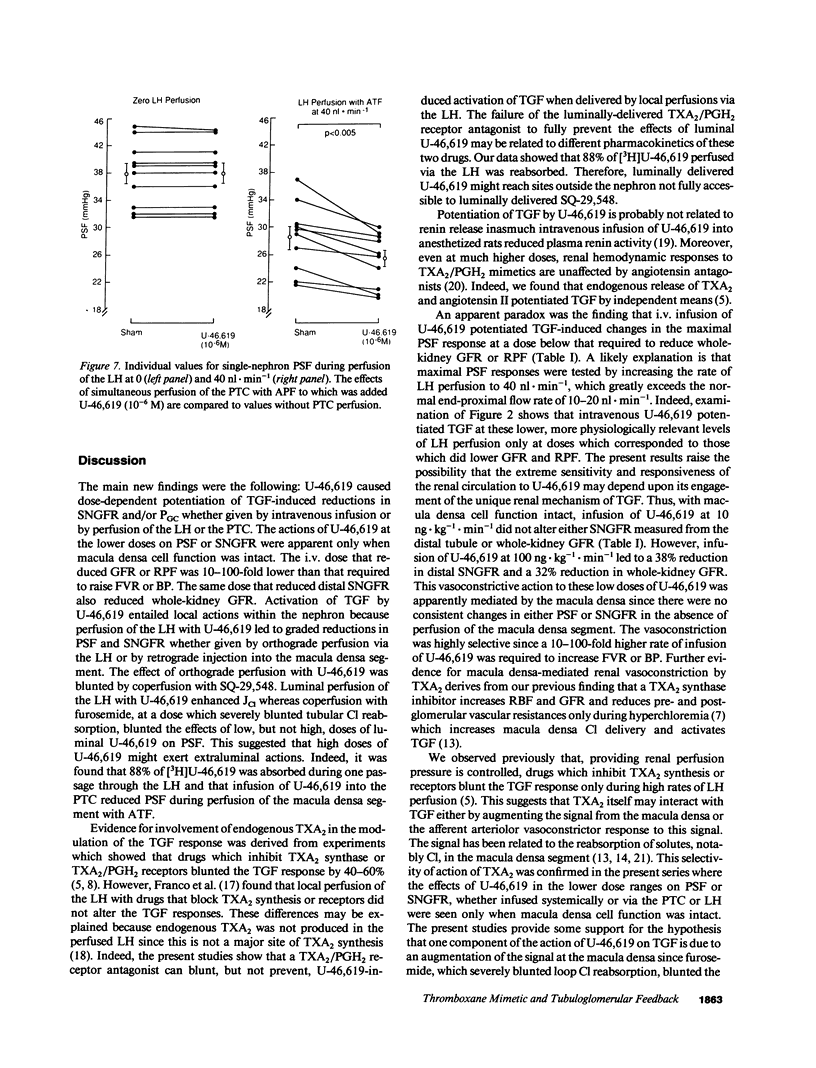
Abstract
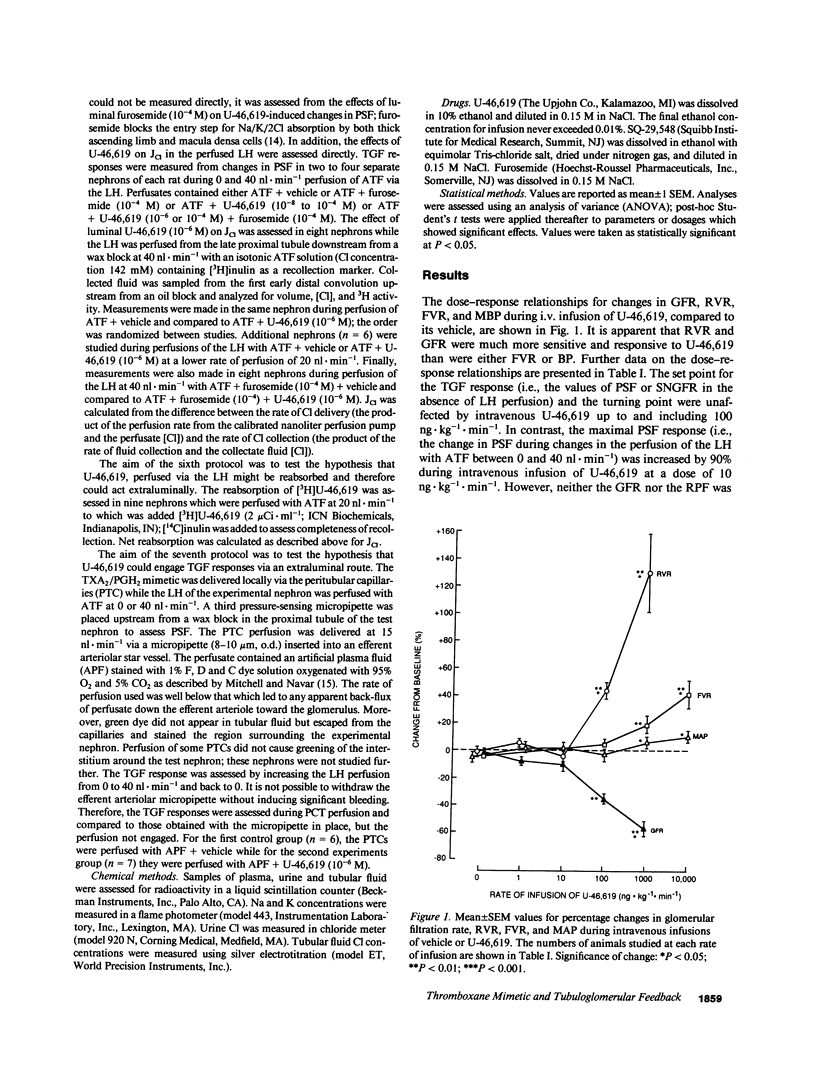
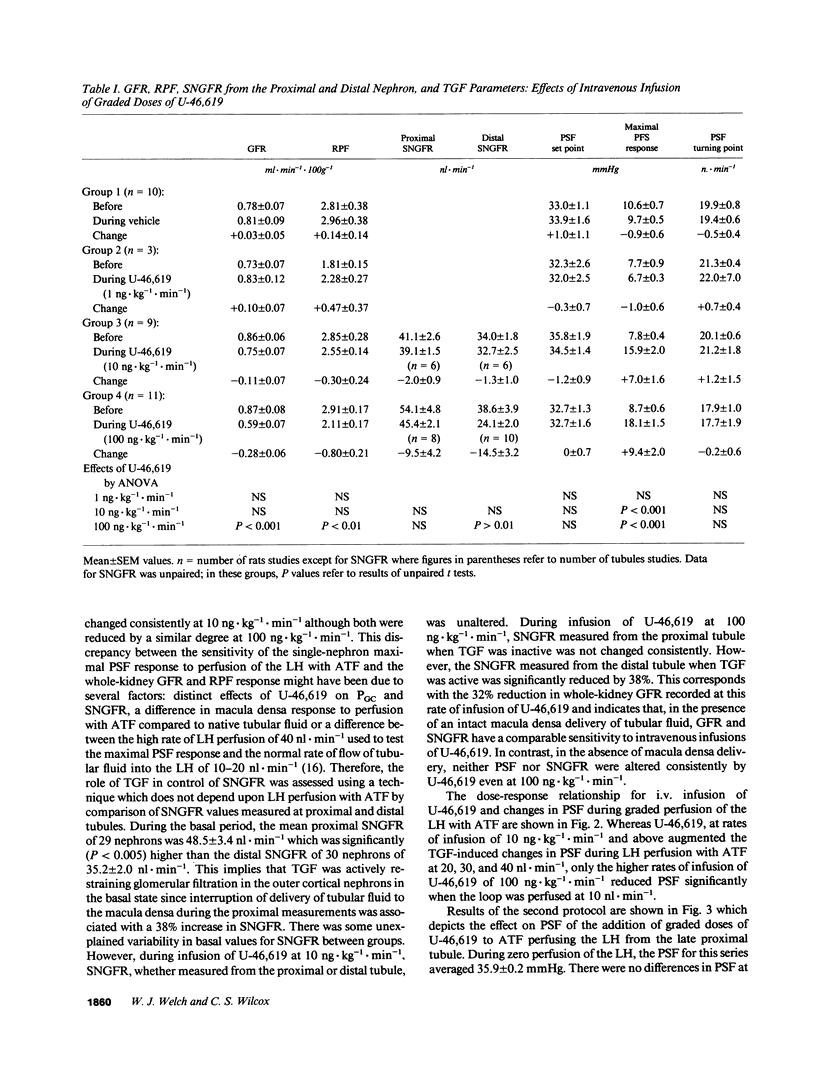
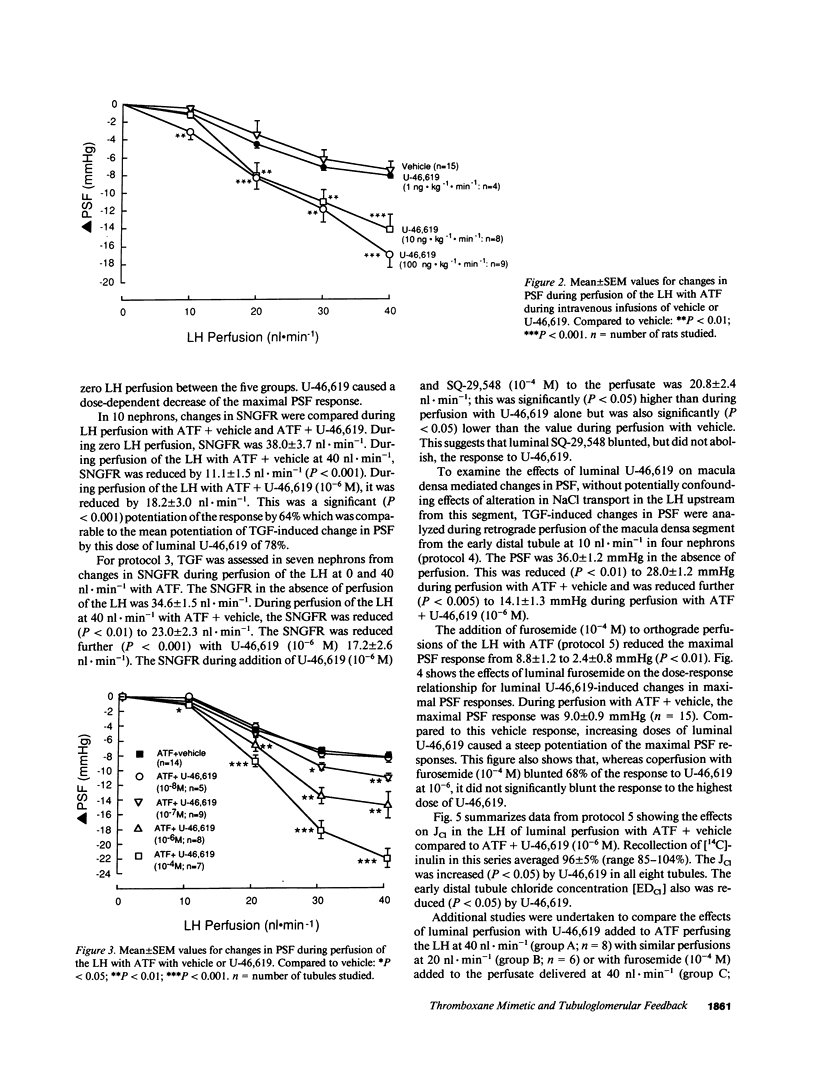
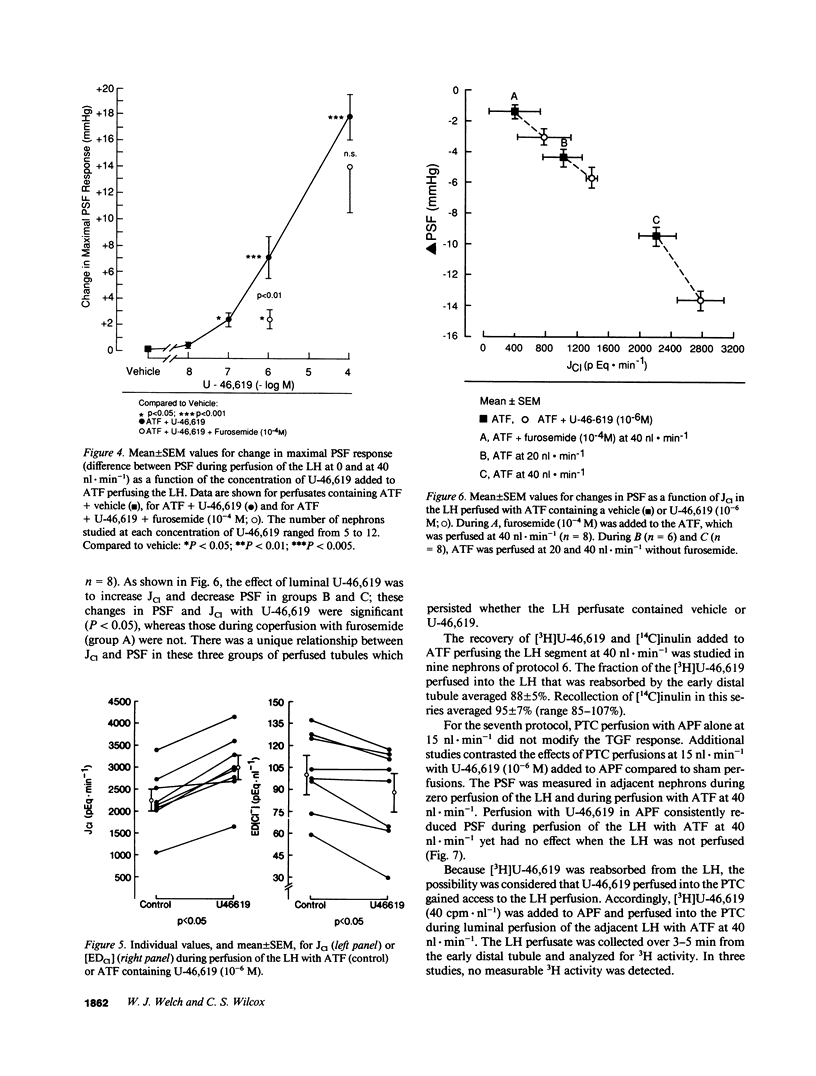
Because endogenous thromboxane A2 (TXA2) potentiates the tubuloglomerular feedback response (TGF), we studied the mechanism of action of TXA2 by using a stable TXA2/prostaglandin (PG) H2 mimetic, U-46,619. Intravenous infusion of U-46,619 at 100 ng.kg-1.min-1 reduced the GFR and the single-nephron (SN)GFR measured from the distal tubule (macula densa function intact), whereas the SNGFR measured from the proximal tubule (macula densa function interrupted) was not changed consistently. 10-100-fold higher rates of infusion of U-46,619 were required to raise blood pressure or femoral vascular resistance. The regulation of glomerular capillary pressure (PGC) by TGF was assessed in anesthetized rats from changes in proximal stop flow pressure (PSF) and/or SNGFR during perfusion of the loop of Henle (LH) with artificial tubular fluid (ATF). Orthograde loop perfusion and retrograde perfusion of U-46,619 into the macula densa segment reduced PSF. Responses to luminal U-46,619 were blunted by a TXA2-PGH2 receptor antagonist. Orthograde loop perfusions with luminal U-46,619 increased net Cl absorption, whereas coperfusion with furosemide (10(-4) M) blunted the response to U-46,619 by 68%. These data indicated that the luminal U-46,619 might increase the signal for TGF activation by increasing Cl reabsorption in macula densa cells. However, since 80 +/- 4% of [3H]U-46,619 perfused via the LH was reabsorbed peritubular capillaries (PTC) were perfused with U-46,619 to test additional extra-luminal actions. PTC perfusion with U-46,619 again increased TGF by reducing PSF selectively only while macula densa function was intact during perfusion of the LH with ATF. Conclusions: (a) TGF is potentiated by U-46,619 given systematically, via the lumen of the LH by orthograde or retrograde perfusions or via the PTC; (b) at the lower doses tested, reduction of PGC and SNGFR by U-46,619 depends on tubular fluid delivery and reabsorption by the macula densa; (c) potentiation of TGF by U-46,619 entails preglomerular vasoconstriction which may be elicited in part by an increased signal due to increased net chloride reabsorption in the LH and presumably macula densa cells and by an increased sensitivity of the arteriole to macula densa-derived signals; (d) activation of TGF may contribute to the selective vasoconstriction of the renal vascular bed by low doses of U-46,619.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blantz R. C., Konnen K. S. Relation of distal tubular delivery and reabsorptive rate to nephron filtration. Am J Physiol. 1977 Oct;233(4):F315–F324. doi: 10.1152/ajprenal.1977.233.4.F315. [DOI] [PubMed] [Google Scholar]
- Briggs J. P., Wright F. S. Feedback control of glomerular filtration rate: site of the effector mechanism. Am J Physiol. 1979 Jan;236(1):F40–F47. doi: 10.1152/ajprenal.1979.236.1.F40. [DOI] [PubMed] [Google Scholar]
- Bullivant E. M., Wilcox C. S., Welch W. J. Intrarenal vasoconstriction during hyperchloremia: role of thromboxane. Am J Physiol. 1989 Jan;256(1 Pt 2):F152–F157. doi: 10.1152/ajprenal.1989.256.1.F152. [DOI] [PubMed] [Google Scholar]
- Bunce K. T., Spraggs C. F. Prostanoid stimulation of anion secretion in guinea-pig gastric and ileal mucosa is mediated by different receptors. Br J Pharmacol. 1990 Dec;101(4):889–895. doi: 10.1111/j.1476-5381.1990.tb14176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting S., Buchanan L. V., Holzgrefe H. H., Fitzpatrick F. A. Pharmacology of synthetic thromboxane A2. Adv Prostaglandin Thromboxane Leukot Res. 1987;17A:192–198. [PubMed] [Google Scholar]
- Farman N., Pradelles P., Bonvalet J. P. PGE2, PGF2 alpha, 6-keto-PGF1 alpha, and TxB2 synthesis along the rabbit nephron. Am J Physiol. 1987 Jan;252(1 Pt 2):F53–F59. doi: 10.1152/ajprenal.1987.252.1.F53. [DOI] [PubMed] [Google Scholar]
- Franco M., Bell P. D., Navar L. G. Evaluation of prostaglandins as mediators of tubuloglomerular feedback. Am J Physiol. 1988 May;254(5 Pt 2):F642–F649. doi: 10.1152/ajprenal.1988.254.5.F642. [DOI] [PubMed] [Google Scholar]
- Friedman P. A., Andreoli T. E. CO2-stimulated NaCl absorption in the mouse renal cortical thick ascending limb of Henle. Evidence for synchronous Na +/H+ and Cl-/HCO3- exchange in apical plasma membranes. J Gen Physiol. 1982 Nov;80(5):683–711. doi: 10.1085/jgp.80.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber J. G., Ellis E., Hollified J., Nies A. S. Effect of prostaglandin endoperoxide analogue on canine renal function, hemodynamics and renin release. Eur J Pharmacol. 1979 Jan 15;53(3):239–246. doi: 10.1016/0014-2999(79)90129-8. [DOI] [PubMed] [Google Scholar]
- Hirata M., Hayashi Y., Ushikubi F., Yokota Y., Kageyama R., Nakanishi S., Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature. 1991 Feb 14;349(6310):617–620. doi: 10.1038/349617a0. [DOI] [PubMed] [Google Scholar]
- Lapointe J. Y., Bell P. D., Hurst A. M., Cardinal J. Basolateral ionic permeabilities of macula densa cells. Am J Physiol. 1991 Jun;260(6 Pt 2):F856–F860. doi: 10.1152/ajprenal.1991.260.6.F856. [DOI] [PubMed] [Google Scholar]
- Mitchell K. D., Navar L. G. Superficial nephron responses to peritubular capillary infusions of angiotensins I and II. Am J Physiol. 1987 May;252(5 Pt 2):F818–F824. doi: 10.1152/ajprenal.1987.252.5.F818. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Briggs J. P., Weber P. C. Tubuloglomerular feedback, prostaglandins, and angiotensin in the autoregulation of glomerular filtration rate. Kidney Int. 1984 Jan;25(1):53–64. doi: 10.1038/ki.1984.8. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Briggs J., Wright F. S. Feedback-mediated reduction of glomerular filtration rate during infusion of hypertonic saline. Kidney Int. 1981 Oct;20(4):462–468. doi: 10.1038/ki.1981.162. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Weihprecht H., Briggs J. P. Inhibition of tubuloglomerular feedback during adenosine1 receptor blockade. Am J Physiol. 1990 Mar;258(3 Pt 2):F553–F561. doi: 10.1152/ajprenal.1990.258.3.F553. [DOI] [PubMed] [Google Scholar]
- Schnermann J., Weihprecht H., Lorenz J. N., Briggs J. P. The afferent arteriole--the target for macula densa-generated signals. Kidney Int Suppl. 1991 Jun;32:S74–S77. [PubMed] [Google Scholar]
- Welch W. J., Wilcox C. S., Dunbar K. R. Modulation of renin by thromboxane: studies with thromboxane synthase inhibitor, receptor antagonists, and mimetic. Am J Physiol. 1989 Oct;257(4 Pt 2):F554–F560. doi: 10.1152/ajprenal.1989.257.4.F554. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Wilcox C. S. Feedback responses during sequential inhibition of angiotensin and thromboxane. Am J Physiol. 1990 Mar;258(3 Pt 2):F457–F466. doi: 10.1152/ajprenal.1990.258.3.F457. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Wilcox C. S. Modulating role for thromboxane in the tubuloglomerular feedback response in the rat. J Clin Invest. 1988 Jun;81(6):1843–1849. doi: 10.1172/JCI113529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. S., Granges F., Kirk G., Gordon D., Giebisch G. Effects of saline infusion on titratable acid generation and ammonia secretion. Am J Physiol. 1984 Sep;247(3 Pt 2):F506–F519. doi: 10.1152/ajprenal.1984.247.3.F506. [DOI] [PubMed] [Google Scholar]
- Wilcox C. S., Roddis S., Peart W. S., Gordon D., Lewis G. P. Intrarenal prostaglandin release: effects of arachidonic acid and hyperchloremia. Kidney Int. 1985 Jul;28(1):43–50. doi: 10.1038/ki.1985.116. [DOI] [PubMed] [Google Scholar]
- Wright F. S., Schnermann J. Interference with feedback control of glomerular filtration rate by furosemide, triflocin, and cyanide. J Clin Invest. 1974 Jun;53(6):1695–1708. doi: 10.1172/JCI107721. [DOI] [PMC free article] [PubMed] [Google Scholar]



