Abstract
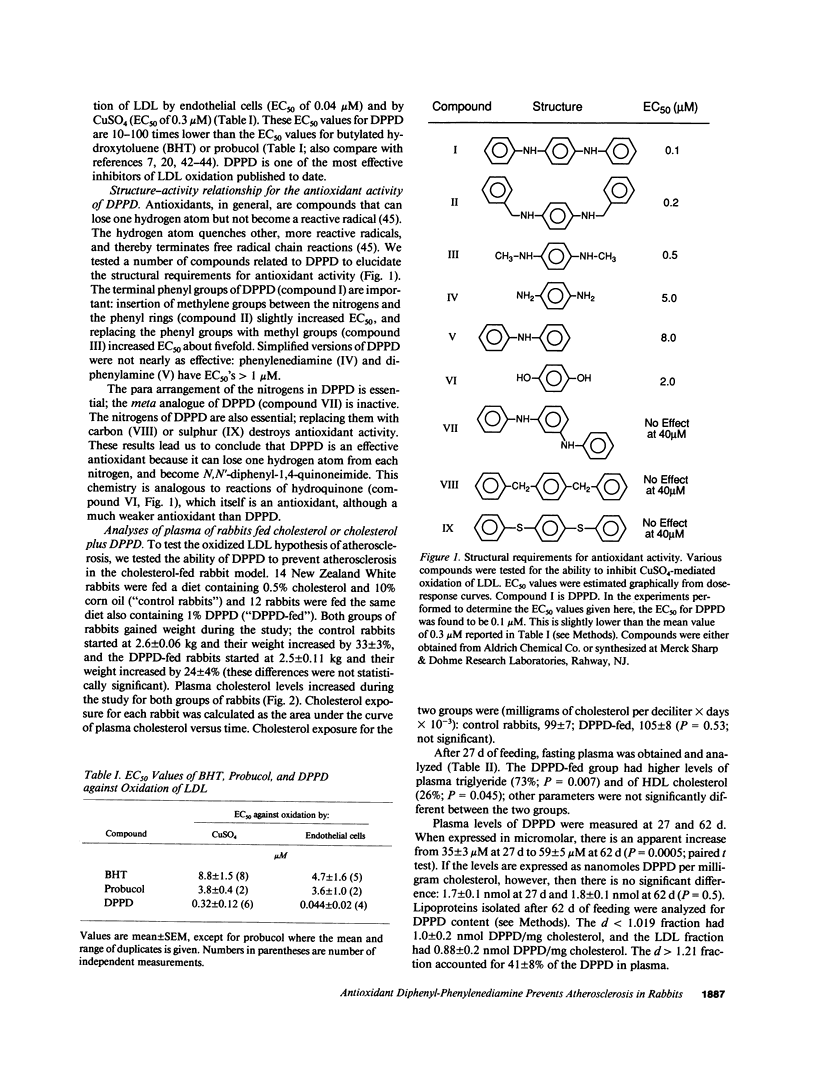
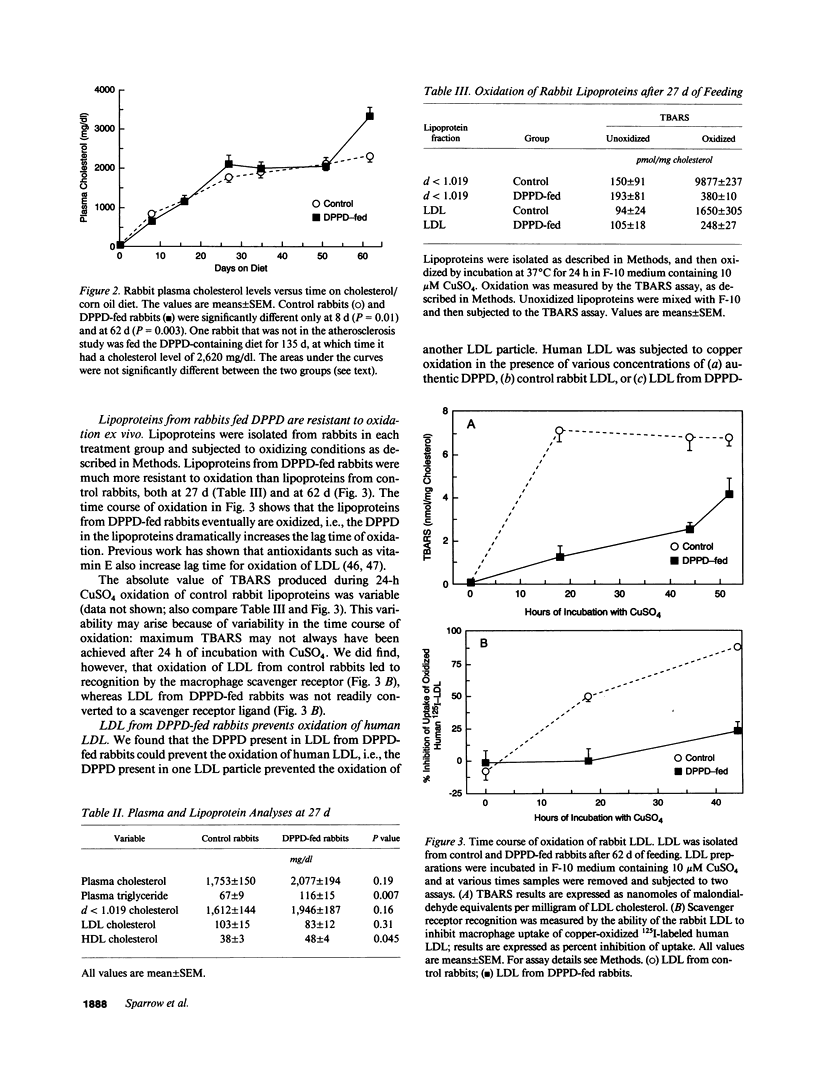
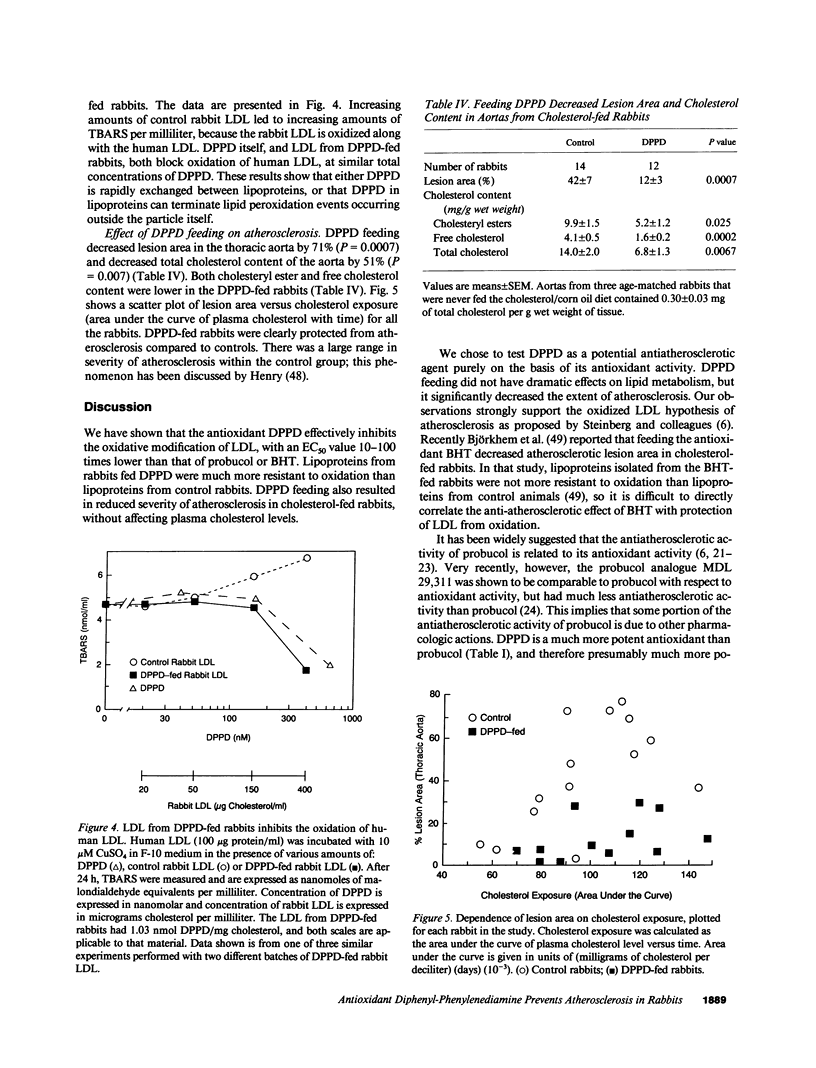
The oxidative modification of low density lipoprotein (LDL) may play an important role in atherosclerosis. We found that the antioxidant N,N'-diphenyl-1,4-phenylenediamine (DPPD) inhibits in vitro LDL oxidation at concentrations much lower than other reported antioxidants. To test whether DPPD could prevent atherosclerosis, New Zealand White rabbits were fed either a diet containing 0.5% cholesterol and 10% corn oil (control group) or the same diet also containing 1% DPPD (DPPD-fed group) for 10 wk. Plasma total cholesterol levels were not different between the two groups, but DPPD feeding increased the levels of triglyceride (73%, P = 0.007) and HDL cholesterol (26%, P = 0.045). Lipoproteins from DPPD-fed rabbits contained DPPD and were much more resistant to oxidation than control lipoproteins. After 10 wk, the DPPD-fed animals had less severe atherosclerosis than did the control animals: thoracic aorta lesion area was decreased by 71% (P = 0.0007), and aortic cholesterol content was decreased by 51% (P = 0.007). Although DPPD cannot be given to humans because it is a mutagen, our results indicate that orally active antioxidants can have antiatherosclerotic activity. This strongly supports the theory that oxidized LDL plays an important role in the pathogenesis of atherosclerosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akeson A. L., Woods C. W., Mosher L. B., Thomas C. E., Jackson R. L. Inhibition of IL-1 beta expression in THP-1 cells by probucol and tocopherol. Atherosclerosis. 1991 Feb;86(2-3):261–270. doi: 10.1016/0021-9150(91)90222-o. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barinaga M. Vitamin C gets a little respect. Science. 1991 Oct 18;254(5030):374–376. doi: 10.1126/science.1925592. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Henriksson-Freyschuss A., Breuer O., Diczfalusy U., Berglund L., Henriksson P. The antioxidant butylated hydroxytoluene protects against atherosclerosis. Arterioscler Thromb. 1991 Jan-Feb;11(1):15–22. doi: 10.1161/01.atv.11.1.15. [DOI] [PubMed] [Google Scholar]
- Breugnot C., Mazière C., Salmon S., Auclair M., Santus R., Morlière P., Lenaers A., Mazière J. C. Phenothiazines inhibit copper and endothelial cell-induced peroxidation of low density lipoprotein. A comparative study with probucol, butylated hydroxytoluene and vitamin E. Biochem Pharmacol. 1990 Nov 1;40(9):1975–1980. doi: 10.1016/0006-2952(90)90226-b. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- Buckley M. M., Goa K. L., Price A. H., Brogden R. N. Probucol. A reappraisal of its pharmacological properties and therapeutic use in hypercholesterolaemia. Drugs. 1989 Jun;37(6):761–800. doi: 10.2165/00003495-198937060-00002. [DOI] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart M. K., Morel D. W., Chisolm G. M., 3rd Monocytes and neutrophils oxidize low density lipoprotein making it cytotoxic. J Leukoc Biol. 1985 Aug;38(2):341–350. doi: 10.1002/jlb.38.2.341. [DOI] [PubMed] [Google Scholar]
- Chan A. C., Pritchard E. T., Choy P. C. Differential effects of dietary vitamin E and antioxidants on eicosanoid synthesis in young rabbits. J Nutr. 1983 Apr;113(4):813–819. doi: 10.1093/jn/113.4.813. [DOI] [PubMed] [Google Scholar]
- Cushing S. D., Berliner J. A., Valente A. J., Territo M. C., Navab M., Parhami F., Gerrity R., Schwartz C. J., Fogelman A. M. Minimally modified low density lipoprotein induces monocyte chemotactic protein 1 in human endothelial cells and smooth muscle cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5134–5138. doi: 10.1073/pnas.87.13.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRAPER H. H., BERGAN J. G., CHIU M., CSALLANY A. S., BOARO A. V. A FURTHER STUDY OF THE SPECIFICITY OF THE VITAMIN E REQUIREMENT FOR REPRODUCTION. J Nutr. 1964 Dec;84:395–400. doi: 10.1093/jn/84.4.395. [DOI] [PubMed] [Google Scholar]
- DRAPER H. H., GOODYEAR S., BARBEE K. D., JOHNSON B. C. A study of the nutritional role of anti-oxidants in the diet of the rat. Br J Nutr. 1958;12(1):89–97. doi: 10.1079/bjn19580012. [DOI] [PubMed] [Google Scholar]
- Ek B., Humble L. Correlation between oxidation of low density lipoproteins and prostacyclin synthesis in cultured smooth muscle cells. Biochem Pharmacol. 1991 Mar 1;41(5):695–699. doi: 10.1016/0006-2952(91)90068-g. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Franceschini G., Sirtori M., Vaccarino V., Gianfranceschi G., Rezzonico L., Chiesa G., Sirtori C. R. Mechanisms of HDL reduction after probucol. Changes in HDL subfractions and increased reverse cholesteryl ester transfer. Arteriosclerosis. 1989 Jul-Aug;9(4):462–469. doi: 10.1161/01.atv.9.4.462. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G. The role of the monocyte in atherogenesis: I. Transition of blood-borne monocytes into foam cells in fatty lesions. Am J Pathol. 1981 May;103(2):181–190. [PMC free article] [PubMed] [Google Scholar]
- Glomset J. A. The plasma lecithins:cholesterol acyltransferase reaction. J Lipid Res. 1968 Mar;9(2):155–167. [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Henry P. D. Calcium antagonists as anti-atherosclerotic agents. Arteriosclerosis. 1990 Nov-Dec;10(6):963–965. doi: 10.1161/01.atv.10.6.963. [DOI] [PubMed] [Google Scholar]
- Jessup W., Rankin S. M., De Whalley C. V., Hoult J. R., Scott J., Leake D. S. Alpha-tocopherol consumption during low-density-lipoprotein oxidation. Biochem J. 1990 Jan 15;265(2):399–405. doi: 10.1042/bj2650399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jialal I., Grundy S. M. Preservation of the endogenous antioxidants in low density lipoprotein by ascorbate but not probucol during oxidative modification. J Clin Invest. 1991 Feb;87(2):597–601. doi: 10.1172/JCI115035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kling L. J., Soares J. H., Jr, Haltman W. A. Effect of vitamin E and synthetic antioxidants on the survival rate of mercury-poisoned Japanese quail. Poult Sci. 1987 Feb;66(2):325–331. doi: 10.3382/ps.0660325. [DOI] [PubMed] [Google Scholar]
- Ku G., Doherty N. S., Schmidt L. F., Jackson R. L., Dinerstein R. J. Ex vivo lipopolysaccharide-induced interleukin-1 secretion from murine peritoneal macrophages inhibited by probucol, a hypocholesterolemic agent with antioxidant properties. FASEB J. 1990 Apr 1;4(6):1645–1653. doi: 10.1096/fasebj.4.6.2318380. [DOI] [PubMed] [Google Scholar]
- Mahley R. W., Innerarity T. L., Brown M. S., Ho Y. K., Goldstein J. L. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980 Nov;21(8):970–980. [PubMed] [Google Scholar]
- Mao S. J., Yates M. T., Parker R. A., Chi E. M., Jackson R. L. Attenuation of atherosclerosis in a modified strain of hypercholesterolemic Watanabe rabbits with use of a probucol analogue (MDL 29,311) that does not lower serum cholesterol. Arterioscler Thromb. 1991 Sep-Oct;11(5):1266–1275. doi: 10.1161/01.atv.11.5.1266. [DOI] [PubMed] [Google Scholar]
- McPherson R., Hogue M., Milne R. W., Tall A. R., Marcel Y. L. Increase in plasma cholesteryl ester transfer protein during probucol treatment. Relation to changes in high density lipoprotein composition. Arterioscler Thromb. 1991 May-Jun;11(3):476–481. doi: 10.1161/01.atv.11.3.476. [DOI] [PubMed] [Google Scholar]
- Minnich A., Zilversmit D. B. Impaired triacylglycerol catabolism in hypertriglyceridemia of the diabetic, cholesterol-fed rabbit: a possible mechanism for protection from atherosclerosis. Biochim Biophys Acta. 1989 Apr 26;1002(3):324–332. doi: 10.1016/0005-2760(89)90346-9. [DOI] [PubMed] [Google Scholar]
- O'Brien K., Nagano Y., Gown A., Kita T., Chait A. Probucol treatment affects the cellular composition but not anti-oxidized low density lipoprotein immunoreactivity of plaques from Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):751–759. doi: 10.1161/01.atv.11.3.751. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Printz D. J., Boyd D., Joy L., Steinberg D. Macrophage oxidation of low density lipoprotein generates a modified form recognized by the scavenger receptor. Arteriosclerosis. 1986 Sep-Oct;6(5):505–510. doi: 10.1161/01.atv.6.5.505. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Quinn M. T., Schwenke D. C., Carew T. E., Steinberg D. Oxidative modification of beta-very low density lipoprotein. Potential role in monocyte recruitment and foam cell formation. Arteriosclerosis. 1989 May-Jun;9(3):398–404. doi: 10.1161/01.atv.9.3.398. [DOI] [PubMed] [Google Scholar]
- Parthasarathy S., Young S. G., Witztum J. L., Pittman R. C., Steinberg D. Probucol inhibits oxidative modification of low density lipoprotein. J Clin Invest. 1986 Feb;77(2):641–644. doi: 10.1172/JCI112349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman R. C., Taylor C. A., Jr Methods for assessment of tissue sites of lipoprotein degradation. Methods Enzymol. 1986;129:612–628. doi: 10.1016/0076-6879(86)29094-1. [DOI] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajavashisth T. B., Andalibi A., Territo M. C., Berliner J. A., Navab M., Fogelman A. M., Lusis A. J. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature. 1990 Mar 15;344(6263):254–257. doi: 10.1038/344254a0. [DOI] [PubMed] [Google Scholar]
- Rannug A., Rannug U., Ramel C. Genotoxic effects of additives in synthetic elastomers with special consideration to the mechanism of action of thiurams and dithiocarbamates. Prog Clin Biol Res. 1984;141:407–419. [PubMed] [Google Scholar]
- Rosenfeld M. E., Khoo J. C., Miller E., Parthasarathy S., Palinski W., Witztum J. L. Macrophage-derived foam cells freshly isolated from rabbit atherosclerotic lesions degrade modified lipoproteins, promote oxidation of low-density lipoproteins, and contain oxidation-specific lipid-protein adducts. J Clin Invest. 1991 Jan;87(1):90–99. doi: 10.1172/JCI115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Tsukada T., Chait A., Bierman E. L., Gown A. M., Ross R. Fatty streak expansion and maturation in Watanabe Heritable Hyperlipemic and comparably hypercholesterolemic fat-fed rabbits. Arteriosclerosis. 1987 Jan-Feb;7(1):24–34. doi: 10.1161/01.atv.7.1.24. [DOI] [PubMed] [Google Scholar]
- Rudel L. L., Morris M. D. Determination of cholesterol using o-phthalaldehyde. J Lipid Res. 1973 May;14(3):364–366. [PubMed] [Google Scholar]
- Sofuni T., Matsuoka A., Sawada M., Ishidate M., Jr, Zeiger E., Shelby M. D. A comparison of chromosome aberration induction by 25 compounds tested by two Chinese hamster cell (CHL and CHO) systems in culture. Mutat Res. 1990 Jun;241(2):175–213. doi: 10.1016/0165-1218(90)90122-i. [DOI] [PubMed] [Google Scholar]
- Sparrow C. P., Parthasarathy S., Steinberg D. A macrophage receptor that recognizes oxidized low density lipoprotein but not acetylated low density lipoprotein. J Biol Chem. 1989 Feb 15;264(5):2599–2604. [PubMed] [Google Scholar]
- Sparrow C. P., Parthasarathy S., Steinberg D. Enzymatic modification of low density lipoprotein by purified lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative modification. J Lipid Res. 1988 Jun;29(6):745–753. [PubMed] [Google Scholar]
- Steinberg D., Parthasarathy S., Carew T. E., Khoo J. C., Witztum J. L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N Engl J Med. 1989 Apr 6;320(14):915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi K. A simple fluorometric assay for lipoperoxide in blood plasma. Biochem Med. 1976 Apr;15(2):212–216. doi: 10.1016/0006-2944(76)90049-1. [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Matsuzawa Y., Yokoyama S., Funahashi T., Yamamura T., Kishino B. Effects of probucol on xanthomata regression in familial hypercholesterolemia. Am J Cardiol. 1986 Jun 27;57(16):29H–35H. doi: 10.1016/0002-9149(86)90434-0. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel P. C., Sowers J. R. Relation between lipids and atherosclerosis: epidemiologic evidence and clinical implications. Am J Cardiol. 1990 Dec 18;66(21):7I–12I. doi: 10.1016/0002-9149(90)91257-7. [DOI] [PubMed] [Google Scholar]



