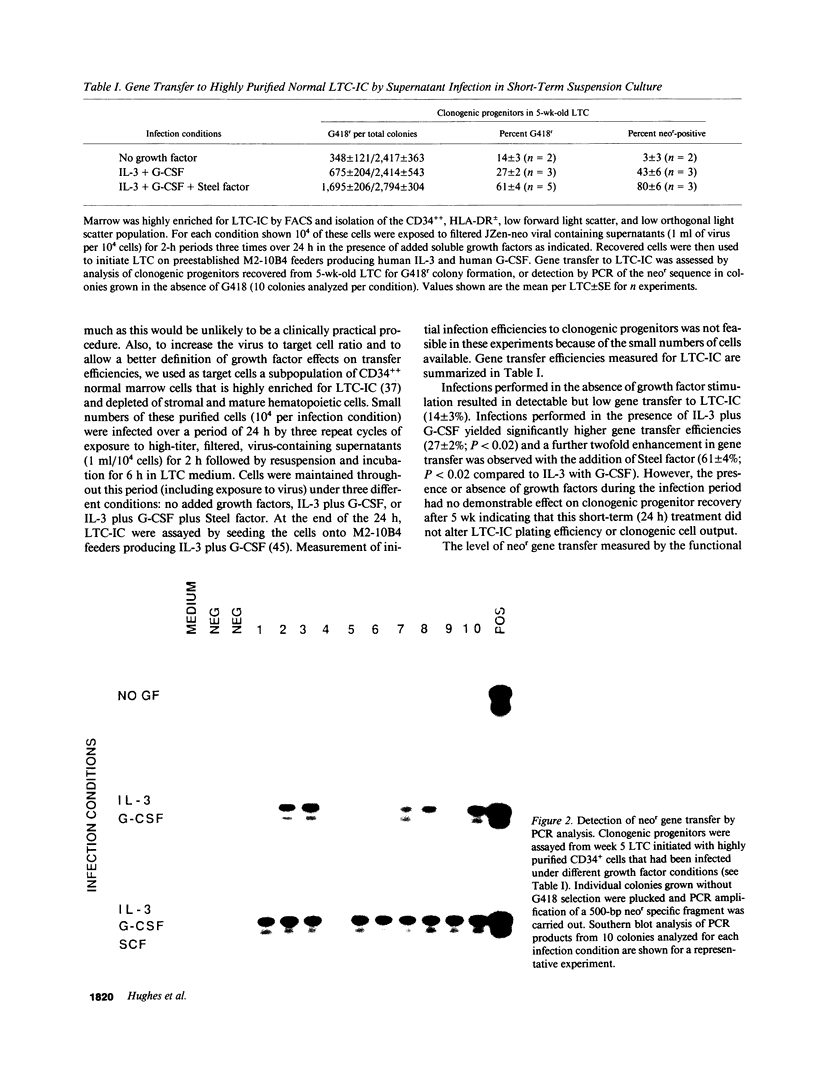
Clinical uses of gene transfer to bone marrow transplants require the establishment of a reproducible method for infecting large numbers of very primitive hematopoietic cells at high efficiency using cell-free retrovirus-containing media. In this study we report the results of experiments with preparations of a high-titer (2-5 x 10(7)/ml) helper-free recombinant neo(r) retrovirus that indicate this goal can now be achieved based on measurements of gene transfer efficiencies to cells referred to as long-term culture initiating cells (LTC-IC) because they give rise to clonogenic cells after greater than or equal to 5 wk in long-term culture (LTC). Intermittent, repeated exposure of normal human marrow mononuclear cells to virus-containing supernatant over a 3-d period of cell maintenance on an IL-3/granulocyte colony-stimulating factor (G-CSF) producing stromal layer resulted in gene transfer efficiencies to LTC-IC of 41%; a level previously obtainable only using co-cultivation infection techniques. Marrow cells enriched greater than or equal to 500-fold for LTC-IC (1-2% pure) by flow cytometry showed gene transfer efficiencies of 27% when infected in a similar fashion over a shorter period (24 h), but in the presence of added soluble IL-3 and G-CSF without stromal feeders, and this increased to 61% when Steel factor was also present during the infection period. By using a less highly enriched population of LTC-IC obtained by a bulk immunoselection technique applicable to large-scale clinical marrow harvests, gene transfer efficiencies to LTC-IC of 40% were achieved and this was increased to 60% by short-term preselection in G418. Southern analysis of DNA from the nonadherent cells produced by these LTC over a 6-wk period provided evidence of clonal evolution of LTC-IC in vitro. Leukemic chronic myelogenous leukemia LTC-IC were also infected at high efficiency using the same supernatant infection strategy with growth factor supplementation. These data demonstrate the feasibility of using cell-free virus preparations for infecting clinical marrow samples suitable for transplantation, as well as for further analysis of human marrow stem cell dynamics in vitro.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apperley J. F., Luskey B. D., Williams D. A. Retroviral gene transfer of human adenosine deaminase in murine hematopoietic cells: effect of selectable marker sequences on long-term expression. Blood. 1991 Jul 15;78(2):310–317. [PubMed] [Google Scholar]
- Bayever E., Haines K., Duprey S., Rappaport E., Douglas S. D., Surrey S. Protection of uninfected human bone marrow cells in long-term culture from G418 toxicity after retroviral-mediated transfer of the NEOr gene. Exp Cell Res. 1988 Nov;179(1):168–180. doi: 10.1016/0014-4827(88)90356-4. [DOI] [PubMed] [Google Scholar]
- Bodine D. M., Karlsson S., Nienhuis A. W. Combination of interleukins 3 and 6 preserves stem cell function in culture and enhances retrovirus-mediated gene transfer into hematopoietic stem cells. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8897–8901. doi: 10.1073/pnas.86.22.8897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., McDonagh K. T., Brandt S. J., Ney P. A., Agricola B., Byrne E., Nienhuis A. W. Development of a high-titer retrovirus producer cell line capable of gene transfer into rhesus monkey hematopoietic stem cells. Proc Natl Acad Sci U S A. 1990 May;87(10):3738–3742. doi: 10.1073/pnas.87.10.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodine D. M., McDonagh K. T., Seidel N. E., Nienhuis A. W. Survival and retrovirus infection of murine hematopoietic stem cells in vitro: effects of 5-FU and method of infection. Exp Hematol. 1991 Mar;19(3):206–212. [PubMed] [Google Scholar]
- Bordignon C., Yu S. F., Smith C. A., Hantzopoulos P., Ungers G. E., Keever C. A., O'Reilly R. J., Gilboa E. Retroviral vector-mediated high-efficiency expression of adenosine deaminase (ADA) in hematopoietic long-term cultures of ADA-deficient marrow cells. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6748–6752. doi: 10.1073/pnas.86.17.6748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel B., Hawley R. G., Mintz B. Long- and short-lived murine hematopoietic stem cell clones individually identified with retroviral integration markers. Blood. 1990 Jun 15;75(12):2267–2270. [PubMed] [Google Scholar]
- Carow C. E., Hangoc G., Cooper S. H., Williams D. E., Broxmeyer H. E. Mast cell growth factor (c-kit ligand) supports the growth of human multipotential progenitor cells with a high replating potential. Blood. 1991 Nov 1;78(9):2216–2221. [PubMed] [Google Scholar]
- Coulombel L., Eaves A. C., Eaves C. J. Enzymatic treatment of long-term human marrow cultures reveals the preferential location of primitive hemopoietic progenitors in the adherent layer. Blood. 1983 Aug;62(2):291–297. [PubMed] [Google Scholar]
- Dick J. E., Kamel-Reid S., Murdoch B., Doedens M. Gene transfer into normal human hematopoietic cells using in vitro and in vivo assays. Blood. 1991 Aug 1;78(3):624–634. [PubMed] [Google Scholar]
- Eaves A. C., Cashman J. D., Gaboury L. A., Kalousek D. K., Eaves C. J. Unregulated proliferation of primitive chronic myeloid leukemia progenitors in the presence of normal marrow adherent cells. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5306–5310. doi: 10.1073/pnas.83.14.5306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaves C. J., Eaves A. C. Erythropoietin (Ep) dose-response curves for three classes of erythroid progenitors in normal human marrow and in patients with polycythemia vera. Blood. 1978 Dec;52(6):1196–1210. [PubMed] [Google Scholar]
- Eglitis M. A., Kantoff P. W., Jolly J. D., Jones J. B., Anderson W. F., Lothrop C. D., Jr Gene transfer into hematopoietic progenitor cells from normal and cyclic hematopoietic dogs using retroviral vectors. Blood. 1988 Mar;71(3):717–722. [PubMed] [Google Scholar]
- Eglitis M. A., Kohn D. B., Moen R. C., Blaese R. M., Anderson W. F. Infection of human hematopoietic progenitor cells using a retroviral vector with a xenotropic pseudotype. Biochem Biophys Res Commun. 1988 Feb 29;151(1):201–206. doi: 10.1016/0006-291x(88)90579-7. [DOI] [PubMed] [Google Scholar]
- Fletcher F. A., Williams D. E., Maliszewski C., Anderson D., Rives M., Belmont J. W. Murine leukemia inhibitory factor enhances retroviral-vector infection efficiency of hematopoietic progenitors. Blood. 1990 Sep 15;76(6):1098–1103. [PubMed] [Google Scholar]
- Fraser C. C., Eaves C. J., Szilvassy S. J., Humphries R. K. Expansion in vitro of retrovirally marked totipotent hematopoietic stem cells. Blood. 1990 Sep 15;76(6):1071–1076. [PubMed] [Google Scholar]
- Fraser C. C., Szilvassy S. J., Eaves C. J., Humphries R. K. Proliferation of totipotent hematopoietic stem cells in vitro with retention of long-term competitive in vivo reconstituting ability. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1968–1972. doi: 10.1073/pnas.89.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hock R. A., Miller A. D. Retrovirus-mediated transfer and expression of drug resistance genes in human haematopoietic progenitor cells. Nature. 1986 Mar 20;320(6059):275–277. doi: 10.1038/320275a0. [DOI] [PubMed] [Google Scholar]
- Hogge D. E., Humphries R. K. Gene transfer to primary normal and malignant human hemopoietic progenitors using recombinant retroviruses. Blood. 1987 Feb;69(2):611–617. [PubMed] [Google Scholar]
- Holland C. A., Rothstein L., Sakakeeny M. A., Anklesaria P., Griffin J. D., Harigaya K., Newburger P. E., Greenberger J. S. Infection of hematopoietic and stromal cells in human continuous bone marrow cultures by a retroviral vector containing the neomycin resistance gene. Acta Haematol. 1989;82(3):136–143. doi: 10.1159/000205362. [DOI] [PubMed] [Google Scholar]
- Hughes P. F., Eaves C. J., Hogge D. E., Humphries R. K. High-efficiency gene transfer to human hematopoietic cells maintained in long-term marrow culture. Blood. 1989 Nov 1;74(6):1915–1922. [PubMed] [Google Scholar]
- Johnson G. R., Gonda T. J., Metcalf D., Hariharan I. K., Cory S. A lethal myeloproliferative syndrome in mice transplanted with bone marrow cells infected with a retrovirus expressing granulocyte-macrophage colony stimulating factor. EMBO J. 1989 Feb;8(2):441–448. doi: 10.1002/j.1460-2075.1989.tb03396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P. W., Flake A. W., Eglitis M. A., Scharf S., Bond S., Gilboa E., Erlich H., Harrison M. R., Zanjani E. D., Anderson W. F. In utero gene transfer and expression: a sheep transplantation model. Blood. 1989 Mar;73(4):1066–1073. [PubMed] [Google Scholar]
- Kantoff P. W., Gillio A. P., McLachlin J. R., Bordignon C., Eglitis M. A., Kernan N. A., Moen R. C., Kohn D. B., Yu S. F., Karson E. Expression of human adenosine deaminase in nonhuman primates after retrovirus-mediated gene transfer. J Exp Med. 1987 Jul 1;166(1):219–234. doi: 10.1084/jem.166.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson S. Treatment of genetic defects in hematopoietic cell function by gene transfer. Blood. 1991 Nov 15;78(10):2481–2492. [PubMed] [Google Scholar]
- Kerk D. K., Henry E. A., Eaves A. C., Eaves C. J. Two classes of primitive pluripotent hemopoietic progenitor cells: separation by adherence. J Cell Physiol. 1985 Oct;125(1):127–134. doi: 10.1002/jcp.1041250117. [DOI] [PubMed] [Google Scholar]
- Kwok W. W., Schuening F., Stead R. B., Miller A. D. Retroviral transfer of genes into canine hemopoietic progenitor cells in culture: a model for human gene therapy. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4552–4555. doi: 10.1073/pnas.83.12.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdorp P. M., Sutherland H. J., Eaves C. J. Selective expression of CD45 isoforms on functional subpopulations of CD34+ hemopoietic cells from human bone marrow. J Exp Med. 1990 Jul 1;172(1):363–366. doi: 10.1084/jem.172.1.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemischka I. R., Raulet D. H., Mulligan R. C. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986 Jun 20;45(6):917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- Lemoine F. M., Humphries R. K., Abraham S. D., Krystal G., Eaves C. J. Partial characterization of a novel stromal cell-derived pre-B-cell growth factor active on normal and immortalized pre-B cells. Exp Hematol. 1988 Sep;16(8):718–726. [PubMed] [Google Scholar]
- Markowitz D., Goff S., Bank A. A safe packaging line for gene transfer: separating viral genes on two different plasmids. J Virol. 1988 Apr;62(4):1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F. H., Suggs S. V., Langley K. E., Lu H. S., Ting J., Okino K. H., Morris C. F., McNiece I. K., Jacobsen F. W., Mendiaz E. A. Primary structure and functional expression of rat and human stem cell factor DNAs. Cell. 1990 Oct 5;63(1):203–211. doi: 10.1016/0092-8674(90)90301-t. [DOI] [PubMed] [Google Scholar]
- Mauch P., Greenberger J. S., Botnick L., Hannon E., Hellman S. Evidence for structured variation in self-renewal capacity within long-term bone marrow cultures. Proc Natl Acad Sci U S A. 1980 May;77(5):2927–2930. doi: 10.1073/pnas.77.5.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliaccio G., Migliaccio A. R., Valinsky J., Langley K., Zsebo K., Visser J. W., Adamson J. W. Stem cell factor induces proliferation and differentiation of highly enriched murine hematopoietic cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7420–7424. doi: 10.1073/pnas.88.16.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Law M. F., Verma I. M. Generation of helper-free amphotropic retroviruses that transduce a dominant-acting, methotrexate-resistant dihydrofolate reductase gene. Mol Cell Biol. 1985 Mar;5(3):431–437. doi: 10.1128/mcb.5.3.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Progress toward human gene therapy. Blood. 1990 Jul 15;76(2):271–278. [PubMed] [Google Scholar]
- Miltenyi S., Müller W., Weichel W., Radbruch A. High gradient magnetic cell separation with MACS. Cytometry. 1990;11(2):231–238. doi: 10.1002/cyto.990110203. [DOI] [PubMed] [Google Scholar]
- Molday R. S., MacKenzie D. Immunospecific ferromagnetic iron-dextran reagents for the labeling and magnetic separation of cells. J Immunol Methods. 1982 Aug 13;52(3):353–367. doi: 10.1016/0022-1759(82)90007-2. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Ikebuchi K., Leary A. G. Humoral regulation of stem cell proliferation. Ann N Y Acad Sci. 1989;554:185–191. doi: 10.1111/j.1749-6632.1989.tb22420.x. [DOI] [PubMed] [Google Scholar]
- Otsuka T., Thacker J. D., Eaves C. J., Hogge D. E. Differential effects of microenvironmentally presented interleukin 3 versus soluble growth factor on primitive human hematopoietic cells. J Clin Invest. 1991 Aug;88(2):417–422. doi: 10.1172/JCI115320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuening F. G., Storb R., Stead R. B., Goehle S., Nash R., Miller A. D. Improved retroviral transfer of genes into canine hematopoietic progenitor cells kept in long-term marrow culture. Blood. 1989 Jul;74(1):152–155. [PubMed] [Google Scholar]
- Snodgrass R., Keller G. Clonal fluctuation within the haematopoietic system of mice reconstituted with retrovirus-infected stem cells. EMBO J. 1987 Dec 20;6(13):3955–3960. doi: 10.1002/j.1460-2075.1987.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead R. B., Kwok W. W., Storb R., Miller A. D. Canine model for gene therapy: inefficient gene expression in dogs reconstituted with autologous marrow infected with retroviral vectors. Blood. 1988 Mar;71(3):742–747. [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Eaves A. C., Dragowska W., Lansdorp P. M. Characterization and partial purification of human marrow cells capable of initiating long-term hematopoiesis in vitro. Blood. 1989 Oct;74(5):1563–1570. [PubMed] [Google Scholar]
- Sutherland H. J., Eaves C. J., Lansdorp P. M., Thacker J. D., Hogge D. E. Differential regulation of primitive human hematopoietic cells in long-term cultures maintained on genetically engineered murine stromal cells. Blood. 1991 Aug 1;78(3):666–672. [PubMed] [Google Scholar]
- Sutherland H. J., Lansdorp P. M., Henkelman D. H., Eaves A. C., Eaves C. J. Functional characterization of individual human hematopoietic stem cells cultured at limiting dilution on supportive marrow stromal layers. Proc Natl Acad Sci U S A. 1990 May;87(9):3584–3588. doi: 10.1073/pnas.87.9.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilvassy S. J., Fraser C. C., Eaves C. J., Lansdorp P. M., Eaves A. C., Humphries R. K. Retrovirus-mediated gene transfer to purified hemopoietic stem cells with long-term lympho-myelopoietic repopulating ability. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8798–8802. doi: 10.1073/pnas.86.22.8798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Van Zant G., Chen J. J., Scott-Micus K. Developmental potential of hematopoietic stem cells determined using retrovirally marked allophenic marrow. Blood. 1991 Feb 15;77(4):756–763. [PubMed] [Google Scholar]
- Zsebo K. M., Wypych J., McNiece I. K., Lu H. S., Smith K. A., Karkare S. B., Sachdev R. K., Yuschenkoff V. N., Birkett N. C., Williams L. R. Identification, purification, and biological characterization of hematopoietic stem cell factor from buffalo rat liver--conditioned medium. Cell. 1990 Oct 5;63(1):195–201. doi: 10.1016/0092-8674(90)90300-4. [DOI] [PubMed] [Google Scholar]