Abstract
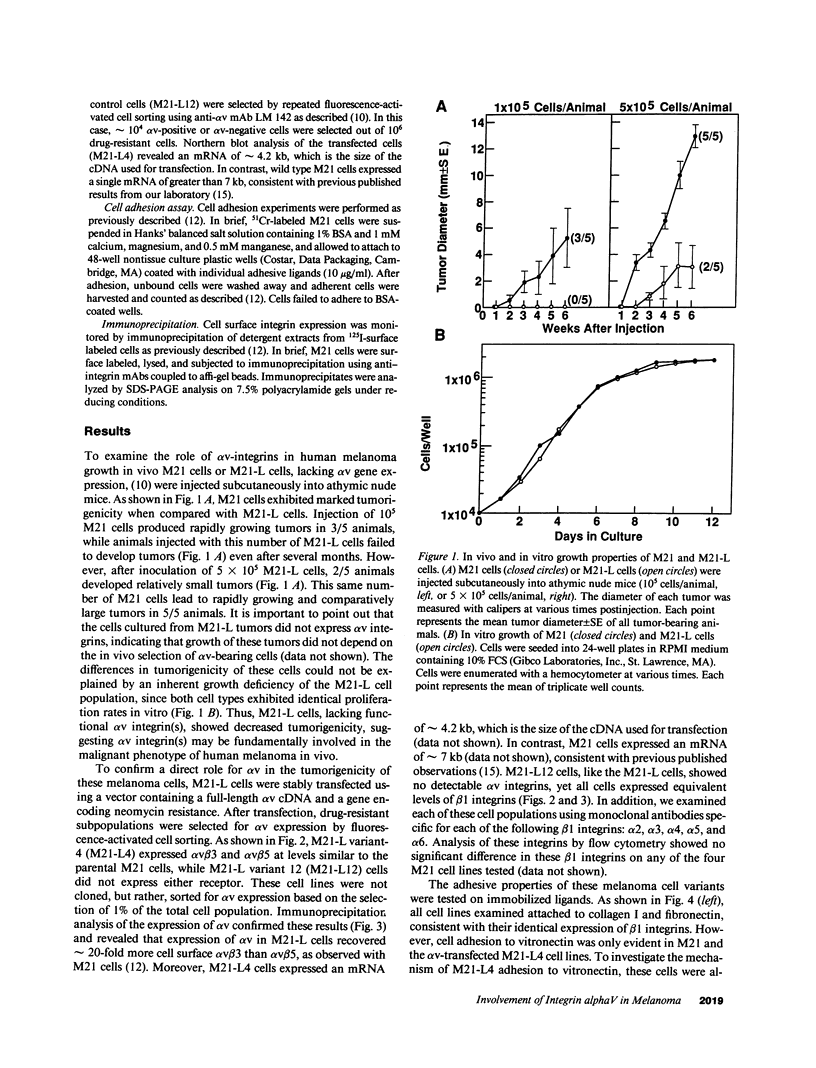
Human melanoma originates in the skin and can lead to wide-spread metastatic disease. Analysis of melanoma biopsy material has shown that the vitronectin receptor, integrin alpha v beta 3, is a specific marker of the most malignant cells, i.e., vertically invasive primary lesions or distant metastases (Albelda, S. M., S. A. Mette, D. E. Elder, R. Stewart, L. Damjanovich, M. Herlyn, and C. A. Buck. 1990. Cancer Res. 50:6757-6764), suggesting a role for this adhesion receptor in the malignant growth of human melanoma tumors. A cell model was established to analyze the role of alpha v integrins on the tumorigenicity of human melanoma. From M21 human melanoma cells, stable variants were selected that lack alpha v gene expression and thus fail to express integrin alpha v beta 3 (M21-L cells). These cells not only lost the ability to attach to vitronectin but showed a dramatic reduction in tumorigenicity when transplanted into athymic nude mice, compared with M21 cells, even though both cell types showed identical beta 1 integrin expression and growth properties in vitro. M21-L cells were stably transfected with a cDNA-encoding alpha v. This resulted in the functional expression of integrin alpha v beta 3 on these cells and completely restored their tumorigenicity. Thus, integrin alpha v gene expression and the resulting adhesive phenotype are directly involved in the proliferation of human melanoma in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Mette S. A., Elder D. E., Stewart R., Damjanovich L., Herlyn M., Buck C. A. Integrin distribution in malignant melanoma: association of the beta 3 subunit with tumor progression. Cancer Res. 1990 Oct 15;50(20):6757–6764. [PubMed] [Google Scholar]
- Barsky S. H., Rao C. N., Williams J. E., Liotta L. A. Laminin molecular domains which alter metastasis in a murine model. J Clin Invest. 1984 Sep;74(3):843–848. doi: 10.1172/JCI111501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodary S. C., McLean J. W. The integrin beta 1 subunit associates with the vitronectin receptor alpha v subunit to form a novel vitronectin receptor in a human embryonic kidney cell line. J Biol Chem. 1990 Apr 15;265(11):5938–5941. [PubMed] [Google Scholar]
- Boukerche H., Berthier-Vergnes O., Bailly M., Doré J. F., Leung L. L., McGregor J. L. A monoclonal antibody (LYP18) directed against the blood platelet glycoprotein IIb/IIIa complex inhibits human melanoma growth in vivo. Blood. 1989 Aug 15;74(3):909–912. [PubMed] [Google Scholar]
- Carter W. G., Wayner E. A., Bouchard T. S., Kaur P. The role of integrins alpha 2 beta 1 and alpha 3 beta 1 in cell-cell and cell-substrate adhesion of human epidermal cells. J Cell Biol. 1990 Apr;110(4):1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan B. M., Matsuura N., Takada Y., Zetter B. R., Hemler M. E. In vitro and in vivo consequences of VLA-2 expression on rhabdomyosarcoma cells. Science. 1991 Mar 29;251(5001):1600–1602. doi: 10.1126/science.2011740. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Honsik C. J., Staffileno L. K., Jung G., Reisfeld R. A. Disialoganglioside GD3 on human melanoma serves as a relevant target antigen for monoclonal antibody-mediated tumor cytolysis. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5155–5159. doi: 10.1073/pnas.82.15.5155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheresh D. A., Smith J. W., Cooper H. M., Quaranta V. A novel vitronectin receptor integrin (alpha v beta x) is responsible for distinct adhesive properties of carcinoma cells. Cell. 1989 Apr 7;57(1):59–69. doi: 10.1016/0092-8674(89)90172-4. [DOI] [PubMed] [Google Scholar]
- Cheresh D. A., Spiro R. C. Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem. 1987 Dec 25;262(36):17703–17711. [PubMed] [Google Scholar]
- Fidler I. J. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990 Oct 1;50(19):6130–6138. [PubMed] [Google Scholar]
- Fitzgerald L. A., Poncz M., Steiner B., Rall S. C., Jr, Bennett J. S., Phillips D. R. Comparison of cDNA-derived protein sequences of the human fibronectin and vitronectin receptor alpha-subunits and platelet glycoprotein IIb. Biochemistry. 1987 Dec 15;26(25):8158–8165. doi: 10.1021/bi00399a021. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Ruoslahti E. Elevated levels of the alpha 5 beta 1 fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990 Mar 9;60(5):849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Olden K., Yamada K. M. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986 Jul 25;233(4762):467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- Humphries M. J., Yamada K. M., Olden K. Investigation of the biological effects of anti-cell adhesive synthetic peptides that inhibit experimental metastasis of B16-F10 murine melanoma cells. J Clin Invest. 1988 Mar;81(3):782–790. doi: 10.1172/JCI113384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Cell surface molecules and tumor metastasis. Regulation of metastatic phenotypic diversity. Exp Cell Res. 1984 Jan;150(1):3–22. doi: 10.1016/0014-4827(84)90696-7. [DOI] [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Poste G., Doll J., Fidler I. J. Interactions among clonal subpopulations affect stability of the metastatic phenotype in polyclonal populations of B16 melanoma cells. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6226–6230. doi: 10.1073/pnas.78.10.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. New perspectives in cell adhesion: RGD and integrins. Science. 1987 Oct 23;238(4826):491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Saiki I., Murata J., Iida J., Nishi N., Sugimura K., Azuma I. The inhibition of murine lung metastasis by synthetic polypeptides [poly(arg-gly-asp) and poly(tyr-ile-gly-ser-arg)] with a core sequence of cell adhesion molecules. Br J Cancer. 1989 Feb;59(2):194–197. doi: 10.1038/bjc.1989.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. W., Vestal D. J., Irwin S. V., Burke T. A., Cheresh D. A. Purification and functional characterization of integrin alpha v beta 5. An adhesion receptor for vitronectin. J Biol Chem. 1990 Jul 5;265(19):11008–11013. [PubMed] [Google Scholar]
- Vogel B. E., Tarone G., Giancotti F. G., Gailit J., Ruoslahti E. A novel fibronectin receptor with an unexpected subunit composition (alpha v beta 1). J Biol Chem. 1990 Apr 15;265(11):5934–5937. [PubMed] [Google Scholar]
- Wayner E. A., Orlando R. A., Cheresh D. A. Integrins alpha v beta 3 and alpha v beta 5 contribute to cell attachment to vitronectin but differentially distribute on the cell surface. J Cell Biol. 1991 May;113(4):919–929. doi: 10.1083/jcb.113.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]




