Abstract
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor that mediates the toxicity of environmental chemicals and regulates many physiological functions, including processes in female reproduction. Previous studies demonstrated that Ahr deletion leads to slow ovarian follicle growth because of impaired estradiol production and reduced gonadotropin responsiveness in prepubertal mice. These studies, however, did not determine how Ahr deletion impairs estradiol production or whether the effects of Ahr deletion on follicle growth and estradiol production persist in adulthood. Thus, the present study evaluated the effect of Ahr deletion on steroid precursors in the estradiol biosynthesis pathway. Furthermore, this study evaluated follicle growth and estradiol biosynthesis in wild-type (WT) and Ahr knockout (AhrKO) antral follicles at different stages of sexual maturity. AhrKO antral follicles from prepubertal mice had slower growth, produced lower estradiol levels, and had reduced cyclin D2 (Ccnd2) expression compared to WT follicles. AhrKO follicles from adult mice, however, produced higher androgen levels and expressed higher levels of Ccnd2 compared to WT follicles. Furthermore, AhrKO follicles from adult mice had growth to that of WT follicles. These findings suggest that the AHR regulates follicle growth by altering factors involved in the estradiol biosynthesis pathway as well as key regulators of follicle growth and that this role of AHR depends on stage of sexual maturity.
Keywords: androgens, aryl hydrocarbon receptor, estradiol, follicle, ovary
The aryl hydrocarbon receptor regulates ovarian follicle growth and estradiol biosynthesis according to stage of sexual maturity.
INTRODUCTION
The aryl hydrocarbon receptor (AHR) is a ligand-activated transcription factor located in the cytosol of cells [1]. For several years, the AHR was only recognized as a mediator of toxicity caused by polychlorinated dioxins and related compounds, because binding of these toxicants to the AHR initiates a cascade of events that often leads to toxicity [2–4]. Recent studies, however, have revealed that the lack of a functional Ahr in mice results in phenotypic changes that suggest participation of the AHR in physiological functions, including female reproduction [5–9]. Female reproductive functions that may require the AHR signaling pathway include fertility, uterine epithelium cyclic changes, embryo nourishment, maintenance of pregnancy, and normal ovarian function [9].
In the ovary, primordial follicles develop into primary, preantral, and early antral follicles [10]. Early antral follicles become highly responsive to follicle-stimulating hormone (FSH) and luteinizing hormone (LH) to initiate biosynthesis of steroid hormones, such as androgens and estrogens [11]. Steroid hormones are paracrine/autocrine regulators necessary for follicle growth and development to the preovulatory stage [11–13]. Thus, antral follicles are required for fertility because they are the only follicles that are capable of releasing eggs for fertilization and producing steroid hormones that regulate menstrual/estrous cyclicity [10].
The presence of the AHR has been described in female reproductive tissues and all cell types in the ovarian follicle (oocyte, granulosa, and theca cells) [14–16]. Deletion of the Ahr produces alterations in follicle growth [17–19]. Specifically, previous studies using Ahr knockout (AhrKO) mice have shown that AhrKO ovaries on Postnatal Days (PDs) 45–53, a time when mice are cycling, contain approximately 50% fewer antral follicles than wild-type (WT) ovaries [17]. A later study by Barnett et al. [19] investigated why AhrKO ovaries on PDs 45–53 have fewer antral follicles than WT ovaries, and those authors found that AhrKO antral follicles isolated from PD 30 ovaries grow more slowly than WT antral follicles. Thus, the slow AhrKO follicle growth at PD 30 likely leads to fewer antral follicles being present in AhrKO ovaries at PDs 45–53. Barnett et al. [19, 20] further elucidated that AhrKO antral follicles have an impaired ability to grow because of reduced abilities to respond to gonadotropins and to produce estradiol.
Although these previous studies suggest that the AHR regulates estradiol production and follicle growth [17–20], the mechanisms by which the AHR regulates estradiol production are unknown. Thus, the present study evaluated the effect of Ahr deletion on the levels of sex steroid precursors and sex steroids. Furthermore, the present study evaluated the effect of Ahr deletion on the expression of key hormone receptors involved in steroid biosynthesis.
Because previous studies only examined the effects of Ahr deletion on follicle growth and estradiol levels in prepubertal mice, it was not known if effects of Ahr deletion on the ovary persist in adulthood. Thus, the present study also evaluated follicle growth and estradiol biosynthesis in WT and AhrKO antral follicles at different stages of sexual maturity. The expression of cyclin D2 (Ccnd2), a gene that plays a fundamental role in follicle growth and development [21], was also compared in WT and AhrKO antral follicles at different stages of sexual maturity.
MATERIALS AND METHODS
Animals
The AhrKO mice (official symbol Ahrtm1Bra) were generated by Schmidt et al. [6] and are in a C57BL/6J background, along with their WT littermates. All mice used were from breeding colonies currently maintained by our laboratory at the University of Illinois, Urbana-Champaign, Veterinary Medicine Animal Facility. Mouse colonies were provided with food and water for ad libitum consumption and were maintained in a temperature- and light-controlled room (24 ± 1°C, 12L:12D photoperiod) with 35% ± 4% relative humidity. Cohorts of homozygous mice (WT [Ahr+/+] and AhrKO [Ahr−/−]) used in each experiment were generated by intercrossing either heterozygous (Ahr+/−) female and male mice or heterozygous Ahr+/− female mice with AhrKO male mice. Mouse genetic screening was performed using ear tissue punches as previously described [17, 18]. All animal care, euthanasia, and tissue collection were approved by the Institutional Animal Use and Care Committee at the University of Illinois.
Antral Follicle Isolation
Female WT and AhrKO mice were euthanized by CO2 inhalation on PDs 30–32, 50–54, and 90–94, and ovaries were removed and placed in α-minimal essential media (α-MEM). WT and AhrKO mice on PDs 30–32 were considered to be of a prepubertal age, as evidenced by lack of a vaginal opening and lack of regular estrous cyclicity. WT and AhrKO mice on PDs 50–54 and 90–94, considered to be adult mice, were monitored daily for estrous cycles via vaginal swabs and were euthanized on estrus. An estrous stage was indicated if the vaginal swab contained predominantly keratinized cells.
Follicles with a diameter of 260–400 μm were mechanically isolated from WT and AhrKO ovaries and cleaned of both interstitial tissue and small follicles using fine watchmaker forceps under a dissecting microscope [22]. Follicles of 260–400 μm were used because their size correlates with the histological appearance of early antral follicles containing multiple granulosa cells layers, a theca cell layer, and an antral space [22]. Upon isolation, early antral follicles were subjected to follicle culture for in vitro follicle growth evaluation or were snap-frozen and stored at −80°C until quantitative PCR (qPCR) analyses as described below.
Measurement of In Vitro Follicle Growth
At least 20 early antral follicles from two to three WT and two to three AhrKO mice on PDs 30–32, 50–54, and 90–94 per experiment were isolated as described above and cultured individually in wells of a 96-well culture plate for 7 days as described previously [19, 22]. Follicle growth patterns were examined by measuring follicle diameters in perpendicular axes every 24 h using an inverted microscope equipped with a calibrator ocular micrometer [22]. Three separate cultures per age-matched WT and AhrKO mice were performed. Culture media were collected on Days 3 and 7 and subjected to measurements of steroid hormone levels as described below.
Measurement of In Vivo Follicle Growth
Ovaries were harvested from four to five WT and four to five AhrKO mice on PDs 90–94. This age range was selected because it is when mice are considered to be cycling adults [10] and when general health problems are not present [23]. Ovaries (one per mouse) were collected and preserved by immersion in Dietrich fixative and were stored at room temperature. Ovaries were then dehydrated in ethanol series, cleared, infiltrated with paraffin wax, and serially sectioned (thickness, 8 μm) throughout the entire ovary. Sections were mounted on slides and stained in hematoxylin with eosin using standard procedures.
Every 10th section of the entire ovary was used to count follicle populations as described previously [17, 18]. Follicles were considered to be primordial if they contained an oocyte surrounded by a single layer of fusiform granulosa cells or by an incomplete layer of cuboidal cells. Follicles were considered to be primary if they contained an oocyte surrounded by a fully formed, single layer of cuboidal granulosa cells. Follicles were considered to be preantral if they contained an oocyte surrounded by two to five layers of granulosa cells and no antral space. Follicles were considered to be antral if they contained an oocyte surrounded by more than five layers of granulosa cells and an antral space. To avoid double-counting follicles, only follicles containing an oocyte with a visible nucleus were counted. To avoid bias, all ovaries were analyzed without knowledge of genotype. Numbers of follicles counted per ovary were multiplied by 10 to obtain an estimate of total follicle number per ovary.
Measurement of Steroid Hormone Levels
Culture media samples were subjected to ELISA using kits for measurement of dehydroepiandrosterone (DHEA; Alpco Diagnostics), androstenedione (DRG International, Inc.), testosterone (Alpco Diagnostics), and estradiol (Calbiotech). Samples were diluted 1:4 (v/v) for androstenedione and 1:10 (v/v) for testosterone analyses. Samples collected on Days 3 and 7 were diluted 1:10 and 1:100, respectively, for estradiol assays. All ELISA procedures were performed according to the manufacturer's protocol. α-MEM was used as background control. Positive controls containing known amounts of specific hormones (provided by the manufacturer) were included in every assay. All samples were run in duplicate, and values were calculated by multiplying by the corresponding dilution factor. Final values were obtained by the sum of hormone levels on Days 3 and 7 of follicle culture. The minimum detection limits, as determined by the ELISA kit manufacturers, were 0.005 ng/ml for DHEA, 0.019 ng/ml for androstenedione, 0.022 ng/ml for testosterone, and 10 pg/ml for estradiol. No samples were below the limit of detection. Intra- and interassay coefficients of variation for all assays were less than 5%.
Topical Autoradiography
Topical autoradiography was performed according to the method described by Oxberry and Greenwald [24] and by Roby [25]. Ovaries were harvested from three WT and three AhrKO mice on PDs 30–32, 50–54, and 90–94 and then frozen in liquid nitrogen, embedded in optimal cutting temperature medium, and cut in a cryostat. The frozen sections (thickness, 10 μm) were placed on Superfrost Plus slides (Fisher Scientific, Pittsburgh, PA), air-dried, fixed in picric acid-formaldehyde, and stored in a desiccator at −80°C until subjected to topical autoradiography of human chorionic gonadotropin (hCG) or FSH receptors. After bringing the slides to room temperature, 125I-labeled hormone (hCG CR-127 or rat FSH-I-9; 10 000 cpm of 20 μCi/mg) was added to the tissue section in 0.2 ml of PBS containing 0.1% bovine serum albumin and incubated for 2 h at 37°C. The hCG and recombinant FSH obtained from the National Hormone and Pituitary Program were iodinated using the lactoperoxidase method [26, 27]. Control, nonspecific binding sections had 10 IU of unlabeled homologous hormone in addition to the radiolabeled hormone. Following incubation, the tissue sections were postfixed in 4% paraformaldehyde and rinsed in PBS. The slides were dipped in autoradiographic emulsion, dried, stored at 4°C for 5 days when using 125I-labeled rat FSH or for 12 days when using 125I-labeled hCG CR-127, and then developed through routine photographic steps of Dektol, Stop, and Fix. Next, the slides were washed and stained with nuclear fast red and picric acid. Tissue sections were photographed on an Olympus 1X71 inverted microscope with an Olympus DP71 digital camera and software. Percentage area occupied by grains in antral follicles was analyzed using ImageJ 1.41o (www.nih.gov/). Data were analyzed using GraphPad Prism software (Version 5.0C; GraphPad Software, La Jolla, CA), and differences in antral follicle grain density/binding between WT and AhrKO ovaries of the same age were evaluated.
Real-Time qPCR
Between 15 and 18 early antral follicles from three to six WT and three to six AhrKO mice on PDs 30–32, 50–54, and 90–94 were isolated as described above and subjected to qPCR. Total RNA (1–2 μg) was extracted from the follicles using the RNeasy Mini Kit (Qiagen, Inc.) and then converted to cDNA using an Omniscript RT kit (Qiagen, Inc.) and random primers (Invitrogen) according to the manufacturers' protocols. The cDNA was amplified by qPCR as described previously [19, 28] using an MJ Research Chromo4 Real Time PCR machine (MJ Research, Inc.) and accompanying software according to the manufacturer's instructions. To allow analysis of the amount of cDNA in the exponential phase, a standard curve from five serial dilutions was generated using cDNA from a pool of WT and AhrKO antral follicles. Specific qPCR primers for the genes of interest and annealing temperatures are listed in Table 1. SYBR Green (Finnzymes, c/o New England) was used as dye for all qPCR analyses. A melting curve was generated at 55–90°C to confirm the generation of a single product, and PCR products were loaded in 3% agarose gel to confirm the product size according to Table 1. Actin, beta (Actb) was used for each sample as an internal control. Relative numbers were generated by the qPCR software per sample, and final values were calculated as the ratio of the gene of interest to Actb for each sample.
TABLE 1.
Primers used in quantitative real-time polymerase chain reaction (qPCR).
Statistical Analysis
The data were analyzed using SAS 9.2 (Statistical Analysis System Institute, Inc.). Independent t-tests were used to compare the numbers of follicles per ovary between WT and AhrKO samples. General linear model (GLM) with repeated measures was used for comparisons over time in follicle cultures. GLM was also used to investigate the effects of age, genotype, and their interaction on the total change in follicle diameter, Ccnd2, hormone levels, steroid hormone receptors, and gonadotropin binding receptors. If the global tests from GLM were significant, Tukey tests were used for pairwise comparisons. Data were expressed as the mean ± SEM. Statistical significance was assigned at P ≤ 0.05.
RESULTS
Effect of Ahr Deletion on Ovarian Follicle Growth and Ccnd2
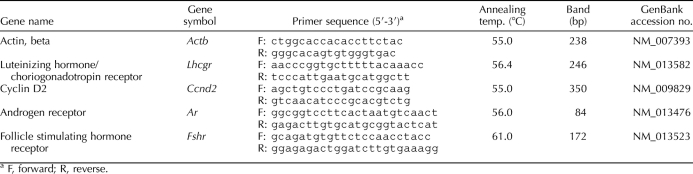
Previous studies demonstrated that follicles isolated from AhrKO ovaries on PD 30 grow more slowly than WT follicles in vitro [19]. In the present study, follicle growth was assessed at different ages of sexual maturity to evaluate whether the effect of the Ahr deletion on follicle growth persists in adulthood (Fig. 1). Similar to previously reported work [19], AhrKO follicles on PDs 30–32 had smaller diameters than WT follicles beginning on Day 4 of culture and persisting until the end of culture (Fig. 1A). AhrKO follicles from both PD 50–54 (Fig. 1B) and PD 90–94 (Fig. 1C) ovaries, however, had diameters similar to those of WT follicles at all time points.
FIG. 1.
In vitro growth of WT and AhrKO antral follicles. Early antral follicles were isolated from WT and AhrKO ovaries on PDs 30–32 (before vaginal opening; A), PDs 50–54 (estrus; B), and PDs 90–94 (estrus; C), and were cultured in supplemented media. WT and AhrKO follicle growth was evaluated by measuring follicle diameter in perpendicular axes every 24 h for 7 days. Total percentage change in WT and AhrKO follicle diameters from Day 0 to Day 7 of culture is shown (D). Each bar represents the mean ± SEM from three separate cultures, each with at least 20 follicles per genotype. In A, asterisks above the bars indicate statistically significant differences between WT and AhrKO follicles (P ≤ 0.05). In D, the letter “a” above the bar indicates a significant difference between WT and AhrKO follicles on PDs 30–32 (P ≤ 0.05). The letter “b” indicates significant difference between WT follicles on PDs 30–32 and WT follicles on PDs 90–94 (P ≤ 0.05).
To compare the total change in growth of WT and AhrKO follicles with age, the percentage change in follicle diameter from Day 0 to Day 7 of culture was compared in WT and AhrKO follicles at PDs 30–32, 50–54, and 90–94 (Fig. 1D). The data indicate that the total percentage change in follicle diameter differs between WT and AhrKO follicles with age. Specifically, the total change in follicle diameter in AhrKO follicles was less than that of WT follicles on PDs 30–32, but not on PDs 50–54 and PDs 90–94 (Fig. 1D). Interestingly, the percentage change in growth of WT follicles declined over time (i.e., the percentage change in growth of WT follicles at PDs 90–94 was less than the percentage change in growth of WT follicles at PDs 30–32), whereas it remained stable over time in AhrKO follicles (i.e., the percentage change in growth was similar in AhrKO follicles at all time points).
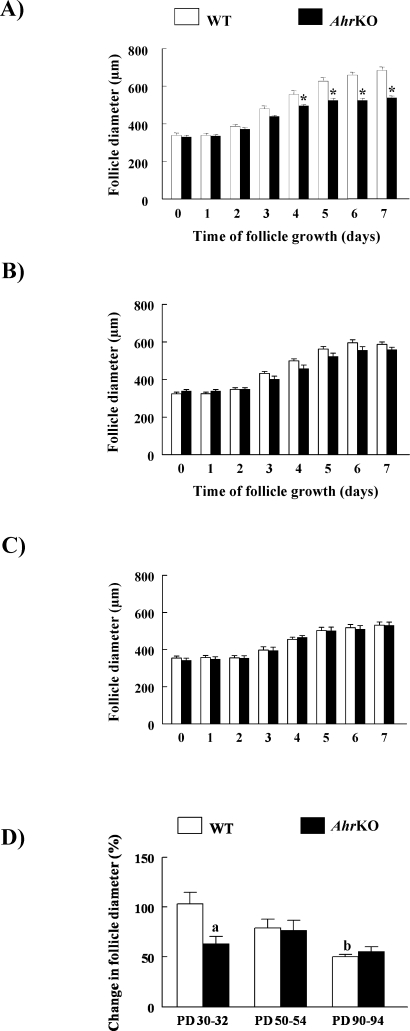
To confirm whether follicle growth was similar in WT and AhrKO ovaries on PDs 90–94, in vivo follicle growth was evaluated in ovarian sections by counting the relative numbers of follicles at each stage of development (Fig. 2). Similar numbers of primordial, primary, preantral, and antral follicles were observed in WT and AhrKO ovaries on PDs 90–94 (Fig. 2).
FIG. 2.
Follicle numbers in WT and AhrKO ovaries from adult ovaries. Ovaries were collected from WT and AhrKO mice on PDs 90–94 during estrus, and complete serial sections were prepared for histological examination of the total numbers of primordial, primary, preantral, and antral follicles as described in Materials and Methods. Each bar represents the mean ± SEM (n = 4–5 ovaries per genotype).
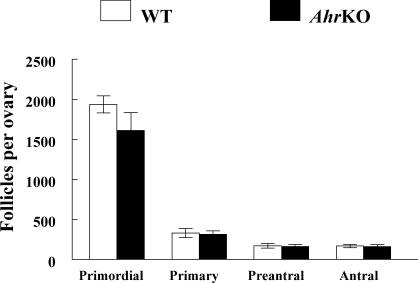
Because in vitro follicle growth differed between WT and AhrKO follicles on PDs 30–32, but at not older ages, the levels of Ccnd2 mRNA, a cell-cycle regulator that promotes the granulosa cell proliferation necessary for follicle growth [21], was compared in WT and AhrKO early antral follicles collected at different ages of sexual maturity (Fig. 3). The data indicate that the levels of Ccnd2 mRNA significantly differed between WT and AhrKO follicles with age. Specifically, on PDs 30–32, AhrKO antral follicles had 2.2-fold lower levels of Ccnd2 mRNA compared to WT follicles, but this difference did not exist on PDs 50–54. On PDs 90–94, AhrKO antral follicles had 4.6-fold higher levels of Ccnd2 mRNA compared to WT follicles. Furthermore, AhrKO follicles on PDs 90–94 had increased levels of Ccnd2 mRNA compared to AhrKO follicles on PDs 30–32 (Fig. 3).
FIG. 3.
Expression of Ccnd2 in WT and AhrKO early antral follicles. Ovaries were removed from WT and AhrKO mice on PDs 30–32 (before vaginal opening), PDs 50–54 (estrus), and PDs 90–94 (estrus). The early antral follicles were immediately isolated from the ovaries and subjected to extraction of RNA. The RNA was subjected to qPCR analysis for Ccnd2 as described in Materials and Methods. All values represent relative expression units of Ccnd2 normalized to Actb as a loading control. Each bar represents the mean ± SEM (n = 3–6 mice/genotype and 15–18 follicles/mouse at each selected age). The letter “a” above the bar indicates a significant difference between WT and AhrKO follicles on PDs 30–32 (P ≤ 0.05). The letter “b” above the bar indicates a significant difference between WT and AhrKO follicles on PDs 90–94 (P ≤ 0.05). The letter “c” above the bar indicates a significant difference between AhrKO follicles on PDs 90–94 and AhrKO follicles on PDs 30–32 (P ≤ 0.05).
Effect of Ahr Deletion on Follicle-Produced Hormones
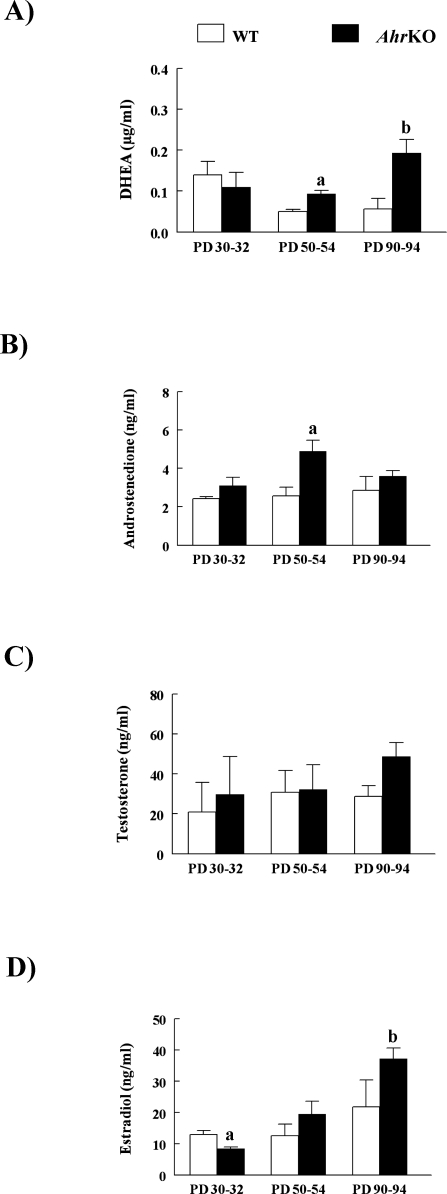
Because granulosa cell proliferation and follicle growth are stimulated by estradiol [21], the abilities of WT and AhrKO follicles to produce estradiol and estradiol precursors were compared by measuring levels of DHEA, androstenedione, testosterone, and estradiol in media from cultured follicles on PDs 30–32, 50–54, and 90–94 (Fig. 4). The levels of DHEA (Fig. 4A), androstenedione (Fig. 4B), and estradiol (Fig. 4D) significantly differed between media samples from WT and AhrKO follicle cultures with age. Specifically, DHEA levels were similar in media samples from WT and AhrKO follicle cultures on PDs 30–32 but were 1.8-fold and 3.4-fold higher in media samples from AhrKO follicle cultures compared to WT follicle cultures on PDs 50–54 and 90–94, respectively (Fig. 4A). Androstenedione levels were similar in media samples from WT and AhrKO follicle cultures on PDs 30–32 and 90–94 but were 1.9-fold higher in media samples from AhrKO follicle cultures compared to WT follicle cultures on PDs 50–54 (Fig. 4B). Estradiol levels were 1.5-fold lower in media samples from AhrKO follicle cultures compared to media samples from WT follicle cultures on PDs 30–32. On PDs 50–54 and 90–94, however, media samples had similar levels of estradiol in WT and AhrKO follicle cultures. Furthermore, media samples from AhrKO follicle cultures on PDs 90–94 had increased estradiol levels compared to media samples from AhrKO follicle cultures on PDs 30–32 (Fig. 4D). Testosterone levels in media samples from WT and AhrKO follicle cultures were similar and did not differ with age (Fig. 4C).
FIG. 4.
Hormone levels in media samples from WT and AhrKO follicle cultures. Early antral follicles from WT and AhrKO ovaries on PDs 30–32 (before vaginal opening), PDs 50–54 (estrus), and PDs 90–94 (estrus) were placed in culture for 7 days. Media samples were collected from follicle cultures and subjected to ELISA to measure levels of DHEA (A), androstenedione (B), testosterone (C), and estradiol (D). Each bar represents the mean ± SEM from three separate cultures, each with at least three separate cultured follicles per genotype at each selected age. In A, the letter “a” above the bar indicates a significant difference between WT and AhrKO samples on PDs 50–54 (P ≤ 0.05), and the letter “b” above the bar indicates a significant difference between WT and AhrKO samples on PDs 90–94 (P ≤ 0.05). In B, the letter “a” above the bar indicates a significant difference between WT and AhrKO samples on PDs 50–54 (P ≤ 0.05). In D, the letter “a” indicates a significant difference between WT and AhrKO samples on PDs 30–32 (P ≤ 0.05), and the letter “b” indicates a significant difference between AhrKO samples on PDs 90–94 and AhrKO samples on PDs 30–32 (P ≤ 0.05).
Effect of Ahr Deletion on Genes Encoding Receptors Involved in Follicle Steroidogenesis
The mRNA expression of genes encoding receptors involved in the synthesis of sex steroid hormone precursors and sex steroids was compared in WT and AhrKO early antral follicles isolated from mice on PDs 30–32, 50–54 and 90–94 (Fig. 5). The levels of luteinizing hormone/choriogonadotropin receptor (Lhcgr) mRNA (Fig. 5A), androgen receptor (Ar) mRNA (Fig. 5B), and Fshr mRNA (Fig. 5C) were significantly different between WT and AhrKO follicles with age. Specifically, the levels of Lhcgr mRNA were 10.8-fold lower in AhrKO follicles compared to WT follicles on PDs 30–32, but this difference did not exist on PDs 50–54. On PDs 90–94, the levels of Lhcgr mRNA were 6.6-fold lower in AhrKO follicles compared to WT follicles (Fig. 5A). The levels of Ar mRNA were similar in WT and AhrKO antral follicles on PDs 30–32 but were 1.8-fold lower in AhrKO follicles compared to WT follicles on PDs 50–54. In contrast, the levels of Ar mRNA were 2.2-fold higher in AhrKO follicles compared to WT follicles on PDs 90–94. Furthermore, the levels of Ar mRNA were lower in WT follicles on PDs 90–94 compared to WT follicles on PDs 30–32 and 50–54 (Fig. 5B). The levels of Fshr mRNA were 1.5-fold lower in AhrKO follicles compared to WT follicles on PDs 30–32 but were similar in WT and AhrKO follicles on both PDs 50–54 and PDs 90–94. In addition, AhrKO follicles on PDs 50–54 had increased levels of Fshr mRNA compared to AhrKO follicles on PDs 30–32 (Fig. 5C).
FIG. 5.
Expression of mRNA encoding follicular receptors involved in estradiol biosynthesis. Ovaries were removed from WT and AhrKO mice on PDs 30–32 (before vaginal opening), PDs 50–54 (estrus), and PDs 90–94 (estrus). The early antral follicles were immediately isolated from the ovaries and subjected to extraction of RNA. The RNA was subjected to qPCR analysis for Lhcgr (A), Ar (B), and Fshr (C) as described in Materials and Methods. All values represent relative expression units of the gene of interest normalized to Actb as a loading control. Each bar represents the mean ± SEM relative units (n = 3–6 mice/genotype and at least 15–18 follicles/mouse at each selected age). In A, the letter “a” above the bar indicates a significant difference between WT and AhrKO samples on PDs 30–32 (P ≤ 0.05), and the letter “b” above the bar indicates a significant difference between WT and AhrKO follicles on PDs 90–94 (P ≤ 0.05). In B, the letter “a” above the bar indicates a significant difference between WT and AhrKO follicles on PDs 50–54 (P ≤ 0.05), the letter “b” above the bar indicates a significant difference between WT follicles on PDs 90–94 and WT follicles on PDs 30–32 (P ≤ 0.05), as well as a significant difference between WT follicles on PDs 90–94 and WT follicles on PDs 50–54 (P ≤ 0.05). The letter “c” above the bar indicates a significant difference between WT and AhrKO follicles on PDs 90–94 (P ≤ 0.05). In C, the letter “a” indicates a significant difference between WT and AhrKO follicles on PDs 30–32 (P ≤ 0.05), and the letter “b” indicates a significant difference between AhrKO follicles on PDs 50–54 and AhrKO follicles on PDs 30–32 (P ≤ 0.05).
Effect of Ahr Deletion on Functional Binding Ability of FSHR and LHCGR in Follicles
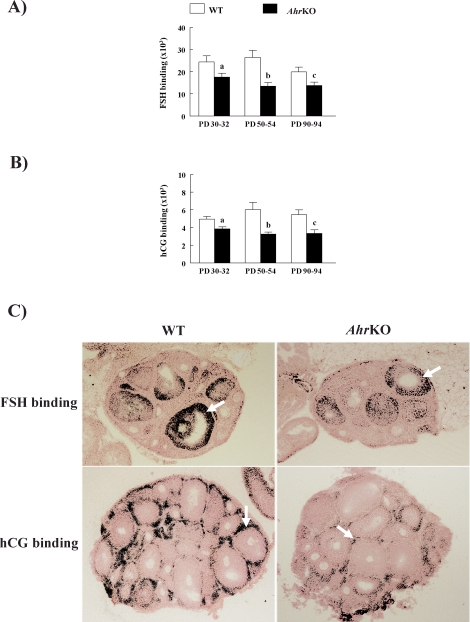
Because the synthesis of sex steroid hormone precursors and sex steroids in antral follicles are promoted by FSH and LH binding to their corresponding receptors in the follicle, the functional binding-abilities of both FSHR and LHCGR were compared in WT and AhrKO early antral follicles from ovaries on PDs 30–32, 50–54, and 90–94 (Fig. 6). The abilities of WT and AhrKO follicles to bind both 125I-radiolabeled FSH (Fig. 6A) and 125I-radiolabeled hCG (Fig. 6B) differed by genotype but not by age. Specifically, AhrKO early antral follicles showed reduced ability to bind both 125I-radiolabeled FSH (Fig. 6A) and 125I-radiolabeled hCG (Fig. 6B) compared to WT early antral follicles at all evaluated ages. Furthermore, 125I-radiolabeled FSH was predominately localized in the granulosa cells of both WT and AhrKO follicles (Fig. 6C, top), whereas 125I-radiolabeled hCG was mainly in theca cells of WT and AhrKO follicles (Fig. 6C, bottom).
FIG. 6.
Effect of Ahr deletion on FSHR and LHCGR in antral follicles. Ovaries were removed from WT and AhrKO mice on PDs 30–32 (before vaginal opening), PDs 50–54 (estrus), and PDs 90–94 (estrus) and subjected to topical autoradiography as described in Materials and Methods. A) 125I-labeled rat FSH was used to localize FSHR binding sites. B) 125I-labeled hCG CR-127 (LH analog) was used to localize LHCGR binding sites. Arrows point to antral follicle grain/density binding for FSHR (top) and LHCGR (bottom). The letter “a” above bars indicates a significant difference between WT and AhrKO ovaries on PDs 30–32 (P ≤ 0.05). The letter “b” above bars indicates a significant difference between WT and AhrKO ovaries on PDs 50–54 (P ≤ 0.05). The letter “c” above bars indicates a significant difference between WT and AhrKO ovaries on PDs 90–94 (P ≤ 0.05). C) Representative micrographs of ovaries on PDs 30–32 collected from each genotype (original magnification ×50) and incubated with 125I-labeled rat FSH (top) or 125I-labeled hCG CR-127 (bottom).
DISCUSSION
The present study describes the dynamics of follicle growth in AhrKO mice and demonstrates differences in follicle growth and estradiol biosynthesis between sexually immature and sexually mature AhrKO mice (Fig. 7). Previous studies demonstrated that AhrKO ovaries on PD 30 have similar numbers of antral follicles compared to WT ovaries [17, 18]. When follicle growth patterns were compared in WT and AhrKO antral follicles from ovaries on PD 30 using in vitro follicle cultures, the present study and that by Barnett et al. [19] found that AhrKO antral follicles grow more slowly than WT follicles. This pattern of slow follicle growth in AhrKO follicles may explain why almost half as many follicles reach the antral stage by PDs 45–53 in AhrKO ovaries as in WT ovaries [17, 18]. This slow growth of AhrKO antral follicles, however, seems to occur only in the early reproductive ages, because the present study further shows that WT and AhrKO antral follicles have similar growth patterns on PDs 50–54 and 90–94 in vitro, as well as similar numbers of antral follicles in WT and AhrKO ovaries on PD 90 in vivo.
FIG. 7.
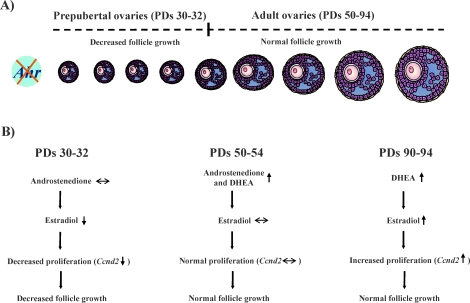
The impact of the AHR on follicle growth and estradiol biosynthesis. A) The lack of the Ahr reduces antral follicle growth in prepubertal ovaries (PDs 30–32), but follicle growth returns to WT levels in adulthood (PDs 50–94). B) Decreased follicle growth in AhrKO antral follicles on PDs 30–32 may be caused by decreased estradiol levels and decreased Ccnd2, a main regulator of granulosa cell proliferation. Between PD 32 and PD 50, sex steroid biosynthesis becomes modified in AhrKO ovaries, resulting in higher levels of androstenedione and DHEA. This may help restore estradiol levels and Ccnd2 expression in AhrKO follicles and, thus, return AhrKO follicle growth to WT levels. Even though follicle growth is normal in AhrKO follicles on PDs 90–94, sex steroid biosynthesis in AhrKO follicles also varies from PD 53 to PD 90, because AhrKO follicles on PDs 90–94 produce high levels of DHEA. As a result, estradiol production by AhrKO follicles increases at this time compared to estradiol production by AhrKO follicles at PDs 30–32, which may promote increases in the levels of Ccnd2.
Whereas all the reasons for the slow antral follicle growth in AhrKO ovaries compared to WT ovaries are unknown, Barnett et al. [19] showed that the slow AhrKO follicle growth may be caused by reduced estradiol biosynthesis and that estradiol replacement restores AhrKO follicle growth to WT levels. The present study expands these previous findings, because it examined whether the reduced estradiol biosynthesis by AhrKO follicles was caused by reduced steroid precursor production by AhrKO follicles compared to WT follicles. The present study also expands previous studies by determining whether any differences in the production of steroid hormone precursors and estradiol between WT and AhrKO follicles from prepubertal mice persist in adulthood. Collectively, the data indicate that the levels of the selected steroid hormone precursors are not significantly lower in AhrKO mice compared to WT mice (Fig. 7). Thus, it seems unlikely that the low levels of estradiol produced by AhrKO follicles at PDs 30–32 are caused by low levels of steroid precursors produced by AhrKO follicles compared to WT follicles at this time. Interestingly, the levels of androgens (DHEA and androstenedione) produced by AhrKO follicles from adult mice are significantly higher than those produced by WT follicles from adult mice. Because levels of estradiol produced by AhrKO follicles are restored by WT levels around this same time, it is possible one mechanism that leads to restoration of estradiol levels involves an increase in androgen production followed by conversion of the androgens to estradiol. Estradiol may then up-regulate Ccnd2 to maintain normal follicle growth (Fig. 7). This idea is supported by studies showing the proliferative effects of estrogens via regulating Ccnd2 in granulosa cells [21, 29, 30].
The reasons for differences in growth and estradiol biosynthesis between WT and AhrKO follicles with stage of sexual maturity are unknown. Interestingly, the percentage change in diameter in WT follicles, but not in AhrKO follicles, declines with age, suggesting that WT follicles are perhaps losing some capacity to grow over time compared to AhrKO follicles. This in turn could lead to WT follicles and AhrKO follicles eventually having similar growth rates. Several studies indicate that immature follicles are more responsive than mature follicles to gonadotropin stimulation [31–33]. Thus, it is possible that WT follicles from PD 30–32 ovaries are more responsive to gonadotropins than WT follicles from PD 50–94 ovaries, and that this results in increased growth of WT follicles from PD 30–32 ovaries compared to WT follicles from PD 50–94 ovaries. In addition, previous studies indicate that whereas circulating levels of both FSH and LH are normal in adult AhrKO mice [8, 20], AhrKO ovaries have reduced gonadotropin responsiveness compared to WT ovaries [20]. Furthermore, in the present study, we observed a reduced binding capability of both FSHR and LHCGR in AhrKO ovaries compared to that in WT ovaries. Therefore, it is possible that the reduced FSHR and LHCGR binding capacity and gonadotropin responsiveness of AhrKO follicles leads to decreased expression of genes required for normal follicle growth and estradiol biosynthesis. This hypothesis is supported by studies indicating that FSH plays a role in the regulation of some steroidogenic genes, such as Cyp19a1 [34, 35], Cyp11a1 [36], as well as Lhcgr and Fshr [35]. It is also supported by data from Baba et al. [8], who showed that the AHR is required for the proper expression of Cyp19a1 in immature ovaries primed with external hormones.
Although we observed reduced binding capacity both FSHR and LHCGR in AhrKO ovaries compared to WT ovaries, expression of Lhcgr and Fshr was not always lower in AhrKO ovaries compared to WT ovaries. These data suggest that that the protein and mRNA expression of both receptors are not correlated completely. Whereas mRNA expression of both Lhcgr and Fshr in AhrKO follicles returns to WT levels by PDs 50–54, corresponding binding capacity remains diminished in the AhrKO follicles. These findings suggest that the transcription of both receptors may be more directly modulated by the hormonal changes observed in the AhrKO follicles from PDs 50–54 than protein translation. However, further studies are needed to test this hypothesis. Similar uncoupled transcription and translation have also been described for estrogen receptors in AhrKO follicles [19].
The normal levels of Fshr mRNA observed in AhrKO follicles on PDs 50–54 in the present study seem to be in contrast to previous findings, which showed via chromatin immunoprecipitation (ChIP) assays that the AHR directly binds to Fshr promoter in adult ovaries [20]. ChIP assays, however, only measure binding of proteins to a promoter region of a target gene; they do not measure transcription directly [37]. Thus, taken together, the data suggest that the AHR binds to the Fshr, but that the binding does not alter transcription of the Fshr at all ages.
In our study, we observed lower levels of Ar mRNA on PDs 50–54 in AhrKO follicles compared to WT follicles. It is possible that increases in DHEA and androstenedione on PDs 50–54 in AhrKO follicles lead to down-regulation of the Ar at PDs 50–54. At PDs 90–94, however, AhrKO follicles showed an increase in the levels of Ar mRNA. The reasons for this are unknown, but studies have demonstrated both an up-regulation of Ar mRNA in ventral prostate from castrated rats (testosterone deprivation) and a down-regulation of Ar mRNA in ventral prostate from castrated rats after treatment with testosterone propionate [38]. Thus, it is possible that factors in the AhrKO ovaries cause either an up-regulation or a down-regulation of Ar mRNA, depending on the age of the animal.
The restoration of growth and estradiol production in AhrKO antral follicles to WT levels by PDs 50–54 is consistent with data obtained from in vivo studies of follicle numbers and estradiol production. Specifically, our in vitro data indicate that growth of early antral follicles is slow in AhrKO ovaries compared to WT ovaries on PDs 30–32. This slow growth of AhrKO follicles likely results in the reduced number of antral follicles observed in AhrKO ovaries compared to WT ovaries by PDs 45–53 [17]. Because antral follicles produce estradiol, the reduced number of antral follicles in AhrKO mice may lead to the low levels of circulating estradiol observed in AhrKO mice compared to WT mice on PDs 30–90 [19]. Furthermore, our in vitro data indicate that the growth of AhrKO follicles is similar to WT levels by PDs 50–54. This growth pattern likely leads to the similar number of antral follicles observed in WT and AhrKO ovaries by PD 90. It also likely means that after antral follicle numbers in AhrKO ovaries are restored to WT levels, circulating estradiol levels in AhrKO mice are restored to WT levels at some point. In fact, our data indicate that WT and AhrKO mice have similar levels of circulating estradiol on PD 180 (data not shown). Taken together, the data obtained in the present study suggest that the AHR may play a role in regulating follicle growth and estradiol biosynthesis in prepubertal mice but not in adult mice. Future studies should address the molecular and biochemical mechanisms by which the AHR regulates follicle growth and estradiol biosynthesis in prepubertal mice. Such studies will lead to a better understanding of the physiological role of the AHR.
Acknowledgments
The authors acknowledge Candace Mainor for her initial help with qPCR, Sharon Meachum for her technical assistance, Jackye Peretz for her help in genotyping and maintaining the AhrKO mouse colony, and Liying Gao for her help in designing qPCR primers for Fshr. The authors also acknowledge Dr. Hsin-Yi Weng (Department of Pathobiology, University of Illinois) for her help and advice in statistical analysis. In addition, the authors thank the reviewers of this manuscript for their constructive criticism, which undoubtedly improved this paper.
Footnotes
Supported by NIH R01HD047275 (to J.A.F.) and NIH MARC Predoctoral Fellowship F31 GM072195 (to K.R.B.-R.).
REFERENCES
- Rowlands JC, Gustafsson JA.Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol 1997; 27: 109–134. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E.Chlorinated dibenzo-p-dioxins: potent inducers of delta-aminolevulinic acid synthetase and aryl hydrocarbon hydroxylase. II. A study of the structure-activity relationship. Mol Pharmacol 1973; 9: 736–747. [PubMed] [Google Scholar]
- Poland A, Glover E, Kende AS.Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem 1976; 251: 4936–4946. [PubMed] [Google Scholar]
- Safe SH.Modulation of gene expression and endocrine response pathways by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related compounds. Pharmacol Ther 1995; 67: 247–281. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ.Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 1995; 268: 722–726. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA.Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A 1996; 93: 6731–6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott BD, Schmid JE, Pitt JA, Buckalew AR, Wood CR, Held GA, Diliberto JA.Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol Appl Pharmacol 1999; 155: 62–70. [DOI] [PubMed] [Google Scholar]
- Baba T, Mimura J, Nakamura N, Harada N, Yamamoto M, Morohashi K, Fujii-Kuriyama Y.Intrinsic function of the aryl hydrocarbon (dioxin) receptor as a key factor in female reproduction. Mol Cell Biol 2005; 25: 10040–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Ochoa I, Karman BN, Flaws JA.The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol 2009; 77: 547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfield AN.Development of follicles in the mammalian ovary. Int Rev Cytol 1991; 124: 43–101. [DOI] [PubMed] [Google Scholar]
- Drummond AE.The role of steroids in follicular growth. Reprod Biol Endocrinol 2006; 4: 16–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AA, Gosden RG, Allison V, Spears N.Effect of androgens on the development of mouse follicles growing in vitro. J Reprod Fertil 1998; 113: 27–33. [DOI] [PubMed] [Google Scholar]
- Hickey TE, Marrocco DL, Amato F, Ritter LJ, Norman RJ, Gilchrist RB, Armstrong DT.Androgens augment the mitogenic effects of oocyte-secreted factors and growth differentiation factor 9 on porcine granulosa cells. Biol Reprod 2005; 73: 825–832. [DOI] [PubMed] [Google Scholar]
- Robles R, Morita Y, Mann KK, Perez GI, Yang S, Matikainen T, Sherr DH, Tilly JL.The aryl hydrocarbon receptor, a basic helix-loop-helix transcription factor of the PAS gene family, is required for normal ovarian germ cell dynamics in the mouse. Endocrinology 2000; 141: 450–453. [DOI] [PubMed] [Google Scholar]
- Hasan A, Fischer B.Epithelial cells in the oviduct and vagina and steroid-synthesizing cells in the rabbit ovary express AhR and ARNT. Anat Embryol (Berl) 2003; 207: 9–18. [DOI] [PubMed] [Google Scholar]
- Baldridge MG, Hutz RJ.Autoradiographic localization of aromatic hydrocarbon receptor (AHR) in rhesus monkey ovary. Am J Primatol 2007; 69: 681–691. [DOI] [PubMed] [Google Scholar]
- Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA.Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci 2000; 56: 382–388. [DOI] [PubMed] [Google Scholar]
- Benedict JC, Miller KP, Lin TM, Greenfeld C, Babus JK, Peterson RE, Flaws JA.Aryl hydrocarbon receptor regulates growth, but not atresia, of mouse preantral and antral follicles. Biol Reprod 2003; 68: 1511–1517. [DOI] [PubMed] [Google Scholar]
- Barnett KR, Tomic D, Gupta RK, Miller KP, Meachum S, Paulose T, Flaws JA.The aryl hydrocarbon receptor affects mouse ovarian follicle growth via mechanisms involving estradiol regulation and responsiveness. Biol Reprod 2007; 76: 1062–1070. [DOI] [PubMed] [Google Scholar]
- Barnett KR, Tomic D, Gupta RK, Babus JK, Roby KF, Terranova PF, Flaws JA.The aryl hydrocarbon receptor is required for normal gonadotropin responsiveness in the mouse ovary. Toxicol Appl Pharmacol 2007; 223: 66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond AE, Findlay JK.The role of estrogen in folliculogenesis. Mol Cell Endocrinol 1999; 151: 57–64. [DOI] [PubMed] [Google Scholar]
- Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA.Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci 2005; 88: 213–221. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Ward JM, Sundberg JP, Gonzalez FJ.Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol 1997; 34: 605–614. [DOI] [PubMed] [Google Scholar]
- Oxberry BA, Greenwald GS.An autoradiographic study of the binding of 125I-labeled follicle-stimulating hormone, human chorionic gonadotropin and prolactin to the hamster ovary throughout the estrous cycle. Biol Reprod 1982; 27: 505–516. [DOI] [PubMed] [Google Scholar]
- Roby KF.Alterations in follicle development, steroidogenesis, and gonadotropin receptor binding in a model of ovulatory blockade. Endocrinology 2001; 142: 2328–2335. [DOI] [PubMed] [Google Scholar]
- Catt KJ, Ketelslegers J, Dufau ML.Receptors for gonadotropic hormones. Blecher M.Methods in Receptor Research. New York:Marcel Dekker;1976: 175–250. [Google Scholar]
- Kim I, Greenwald GS.Occupied and unoccupied FSH receptors in follicles of cyclic, hypophysectomized or hypophysectomized/gonadotropin-treated hamsters. Mol Cell Endocrinol 1986; 44: 141–145. [DOI] [PubMed] [Google Scholar]
- Tomic D, Miller KP, Kenny HA, Woodruff TK, Hoyer P, Flaws JA.Ovarian follicle development requires Smad3. Mol Endocrinol 2004; 18: 2224–2240. [DOI] [PubMed] [Google Scholar]
- Robker RL, Richards JS.Hormone-induced proliferation and differentiation of granulosa cells: a coordinated balance of the cell cycle regulators cyclin D2 and p27Kip1. Mol Endocrinol 1998; 12: 924–940. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGinnis LK, Biggers JD, Eppig JJ, Bronson RT, et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature 1996; 384: 470–474. [DOI] [PubMed] [Google Scholar]
- Zarrow MX, Wilson ED.The influence of age on superovulation in the immature rat and mouse. J Reprod Fertil 1961; 94: 492–496. [Google Scholar]
- Neal P, Baker TG.Response of mouse ovaries in vivo and in organ culture to pregnant mare's serum gonadotrophin and human chorionic gonadotrophin. III. Effect of age. J Reprod Fertil 1974; 39: 411–414. [DOI] [PubMed] [Google Scholar]
- Neal P, Challoner S.The development of the mouse ovary and its response to exogenous gonadotropins. J Reprod Fertil 1975; 45: 449–454. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick SL, Richards JS.Regulation of cytochrome P450 aromatase messenger ribonucleic acid and activity by steroids and gonadotropins in rat granulosa cells. Endocrinology 1991; 129: 1452–1462. [DOI] [PubMed] [Google Scholar]
- Liu Z, Rudd MD, Hernandez-Gonzalez I, Gonzalez-Robayna I, Fan HY, Zeleznik AJ, Richards JS.FSH and FOXO1 regulate genes in the sterol/steroid and lipid biosynthetic pathways in granulosa cells. Mol Endocrinol 2009; 23: 649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring NB, Durica JM, Lifka J, Hedin L, Ratoosh SL, Miller WL, Orly J, Richards JS.Cholesterol side-chain cleavage P450 messenger ribonucleic acid: evidence for hormonal regulation in rat ovarian follicles and constitutive expression in corpora lutea. Endocrinology 1987; 120: 1942–1950. [DOI] [PubMed] [Google Scholar]
- Kuo MH, Allis CD.In vivo cross-linking and immunoprecipitation for studying dynamic protein:DNA associations in a chromatin environment. Methods 1999; 19: 425–433. [DOI] [PubMed] [Google Scholar]
- Quarmby VE, Yarbrough WG, Lubahn DB, French FS, Wilson EM.Autologous down-regulation of androgen receptor messenger ribonucleic acid. Mol Endocrinol 1990; 4: 22–28. [DOI] [PubMed] [Google Scholar]