Abstract
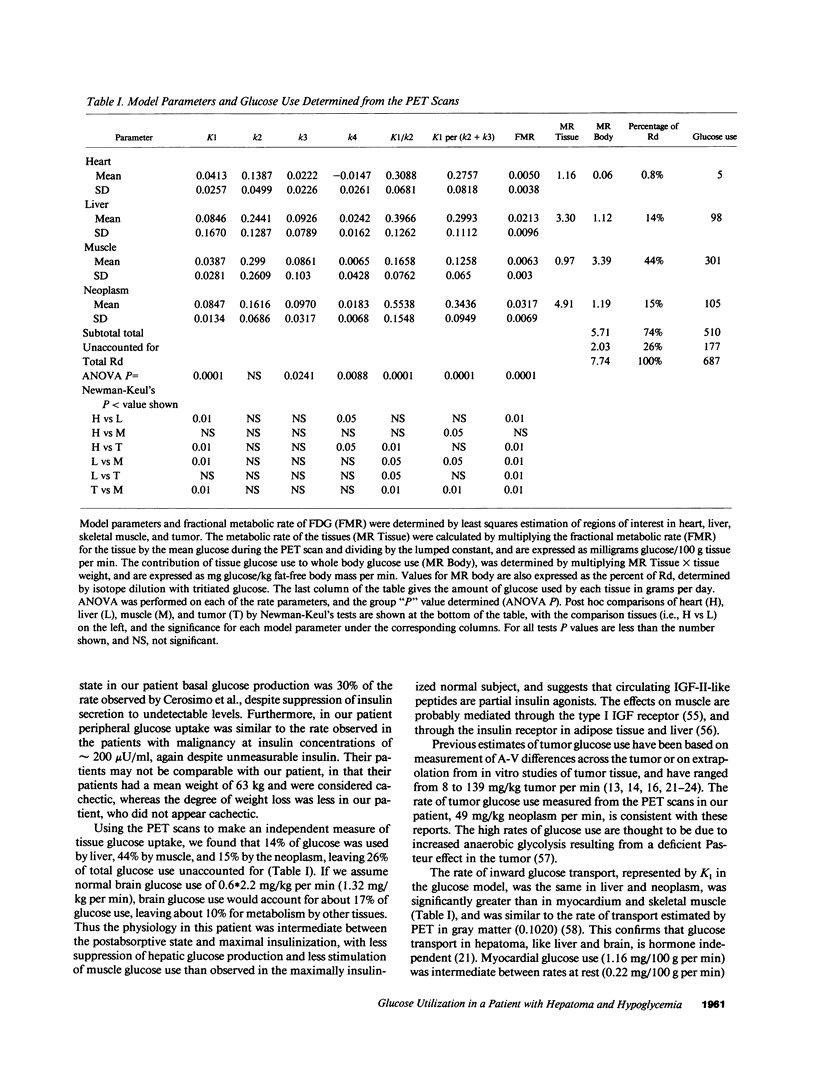
Tumor glucose use in patients with non-islet-cell tumors has been difficult to measure, particularly in hepatoma, because of hepatic involvement by neoplasm. We studied a patient with nonhepatic recurrence of hepatoma after successful liver transplantation. Tumor tissue contained messenger RNA for insulin-like growth factor-II (IGF-II), and circulating high molecular weight components and E-peptide of IGF-II were increased. Glucose use measured by isotope dilution with [3-3H]glucose was 7.94 mg/kg fat-free mass per min, and splanchnic glucose production was 0.93 mg/kg fat-free mass per min. Glucose uptake and glucose model parameters were independently measured in tissues by positron emission tomography with 18F-fluoro-2-deoxy-D-glucose. Glucose uptake by heart muscle, liver, skeletal muscle, and neoplasm accounted for 0.8, 14, 44, and 15% of total glucose use, respectively. Model parameters in liver and neoplasm were not significantly different, and glucose transport and phosphorylation were twofold and fourfold greater than in muscle. This suggests that circulating IGF-II-like proteins are partial insulin agonists, and that hypoglycemia in hepatoma with IGF-II production is predominantly due to glucose uptake by skeletal muscle and suppression of glucose production.
Full text
PDF

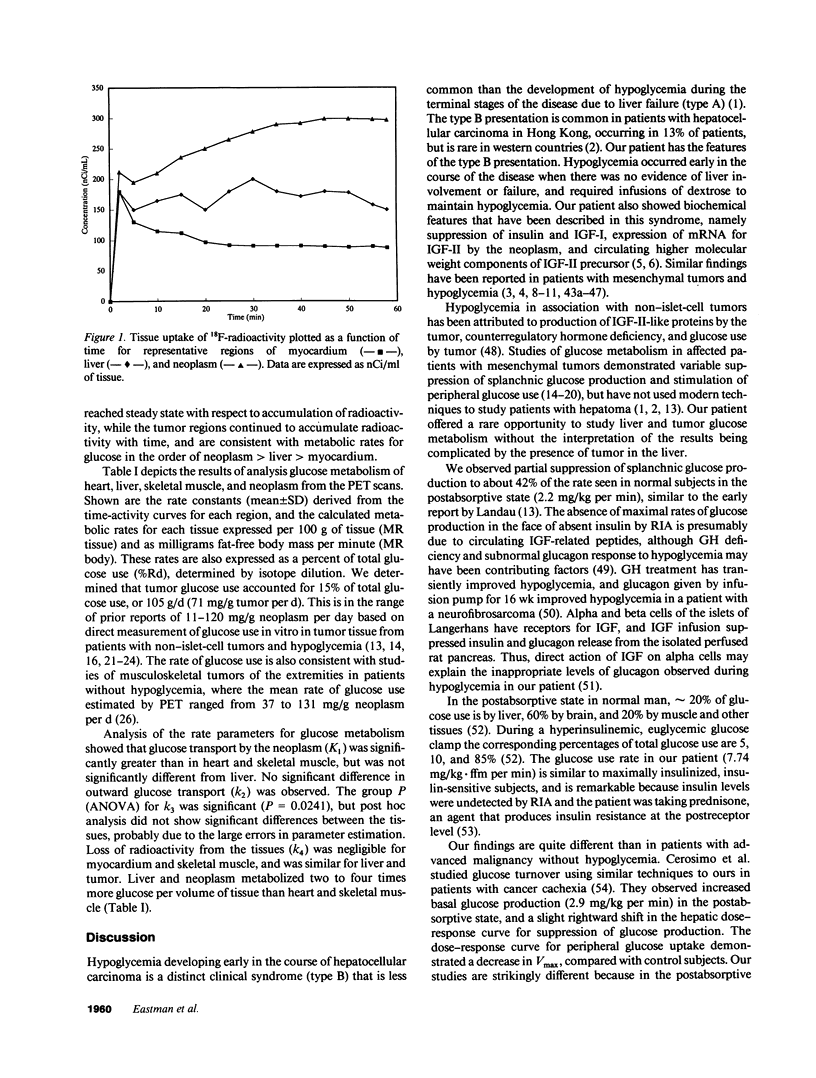


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUGUST J. T., HIATT H. H. Severe hypoglycemia secondary to a nonpancreatic fibrosarcoma with insulin activity. N Engl J Med. 1958 Jan 2;258(1):17–20. doi: 10.1056/NEJM195801022580104. [DOI] [PubMed] [Google Scholar]
- Araujo L. I., Camici P., Spinks T. J., Jones T., Maseri A. Abnormalities in myocardial metabolism in patients with unstable angina as assessed by positron emission tomography. Cardiovasc Drugs Ther. 1988 May;2(1):41–46. doi: 10.1007/BF00054251. [DOI] [PubMed] [Google Scholar]
- Benn J. J., Firth R. G., Sönksen P. H. Metabolic effects of an insulin-like factor causing hypoglycaemia in a patient with a haemangiopericytoma. Clin Endocrinol (Oxf) 1990 Jun;32(6):769–780. doi: 10.1111/j.1365-2265.1990.tb00924.x. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Poulos J., Ittoop O., Pories W. J., Flickinger E. G., Sinha M. K. Insulin-like growth factor I binding in hepatocytes from human liver, human hepatoma, and normal, regenerating, and fetal rat liver. J Clin Invest. 1988 Apr;81(4):976–981. doi: 10.1172/JCI113451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cersosimo E., Pisters P. W., Pesola G., Rogatko A., Vydelingum N. A., Bajorunas D., Brennan M. F. The effect of graded doses of insulin on peripheral glucose uptake and lactate release in cancer cachexia. Surgery. 1991 Apr;109(4):459–467. [PubMed] [Google Scholar]
- Chandalia H. B., Boshell B. R. Hypoglycemia associated with extrapancreatic tumors. Report of two cases with studies on its pathogenesis. Arch Intern Med. 1972 Mar;129(3):447–456. doi: 10.1001/archinte.129.3.447. [DOI] [PubMed] [Google Scholar]
- Chowdhury F., Bleicher S. J. Studies of tumor hypoglycemia. Metabolism. 1973 May;22(5):663–674. doi: 10.1016/0026-0495(73)90238-2. [DOI] [PubMed] [Google Scholar]
- Cohn S. H., Vartsky D., Yasumura S., Sawitsky A., Zanzi I., Vaswani A., Ellis K. J. Compartmental body composition based on total-body nitrogen, potassium, and calcium. Am J Physiol. 1980 Dec;239(6):E524–E530. doi: 10.1152/ajpendo.1980.239.6.E524. [DOI] [PubMed] [Google Scholar]
- Cowan J. S., Hetenyi G., Jr Glucoregulatory responses in normal and diabetic dogs recorded by a new tracer method. Metabolism. 1971 Apr;20(4):360–372. doi: 10.1016/0026-0495(71)90098-9. [DOI] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Daughaday W. H., Emanuele M. A., Brooks M. H., Barbato A. L., Kapadia M., Rotwein P. Synthesis and secretion of insulin-like growth factor II by a leiomyosarcoma with associated hypoglycemia. N Engl J Med. 1988 Dec 1;319(22):1434–1440. doi: 10.1056/NEJM198812013192202. [DOI] [PubMed] [Google Scholar]
- Daughaday W. H., Kapadia M. Significance of abnormal serum binding of insulin-like growth factor II in the development of hypoglycemia in patients with non-islet-cell tumors. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6778–6782. doi: 10.1073/pnas.86.17.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughaday W. H., Trivedi B., Kapadia M. Measurement of insulin-like growth factor II by a specific radioreceptor assay in serum of normal individuals, patients with abnormal growth hormone secretion, and patients with tumor-associated hypoglycemia. J Clin Endocrinol Metab. 1981 Aug;53(2):289–294. doi: 10.1210/jcem-53-2-289. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Dohm G. L., Elton C. W., Raju M. S., Mooney N. D., DiMarchi R., Pories W. J., Flickinger E. G., Atkinson S. M., Jr, Caro J. F. IGF-I--stimulated glucose transport in human skeletal muscle and IGF-I resistance in obesity and NIDDM. Diabetes. 1990 Sep;39(9):1028–1032. doi: 10.2337/diab.39.9.1028. [DOI] [PubMed] [Google Scholar]
- Eastman R. C., Carson R. E., Gordon M. R., Berg G. W., Lillioja S., Larson S. M., Roth J. Brain glucose metabolism in noninsulin-dependent diabetes mellitus: a study in Pima Indians using positron emission tomography during hyperinsulinemia with euglycemic glucose clamp. J Clin Endocrinol Metab. 1990 Dec;71(6):1602–1610. doi: 10.1210/jcem-71-6-1602. [DOI] [PubMed] [Google Scholar]
- Frerichs H., Willms B., Kasper H., Creutzfeldt C., Creutzfeldt W. Contribution to the pathogenesis of tumour hypoglycaemia. Eur J Clin Invest. 1970 Mar;1(1):2–11. doi: 10.1111/j.1365-2362.1970.tb00590.x. [DOI] [PubMed] [Google Scholar]
- HORECKER B. L., HIATT H. H. Pathways of carbohydrate metabolism in normal and neoplastic cells. N Engl J Med. 1958 Jan 23;258(4):177–contd. doi: 10.1056/NEJM195801232580406. [DOI] [PubMed] [Google Scholar]
- Huang S. C., Phelps M. E., Hoffman E. J., Sideris K., Selin C. J., Kuhl D. E. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol. 1980 Jan;238(1):E69–E82. doi: 10.1152/ajpendo.1980.238.1.E69. [DOI] [PubMed] [Google Scholar]
- Hyodo T., Megyesi K., Kahn C. R., McLean J. P., Friesen H. G. Adrenocortical carcinoma and hypoglycemia: evidence for production of nonsuppressible insulin-like activity by the tumor. J Clin Endocrinol Metab. 1977 Jun;44(6):1175–1184. doi: 10.1210/jcem-44-6-1175. [DOI] [PubMed] [Google Scholar]
- Insel P. A., Liljenquist J. E., Tobin J. D., Sherwin R. S., Watkins P., Andres R., Berman M. Insulin control of glucose metabolism in man: a new kinetic analysis. J Clin Invest. 1975 May;55(5):1057–1066. doi: 10.1172/JCI108006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R. The riddle of tumour hypoglycaemia revisited. Clin Endocrinol Metab. 1980 Jul;9(2):335–360. doi: 10.1016/s0300-595x(80)80037-5. [DOI] [PubMed] [Google Scholar]
- Kern K. A., Brunetti A., Norton J. A., Chang A. E., Malawer M., Lack E., Finn R. D., Rosenberg S. A., Larson S. M. Metabolic imaging of human extremity musculoskeletal tumors by PET. J Nucl Med. 1988 Feb;29(2):181–186. [PubMed] [Google Scholar]
- Kreisberg R. A., Hershman J. M., Spenney J. G., Boshell B. R., Pennington L. F. Biochemistry of extrapancreatic tumor hypoglycemia. Diabetes. 1970 Apr;19(4):248–258. doi: 10.2337/diab.19.4.248. [DOI] [PubMed] [Google Scholar]
- LANDAU B. R., WILLS N., CRAIG J. W., LEONARDS J. R., MORIWAKI T. The mechanism of hepatoma-induced hypoglycemia. Cancer. 1962 Nov-Dec;15:1188–1196. doi: 10.1002/1097-0142(196211/12)15:6<1188::aid-cncr2820150616>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Lowe W. L., Jr, Adamo M., Werner H., Roberts C. T., Jr, LeRoith D. Regulation by fasting of rat insulin-like growth factor I and its receptor. Effects on gene expression and binding. J Clin Invest. 1989 Aug;84(2):619–626. doi: 10.1172/JCI114207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe W. L., Jr, Lasky S. R., LeRoith D., Roberts C. T., Jr Distribution and regulation of rat insulin-like growth factor I messenger ribonucleic acids encoding alternative carboxyterminal E-peptides: evidence for differential processing and regulation in liver. Mol Endocrinol. 1988 Jun;2(6):528–535. doi: 10.1210/mend-2-6-528. [DOI] [PubMed] [Google Scholar]
- Lowe W. L., Jr, Roberts C. T., Jr, Lasky S. R., LeRoith D. Differential expression of alternative 5' untranslated regions in mRNAs encoding rat insulin-like growth factor I. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8946–8950. doi: 10.1073/pnas.84.24.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe W. L., Jr, Roberts C. T., Jr, LeRoith D., Rojeski M. T., Merimee T. J., Fui S. T., Keen H., Arnold D., Mersey J., Gluzman S. Insulin-like growth factor-II in nonislet cell tumors associated with hypoglycemia: increased levels of messenger ribonucleic acid. J Clin Endocrinol Metab. 1989 Dec;69(6):1153–1159. doi: 10.1210/jcem-69-6-1153. [DOI] [PubMed] [Google Scholar]
- MacGorman L. R., Rizza R. A., Gerich J. E. Physiological concentrations of growth hormone exert insulin-like and insulin antagonistic effects on both hepatic and extrahepatic tissues in man. J Clin Endocrinol Metab. 1981 Sep;53(3):556–559. doi: 10.1210/jcem-53-3-556. [DOI] [PubMed] [Google Scholar]
- Marchesini G., Bianchi G. Carbohydrate metabolism in hepatocellular carcinoma: where does the glucose go? Hepatology. 1989 Aug;10(2):253–255. doi: 10.1002/hep.1840100221. [DOI] [PubMed] [Google Scholar]
- McFadzean A. J., Yeung R. T. Further observations on hypoglycaemia in hepatocellular carcinoma. Am J Med. 1969 Aug;47(2):220–235. doi: 10.1016/0002-9343(69)90148-x. [DOI] [PubMed] [Google Scholar]
- Megyesi K., Kahn C. R., Roth J., Gorden P. Hypoglycemia in association with extrapancreatic tumors: demonstration of elevated plasma NSILA-s by a new radioreceptor assay. J Clin Endocrinol Metab. 1974 May;38(5):931–934. doi: 10.1210/jcem-38-5-931. [DOI] [PubMed] [Google Scholar]
- Merimee T. J. Insulin-like growth factors in patients with nonislet cell tumors and hypoglycemia. Metabolism. 1986 Apr;35(4):360–363. doi: 10.1016/0026-0495(86)90155-1. [DOI] [PubMed] [Google Scholar]
- Mossberg K. A., Rowe R. W., Tewson T. J., Taegtmeyer H. Rabbit hindlimb glucose uptake assessed with positron-emitting fluorodeoxyglucose. J Appl Physiol (1985) 1989 Oct;67(4):1569–1577. doi: 10.1152/jappl.1989.67.4.1569. [DOI] [PubMed] [Google Scholar]
- Møller N., Blum W. F., Mengel A., Hansen L. B., Alberti K. G., Schmitz O. Basal and insulin stimulated substrate metabolism in tumour induced hypoglycaemia; evidence for increased muscle glucose uptake. Diabetologia. 1991 Jan;34(1):17–20. doi: 10.1007/BF00404019. [DOI] [PubMed] [Google Scholar]
- Nissan S., Bar-Maor A., Shafrir E. Hypoglycemia associated with extrapancreatic tumors. Two cases with biochemical investigations of glucose and fatty acid metabolism. N Engl J Med. 1968 Jan 25;278(4):177–183. doi: 10.1056/NEJM196801252780402. [DOI] [PubMed] [Google Scholar]
- PERKOFF G. T., SIMONS E. L. HYPOGLYCEMIA IN A PATIENT WITH A FIBROUS TUMOR. STUDIES OF THE MECHANISM OF THE HYPOGLYCEMIA. Arch Intern Med. 1963 Oct;112:589–593. doi: 10.1001/archinte.1963.03860040185018. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Blasberg R. G., Fenstermacher J. D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983 Mar;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Blasberg R. G. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985 Dec;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Perdue J. F., Chan J. K., Thibault C., Radaj P., Mills B., Daughaday W. H. The biochemical characterization of detergent-solubilized insulin-like growth factor II receptors from rat placenta. J Biol Chem. 1983 Jun 25;258(12):7800–7811. [PubMed] [Google Scholar]
- Phelps M. E., Huang S. C., Hoffman E. J., Selin C., Sokoloff L., Kuhl D. E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979 Nov;6(5):371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Ratib O., Phelps M. E., Huang S. C., Henze E., Selin C. E., Schelbert H. R. Positron tomography with deoxyglucose for estimating local myocardial glucose metabolism. J Nucl Med. 1982 Jul;23(7):577–586. [PubMed] [Google Scholar]
- Rizza R. A., Mandarino L. J., Gerich J. E. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982 Jan;54(1):131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- Ron D., Powers A. C., Pandian M. R., Godine J. E., Axelrod L. Increased insulin-like growth factor II production and consequent suppression of growth hormone secretion: a dual mechanism for tumor-induced hypoglycemia. J Clin Endocrinol Metab. 1989 Apr;68(4):701–706. doi: 10.1210/jcem-68-4-701. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Shapiro E. T., Bell G. I., Polonsky K. S., Rubenstein A. H., Kew M. C., Tager H. S. Tumor hypoglycemia: relationship to high molecular weight insulin-like growth factor-II. J Clin Invest. 1990 May;85(5):1672–1679. doi: 10.1172/JCI114619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert C. K., Rossini A. A., Ghazvinian S., Widrich W. C., Marks L. J., Sawin C. T. Tumor hypoglycemia: deficient splanchnic glucose output and deficient glucagon secretion. Diabetes. 1976 Mar;25(3):202–206. doi: 10.2337/diab.25.3.202. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Teale J. D., Marks V. Inappropriately elevated plasma insulin-like growth factor II in relation to suppressed insulin-like growth factor I in the diagnosis of non-islet cell tumour hypoglycaemia. Clin Endocrinol (Oxf) 1990 Jul;33(1):87–98. doi: 10.1111/j.1365-2265.1990.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Unger R. H. The riddle of tumor hypoglycemia. Am J Med. 1966 Mar;40(3):325–330. doi: 10.1016/0002-9343(66)90127-6. [DOI] [PubMed] [Google Scholar]
- Van Schravendijk C. F., Foriers A., Van den Brande J. L., Pipeleers D. G. Evidence for the presence of type I insulin-like growth factor receptors on rat pancreatic A and B cells. Endocrinology. 1987 Nov;121(5):1784–1788. doi: 10.1210/endo-121-5-1784. [DOI] [PubMed] [Google Scholar]
- Wasada T., Hizuka N., Yamamoto M., Haruki K., Ikejiri K., Oka Y., Asano T., Aiba M., Hirata Y. An insulin-like growth factor II-producing histiocytoma associated with hypoglycemia: analysis of the peptide, its gene expression, and glucose transporter isoforms. Metabolism. 1992 Mar;41(3):310–316. doi: 10.1016/0026-0495(92)90277-h. [DOI] [PubMed] [Google Scholar]
- Wu J. C., Daughaday W. H., Lee S. D., Hsiao T. S., Chou C. K., Lin H. D., Tsai Y. T., Chiang B. N. Radioimmunoassay of serum IGF-I and IGF-II in patients with chronic liver diseases and hepatocellular carcinoma with or without hypoglycemia. J Lab Clin Med. 1988 Nov;112(5):589–594. [PubMed] [Google Scholar]
- Zapf J., Morell B., Walter H., Laron Z., Froesch E. R. Serum levels of insulin-like growth factor (IGF) and its carrier protein in various metabolic disorders. Acta Endocrinol (Copenh) 1980 Dec;95(4):505–517. doi: 10.1530/acta.0.0950505. [DOI] [PubMed] [Google Scholar]
- Zapf J., Schmid C., Guler H. P., Waldvogel M., Hauri C., Futo E., Hossenlopp P., Binoux M., Froesch E. R. Regulation of binding proteins for insulin-like growth factors (IGF) in humans. Increased expression of IGF binding protein 2 during IGF I treatment of healthy adults and in patients with extrapancreatic tumor hypoglycemia. J Clin Invest. 1990 Sep;86(3):952–961. doi: 10.1172/JCI114797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapf J., Walter H., Froesch E. R. Radioimmunological determination of insulinlike growth factors I and II in normal subjects and in patients with growth disorders and extrapancreatic tumor hypoglycemia. J Clin Invest. 1981 Nov;68(5):1321–1330. doi: 10.1172/JCI110379. [DOI] [PMC free article] [PubMed] [Google Scholar]



