Abstract
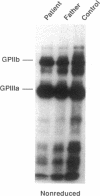
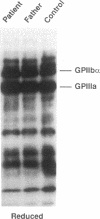
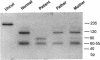
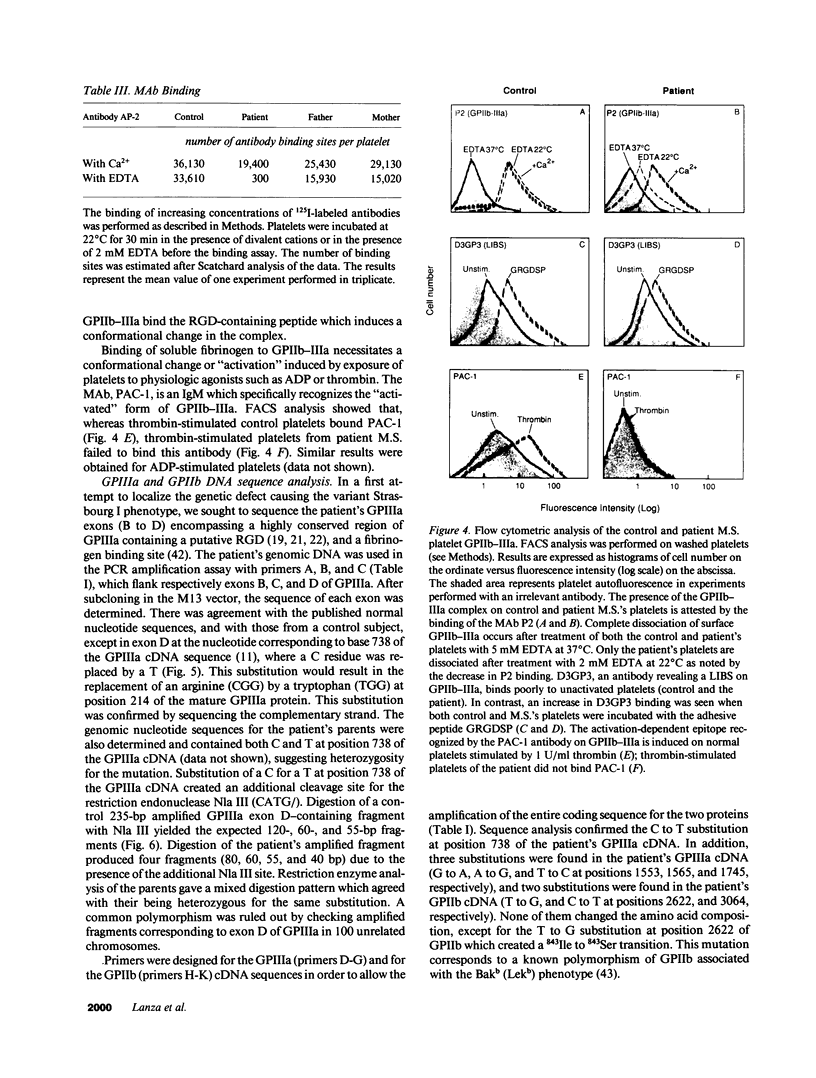
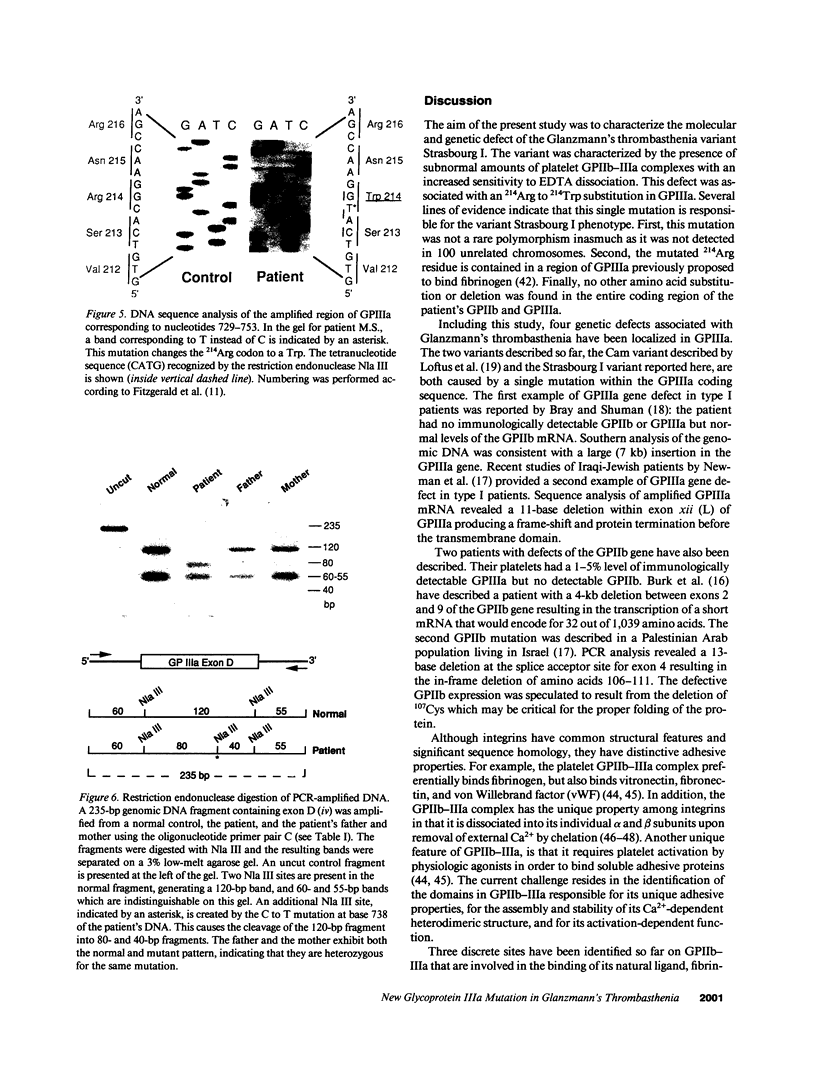
We describe a new variant of Glanzmann's thrombasthenia (variant Strasbourg I). The patient (M.S.) showed an absence of platelet aggregation to ADP, thrombin, and collagen, and a decreased clot retraction. Platelet fibrinogen was approximately 20% of normal levels. ADP-stimulated platelets bound markedly reduced amounts of soluble fibrinogen and platelet adhesion to surface-bound fibrinogen was defective. Normal to subnormal amounts of glycoprotein (GP) IIb-IIIa (alpha IIb beta 3) complexes, the platelet fibrinogen receptor, were revealed by SDS-PAGE, crossed immunoelectrophoresis, and antibody binding. However, the complexes were unusually sensitive to dissociation with EDTA at room temperature. Furthermore, flow cytometry showed that the platelets failed to bind the activation-dependent monoclonal antibody, PAC-1, after stimulation. In contrast, an RGDS-containing peptide induced significant binding of the anti-ligand-induced binding site antibody, D3GP3, suggesting the presence of a functional RGD binding domain on the patient's GPIIb-IIIa complex. Sequence analysis was performed after polymerase chain reaction amplification of selected patient's GPIIIa exons, and of the patient's platelet GPIIb and GPIIIa mRNAs. A point mutation (C to T) was localized in exon D (iv) of GPIIIa that resulted in an 214Arg to 214Trp amino acid substitution. The defect has been inherited from the parents who are heterozygous for the same mutation. This substitution points to an essential amino acid in a region of GPIIIa involved in the binding of fibrinogen and influencing the Ca(2+)-dependent stability of the GPIIb-IIIa complex.
Full text
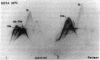
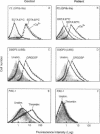
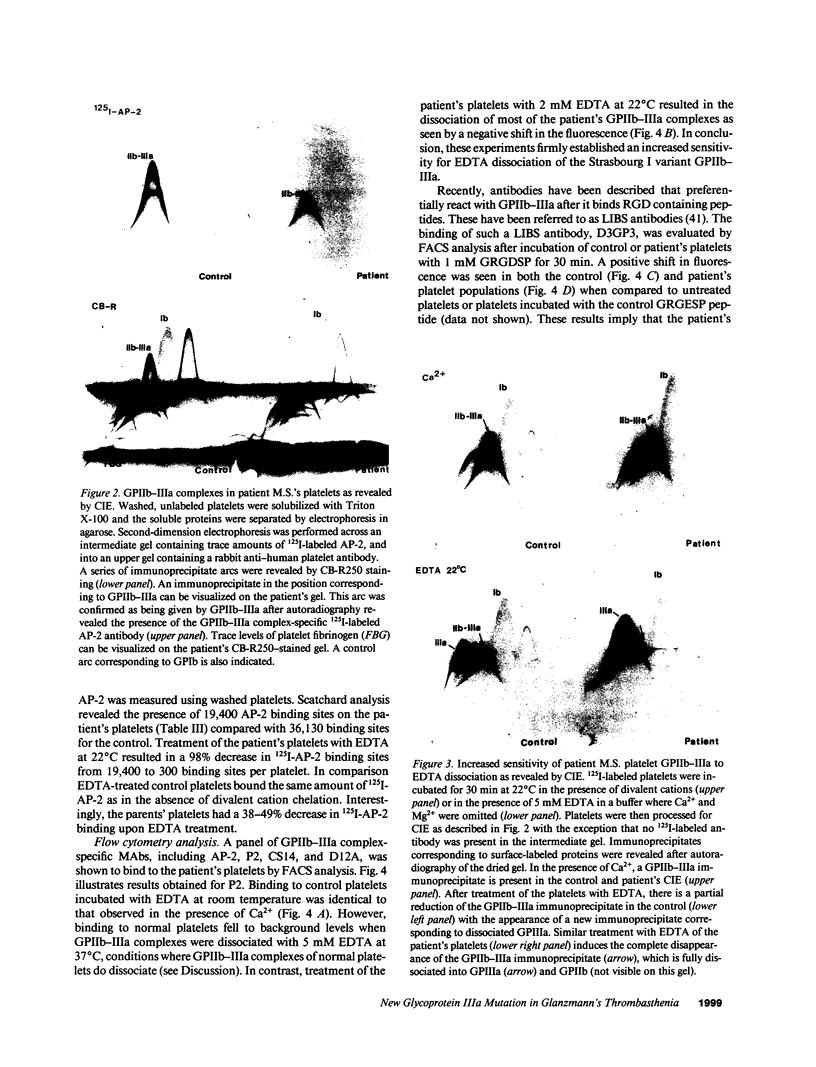
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argraves W. S., Suzuki S., Arai H., Thompson K., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987 Sep;105(3):1183–1190. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajt M. L., Ginsberg M. H., Frelinger A. L., 3rd, Berndt M. C., Loftus J. C. A spontaneous mutation of integrin alpha IIb beta 3 (platelet glycoprotein IIb-IIIa) helps define a ligand binding site. J Biol Chem. 1992 Feb 25;267(6):3789–3794. [PubMed] [Google Scholar]
- Bird A. P. DNA methylation and the frequency of CpG in animal DNA. Nucleic Acids Res. 1980 Apr 11;8(7):1499–1504. doi: 10.1093/nar/8.7.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass L. F., Shattil S. J., Kunicki T. J., Bennett J. S. Effect of calcium on the stability of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Jul 5;260(13):7875–7881. [PubMed] [Google Scholar]
- Bray P. F., Shuman M. A. Identification of an abnormal gene for the GPIIIa subunit of the platelet fibrinogen receptor resulting in Glanzmann's thrombasthenia. Blood. 1990 Feb 15;75(4):881–888. [PubMed] [Google Scholar]
- Burk C. D., Newman P. J., Lyman S., Gill J., Coller B. S., Poncz M. A deletion in the gene for glycoprotein IIb associated with Glanzmann's thrombasthenia. J Clin Invest. 1991 Jan;87(1):270–276. doi: 10.1172/JCI114982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave J. P., Hemmendinger S., Beretz A., Sutter-Bay A., Launay J. L'agrégation plaquettaire: outil d'investigation clinique et d'étude pharmacologique. Méthodologie. Ann Biol Clin (Paris) 1983;41(3):167–179. [PubMed] [Google Scholar]
- Charo I. F., Nannizzi L., Phillips D. R., Hsu M. A., Scarborough R. M. Inhibition of fibrinogen binding to GP IIb-IIIa by a GP IIIa peptide. J Biol Chem. 1991 Jan 25;266(3):1415–1421. [PubMed] [Google Scholar]
- Cheresh D. A., Berliner S. A., Vicente V., Ruggeri Z. M. Recognition of distinct adhesive sites on fibrinogen by related integrins on platelets and endothelial cells. Cell. 1989 Sep 8;58(5):945–953. doi: 10.1016/0092-8674(89)90946-x. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Coller B. S., Seligsohn U., Zivelin A., Zwang E., Lusky A., Modan M. Immunologic and biochemical characterization of homozygous and heterozygous Glanzmann thrombasthenia in the Iraqi-Jewish and Arab populations of Israel: comparison of techniques for carrier detection. Br J Haematol. 1986 Apr;62(4):723–735. doi: 10.1111/j.1365-2141.1986.tb04096.x. [DOI] [PubMed] [Google Scholar]
- Cooney K. A., Nichols W. C., Bruck M. E., Bahou W. F., Shapiro A. D., Bowie E. J., Gralnick H. R., Ginsburg D. The molecular defect in type IIB von Willebrand disease. Identification of four potential missense mutations within the putative GpIb binding domain. J Clin Invest. 1991 Apr;87(4):1227–1233. doi: 10.1172/JCI115123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Burke T. A., Plow E. F. The ligand binding site of the platelet integrin receptor GPIIb-IIIa is proximal to the second calcium binding domain of its alpha subunit. J Biol Chem. 1990 Feb 25;265(6):3440–3446. [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Matsueda G. R., Plow E. F. A discrete sequence in a platelet integrin is involved in ligand recognition. Nature. 1991 Mar 7;350(6313):66–68. doi: 10.1038/350066a0. [DOI] [PubMed] [Google Scholar]
- Du X. P., Plow E. F., Frelinger A. L., 3rd, O'Toole T. E., Loftus J. C., Ginsberg M. H. Ligands "activate" integrin alpha IIb beta 3 (platelet GPIIb-IIIa). Cell. 1991 May 3;65(3):409–416. doi: 10.1016/0092-8674(91)90458-b. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Phillips D. R. Calcium regulation of the platelet membrane glycoprotein IIb-IIIa complex. J Biol Chem. 1985 Sep 15;260(20):11366–11374. [PubMed] [Google Scholar]
- Fitzgerald L. A., Poncz M., Steiner B., Rall S. C., Jr, Bennett J. S., Phillips D. R. Comparison of cDNA-derived protein sequences of the human fibronectin and vitronectin receptor alpha-subunits and platelet glycoprotein IIb. Biochemistry. 1987 Dec 15;26(25):8158–8165. doi: 10.1021/bi00399a021. [DOI] [PubMed] [Google Scholar]
- Fitzgerald L. A., Steiner B., Rall S. C., Jr, Lo S. S., Phillips D. R. Protein sequence of endothelial glycoprotein IIIa derived from a cDNA clone. Identity with platelet glycoprotein IIIa and similarity to "integrin". J Biol Chem. 1987 Mar 25;262(9):3936–3939. [PubMed] [Google Scholar]
- Fournier D. J., Kabral A., Castaldi P. A., Berndt M. C. A variant of Glanzmann's thrombasthenia characterized by abnormal glycoprotein IIb/IIIa complex formation. Thromb Haemost. 1989 Nov 24;62(3):977–983. [PubMed] [Google Scholar]
- Frelinger A. L., 3rd, Lam S. C., Plow E. F., Smith M. A., Loftus J. C., Ginsberg M. H. Occupancy of an adhesive glycoprotein receptor modulates expression of an antigenic site involved in cell adhesion. J Biol Chem. 1988 Sep 5;263(25):12397–12402. [PubMed] [Google Scholar]
- George J. N., Caen J. P., Nurden A. T. Glanzmann's thrombasthenia: the spectrum of clinical disease. Blood. 1990 Apr 1;75(7):1383–1395. [PubMed] [Google Scholar]
- George J. N., Nurden A. T., Phillips D. R. Molecular defects in interactions of platelets with the vessel wall. N Engl J Med. 1984 Oct 25;311(17):1084–1098. doi: 10.1056/NEJM198410253111705. [DOI] [PubMed] [Google Scholar]
- Giannelli F., Green P. M., High K. A., Lozier J. N., Lillicrap D. P., Ludwig M., Olek K., Reitsma P. H., Goossens M., Yoshioka A. Haemophilia B: database of point mutations and short additions and deletions. Nucleic Acids Res. 1990 Jul 25;18(14):4053–4059. doi: 10.1093/nar/18.14.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Frelinger A. L., Lam S. C., Forsyth J., McMillan R., Plow E. F., Shattil S. J. Analysis of platelet aggregation disorders based on flow cytometric analysis of membrane glycoprotein IIb-IIIa with conformation-specific monoclonal antibodies. Blood. 1990 Nov 15;76(10):2017–2023. [PubMed] [Google Scholar]
- Ginsberg M. H., Lightsey A., Kunicki T. J., Kaufmann A., Marguerie G., Plow E. F. Divalent cation regulation of the surface orientation of platelet membrane glycoprotein IIb. Correlation with fibrinogen binding function and definition of a novel variant of Glanzmann's thrombasthenia. J Clin Invest. 1986 Oct;78(4):1103–1111. doi: 10.1172/JCI112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg M. H., Loftus J. C., Plow E. F. Cytoadhesins, integrins, and platelets. Thromb Haemost. 1988 Feb 25;59(1):1–6. [PubMed] [Google Scholar]
- Heidenreich R., Eisman R., Surrey S., Delgrosso K., Bennett J. S., Schwartz E., Poncz M. Organization of the gene for platelet glycoprotein IIb. Biochemistry. 1990 Feb 6;29(5):1232–1244. doi: 10.1021/bi00457a020. [DOI] [PubMed] [Google Scholar]
- Hogervorst F., Kuikman I., von dem Borne A. E., Sonnenberg A. Cloning and sequence analysis of beta-4 cDNA: an integrin subunit that contains a unique 118 kd cytoplasmic domain. EMBO J. 1990 Mar;9(3):765–770. doi: 10.1002/j.1460-2075.1990.tb08171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- KEKWICK R. A., MACKAY M. E., NANCE M. H., RECORD B. R. The purification of human fibrinogen. Biochem J. 1955 Aug;60(4):671–683. doi: 10.1042/bj0600671b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer N., Fitzgerald L. A., Wolf D., Cheresh D. A., Phillips D. R. Adhesive properties of the beta 3 integrins: comparison of GP IIb-IIIa and the vitronectin receptor individually expressed in human melanoma cells. J Cell Biol. 1991 Apr;113(2):451–461. doi: 10.1083/jcb.113.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieffer N., Phillips D. R. Platelet membrane glycoproteins: functions in cellular interactions. Annu Rev Cell Biol. 1990;6:329–357. doi: 10.1146/annurev.cb.06.110190.001553. [DOI] [PubMed] [Google Scholar]
- Kinlough-Rathbone R. L., Mustard J. F., Packham M. A., Perry D. W., Reimers H. J., Cazenave J. P. Properties of washed human platelets. Thromb Haemost. 1977 Apr 30;37(2):291–308. [PubMed] [Google Scholar]
- Kishimoto T. K., O'Connor K., Lee A., Roberts T. M., Springer T. A. Cloning of the beta subunit of the leukocyte adhesion proteins: homology to an extracellular matrix receptor defines a novel supergene family. Cell. 1987 Feb 27;48(4):681–690. doi: 10.1016/0092-8674(87)90246-7. [DOI] [PubMed] [Google Scholar]
- Kouns W. C., Wall C. D., White M. M., Fox C. F., Jennings L. K. A conformation-dependent epitope of human platelet glycoprotein IIIa. J Biol Chem. 1990 Nov 25;265(33):20594–20601. [PubMed] [Google Scholar]
- Kunicki T. J., Pidard D., Rosa J. P., Nurden A. T. The formation of Ca++-dependent complexes of platelet membrane glycoproteins IIb and IIIa in solution as determined by crossed immunoelectrophoresis. Blood. 1981 Aug;58(2):268–278. [PubMed] [Google Scholar]
- Lanza F., Kieffer N., Phillips D. R., Fitzgerald L. A. Characterization of the human platelet glycoprotein IIIa gene. Comparison with the fibronectin receptor beta-subunit gene. J Biol Chem. 1990 Oct 25;265(30):18098–18103. [PubMed] [Google Scholar]
- Laurell C. B. Quantitative estimation of proteins by electrophoresis in agarose gel containing antibodies. Anal Biochem. 1966 Apr;15(1):45–52. doi: 10.1016/0003-2697(66)90246-6. [DOI] [PubMed] [Google Scholar]
- Legrand C., Dubernard V., Nurden A. T. Studies on the mechanism of expression of secreted fibrinogen on the surface of activated human platelets. Blood. 1989 Apr;73(5):1226–1234. [PubMed] [Google Scholar]
- Loftus J. C., O'Toole T. E., Plow E. F., Glass A., Frelinger A. L., 3rd, Ginsberg M. H. A beta 3 integrin mutation abolishes ligand binding and alters divalent cation-dependent conformation. Science. 1990 Aug 24;249(4971):915–918. doi: 10.1126/science.2392682. [DOI] [PubMed] [Google Scholar]
- Lyman S., Aster R. H., Visentin G. P., Newman P. J. Polymorphism of human platelet membrane glycoprotein IIb associated with the Baka/Bakb alloantigen system. Blood. 1990 Jun 15;75(12):2343–2348. [PubMed] [Google Scholar]
- McEver R. P., Bennett E. M., Martin M. N. Identification of two structurally and functionally distinct sites on human platelet membrane glycoprotein IIb-IIIa using monoclonal antibodies. J Biol Chem. 1983 Apr 25;258(8):5269–5275. [PubMed] [Google Scholar]
- McLean J. W., Vestal D. J., Cheresh D. A., Bodary S. C. cDNA sequence of the human integrin beta 5 subunit. J Biol Chem. 1990 Oct 5;265(28):17126–17131. [PubMed] [Google Scholar]
- Mustard J. F., Kinlough-Rathbone R. L., Packham M. A., Perry D. W., Harfenist E. J., Pai K. R. Comparison of fibrinogen association with normal and thrombasthenic platelets on exposure to ADP or chymotrypsin. Blood. 1979 Nov;54(5):987–993. [PubMed] [Google Scholar]
- Newman P. J., Seligsohn U., Lyman S., Coller B. S. The molecular genetic basis of Glanzmann thrombasthenia in the Iraqi-Jewish and Arab populations in Israel. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3160–3164. doi: 10.1073/pnas.88.8.3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurden A. T., Caen J. P. An abnormal platelet glycoprotein pattern in three cases of Glanzmann's thrombasthenia. Br J Haematol. 1974 Oct;28(2):253–260. doi: 10.1111/j.1365-2141.1974.tb06660.x. [DOI] [PubMed] [Google Scholar]
- Nurden A. T., Rosa J. P., Fournier D., Legrand C., Didry D., Parquet A., Pidard D. A variant of Glanzmann's thrombasthenia with abnormal glycoprotein IIb-IIIa complexes in the platelet membrane. J Clin Invest. 1987 Mar;79(3):962–969. doi: 10.1172/JCI112907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Agin P. P. Platelet membrane defects in Glanzmann's thrombasthenia. Evidence for decreased amounts of two major glycoproteins. J Clin Invest. 1977 Sep;60(3):535–545. doi: 10.1172/JCI108805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips D. R., Charo I. F., Parise L. V., Fitzgerald L. A. The platelet membrane glycoprotein IIb-IIIa complex. Blood. 1988 Apr;71(4):831–843. [PubMed] [Google Scholar]
- Pidard D., Didry D., Kunicki T. J., Nurden A. T. Temperature-dependent effects of EDTA on the membrane glycoprotein IIb-IIIa complex and platelet aggregability. Blood. 1986 Mar;67(3):604–611. [PubMed] [Google Scholar]
- Pidard D., Frelinger A. L., Bouillot C., Nurden A. T. Activation of the fibrinogen receptor on human platelets exposed to alpha chymotrypsin. Relationship with a major proteolytic cleavage at the carboxyterminus of the membrane glycoprotein IIb heavy chain. Eur J Biochem. 1991 Sep 1;200(2):437–447. doi: 10.1111/j.1432-1033.1991.tb16202.x. [DOI] [PubMed] [Google Scholar]
- Pidard D., Montgomery R. R., Bennett J. S., Kunicki T. J. Interaction of AP-2, a monoclonal antibody specific for the human platelet glycoprotein IIb-IIIa complex, with intact platelets. J Biol Chem. 1983 Oct 25;258(20):12582–12586. [PubMed] [Google Scholar]
- Poncz M., Eisman R., Heidenreich R., Silver S. M., Vilaire G., Surrey S., Schwartz E., Bennett J. S. Structure of the platelet membrane glycoprotein IIb. Homology to the alpha subunits of the vitronectin and fibronectin membrane receptors. J Biol Chem. 1987 Jun 25;262(18):8476–8482. [PubMed] [Google Scholar]
- Ramaswamy H., Hemler M. E. Cloning, primary structure and properties of a novel human integrin beta subunit. EMBO J. 1990 May;9(5):1561–1568. doi: 10.1002/j.1460-2075.1990.tb08275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randi A. M., Rabinowitz I., Mancuso D. J., Mannucci P. M., Sadler J. E. Molecular basis of von Willebrand disease type IIB. Candidate mutations cluster in one disulfide loop between proposed platelet glycoprotein Ib binding sequences. J Clin Invest. 1991 Apr;87(4):1220–1226. doi: 10.1172/JCI115122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa J. P., Bray P. F., Gayet O., Johnston G. I., Cook R. G., Jackson K. W., Shuman M. A., McEver R. P. Cloning of glycoprotein IIIa cDNA from human erythroleukemia cells and localization of the gene to chromosome 17. Blood. 1988 Aug;72(2):593–600. [PubMed] [Google Scholar]
- Shattil S. J., Cunningham M., Hoxie J. A. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood. 1987 Jul;70(1):307–315. [PubMed] [Google Scholar]
- Smith J. W., Cheresh D. A. The Arg-Gly-Asp binding domain of the vitronectin receptor. Photoaffinity cross-linking implicates amino acid residues 61-203 of the beta subunit. J Biol Chem. 1988 Dec 15;263(35):18726–18731. [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Arai H., Languino L. R., Pierschbacher M. D., Ruoslahti E. Amino acid sequence of the vitronectin receptor alpha subunit and comparative expression of adhesion receptor mRNAs. J Biol Chem. 1987 Oct 15;262(29):14080–14085. [PubMed] [Google Scholar]
- Suzuki S., Naitoh Y. Amino acid sequence of a novel integrin beta 4 subunit and primary expression of the mRNA in epithelial cells. EMBO J. 1990 Mar;9(3):757–763. doi: 10.1002/j.1460-2075.1990.tb08170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taub R., Gould R. J., Garsky V. M., Ciccarone T. M., Hoxie J., Friedman P. A., Shattil S. J. A monoclonal antibody against the platelet fibrinogen receptor contains a sequence that mimics a receptor recognition domain in fibrinogen. J Biol Chem. 1989 Jan 5;264(1):259–265. [PubMed] [Google Scholar]
- Youssoufian H., Kazazian H. H., Jr, Phillips D. G., Aronis S., Tsiftis G., Brown V. A., Antonarakis S. E. Recurrent mutations in haemophilia A give evidence for CpG mutation hotspots. 1986 Nov 27-Dec 3Nature. 324(6095):380–382. doi: 10.1038/324380a0. [DOI] [PubMed] [Google Scholar]
- Zimrin A. B., Eisman R., Vilaire G., Schwartz E., Bennett J. S., Poncz M. Structure of platelet glycoprotein IIIa. A common subunit for two different membrane receptors. J Clin Invest. 1988 May;81(5):1470–1475. doi: 10.1172/JCI113478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimrin A. B., Gidwitz S., Lord S., Schwartz E., Bennett J. S., White G. C., 2nd, Poncz M. The genomic organization of platelet glycoprotein IIIa. J Biol Chem. 1990 May 25;265(15):8590–8595. [PubMed] [Google Scholar]
- de la Salle C., Baas M. J., Grunebaum L., Wiesel M. L., Blanco A., Gialeraki R., Mandalaki T., Cazenave J. P. Carrier detection in hemophilia using pedigree analysis coagulation tests and DNA probes. Nouv Rev Fr Hematol. 1989;31(3):193–202. [PubMed] [Google Scholar]