Summary
Because of recent insights into the pathogenesis of age-related bone loss, we investigated whether intermittent parathyroid hormone (PTH) administration antagonizes the molecular mechanisms of the adverse effects of aging on bone. PTH produced a greater increase in vertebral trabecular bone mineral density and bone volume as well as a greater expansion of the endocortical bone surface in the femur of 26 as compared to 6 month old female C57BL/6 mice. Moreover, PTH increased trabecular connectivity in vertebrae and the toughness of both vertebrae and femora in old, but not young, mice. PTH also increased the rate of bone formation and reduced osteoblast apoptosis to a greater extent in the old mice. Most strikingly, PTH reduced reactive oxygen species (ROS), p66Shc phosphorylation and expression of the lipoxygenase Alox15; and it increased glutathione and stimulated Wnt signaling in bone of old mice. PTH also antagonized the effects of oxidative stress on p66Shc phosphorylation, FoxO transcriptional activity, osteoblast apoptosis, and Wnt signaling in vitro. In contrast, administration of the antioxidants N-acetyl cysteine or pegylated catalase reduced osteoblast progenitors, and attenuated proliferation and Wnt signaling. These results suggest that PTH has a greater bone anabolic efficacy in old age because in addition to its other positive actions on bone formation it antagonizes the age-associated increase in oxidative stress and its adverse effects on the birth and survival of osteoblasts. On the other hand, ordinary antioxidants cannot restore bone mass in old age because they slow remodeling and attenuate osteoblastogenesis by interfering with Wnt signaling.
Keywords: osteoporosis, parathyroid hormone, oxidative stress, osteoblasts, bone formation
Introduction
A series of remarkable observations, beginning a century ago with the demonstration of a bone anabolic effect of parathyroid gland extracts in rats, culminated in studies showing that daily injections of synthetic parathyroid hormone (PTH) increase bone mass and reduce the incidence of fractures in post-menopausal women and hypogonadal men (Potts, 2005). As a result, intermittent PTH administration was approved by the Food and Drug Administration as a therapy for osteoporosis capable of restoring bone mass, as opposed to alternative anti-catabolic therapies that only prevent further bone loss (Hodsman et al, 2005; Riggs & Parfitt, 2005). Intermittent PTH is also an effective treatment for glucocorticoid-induced osteoporosis (Saag et al, 2009) – a condition that, like old age, is characterized by a decrease in bone formation. Prompted by new insights into the cellular and molecular mechanisms responsible for the decline of bone mass and strength with advancing age (Manolagas, 2010), we investigated whether PTH represents a mechanistically rational therapy for osteoporosis in the elderly.
The histologic hallmark of age-related bone loss in both humans and mice is a decline in wall thickness, an index of the amount of bone made by each team of osteoblasts during an episode of bone remodeling (Lips et al, 1978; Almeida et al, 2007b). Because bone formation is largely determined by osteoblast number (Manolagas, 2000), the decline in wall thickness must reflect decreased differentiation and survival of osteoblasts. Although sex steroid deficiency contributes to osteoporosis, it is now clear that loss of trabecular bone begins as early as the 3rd decade of life in both men and women (Riggs et al, 2008) – well before the decline in sex steroid production. Moreover, in female and male C57BL/6 mice, which do not exhibit a significant decline in estrogen or androgen production with aging, loss of bone mass and strength begins at 4–8 months of age and continues until at least 31 months of age (Almeida et al, 2007b). The development of this osteoporotic phenotype is associated with a progressive increase in the prevalence of osteoblast apoptosis, and a decrease in osteoblast number, wall width and bone formation rate.
Oxidative stress may be a fundamental mechanism of the adverse effects of aging on bone mass and strength, as is the case in other tissues (Manolagas, 2010). Indeed, the skeletal changes in aging C57BL/6 mice are accompanied by a progressive increase in reactive oxygen species (ROS) in the bone marrow together with an increase in bone itself of phosphorylated p66Shc (Almeida et al, 2007b), a marker and amplifier of oxidative stress (Pinton et al, 2007). ROS activate protein kinase C-β (PKCβ) which phosphorylates cytoplasmic p66Shc. Phosphorylated p66Shc is then translocated to the mitochondria where it acts as a redox enzyme, catalyzing the reduction of O2 to H2O2 during electron transfer from cytochrome c (Giorgio et al, 2005). Increased lipid oxidation also contributes to and amplifies oxidative stress in the skeleton (Almeida et al, 2009). Thus, ROS as well as lipoxygenases like Alox15 oxidize polyunsaturated fatty acids, resulting in the formation of unstable hydroperoxy derivatives that give rise to strong pro-oxidants like 4-hydroxynonenal (4-HNE) via a nonenzymatic reaction (Schneider et al, 2008). Finally, an age-related rise in endogenous glucocorticoids may also generate ROS (Almeida et al, 2008). ROS greatly influence the generation and survival of osteoclasts, osteoblasts, and osteocytes (Almeida et al, 2007b). Moreover, defense against oxidative stress by the Forkhead Box O (FoxO) family of transcription factors is indispensable for skeletal homeostasis as evidenced by the increased oxidative stress and osteoblast apoptosis, and the bone loss, that occurs following global deletion of FoxO1, 3 and 4 in 3 month old mice (Ambrogini et al, 2010).
In addition to increased levels of ROS, diminished Wnt signaling accompanies bone loss in aging mice. Wnt ligands bind to co-receptors comprised of Frizzled and either low density lipoprotein 5 (LRP5) or LRP6 on the cell surface, resulting in the stabilization of β-catenin that would otherwise be degraded. β-catenin then migrates to the nucleus and serves as a partner with members of the T cell factor (Tcf) family of transcription factors to regulate the synthesis of genes that promote osteoblastogenesis and inhibit osteoblast apoptosis (Rodda & McMahon, 2006; Bodine, 2008; Almeida et al, 2005). The reduction in Wnt signaling in the skeleton with advancing age may be explained by ROS-mediated activation of FoxOs resulting in diversion of ß-catenin from Tcf- to FoxO-mediated transcription (Almeida et al, 2007a). In addition, lipid oxidation generates ligands for peroxisome proliferator activated receptor γ (PPARγ), which attenuates Wnt signaling by sequestrating β-catenin (Almeida et al, 2009).
Intermittent PTH stimulates bone formation by increasing the number of osteoblasts at sites of bone remodeling, where old bone is replaced with new by teams of osteoclasts and osteoblasts (Compston, 2007; Lindsay et al, 2006; Ma et al, 2006). The increase in osteoblast number has been explained by attenuation of osteoblast apoptosis (Jilka et al, 1999; Bellido et al, 2003), as well as stimulation of osteoblast differentiation (Jilka, 2007). The pro-differentiating effects of PTH evidently predominate in the anabolic effect of the hormone on periosteal bone where, unlike the situation in trabecular bone, only a small percentage of osteoblasts die by apoptosis (Jilka et al, 2009). Recent evidence indicates that the anabolic effect of intermittent PTH is mediated by increased Wnt signaling. Specifically, PTH downregulates Wnt antagonists including sclerostin (Bellido et al, 2005; Keller & Kneissel, 2005; O'Brien et al, 2008; Kramer et al, 2010b), secreted Frizzled related protein 1 (Sfrp1) (Bodine et al, 2007), and Dickkopf 1 (Dkk1) (Guo et al, 2010a). In addition, PTH directly activates the Wnt co-receptor LRP6 (Wan et al, 2008). Consistent with an important role of Wnt signaling in the mediation of the anabolic effects of PTH, mice lacking Sfrp1 or over-expressing Dkk1, as well as mice lacking or overexpressing sclerostin, exhibit a diminished anabolic response to daily injections of PTH (Kramer et al, 2010a).
Based on the above lines of evidence, we have tested the hypothesis that the efficacy of PTH in old age may result from its ability to interfere with age-related mechanistic culprits such as oxidative stress. To this end, we have compared the efficacy of intermittent PTH administration in young vs. old C57BL/6 mice and searched for potential mechanistic differences between the two groups. In addition, we have compared the effects of PTH to those of antioxidants such as N-acetyl cysteine (NAC) and catalase.
Results
Increased efficacy of intermittent PTH on vertebrae of 26 month old mice
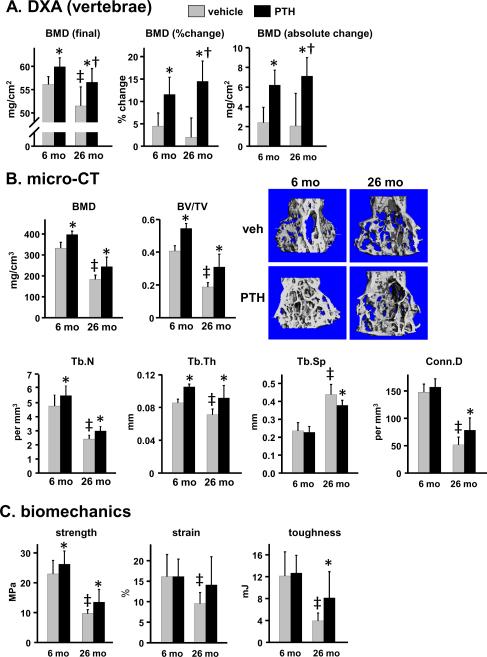
Mice were given daily injections of 100 ng/g PTH(1–34), or vehicle control, for 28 days. The lumbar spine of vehicle-treated 26 month old mice female C57BL/6 mice exhibited a lower bone mineral density (BMD) as compared to 6 month old controls, determined by dual energy X-ray absorptiometry (DXA) (Fig. 1A). Micro-computed tomography (micro-CT) analysis of vertebral bone (L4) revealed a lower trabecular BMD, and a lower ratio of trabecular bone volume per tissue volume (BV/TV) in old mice (Fig. 1B). The age-related decline in BV/TV was due to a reduction in the number and thickness of the trabeculae and increased trabecular spacing, and was associated with decreased trabecular connectivity. Compression testing of L5 demonstrated that aging reduced vertebral bone strength, strain (the amount of deformation tolerated before breaking), and toughness (the energy required to break the bone, an index of fracture resistance) (Fig. 1C).
Figure 1.
Enhanced efficacy of intermittent PTH in vertebral trabecular bone of young vs. old old mice. Female C57BL/6 mice (N=9–12 per group) were given daily injections of vehicle or 100 ng/g PTH(1–34) for 28 days. (A) BMD was measured at the beginning and end of the experiment to allow calculation of % change and absolute magnitude of change caused by PTH. (B) Trabecular BMD, bone volume/tissue volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp) and connectivity density of the trabeculae (Conn.D) were determined in L4. Representative micro-CT images from each group of animals are shown. (C) Strength (load tolerated at the breaking point adjusted for bone size), strain (deformation tolerated before breaking), and toughness (energy required to break the bone) were measured in L5. Statistical analysis was done by 2-way ANOVA, except for BV/TV, Tb.Th, and Tb.N due to non-normal distribution of data. These parameters were analyzed by Kruskal-Wallis ANOVA on ranks. * p<0.05 vs. respective vehicle control; † p<0.05 vs. 6 month PTH response; ‡ p<0.05 vs. 6 month vehicle control.
PTH increased spinal BMD in both 6 and 26 month old mice as determined by DXA (Fig. 1A). In old mice, PTH restored spinal BMD almost to the level of untreated young mice. The magnitude of the increase in BMD in 26 month old mice was significantly greater than in 6 month old mice whether expressed as the final BMD, or as the percent change or absolute change in BMD from baseline (Fig. 1A). Determination of the effect of PTH on the trabecular bone compartment of L4 using micro-CT showed that the effect of PTH on trabecular BMD and BV/TV was similar in young and old mice (Fig. 1B). However, the percentage increase was greater in old mice because of a lower starting value (BMD: 20% increase in 6 month vs. 33% increase in 26 month old mice; BV/TV: 35% increase in 6 month vs. 64% increase in 26 month old mice). The PTH-stimulated increase in vertebral trabecular bone volume in both young and old mice was due to increased trabecular number and trabecular thickness. Interestingly, however, PTH caused a reduction in trabecular spacing and an increase in trabecular connectivity only in 26 month old animals, indicating that PTH causes distinctive architectural changes in the vertebral trabecular bone of aged mice. PTH produced an equivalent increase in strength in young and old mice (Fig. 1C), but had no effect on strain in either young or old mice. However, consistent with the age-specific architectural changes, PTH increased toughness by 106% only in old mice.
Increased efficacy of intermittent PTH on femora of 26 month old mice
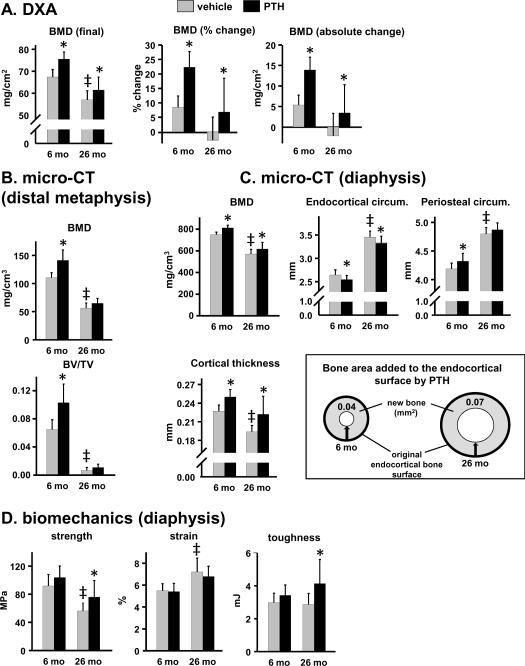
Femoral bone of aged mice exhibited a lower BMD than young mice (Fig. 2A,B). Moreover, metaphyseal trabecular bone was scant by 26 months (Fig. 2B). Femoral bone size increased with age consistent with our earlier report (Almeida et al, 2007b). Thus, femora of 26 month old mice exhibited a greater periosteal circumference, and a corresponding increase in endocortical circumference, measured at the diaphysis (Fig. 2C). Nevertheless, cortical bone thickness was lower in 26 month old mice, and there was a corresponding decrease in diaphyseal cortical bone strength as determined by 3-point bending (Fig. 2D). The femora of 26 month old animals tolerated more strain as compared to that of 6 month old animals, probably due to the greater moment of inertia, i.e. the femoral cortical bone mass in old mice was distributed further away from the central axis of the bone as reflected by the increased periosteal and endocortical circumference. However, this change was insufficient to improve femoral toughness.
Figure 2.
PTH increases femoral strength only in old mice. Measurements were made on femora from the experiment described in Fig. 1. (A) Determination of femoral BMD by DXA. (B) Determination of trabecular bone architecture of the distal metaphysis by micro-CT. (C) Determination of cortical bone architecture at the diaphysis by micro-CT. The diagram represents a cross-sectional view of the femoral diaphysis, and depicts the bone area added to the endocortical surface of 6 month and 26 month old mice in mice receiving PTH. Arrows indicate deposition of new bone onto the original endocortical surface, resulting in a reduction in the endocortical circumference. (D) Biomechanical properties of the femoral diaphysis. * p<0.05 vs. respective vehicle control. † p<0.05 vs. 6 month PTH response by; ‡ p<0.05 vs. 6 month vehicle control, by 2-way ANOVA.
Intermittent PTH caused an equivalent increase in femoral BMD in both young and old mice (Fig. 2A). In young mice, PTH increased bone mass in both metaphyseal trabecular bone and diaphyseal cortical bone of the femur, whereas in old mice the increase in bone mass was restricted to cortical bone (Fig. 2B,C). Failure to detect an anabolic effect of PTH in trabecular bone at this site in aged mice is explained by the very limited surface upon which new bone formation can take place.
The magnitude of the PTH-stimulated increase in BMD and cortical thickness at the femoral diaphysis was similar in young and old mice (Fig. 2C). In young mice, PTH added bone to both the endocortical and periosteal surface as reflected by a decrease in endocortical circumference and an increase in periosteal circumference, respectively. In old mice, however, PTH added bone only to the endocortical surface as reflected by the decrease in endocortical circumference. The amount of bone added by PTH to the endocortical surface was determined by calculating the reduction in marrow area in vehicle vs. PTH-treated animals. Marrow area was calculated from the endocortical circumference, assuming a circular shape of the diaphyseal cross section. As illustrated in the diagram of Fig. 2C, the bone area added in old mice was 75% greater than that in young mice due to larger diameter of femoral bone in old mice. Consistent with this, intermittent PTH administration caused a significant increase (35%) in femoral strength only in the older mice (Fig. 2D). PTH had no effect on femoral strain at either age; however, femoral toughness increased by 15% in the old mice receiving PTH.
PTH stimulates the rate of bone formation, inhibits osteoblast apoptosis, and stimulates Wnt signaling more potently in 26 month old mice
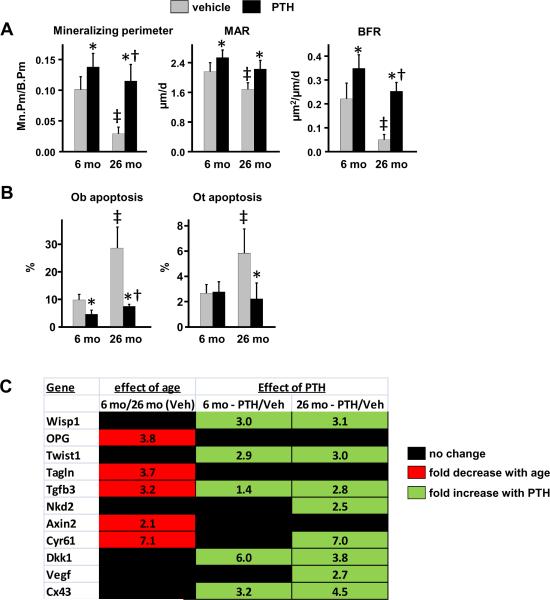
Bone formation was lower in vertebral trabecular bone of vehicle-treated 26 month old mice as compared to 6 month old mice, as measured by the mineralizing (tetracycline-labeled) perimeter (Mn.Pm/B.Pm) (Fig. 3A). Mineral apposition rate (MAR), the distance between the tetracycline labels, was also significantly lower in 26 month old mice. Overall bone formation rate (MAR × Mn.Pm/B.Pm) was therefore significantly reduced in old mice.
Figure 3.
Increased efficacy of intermittent PTH on stimulation of bone formation rate, inhibition of osteoblast apoptosis, and stimulation of Wnt signaling in vertebral bone of 26 month old mice. Vertebral bone sections (N=6 per group) from the experiment described in Fig. 1 were used to quantify (A) mineralizing perimeter/bone perimeter (Mn.Pm/B.Pm), mineral apposition rate (MAR) and bone formation rate (BFR) using tetracycline labeling, and (B) the prevalence of apoptotic osteoblasts (Ob) and osteocytes (Ot) using ISEL-labeling. (A,B) Statistical analysis by 2-way ANOVA. *, p<0.05 vs respective vehicle control; †, p<0.05 vs. 6 month PTH response by 2-way ANOVA; ‡, p<0.05 vs. 6 month vehicle control. (C) Expression of Wnt target genes in vertebral bone (N=9–12/group). The data shown represents the fold decrease (red) in transcript expression from 6 month to 26 months of age; and the fold increase (green) in expression in PTH-treated 6 month and 26 month old mice compared to respective vehicle (Veh) controls. Expression levels were normalized to GAPDH. Only statistically significant (p<0.05) changes are shown, as determined by one-way ANOVA for each transcript.
PTH increased mineralizing perimeter to a greater extent in old than in young mice, reaching a value indistinguishable from that observed in young mice receiving vehicle (Fig. 3A). MAR was increased to the same extent by PTH in both young and old mice. Thus, the magnitude of the PTH-stimulated increase in bone formation rate was greater in old as compared to young mice.
We next examined the effect of intermittent PTH on the prevalence of osteoblast apoptosis, which is known to be tightly linked to the anabolic effect of the hormone (Bellido et al, 2003; Weinstein et al, 2010a). Apoptotic osteoblasts, detected by in situ end-labeling (ISEL), were increased by approximately 3-fold in old as compared to young mice (Fig. 3B), similar to our previous report (Almeida et al, 2007b). Intermittent PTH reduced the prevalence of osteoblast apoptosis in both young and old mice, but the magnitude of the reduction was greater in old mice. Notably, PTH reduced osteoblast apoptosis to a level below that seen in young mice receiving vehicle. PTH also reversed the age-related increase in apoptotic osteocytes (Fig. 3B). Because osteocytes are long-lived cells, the PTH-induced decrease in the percentage exhibiting ISEL stain in bone of old mice reflects addition of new bone with viable osteocytes on top of old bone containing dead or dying ones.
We next measured Wnt signaling by quantifying the expression of several established Wnt target genes in vertebral bone (Jackson et al, 2005; Glass II et al, 2005; Chamorro et al, 2005; van der Heyden et al, 1998). The rationale underlying this approach is based on published evidence that the expression of Wnt target genes is concordant with the expression of β-galactosidase in bone of TOPGAL mice bearing a β-galactosidase transgene under the control of a β-catenin/Tcf-responsive promoter (Guo et al, 2010a). Consistent with earlier evidence for diminished Wnt signaling in aged mice (Almeida et al, 2007a), expression of Axin2, OPG, Tagln, Tgfb3 and Cyr61 were lower in 26 month as compared to 6 month old mice (Fig. 3C). PTH increased expression of Wisp1, Twist1 and Tgfb3 in both young and old mice. Interestingly, PTH also increased expression of Nkd2 and Cyr6, but only in old mice.
Intermittent PTH attenuates the age-related increase in oxidative stress and osteoblast apoptosis
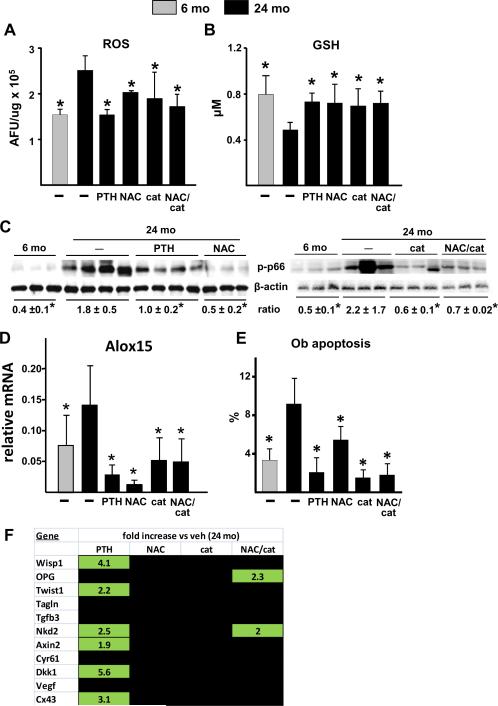
We next investigated whether the greater efficacy of intermittent PTH in aged mice is associated with interference with age-specific mechanisms of osteoporosis, namely oxidative stress. Twenty-four month old female C57BL/6 mice were given daily injections of 100 ng/g PTH(1–34), or the antioxidants NAC (100 mg/kg of chow) or pegylated catalase (200 μg/g by daily injection), or the combination of NAC and catalase, for 28 days. Vehicle-injected 6 and 24 month old mice served as controls. Remarkably, intermittent PTH reversed the increase in ROS and decrease in glutathione in old mice to the levels seen in young mice (Fig. 4A,B). PTH also attenuated the age-related increase in phosphorylated p66Shc (Fig. 4C). These antioxidant effects of PTH were indistinguishable from those of NAC, catalase, or their combination.
Figure 4.
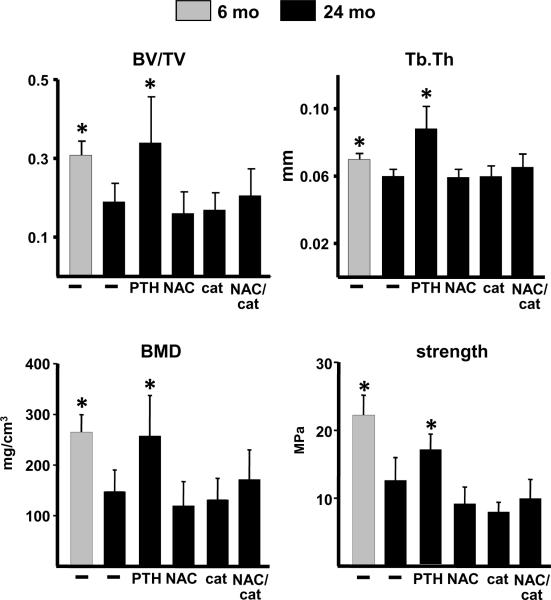
PTH and antioxidants reduce the age-related increase in oxidative stress and osteoblast apoptosis. Six month old female C57BL/6 mice were injected daily with saline (−). 24 month old mice were injected daily with saline (−), 100 ng/g PTH(1–34) or 200 μg/g pegylated catalase (cat), or fed a diet containing 100 mg/kg NAC, or given both NAC and catalase (NAC/cat) for 28 days (N=9–12/group). ROS (A) and glutathione (GSH) (B) were quantified in cells flushed from femoral bone marrow (AFU, arbitrary fluorescence units) (N=4/group). (C) Phosphorylated p66Shc in vertebral lysates. Two blots were performed to accommodate all treatment groups. Each lane represents a vertebral lysate from a single animal. (D) Alox15 mRNA in vertebral extracts (N=9–12/group). (E) The prevalence of osteoblast apoptosis was determined in vertebral bone sections (N=5 per group). * p<0.05 vs. 24 month old vehicle control by one-way ANOVA. (F) Expression of Wnt target genes in vertebral bone (N=9–12/group). The data shown represents the fold decrease (red) in transcript expression from 6 month to 24 months of age; and the fold increase (green) in expression in 26 month old mice given PTH or antioxidants compared to vehicle controls. Expression levels were normalized to GAPDH. Only statistically significant (p<0.05) changes are shown, as determined by one-way ANOVA for each transcript.
Similar to phosphorylated p66Shc, the lipoxygenase Alox15 is both induced by and produces oxidative stress (Seiler et al, 2008; Almeida et al, 2009); and its expression in the murine skeleton increases with advancing age (Almeida et al, 2009). Strikingly, PTH, as well as NAC, catalase, or their combination, completely reversed the age-related increase in Alox15 expression to the levels found in the younger mice (Fig. 4D). NAC, catalase, and the combination, also reduced the prevalence of osteoblast apoptosis in vertebral cancellous bone of 24 month old mice to the level of 6 month old mice (Fig. 4E). More important, the pro-survival effect of these antioxidants on osteoblast apoptosis was indistinguishable from that of daily PTH administration. On the other hand, analysis of the same panel of Wnt target genes as in Fig. 3C revealed that whereas PTH increased the expression of 6 Wnt target genes in 24 month old mice (Fig. 4F), administration of NAC or catalase had no effect on any of the tested transcripts; albeit the combination of NAC and catalase increased the expression of 2 of them. Similarly, PTH caused the expected increase in vertebral trabecular bone volume, bone density and trabecular thickness, as well as vertebral compression strength, but the antioxidants did not (Fig. 5).
Figure 5.
PTH and but not antioxidants increases bone mass. Trabecular bone volume (BV/TV) and bone density (BMD), trabecular thickness (Tb.Th) and compression strength, was determined in vertebral bone from the experiment described in Fig. 4 (N= 9–12 per group). * p<0.05 vs. 24 month old vehicle control by one-way ANOVA.
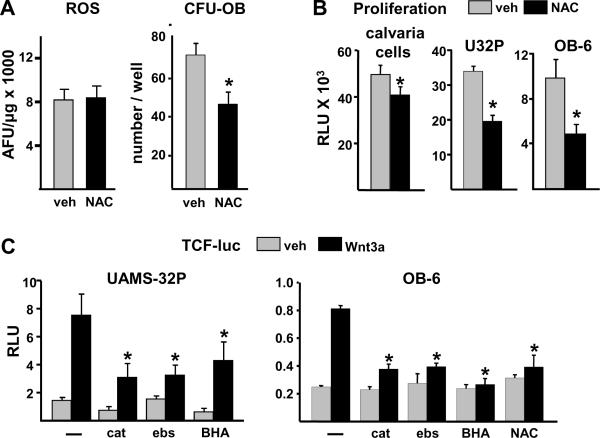
Antioxidants reduce osteoblast progenitors in vivo and suppress proliferation and Wnt signaling in vitro
Cellular redox status plays an important role in intracellular signaling pathways, including activation of β-catenin (Funato et al, 2006; Janssen-Heininger et al, 2008). We therefore suspected that the inability of antioxidant therapy to increase bone mass in 24 month old mice was due at least in part to interference with the signaling pathways involved in the proliferation and differentiation of osteoblast progenitors. To investigate this issue, 5 month old male C57BL/6 mice were given daily injections of NAC for 4 weeks. NAC did not reduce the level of ROS in 5 month old mice (Fig. 6A), but did cause a significant decline the number of early osteoblast progenitors in the bone marrow, assayed as colony forming unit-osteoblasts (CFU-OB). NAC also inhibited the proliferation of osteoblastic cells derived from neonatal calvaria (Fig. 6B), as well as the proliferation of UAMS-32P and OB-6 cells (Fig. 6B), which exhibit properties of osteoblast progenitors in the early and late stages of differentiation, respectively (Galli et al, 2009; Lecka-Czernik et al, 1999). Furthermore, several antioxidants including catalase, and the ROS scavengers ebselen and butylated hydroxyanisole (BHA), as well as NAC, attenuated Wnt3a-induced activation of Tcf/β-catenin-mediated transcription in these cells as measured by a transfected Tcf-luciferase reporter construct (Fig. 6C).
Figure 6.
Antioxidants reduce osteoblast progenitors, and suppress replication and Wnt-signaling of cultured osteoblastic cells. (A) Five month old male C57BL/6 mice were injected daily with PBS (veh) or 100 μg/g of NAC, (N=4 mice per group) for 28 days. ROS and early osteoblast progenitors [colony forming unit-osteoblasts (CFU-OB)] were determined. * p<0.05 vs. vehicle control by Student's t-test. (B) Proliferation (relative light units, RLU) of osteoblastic cells isolated from neonatal murine calvaria, and of UAMS-32P and OB-6 cells, after 24 hour incubation with PBS (veh) or 1 mM NAC. Bars represent the mean of triplicate determinations. * p<0.05 vs. vehicle control by Student's t-test. (C) Luciferase activity (RLU) in UAMS-32P or OB-6 cells transfected with a Tcf-luc reporter construct after pretreatment for 1 hour with PBS (−), 600 U/ml pegylated catalase (cat), 20 μM ebselen (ebs), 50 μM BHA, or 1 mM NAC, then with saline (veh) or Wnt3a (50 ng/ml) for 24 h. Bars represent the mean of triplicate determinations. * p<0.05 vs. Wnt3a alone by one-way ANOVA.
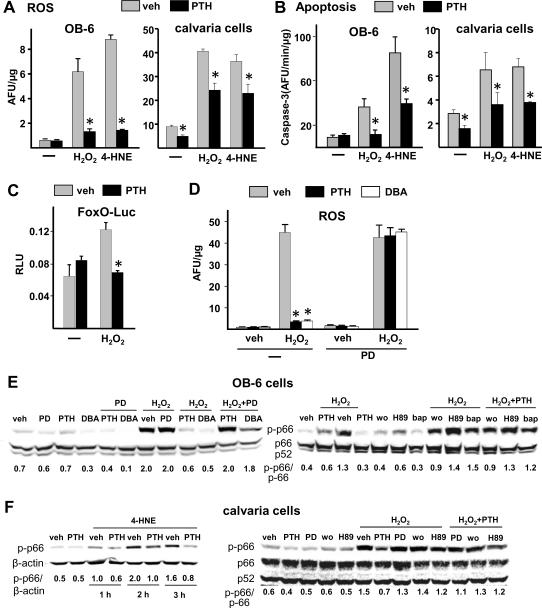
PTH attenuates the rise in intracellular ROS, p66Shc phosphorylation, and FoxO transcriptional activity induced by oxidative stress in osteoblastic cells
Consistent with the in vivo studies, preincubation of OB-6 or calvaria cells for 1 hour with PTH attenuated the rise in intracellular ROS levels induced by H2O2 or 4-HNE (Fig. 7A). Moreover, PTH attenuated H2O2- and 4-HNE-induced apoptosis as determined by caspase-3 activity in both cell preparations (Fig. 7B). PTH also prevented the H2O2-induced activation of FoxO-mediated transcription, as measured in UAMS-32 cells transfected with a FoxO-sensitive luciferase reporter construct (Fig. 7C).
Figure 7.
Antioxidant effects of PTH in cultured osteoblastic cells. (A,B) Osteoblastic OB-6 cells or osteoblastic cells derived from neonatal murine calvaria were preincubated for 1 hour with 0.01% acetic acid (veh), 50 nM PTH(1–34), or 1 mM NAC, followed by addition of saline (−), 100 μM H2O2 or 20 μM 4-HNE. Intracellular ROS were quantified 10 minutes later (A). Caspase-3 activity was measured in separate cultures 6 hours later (B). (C) Luciferase activity (RLU) in UAMS-32 cells transfected with a FoxO-luc reporter construct after pretreatment for 1 hour with PBS (veh) or 50 nM PTH, followed by incubation with saline (−) or 100 μM H2O2 for 24 hours. (D–F) OB-6 cells or calvaria cells were preincubated for 1 hour with saline (−), 50 μg/ml PD98059 (PD), 50 μM H89, 100 ng/ml of BAPTA (bap), or 30 nM wortmannin (wo); then for an additional hour with 0.01% acetic acid (veh), 50 nM PTH, or 1 mM dibutyryl cAMP (DBA), as indicated. Intracellular ROS was determined 10 minutes after addition of 100 μM H2O2 (D). Phosphorylated p66Shc levels were quantified in lysates prepared 30 minutes after addition of 100 μM H2O2 (E,F), or at 1, 2 or 3 hours after addition of 40 μM 4-HNE (F). * p<0.05 vs. respective vehicle control by one-way ANOVA.
We next investigated the pathways involved in the antioxidant effect of PTH. Dibutyryl-cAMP (DBA) was as effective as PTH in preventing the H2O2-induced increase in ROS (Fig. 7D), indicating that the antioxidant actions of the hormone require activation of Gsα. Interestingly, addition of the mitogen-activated protein kinase kinase (MEK) inhibitor PD98059 also prevented the inhibitory effect of PTH or DBA on the H2O2-induced rise in intracellular ROS, implicating the mitogen-activated protein (MAP) kinase pathway as well.
Preincubation of OB-6 cells or calvaria cells with PTH also prevented the increase in phosphorylated p66Shc caused by H2O2 or 4-HNE (Fig. 7E–F). The 4-HNE-induced rise in phosphorylated p66Shc was prevented by PTH for as long as 3 hours in calvaria cells (Fig. 7F). As in the case of induction of ROS, the damping effect of PTH on ROS-induced p66Shc phosphorylation was reproduced by DBA and prevented by PD98059 in OB-6 cells and in calvaria cells (Fig. 7E,F). Similarly, the phosphoinositide 3-kinase (PI-3 kinase) inhibitor wortmannin, the protein kinase A (PKA) inhibitor H89, and the intracellular calcium chelator 1,2-bis(o-aminophenoxy)ethane-N,N,N',N'-tetraacetic acid (BAPTA), each attenuated the ability of PTH to prevent H2O-induced p66Shc phosphorylation. Thus, PTH interferes with p66Shc-mediated amplification of oxidative stress induced by H2O2- or 4-HNE via activation of PKA, MAP kinase, and PI-3 kinase signaling cascades.
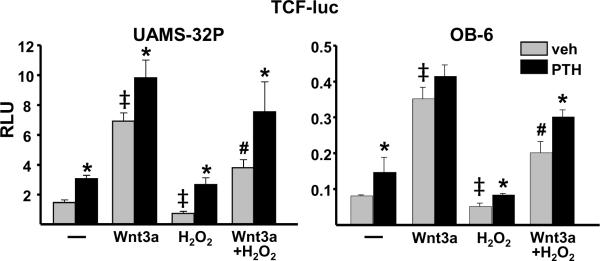
PTH attenuates the inhibitory effect of oxidative stress on β-catenin/Tcf-mediated transcription
We have previously shown that oxidative stress inhibits Wnt signaling by activating FoxOs and diverting β-catenin from Tcf- to FoxO-mediated transcription (Almeida et al, 2007a). Prompted by the evidence that PTH rapidly generates antioxidant signals that prevent FoxO activation, we investigated whether PTH could reverse the inhibitory effect of oxidative stress on β-catenin/Tcf-mediated transcription. PTH or Wnt3a alone stimulated Tcf-luc activity in UAMS-32P or OB-6 cells transfected with a β-catenin/Tcf-sensitive promoter (Fig. 8). The combination of both agents had an additive effect on Tcf-luc activity in UAMS-32P cells but not in OB-6 cells. More important, PTH attenuated the H2O2-induced suppression of basal as well as Wnt3a-stimulated Tcf-luc activity in both cell lines, albeit PTH did not completely reverse the negative effect of oxidative stress on Wnt signaling. Nevertheless, the magnitude of the PTH-stimulated increase in Tcf-luc activity was greater in the presence of H2O2 in Wnt3a-treated UAMS-32P cells (basal: 1.4-fold; H2O2: 2.0-fold). In Wnt3a-treated OB-6 cells, PTH caused only a nonsignificant 1.2-fold increase in Tcf-luc under basal conditions, but significantly raised Wnt signaling by 1.5-fold in the presence of H2O2.
Figure 8.
PTH attenuates the inhibitory effect of oxidative stress on β-catenin/Tcf-mediated transcription. Luciferase activity (relative light units, RLU) in UAMS-32P or OB-6 cells transfected with a Tcf-luc reporter construct after pretreatment for 1 hour with PBS (veh) or 50 nM PTH, then with saline (−), 50 ng/ml Wnt3a (50 ng/ml), 100 μM H2O2, or the combination of Wnt3a and H2O2 for 24 h. Bars represent the mean of triplicate determinations. * p<0.05 vs. respective vehicle control, ‡ <0.05 vs. vehicle alone, # p<0.05 vs. Wnt3a alone by one-way ANOVA.
Discussion
Intermittent PTH is an effective anabolic therapy in aged osteoporotic humans and animals (Marcus et al, 2003; Hodsman et al, 2005; Knopp et al, 2005). We have therefore hypothesized that PTH counteracts the pathophysiologic mechanisms that are responsible for the reduction in the number of osteoblasts and osteocytes - the seminal pathogenetic changes associated with involutional osteoporosis (Manolagas, 2010; Manolagas & Parfitt, 2010). The results presented in this manuscript demonstrate that in vertebral cancellous bone, PTH increased bone mass and bone formation rate to a greater extent in old than in young mice, confirming an earlier report (Knopp et al, 2005). In addition, our findings show that PTH fully reversed the age-related increase in osteoblast apoptosis. PTH also increased trabecular connectivity in vertebrae of aged mice, consistent with similar effects in postmenopausal women (Brennan et al, 2009; Recker et al, 2009). Strikingly, this latter effect was absent in young mice. An increase in trabecular connectivity may be responsible for the increase in vertebral toughness, which was also seen only in old mice. Consistent with this contention, trabecular connectivity is inversely related to vertebral fractures in post-menopausal women (Kleerekoper et al, 1985). Further, in the present work, PTH added a greater amount of bone to the endocortical surface, and increased the toughness, of femora of old mice. Resistance to bending of long bones increases in proportion to the fourth power of the radius. Hence, the ability of PTH to increase femoral toughness only in old mice is likely due to addition of new bone to the endocortical surface of femora with increased width.
We have also elucidated here a previously unrecognized antioxidant property of intermittent PTH that is associated with its increased anabolic efficacy in old mice. Indeed, intermittent PTH reduced ROS and increased glutathione, and reduced the level of phosphorylation of p66Shc, in old mice as effectively as classical antioxidants like NAC and catalase. Oxidative stress evidently plays an important role in the increased osteoblast apoptosis seen in aged mice, as evidenced by the finding of increased osteoblast survival following administration of NAC or catalase. Oxidative stress also compromises osteoblast differentiation, at least in part by inhibiting Wnt signaling (Almeida et al, 2007a; Almeida et al, 2009). Therefore, the increased anabolic efficacy of PTH in old mice may be due at least in part to attenuation of the oxidative stress that compromises osteoblast differentiation and survival in old age. Conversely, the antioxidant actions of PTH would have less of an impact on bone formation in young animals in which oxidative stress is low (Almeida et al, 2007b). Although we have not measured ROS in young mice treated with PTH, we found that NAC had no effect on the level of ROS in 5 mo old mice. Thus, it is unlikely that PTH would reduce ROS in young mice. Nevertheless, we cannot exclude the possibility that the anabolic effect of intermittent PTH in young mice depends in part on subtle changes in redox signaling that cannot be detected with the methodology used herein.
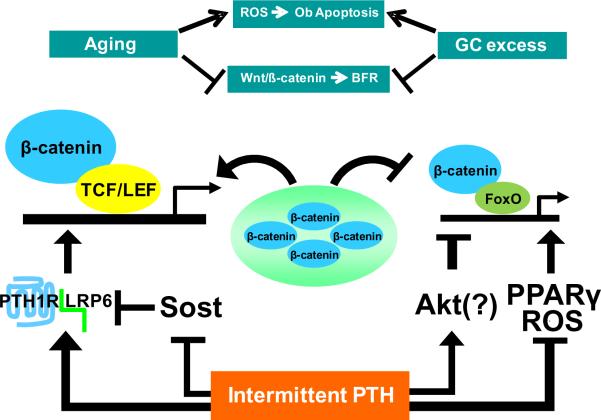
Mounting evidence implicates a critical role of PTH-induced Wnt signaling in the anabolic effect of the hormone (Kramer et al, 2009; Bodine et al, 2007; Kramer et al, 2010a). Specifically, PTH stimulates Wnt/β-catenin signaling by activating LRP6 (Wan et al, 2008) and by inhibiting the synthesis of sclerostin and other Wnt antagonists (Bellido et al, 2005; Keller & Kneissel, 2005; Bodine et al, 2007; Guo et al, 2010a) (Fig. 9). In the present paper, we found that the expression of a set of Wnt target genes was stimulated by PTH in old mice. Moreover, our in vitro experiments demonstrated that PTH attenuates the decrease in Wnt signaling caused by oxidative stress. Indeed, the magnitude of the PTH-stimulated increase in Wnt signaling was augmented in H2O2-treated cells. A similar response to PTH may occur in the skeleton of aged mice, perhaps because the antioxidant property of the hormone antagonizes pathways that would otherwise reduce β-catenin availability, such as ROS activation of FoxOs (Ambrogini et al, 2010; Almeida et al, 2007a) and activation of PPARγ by oxidized lipids (Almeida et al, 2009) (Fig. 9). The ability of PTH to activate Akt (Weinstein et al, 2010a), which phosphorylates FoxOs and thereby prevents their entry into the nucleus, may further suppress FoxO activation, but this has not yet been demonstrated. Further studies are required to establish whether the antioxidant property of PTH contributes to its increased efficacy in aged mice, and if so whether augmentation of hormone-induced Wnt signaling is involved.
Figure 9.
Potential mechanisms involved in the reversal of the age-related decline in Wnt signaling by intermittent PTH. The increased levels of ROS and endogenous glucocorticoids that occur with advancing aging stimulate osteoblast apoptosis and inhibit Wnt signaling leading to decreased bone formation. Daily injections of PTH reduce oxidative stress, and increase Wnt signaling by activating LRP6 and by suppressing expression of Wnt antagonists such as sclerostin. By reducing oxidative stress and lipid oxidation, PTH-stimulated Wnt signaling in aged mice is further enhanced by reducing diversion of β-catenin to FoxOs, and by preventing PPARγ-mediated degradation of β-catenin. Activation of Akt by PTH may further lower activated FoxO levels. Ob, osteoblast; BFR, bone formation rate; GC, glucocorticoids; TCF/LEF, T cell factor/lymphoid enhancer factor; PTH1R, parathyroid hormone 1 receptor; Sost, the gene encoding sclerostin.
In the work described herein, PTH prevented the H2O2- and 4-HNE-induced rise in intracellular ROS and p66Shc phosphorylation in osteoblastic cells in vitro; and in studies reported earlier by our group, we have shown that p66Shc is indispensible for H2O2-induced apoptosis in osteoblastic cells (Almeida et al, 2010). Therefore, attenuation of H2O2-induced phosphorylation of p66Shc must be a critical mechanism of the anti-apoptotic effect of the hormone. PKA, MAP kinase, and PI-3-kinase/intracellular Ca+2 signaling are stimulated by PTH (Abou-Samra et al, 1992; Chen et al, 2004; Guo et al, 2010b) and inhibition of each of these pathways with pharmacologic inhibitors in the present work prevented the ability of PTH to attenuate ROS-induced p66Shc phosphorylation, indicating that all these kinases are upstream of the effects of PTH on p66Shc. However, additional work will be required to establish whether these PTH-activated pathways lead to inhibition of PKCβ-mediated phosphorylation of p66Shc or to dephosphorylation of phospho-p66Shc by phosphatases. Alternatively, PTH might prevent the initial rise in ROS by stimulating the synthesis of one or more antioxidant enzymes. Any one of these mechanisms could also account for the ability of PTH to prevent H2O2-induced activation of FoxO-mediated transcription in osteoblastic cells. In the context of this discussion, it is worth noting that similar to PTH, estrogen prevents ROS-induced activation of p66Shc and apoptosis in osteoblastic cells via a mechanism involving MAP kinase and PKCβ (Almeida et al, 2007b; Almeida et al, 2010).
Intermittent administration of PTH to old mice also suppressed the expression of Alox15, another amplifier of oxidative stress. Alox15 indirectly increases ROS by acting on polyunsaturated fatty acids to form peroxidized fatty acid derivatives that subsequently give rise to pro-oxidants like 4-HNE (Schneider et al, 2008). Hence, attenuation of Alox15 expression by PTH may reduce this important source of ROS (Almeida et al, 2009). Oxidative stress itself stimulates Alox15 expression in cultured neuronal cells (Seiler et al, 2008); and other conditions leading to oxidative stress in the skeleton, like ovariectomy (Almeida et al, 2007b) and deletion of FoxOs 1, 3 and 4 (Ambrogini et al, 2010), increased Alox15 expression in the murine skeleton (unpublished observations). It is therefore likely that the inhibition of Alox15 expression by intermittent PTH observed in the present report is secondary to the antioxidant effect of the hormone.
Unlike PTH, administration of NAC and/or catalase did not increase vertebral bone mass or strength in aged mice, despite the ability of these agents to suppress ROS and osteoblast apoptosis. Similarly, these antioxidants could not recapitulate the dramatic increase in Wnt signaling seen with intermittent PTH. To the contrary, antioxidants exerted negative effects on osteoblastogenesis as evidenced by reduction of osteoblast progenitors in vivo, and inhibition of osteoblastic cell proliferation and Wnt signaling in vitro. Consistent with our findings, antioxidants promote the binding of the redox-sensitive protein nucleoredoxin to Dishelleved, thereby inhibiting the activity of this crucial mediator of β-catenin stabilization (Funato et al, 2006). This mechanism can at least theoretically override the positive effect of antioxidants on Wnt/β-catenin signaling resulting from abrogation of ROS-induced FoxO activation (Almeida et al, 2009; Almeida et al, 2007a). Perhaps more importantly, antioxidants inhibit the generation and activity of osteoclasts, leading to a reduction in bone remodeling (Lean et al, 2003; Almeida et al, 2007b). While effective in preserving bone mass in states of high remodeling such as estrogen deficiency, anti-remodeling agents reduce the anabolic efficacy of intermittent PTH (Samadfam et al, 2007; Hodsman et al, 2005). Moreover, we have recently shown that the generation and recruitment of sufficient osteoblasts to refill osteoclastic resorption cavities requires factors liberated by osteoclasts from the bone matrix and/or produced by osteoclasts themselves (Jilka et al, 2010). Thus, classical antioxidants are evidently purely anti-remodeling agents quite distinct from anabolic regimens such as intermittent PTH.
Intermittent PTH is an effective therapy for the treatment of glucocorticoid-induced osteoporosis (Saag et al, 2009). PTH anabolism in mice receiving excess glucocorticoids is closely associated with attenuation of the increase in osteoblast apoptosis (Weinstein et al, 2010a). Moreover, intermittent PTH prevents the negative effects of glucocorticoids on Tcf/β-catenin-mediated transcription. Glucocorticoid levels in mice increase with age, and the adverse effects of both glucocorticoid excess and aging on osteoblast apoptosis are reduced by overexpression of the glucocorticoid inactivating enzyme 11β-hydroxysteriod dehydrogenase 2 (11β-HSD2) in these cells (Jia et al, 2006; Weinstein et al, 2010b). Thus, besides oxidative stress, intermittent PTH may antagonize the negative effects of endogenous glucocorticoids, as it does with exogenous glucocorticoids (Fig. 9).
Old age or glucocorticoid excess leads to decreased vasculature and hydration of the skeleton, two evidently interdependent determinants of bone strength (Weinstein et al, 2010b). Moreover, the effects of aging or glucocorticoid excess on the vasculature and skeletal hydration are also attenuated by overexpression of 11β-HSD2 in osteoblasts and osteocytes, implicating these two cell types in the regulation of bone vascularization and hydration. Interestingly, both intermittent (Langer et al, 2009) and continuous (Jilka et al, 2010) elevation of PTH in mice increase the vasculature adjacent to the bone surface. Thus, the increase in osteoblast number, and the addition of new bone containing viable osteocytes, in aged mice receiving PTH may contribute to increased bone strength by promoting the development of new blood vessels.
In conclusion, the findings of the present report elucidate for the first time that old age and the associated increase in oxidative stress is a critical determinant of the responsiveness of the skeleton to an anti-osteoporotic therapy such as intermittent PTH. Specifically, our results suggest that PTH has a superior anabolic effect in old, as compared to young, age because in addition to its other positive actions on bone formation it antagonizes the age-associated increase in oxidative stress and its adverse effects on the birth and survival of osteoblasts. In contrast, ordinary antioxidants cannot restore bone mass in the setting of aging because they slow remodeling by suppressing both osteoclastogenesis and osteoblastogenesis.
Experimental Procedures
Animals
Female C57BL/6 mice, aged 6, 24 or 26 mo, were obtained from Harlan Inc. (Indianapolis, IN) maintained with support from the National Institute of Aging. Bone growth, as measured by femoral length and total body BMD, plateaus at approximately 6 months of age in C57BL/6 mice (Glatt et al, 2007). Therefore, we used 6 month old mice as the “young” controls to minimize confounding effects of increasing bone size and density during growth on differences in the efficacy of intermittent PTH in young vs. old mice. Animal use protocols were approved by the Institutional Animal Care and Use Committees of the University of Arkansas for Medical Sciences, and the Central Arkansas Veterans Healthcare System. Mice were electronically tagged (Biomedic Data System Inc., Maywood, NJ, USA) and maintained under standard laboratory conditions with a 12 hour dark, 12 hour light cycle and a constant temperature of 20 C and humidity of 48%. Mice were fed a standard rodent diet (Harlan Teklad 22/5) containing 22% protein, 1.13% calcium, and 0.94% phosphorus. Synthetic human PTH(1–34) (Bachem California, Inc., Torrance, CA) was injected daily, i.p., at 100 ng/g body weight for 28 days. Weight changes ranged from 2–12% during the course of the experiment. In a second experiment, 24 month old female were injected daily with PTH as above, 200μg/g pegylated catalase (Sigma-Alrich, St. Louis, MO), or vehicle (PBS), or fed a a diet containing 100 mg/kg of chow of N-acetyl cysteine (NAC) (Research Diets, New Brunswick, NJ), for 28 days. Based on an average food consumption of 4 g of chow per day, mice ingested approximately 13 μg NAC/g body weight per day. Vehicle-injected 6 month old mice served as controls. In a third experiment, 5 month old male C57BL/6 mice were injected twice daily with NAC (100 mg/kg body weight), or saline.
BMD, bone architecture and strength measurements
The BMD of lumbar vertebrae was determined before and after PTH administration with a PIXImus densitometer (GE-Lunar Corp., Madison, WI, USA), as previously described (Almeida et al, 2007b). Measurements of trabecular architecture of vertebral (L4), distal femoral metaphyseal bone, and the architecture of femoral bone at the mid-diaphysis, were done with a Scanco μCT40 instrument as previously described (Martin-Millan et al, 2010; Weinstein et al, 2010b). Endocortical and periosteal circumference at the femoral diaphysis were measured by tracing printed micro-CT images, using the OsteoMeasure Analysis System (Osteometrics Inc., Decatur, GA). Cortical bone area at the diaphysis was determined by subtraction of inner (non-bone) area calculated from the endocortical circumference from the total (bone + non-bone) area calculated from the periosteal circumference, assuming a circular shape. The load bearing properties of lumbar vertebra L5 were measured using a single column material testing machine and a calibrated tension/compression load cell (Model 5542; Instron Corp., Canton, MA) as previously described (Weinstein et al, 2010b). Femoral strength was measured by 3-point bending using a miniature bending apparatus as previously described (Weinstein et al, 2010b).
Histomorphometry
After fixation in Millonigs, lumbar vertebrae (L1–L3) were embedded nondecalcified in methylmethacrylate by an automated procedure using a Tissue-Tek-VIP machine as we have previously described (Almeida et al, 2007b). Histomorphometric examination of undecalcified longitudinal bone sections were done with the OsteoMeasure Analysis System (OsteoMetrics, Inc., Decatur, GA, USA) as previously described (Bellido et al, 2003). Cancellous measurements were made in the entire secondary spongiosa of 3 vertebrae. Variables were measured and reported using terminology recommended by the Histomorphometry Nomenclature Committee of the American Society for Bone and Mineral Research (Parfitt et al, 1987). Detection and quantification of apoptotic osteoblasts and osteoctyes by in situ nick-end labeling (ISEL) was performed as previously described (Bellido et al, 2003; Jilka et al, 2007; Weinstein et al, 2010b).
Other skeletal assays
Phosphorylated p66Shc was quantified in vertebral bone extracts by Western blotting using a mouse monoclonal antibody that recognizes Ser36 phosphorylated p66Shc (EMD Biosciences, Calbiochem, La Jolla, CA) as previously described (Almeida et al, 2007b). Phosphorylated p66Shc levels were normalized to β-actin using an antibody from Sigma, or to total p66Shc using an antibody from BD Biosciences (La Jolla, CA). ROS were quantified as previously described (Almeida et al, 2007b) using bone marrow cells flushed from femurs and washed with PBS. The marrow content of reduced glutathione was determined using a kit from Cayman Chemical (Ann Arbor, MI). Quantification of transcripts in vertebral bone was determined by TaqMan PCR of total RNA isolated as previously described (Almeida et al, 2009), using primer and probe pair sets from Applied Biosystems (Foster City, CA). Relative mRNA level was calculated by normalizing to the housekeeping gene ribosomal S2, or GAPDH, using the ΔCt method (Livak & Schmittgen, 2001).
Cell cultures and assays
Osteoblastic cells were obtained from neonatal (3–6 days old) murine calvaria by sequential collagenase digestion and cultured as previously described (Jilka, 1986; Horowitz et al, 1989). The number of colony-forming osteoblast (CFU-OB) progenitors in the femoral bone marrow was determined by culturing femoral marrow cell isolates in α-MEM supplemented with 15% FBS, 1% PGS and 1 mM ascorbate-2-phosphate for 20 days. The number of colonies containing osteoblastic cells capable of elaborating a mineralized matrix was determined after Von Kossa staining (Di Gregorio et al, 2001). Osteoblastic UAMS-32, UAMS-32P, and OB-6 cells were cultured as previously described (Galli et al, 2009; Lecka-Czernik et al, 1999). The level of phosphorylated p66Shc and ROS in osteoblastic cells were quantified as described above. Apoptosis of cultured osteoblastic cells was measured by caspase-3 activity using a fluorogenic substrate (Bellido et al, 2003). Assays described earlier (Almeida et al, 2009) were used to measure cell proliferation by BrdU incorporation. NAC, catalase, ebselen (2-phenyl-1, 2-benzisoselenazol-3(2H)-one), H89, BAPTA, BHA, and H2O2 were obtained from Sigma-Aldrich. PD98059 was from Cell Signaling Technology (Boston, MA). 4-HNE was obtained from Cayman Chemical.
Transient transfection and luciferase assay
pcDNA was purchased from Invitrogen (Carlsbad, CA). A reporter plasmid carrying 3 Tcf binding sites upstream of a minimal c-fos promoter driving the firefly Luciferase gene (TOPFLASH) (He et al, 1998) was provided by B. Vogelstein, Johns Hopkins University Medical Institutions, Baltimore, MD. A reporter plasmid containing 6 copies of daf-16 family protein binding element (FoxO-luc) was provided by B. Burgering, University Medical Center, Utrecht, Netherlands. To assay Tcf/β-catenin- or FoxO-mediated transcription, cells were transfected with the relevant reporter plasmid using Lipofectamine Plus (Invitrogen). Luciferase activity was determined 24 hours later using the Dual-Luciferase Reporter® assay system (Promega, Madison, WI), according to the manufacturer's instructions. Light intensity was measured with luminometer and the luciferase activity was divided by the Renilla activity (control reporter) to normalize for transfection efficiency.
Statistics
All values are reported as the mean ± s.d. The SigmaPlot (SPSS Science) software package was used for statistical analysis. Data were analyzed by 2-Way ANOVA to detect age-specific significant differences in the response to PTH. Some data were not normally distributed, and could not be normalized with commonly used transformations. In these cases, data were analyzed by Kruskal-Wallis ANOVA on Ranks. One-way ANOVA, or Student's 2-tailed t-test, were used for other analyses as indicated. The Holm-Sidak method was used to detect significant differences among treatment groups following ANOVA.
Acknowledgements
This work was supported by the Department of Veterans Affairs (Merit Reviews to R.L.J., R.S.W. and S.C.M.), the National Institutes of Health (P01 AG013918 to S.C.M.), and Tobacco Settlement Funds provided by the UAMS College of Medicine. E.A. is supported by a PhD fellowship from the University of Pisa, Italy. The authors thank S. Berryhill, L. Climer, T. Chambers, A. DeLoose, K. Vyas, A. Warren, W. Webb, C. Wiggins III, and R. Wynne, for their contributions to this work.
References
- Abou-Samra AB, Jüppner H, Force T, Freeman MW, Kong XF, Schipani E, Urena P, Richards J, Bonventre JV, Potts JT, Kronenberg HM, Segre GV. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc. Natl. Acad. Sci. USA. 1992;89:2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Ambrogini E, Martin-Millan M, Han L, Warren A, Shelton R, Plotkin L, Bellido T, O'Brien CA, Jilka RL, Weinstein RS, Manolagas SC. Induction of oxidative stress and diversion of β-catenin from TCF- to FOXO-mediated transcription by glucocorticoids or TNFα in osteoblastic cells. J. Bone Miner. Res. 2008;23:S170. [Google Scholar]
- Almeida M, Han L, Ambrogini E, Bartell SM, Manolagas SC. Oxidative stress stimulates apoptosis and activated NF-kB in osteoblastic cells via a PKCβ/p66shc siganaling cascade: counter regulation by estrogens or androgens. Mol Endocrinol. 2010 doi: 10.1210/me.2010-0189. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Bellido T, Manolagas SC, Kousteni S. Wnt proteins prevent apoptosis of both uncommitted osteoblast progenitors and differentiated osteoblasts by β-catenin-dependent and -independent signaling cascades involving Src/ERK and phosphatidylinositol 3-kinase/AKT. J Biol Chem. 2005;280:41342–41351. doi: 10.1074/jbc.M502168200. [DOI] [PubMed] [Google Scholar]
- Almeida M, Ambrogini E, Han L, Manolagas SC, Jilka RL. Increased lipid oxidation causes oxidative stress, increased PPARγ expression and diminished pro-osteogenic Wnt signaling in the skeleton. J. Biol. Chem. 2009;284:27438–27448. doi: 10.1074/jbc.M109.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, O'Brien CA, Manolagas SC. Oxidative stress antagonizes Wnt signaling in osteoblast precursors by diverting β-catenin from T cell factor- to Forkhead box O-mediated transcription. J. Biol. Chem. 2007a;282:27298–27305. doi: 10.1074/jbc.M702811200. [DOI] [PubMed] [Google Scholar]
- Almeida M, Han L, Martin-Millan M, Plotkin LI, Stewart SA, Roberson PK, Kousteni S, O'Brien CA, Bellido T, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Skeletal involution and oxidative stress: effects of aging accelerated by loss of sex steroids. J. Biol. Chem. 2007b;282:27285–27297. doi: 10.1074/jbc.M702810200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrogini E, Almeida M, Martin-Millan M, Paik JH, DePinho RA, Han L, Goellner J, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. FoxO-Mediated defense against oxidative stress in osteoblasts is indispensable for skeletal homeostasis in mice. Cell Metabolism. 2010;11:136–146. doi: 10.1016/j.cmet.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O'Brien CA, Manolagas SC, Jilka RL. Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology. 2005;146:4577–4583. doi: 10.1210/en.2005-0239. [DOI] [PubMed] [Google Scholar]
- Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts: a putative explanation for why intermittent administration is needed for bone anabolism. J. Biol. Chem. 2003;278:50259–50272. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- Bodine PV. Wnt signaling control of bone cell apoptosis. Cell Res. 2008;18:248–253. doi: 10.1038/cr.2008.13. [DOI] [PubMed] [Google Scholar]
- Bodine PV, Seestaller-Wehr L, Kharode YP, Bex FJ, Komm BS. Bone anabolic effects of parathyroid hormone are blunted by deletion of the Wnt antagonist secreted frizzled-related protein-1. J Cell Physiol. 2007;210:352–357. doi: 10.1002/jcp.20834. [DOI] [PubMed] [Google Scholar]
- Brennan TC, Rizzoli R, Ammann P. Selective modification of bone quality by PTH, pamidronate, or raloxifene. J Bone Miner. Res. 2009;24:800–808. doi: 10.1359/jbmr.081227. [DOI] [PubMed] [Google Scholar]
- Chamorro MN, Schwartz DR, Vonica A, Brivanlou AH, Cho KR, Varmus HE. FGF-20 and DKK1 are transcriptional targets of β-catenin and FGF-20 is implicated in cancer and development. EMBO J. 2005;24:73–84. doi: 10.1038/sj.emboj.7600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Koh AJ, Datta NS, Zhang J, Keller ET, Xiao G, Franceschi RT, D'Silva NJ, McCauley LK. Impact of the mitogen-activated protein kinase pathway on parathyroid hormone-related protein actions in osteoblasts. J. Biol. Chem. 2004;279:29121–29129. doi: 10.1074/jbc.M313000200. [DOI] [PubMed] [Google Scholar]
- Compston JE. Skeletal actions of intermittent parathyroid hormone: effects on bone remodelling and structure. Bone. 2007;40:1447–1452. doi: 10.1016/j.bone.2006.09.008. [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Yamamoto M, Ali AA, Abe E, Roberson P, Manolagas SC, Jilka RL. Attenuation of the self-renewal of transit-amplifying osteoblast progenitors in the murine bone marrow by 17β-estradiol. J. Clin. Invest. 2001;107:803–812. doi: 10.1172/JCI11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funato Y, Michiue T, Asashima M, Miki H. The thioredoxin-related redox-regulating protein nucleoredoxin inhibits Wnt-beta-catenin signalling through dishevelled. Nat Cell Biol. 2006;8:501–508. doi: 10.1038/ncb1405. [DOI] [PubMed] [Google Scholar]
- Galli C, Fu Q, Wang W, Olsen BR, Manolagas SC, Jilka RL, O'Brien CA. Commitment to the osteoblast lineage is not required for RANKL gene expression. J Biol Chem. 2009;284:12654–12662. doi: 10.1074/jbc.M806628200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio M, Migliaccio E, Orsini F, Paolucci D, Moroni M, Contursi C, Pelliccia G, Luzi L, Minucci S, Marcaccio M, Pinton P, Rizzuto R, Bernardi P, Paolucci F, Pelicci PG. Electron transfer between cytochrome c and p66Shc generates reactive oxygen species that trigger mitochondrial apoptosis. Cell. 2005;122:221–233. doi: 10.1016/j.cell.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Glass DA, II, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Developmental Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J. Bone Miner. Res. 2007;22:1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- Guo J, Liu M, Yang D, Bouxsein ML, Saito H, Galvin S, Kuhstoss SA, Thomas CC, Schipani E, Baron R, Bringhurst FR, Kronenberg HM. Suppression of Wnt signaling by Dkk1 attenuates PTH-mediated stromal cell response and new bone formation. Cell Metabolism. 2010a;11:161–171. doi: 10.1016/j.cmet.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Liu M, Yang D, Bouxsein ML, Thomas CC, Schipani E, Bringhurst FR, Kronenberg HM. Phospholipase C signaling via the parathyroid Hormone (PTH)/PTH-related peptide receptor is essential for normal bone responses to PTH. Endocrinologyen. 2010b doi: 10.1210/en.2009-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Bauer DC, Dempster D, Dian L, Hanley DA, Harris ST, Kendler D, McClung MR, Miller PD, Olszynski WP, Orwoll E, Yuen CK. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–703. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- Horowitz MC, Coleman DL, Flood PM, Kupper TS, Jilka RL. Parathyroid hormone and lipopolysaccharide induce murine osteoblast-like cells to secrete a cytokine indistinguishable from granulocyte-macrophage colony-stimulating factor. J. Clin. Invest. 1989;83:149–157. doi: 10.1172/JCI113852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Vayssiere B, Garcia T, Newell W, Baron R, Roman-Roman S, Rawadi G. Gene array analysis of Wnt-regulated genes in C3H10T1/2 cells. Bone. 2005;36:585–598. doi: 10.1016/j.bone.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, Van d, V Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic. Biol. Med. 2008;45:1–17. doi: 10.1016/j.freeradbiomed.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia D, O'Brien CA, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoclasts to increase their life span and reduce bone density. Endocrinology. 2006;147:5592–5599. doi: 10.1210/en.2006-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL. Parathyroid hormone-stimulated development of osteoclasts in cultures of cells from neonatal murine calvaria. Bone. 1986;7:29–40. doi: 10.1016/8756-3282(86)90149-3. [DOI] [PubMed] [Google Scholar]
- Jilka RL, O'Brien CA, Ali AA, Roberson PK, Weinstein RS, Manolagas SC. Intermittent PTH stimulates periosteal bone formation by actions on post-mitotic preosteoblasts. Bone. 2009;44:275–286. doi: 10.1016/j.bone.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, O'Brien CA, Weinstein RS, Manolagas SC. Continuous elevation of PTH increases the number of osteoblasts via both osteoclast-dependent and - independent mechanisms. J. Bone Miner. Res. 2010 doi: 10.1002/jbmr.145. In Press. doi: 10.1002/jbmr.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Bellido T, Roberson P, Parfitt AM, Manolagas SC. Increased bone formation by prevention of osteoblast apoptosis with parathyroid hormone. J. Clin. Invest. 1999;104:439–446. doi: 10.1172/JCI6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL. Molecular and cellular mechanisms of the anabolic effect of intermittent PTH. Bone. 2007;40:1434–1446. doi: 10.1016/j.bone.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilka RL, Weinstein RS, Parfitt AM, Manolagas SC. Quantifying osteoblast and osteocyte apoptosis: challenges and rewards. J. Bone Miner. Res. 2007;22:1492–1501. doi: 10.1359/jbmr.070518. [DOI] [PubMed] [Google Scholar]
- Keller H, Kneissel M. SOST is a target gene for PTH in bone. Bone. 2005;37:148–158. doi: 10.1016/j.bone.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Kleerekoper M, Villanueva AR, Stanciu J, Rao DS, Parfitt AM. The role of three-dimensional trabecular microstructure in the pathogenesis of vertebral compression fractures. Calcif. Tissue Int. 1985;37:594–597. doi: 10.1007/BF02554913. [DOI] [PubMed] [Google Scholar]
- Knopp E, Troiano N, Bouxsein M, Sun Bh, Lostritto K, Gundberg C, Dziura J, Insogna K. The effect of aging on the skeletal response to intermittent treatment with parathyroid hormone. Endocrinology. 2005;146:1983–1990. doi: 10.1210/en.2004-0770. [DOI] [PubMed] [Google Scholar]
- Kramer I, Keller H, Leupin O, Kneissel M. Does osteocytic SOST suppression mediate PTH bone anabolism? Trends Endocrinol. Metab. 2010a;21:237–244. doi: 10.1016/j.tem.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid hormone (PTH) induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 2009 doi: 10.1359/jbmr.090730. 10.1359/JBMR.090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer I, Loots GG, Studer A, Keller H, Kneissel M. Parathyroid hormone (PTH) induced bone gain is blunted in SOST overexpressing and deficient mice. J. Bone Miner. Res. 2010b;25:178–189. doi: 10.1359/jbmr.090730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer M, Prisby R, Peter Z, Boistel R, Lafage-Proust MH, Peyrin F. Quantitative investigation of bone microvascularization from 3D synchrotron micro-computed tomography in a rat model. Conf. Proc. IEEE Eng Med Biol Soc.; 2009. pp. 1004–1007. [DOI] [PubMed] [Google Scholar]
- Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ. A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest. 2003;112:915–923. doi: 10.1172/JCI18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B, Gubrij I, Moerman EJ, Kajkenova O, Lipschitz DA, Manolagas SC, Jilka RL. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARγ2. J. Cell Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, Nieves JW, Dempster DW. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: early actions of teriparatide. J Bone Miner Res. 2006;21:366–373. doi: 10.1359/JBMR.051109. [DOI] [PubMed] [Google Scholar]
- Lips P, Courpron P, Meunier PJ. Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif. Tissue Res. 1978;26:13–17. doi: 10.1007/BF02013227. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ma YL, Zeng Q, Donley DW, Ste-Marie LG, Gallagher JC, Dalsky GP, Marcus R, Eriksen EF. Teriparatide increases bone formation in modeling and remodeling osteons and enhances IGF-II immunoreactivity in postmenopausal women with osteoporosis. J Bone Miner. Res. 2006;21:855–864. doi: 10.1359/jbmr.060314. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 2000;21:115–137. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- Manolagas SC. From estrogen-centric to aging and oxidative stress: A revised perspective of the pathogenesis of osteoporosis. Endocr Rev. 2010;31:266–300. doi: 10.1210/er.2009-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolagas SC, Parfitt AM. What old means to bone. Trends in Endocrinology & Metabolism. 2010;21:369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus R, Wang O, Satterwhite J, Mitlak B. The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner. Res. 2003;18:18–23. doi: 10.1359/jbmr.2003.18.1.18. [DOI] [PubMed] [Google Scholar]
- Martin-Millan M, Almeida M, Ambrogini E, Han L, Zhao H, Weinstein RS, Jilka RL, O'Brien CA, Manolagas SC. The estrogen receptor-α in osteoclasts mediates the protective effects of estrogens on cancellous but not cortical bone. Mol. Endocrinol. 2010;24:323–334. doi: 10.1210/me.2009-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien CA, Plotkin LI, Galli C, Goellner JJ, Gortazar AR, Allen MR, Robling A, Bouxsein M, Schipani E, Turner CH, Jilka RL, Weinstein RS, Manolagas SC, Bellido T. Control of bone mass and remodeling by PTH receptor signaling in osteocytes. PLoS ONE. 2008;3:e2942. doi: 10.1371/journal.pone.0002942. doi:10.1371/journal.pone.0002942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Marchi S, Orsini F, Migliaccio E, Giorgio M, Contursi C, Minucci S, Mantovani F, Wieckowski MR, Del Sal G, Pelicci PG, Rizzuto R. Protein kinase C β and prolyl isomerase 1 regulate mitochondrial effects of the life-span determinant p66Shc. Science. 2007;315:659–663. doi: 10.1126/science.1135380. [DOI] [PubMed] [Google Scholar]
- Potts JT. Parathyroid hormone: past and present. J. Endocrinol. 2005;187:311–325. doi: 10.1677/joe.1.06057. [DOI] [PubMed] [Google Scholar]
- Recker RR, Bare SP, Smith SY, Varela A, Miller MA, Morris SA, Fox J. Cancellous and cortical bone architecture and turnover at the iliac crest of postmenopausal osteoporotic women treated with parathyroid hormone 1–84. Bone. 2009;44:113–119. doi: 10.1016/j.bone.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ, Robb RA, Camp JJ, Atkinson EJ, McDaniel L, Amin S, Rouleau PA, Khosla S. A population-based assessment of rates of bone loss at multiple skeletal sites: evidence for substantial trabecular bone loss in young adult women and men. J. Bone Miner. Res. 2008;23:205–214. doi: 10.1359/JBMR.071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs BL, Parfitt AM. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J. Bone Miner. Res. 2005;20:177–184. doi: 10.1359/JBMR.041114. [DOI] [PubMed] [Google Scholar]
- Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, Krege JH, Krohn K, Warner MR. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60:3346–3355. doi: 10.1002/art.24879. [DOI] [PubMed] [Google Scholar]
- Samadfam R, Xia Q, Goltzman D. Pretreatment with anticatabolic agents blunts but does not eliminate the skeletal anabolic response to parathyroid hormone in oophorectomized mice. Endocrinology. 2007;148:2778–2787. doi: 10.1210/en.2006-1475. [DOI] [PubMed] [Google Scholar]
- Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: fundamental issues in the mechanisms of lipid peroxidation. J. Biol. Chem. 2008;283:15539–15543. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Bornkamm GW, Schweizer U, Conrad M. Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell Metab. 2008;8:237–248. doi: 10.1016/j.cmet.2008.07.005. [DOI] [PubMed] [Google Scholar]
- van der Heyden MA, Rook MB, Hermans MM, Rijksen G, Boonstra J, Defize LH, Destree OH. Identification of connexin43 as a functional target for Wnt signalling. J. Cell Sci. 1998;111:1741–1749. doi: 10.1242/jcs.111.12.1741. [DOI] [PubMed] [Google Scholar]
- Wan M, Yang C, Li J, Wu X, Yuan H, Ma H, He X, Nie S, Chang C, Cao X. Parathyroid hormone signaling through low-density lipoprotein-related protein 6. Genes Dev. 2008;22:2968–2979. doi: 10.1101/gad.1702708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010a;151:2641–2649. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein RS, Wan C, Liu Q, Wang Y, Almeida M, O'Brien CA, Thostenson J, Roberson PK, Boskey AL, Clemens TL, Manolagas SC. Endogenous glucocorticoids decrease skeletal angiogenesis, vascularity, hydration, and strength in 21-month-old mice. Aging Cell. 2010b;9:147–161. doi: 10.1111/j.1474-9726.2009.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]