Abstract
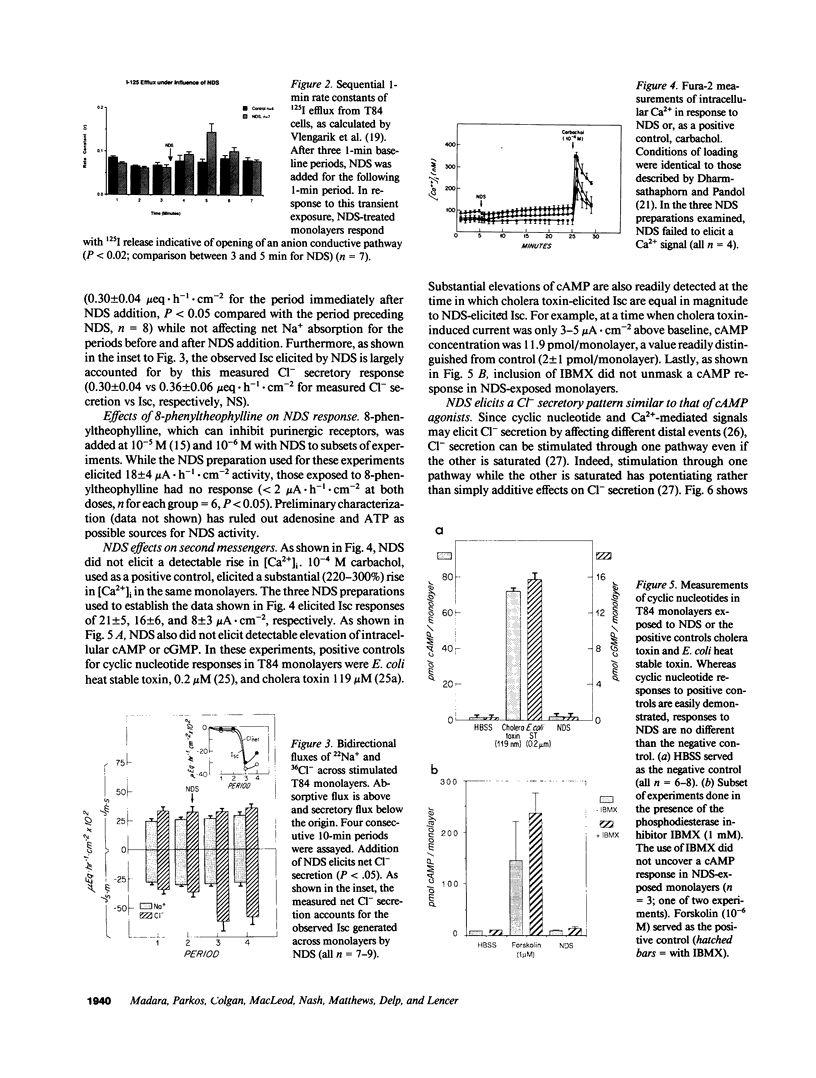
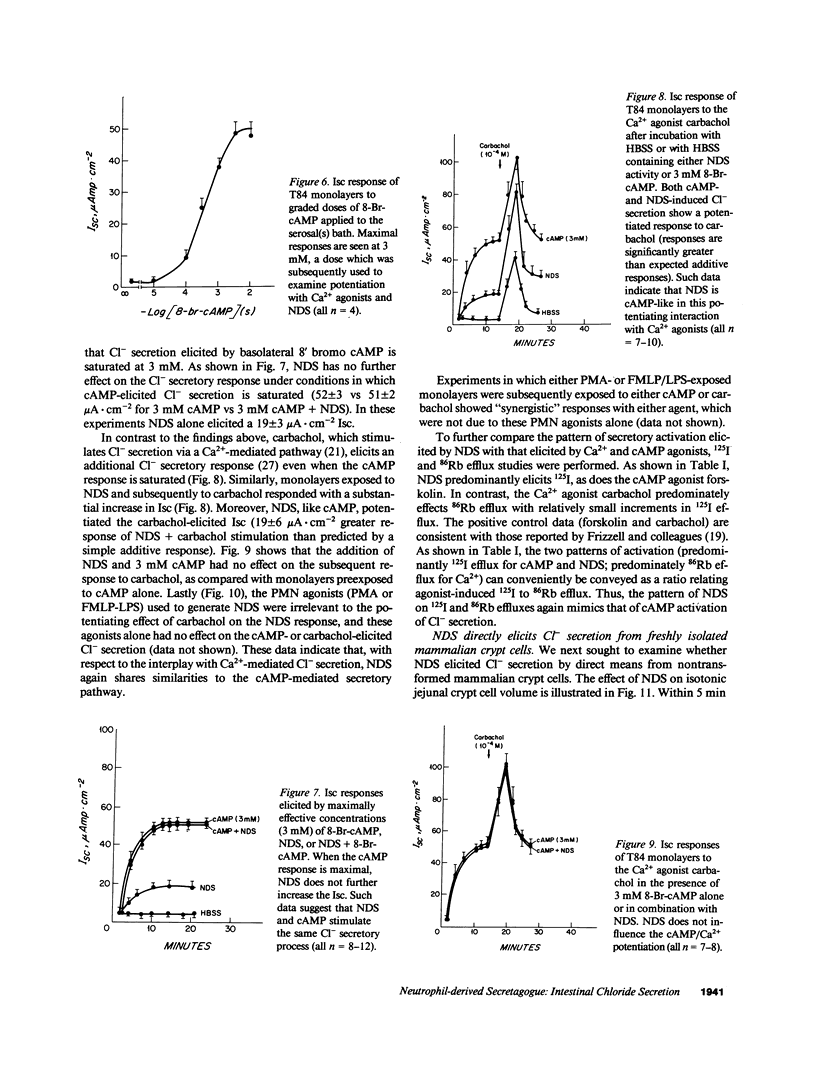
A secreted product of activated neutrophils, NDS (neutrophil-derived secretagogue), elicits a short circuit current (Isc) in epithelial monolayers derived from the human intestinal cell line T84 (J. Clin. Invest. 1991. 87:1474-1477). Here, we identify and characterize the source of this Isc and examine associated signaling pathways. 125I efflux studies suggested that NDS activates an anion conductive channel. Bidirectional 22Na 36Cl flux studies showed that electrogenic Cl- secretion fully accounts for the NDS-induced Isc response. NDS behaved in many respects as a cAMP-mediated secretagogue: NDS did not further increase maximal cAMP-induced Cl- secretion; NDS potentiated Ca(2+)-mediated Cl secretion; and NDS elicited measurable 125I but not 86Rb effluxes. However, NDS did not elicit a detectable rise in intracellular cAMP. Such data suggest that NDS may elicit Cl- secretion by effecting distal events in the cAMP-mediated pathway. Data derived from cell volume assays of isolated guinea pig intestinal crypt cells indicated that NDS also directly elicits Cl- secretion from natural intestinal epithelia. Additionally, since NDS activity is released from PMN by stimuli normally present in the colonic lumen, since NDS is active when applied apically to this model intestinal epithelium, and since the NDS-elicited Isc response is indicative of electrogenic chloride secretion, we speculate NDS may contribute to the secretory diarrhea encountered in many patients with inflammatory intestinal disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett K. E., Cohn J. A., Huott P. A., Wasserman S. I., Dharmsathaphorn K. Immune-related intestinal chloride secretion. II. Effect of adenosine on T84 cell line. Am J Physiol. 1990 May;258(5 Pt 1):C902–C912. doi: 10.1152/ajpcell.1990.258.5.C902. [DOI] [PubMed] [Google Scholar]
- Barrett K. E., Huott P. A., Shah S. S., Dharmsathaphorn K., Wasserman S. I. Differing effects of apical and basolateral adenosine on colonic epithelial cell line T84. Am J Physiol. 1989 Jan;256(1 Pt 1):C197–C203. doi: 10.1152/ajpcell.1989.256.1.C197. [DOI] [PubMed] [Google Scholar]
- Bridges R. J., Worrell R. T., Frizzell R. A., Benos D. J. Stilbene disulfonate blockade of colonic secretory Cl- channels in planar lipid bilayers. Am J Physiol. 1989 Apr;256(4 Pt 1):C902–C912. doi: 10.1152/ajpcell.1989.256.4.C902. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., McRoberts J. A., Mandel K. G., Dharmsathaphorn K. Synergistic action of cyclic adenosine monophosphate- and calcium-mediated chloride secretion in a colonic epithelial cell line. J Clin Invest. 1985 Nov;76(5):1837–1842. doi: 10.1172/JCI112176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick V. S., Mellor D. M., Myers D. B., Selden A. C., Keshavarzian A., Broom M. F., Hobson C. H. Production of peptides inducing chemotaxis and lysosomal enzyme release in human neutrophils by intestinal bacteria in vitro and in vivo. Scand J Gastroenterol. 1988 Jan;23(1):121–128. doi: 10.3109/00365528809093861. [DOI] [PubMed] [Google Scholar]
- Cliff W. H., Frizzell R. A. Separate Cl- conductances activated by cAMP and Ca2+ in Cl(-)-secreting epithelial cells. Proc Natl Acad Sci U S A. 1990 Jul;87(13):4956–4960. doi: 10.1073/pnas.87.13.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Madara J. L. Established intestinal cell lines as model systems for electrolyte transport studies. Methods Enzymol. 1990;192:354–389. doi: 10.1016/0076-6879(90)92082-o. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., McRoberts J. A., Mandel K. G., Tisdale L. D., Masui H. A human colonic tumor cell line that maintains vectorial electrolyte transport. Am J Physiol. 1984 Feb;246(2 Pt 1):G204–G208. doi: 10.1152/ajpgi.1984.246.2.G204. [DOI] [PubMed] [Google Scholar]
- Dharmsathaphorn K., Pandol S. J. Mechanism of chloride secretion induced by carbachol in a colonic epithelial cell line. J Clin Invest. 1986 Feb;77(2):348–354. doi: 10.1172/JCI112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht G., Pothoulakis C., LaMont J. T., Madara J. L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988 Nov;82(5):1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson P. M., Oades Z. G. Stimulation of human neutrophils by soluble and insoluble immunoglobulin aggregates. Secretion of granule constituents and increased oxidation of glucose. J Clin Invest. 1975 Oct;56(4):1053–1061. doi: 10.1172/JCI108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huott P. A., Liu W., McRoberts J. A., Giannella R. A., Dharmsathaphorn K. Mechanism of action of Escherichia coli heat stable enterotoxin in a human colonic cell line. J Clin Invest. 1988 Aug;82(2):514–523. doi: 10.1172/JCI113626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karayalcin S. S., Sturbaum C. W., Wachsman J. T., Cha J. H., Powell D. W. Hydrogen peroxide stimulates rat colonic prostaglandin production and alters electrolyte transport. J Clin Invest. 1990 Jul;86(1):60–68. doi: 10.1172/JCI114715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. B., Nostrant T. T., Appelman H. D. The histopathologic spectrum of acute self-limited colitis (acute infectious-type colitis). Am J Surg Pathol. 1982 Sep;6(6):523–529. doi: 10.1097/00000478-198209000-00004. [DOI] [PubMed] [Google Scholar]
- MacLeod R. J., Hamilton J. R. Regulatory volume increase in mammalian jejunal villus cells is due to bumetanide-sensitive NaKCl2 cotransport. Am J Physiol. 1990 May;258(5 Pt 1):G665–G674. doi: 10.1152/ajpgi.1990.258.5.G665. [DOI] [PubMed] [Google Scholar]
- MacLeod R. J., Hamilton J. R. Separate K+ and Cl- transport pathways are activated for regulatory volume decrease in jejunal villus cells. Am J Physiol. 1991 Mar;260(3 Pt 1):G405–G415. doi: 10.1152/ajpgi.1991.260.3.G405. [DOI] [PubMed] [Google Scholar]
- Madara J. L., Dharmsathaphorn K. Occluding junction structure-function relationships in a cultured epithelial monolayer. J Cell Biol. 1985 Dec;101(6):2124–2133. doi: 10.1083/jcb.101.6.2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S., Parkos C., Nusrat A., Delp C., Madara J. L. In vitro model of intestinal crypt abscess. A novel neutrophil-derived secretagogue activity. J Clin Invest. 1991 Apr;87(4):1474–1477. doi: 10.1172/JCI115156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash S., Stafford J., Madara J. L. Effects of polymorphonuclear leukocyte transmigration on the barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1987 Oct;80(4):1104–1113. doi: 10.1172/JCI113167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omann G. M., Allen R. A., Bokoch G. M., Painter R. G., Traynor A. E., Sklar L. A. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987 Jan;67(1):285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Shapiro M., Matthews J., Hecht G., Delp C., Madara J. L. Stabilization of F-actin prevents cAMP-elicited Cl- secretion in T84 cells. J Clin Invest. 1991 Jun;87(6):1903–1909. doi: 10.1172/JCI115215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglarik C. J., Bridges R. J., Frizzell R. A. A simple assay for agonist-regulated Cl and K conductances in salt-secreting epithelial cells. Am J Physiol. 1990 Aug;259(2 Pt 1):C358–C364. doi: 10.1152/ajpcell.1990.259.2.C358. [DOI] [PubMed] [Google Scholar]
- van Deventer S. J., ten Cate J. W., Tytgat G. N. Intestinal endotoxemia. Clinical significance. Gastroenterology. 1988 Mar;94(3):825–831. doi: 10.1016/0016-5085(88)90261-2. [DOI] [PubMed] [Google Scholar]