Abstract
Toll-like receptors (TLRs) recognize molecular constituents of pathogens and activate host innate immune responses. TLR2 responds to Gram-positive organisms and components of their cell walls. TLR3 responds to double-stranded RNA (an intermediate in viral replication). A mouse macrophage cell line (RAW 264.7) and freshly obtained mouse peritoneal macrophages were treated in tissue culture for 5 or 10 h with either peptidoglycan (PGN; a TLR2 ligand, 1 μg/ml), polyinosinic:cytidylic acid (poly(I:C); a TLR3 ligand, 10 μg/ml), both PGN and poly(I:C), or neither. Total RNA was extracted, and RT-PCR was performed. A mouse model of preterm birth induced by intrauterine injection of TLR ligands was used to test in vivo effects. Compared to stimulation with either PGN or poly(I:C) alone, stimulation of macrophages with both ligands (whether simultaneously or sequentially) resulted in synergistic expression of inflammatory mediators, including inducible nitric oxide synthase, interleukin 1 beta, tumor necrosis factor alpha, and the chemokine CCL5 (RANTES). Using peritoneal macrophages obtained from mutant and control mice, this synergy was determined to be dependent upon TLR2 and the TLR-related intracellular adaptor proteins MYD88 and TICAM1 (TRIF). Simultaneous administration of both PGN and poly(I:C) to pregnant mice also produced dramatic synergy in the occurrence of preterm delivery. These results support a possible role for viral infection in preterm labor. Synergy in the mechanisms of parturition suggests the existence of a “two-hit” trigger mechanism that minimizes responses to stimuli of limited biological significance while providing an efficient amplification strategy for rapid activation of labor in response to multiple or more severe insults.
Keywords: inflammation, innate immunity, macrophage, parturition, pregnancy, preterm birth, toll-like receptors
Activation of both TLR2 and TLR3 has a synergistic effect on preterm delivery and on the expression of inflammatory markers in a mouse model.
INTRODUCTION
Preterm birth is considered to be the most important cause of neonatal morbidity and mortality in developed countries, accounting for 60–80% of neonatal deaths not resulting from congenital anomalies [1]. The mechanisms underlying the onset of parturition in humans, whether physiologic or pathologic, are largely unknown. A significant proportion of preterm births occurs in association with microbial invasion of the gestational compartment, with the highest likelihood (estimated at 50% or more) in cases of extreme prematurity [2, 3]. The mechanisms by which microbial pathogens induce labor have not been fully elucidated; however. evidence supports key roles for several factors, including inflammatory cytokines and prostaglandins [4]. Newer data suggest an important role for signaling by toll-like receptors (TLRs) in bacterially induced labor [5–12].
The TLRs are membrane-bound proteins that activate the innate immune system by recognizing specific molecular signatures of various pathogens. Thus far, 13 TLRs have been identified in mammals. TLR2 mediates cellular responses to Gram-positive organisms via their membrane lipoproteins, glycolipids, and peptidoglycans [13]. TLR3 is involved in the response to viral infection by recognizing double-stranded RNA [14], a replication intermediate of most viruses.
Once a TLR has been engaged by a ligand, a sequence of downstream signaling events occurs to activate the host response [15]. These signaling events progress via two main pathways and are classified according to the participation of an intracellular adaptor protein known as MYD88 (myeloid differentiation primary-response gene 88). All TLRs, with the exception of TLR3, signal via the “MYD88-dependent” pathway. TLR3 does not signal via MYD88 but, rather, uses the “MYD88-independent” pathway, via an alternate adaptor protein known as TICAM1 (TRIF, toll/interleukin [IL] 1 receptor-domain-containing adaptor protein inducing interferon beta [IFNB]). Activation of either of these two pathways leads to expression of distinctive, yet partially overlapping, sets of inflammatory and other mediators, including nuclear factor kapa B (primarily activated via MYD88, with a late phase mediated by TICAM1) and both the chemokine CCL5 (RANTES) and interferon-dependent genes (primarily activated via TICAM1). TLR4 is the only TLR that signals via both the MYD88-dependent and the MYD88-independent pathways [16, 17].
In the present study, we investigate the effects of combined stimulation of the MYD88-dependent and MYD88-independent pathways via activation of TLR2 and TLR3, respectively, both in vitro and in a mouse pregnancy model. Such combined stimulation might occur in nature in at least four scenarios: 1) engagement of TLR4; 2) activation of both TLR3 and another TLR simultaneously by a single organism (e.g., murine cytomegalovirus, herpes simplex virus, and Schistosoma mansoni [18, 19]); 3) superinfection, in which a host is infected simultaneously by more than one microorganism, such as a virus and a bacterium; and 4) activation of TLRs by one of several known, endogenously produced TLR ligands together with an exogenous pathogen. We hypothesize that some cases of preterm birth in women involve one or more of these mechanisms.
MATERIALS AND METHODS
Reagents
Peptidoglycan (PGN), extracted from Staphylococcus aureus (Sigma Chemical Company), was suspended in PBS. Polyinosinic:cytidylic acid (poly(I:C); Amersham Biosciences) was dissolved in diethyl pyrocarbonate-treated water (Invitrogen). Frozen aliquots were thawed and diluted before use in experiments.
Cell Culture
Two sources of macrophages were used for cell culture experiments: 1) the mouse macrophage cell line RAW 264.7 (American Type Culture Collection TIB-71), and 2) thioglycolate-stimulated peritoneal macrophages freshly harvested as previously described [20]. Briefly, mice were injected intraperitoneally with 1 ml of 3% thioglycolate. Three days after injection, peritoneal exudate cells were isolated by washing the peritoneal cavity with 10 ml of ice-cold PBS. These cells were incubated for 2 h in tissue culture plates, and adherent cells were considered to be peritoneal macrophages.
RAW 264.7 cells were cultured in Dulbecco modified Eagle medium (high glucose; 11965–092; Gibco) supplemented with 10% fetal bovine serum, 1% streptomycin, and 1% penicillin in tissue culture flasks at 37°C in 5% CO2/95% air and were passaged every 2 or 3 days to maintain logarithmic growth. Freshly isolated peritoneal macrophages were cultured under similar conditions, supplemented with 10 mM Hepes. Before each experiment, cells (4 × 105 cells/well) were plated in triplicate in six-well plates, incubated for 2 h, and then treated with either PBS, PGN (1 μg/ml), poly(I:C) (5 or 10 μg/ml), or both PGN and poly(I:C). For sequential incubations, medium was removed, and cells were washed three times with PBS before incubation with a second reagent. All tissue culture experiments were performed in triplicate and repeated three times.
Viability of cultured cells was assessed using trypan blue dye exclusion. For RAW 264.7 cells, viability before plating was 97% and 5 h after plating, viability was 95% for control (medium) treatment, 93.1% for PGN, 93.5% for poly(I:C), and 92.5% for PGN plus poly(I:C). For peritoneal macrophages, the corresponding viability values were as follows: preplating: 94%; after plating: control, 90%; PGN, 88.5%; poly(I:C), 89%; PGN plus poly(I:C), 86%. The postplating values are not statistically significantly different from each other.
RT-PCR Analysis
We evaluated the activation of TLR signaling pathways using RT-PCR by measuring the relative quantity of transcripts primarily under control of the MYD88-dependent pathway (IL1B and nitric oxide synthase [NOS2]), the MYD88-independent pathway (CCL5), and both pathways tumor necrosis factor (TNF). At the end of tissue culture experiments, medium was aspirated, and cells were washed once with PBS and then lysed in the wells with TRIzol reagent (Invitrogen) to extract total RNA according to the manufacturer's protocol. The cDNA was prepared using random primers and the Moloney Murine Leukemia Virus reverse transcriptase system (Invitrogen). All PCR primers and probes were purchased from Applied Biosystems (IL1B, Mm00434228; NOS2, Mm00440485; CCL5 (RANTES), Mm01302428; TNF, Mm00443258; TLR2, Mm00442354; TLR3, Mm01207403; Mouse glyceraldehyde 3-phosphate dehydrogenase [GAPDH; 20×], 4452339E). Use of TaqMan PCR Reagent Kits was in accordance with the manufacturer's manual. Reactions occurred in a 10-μl mixture containing 0.5 μl of cDNA. Duplex RT-PCR was performed with one primer pair amplifying the gene of interest and the other an endogenous reference (GAPDH) in the same tube, with the exception of TLR3 (TLR3 and GAPDH were amplified in separate tubes because of relatively weaker performance of the TLR3 primer-probe set). Thermocycler parameters were 50°C for 2 min, 95°C for 10 min, and then 30 to 45 cycles at 95°C for 15 sec and 60°C for 1 min. Semiquantitative analysis of gene expression was done using the comparative CT (ΔΔCT) method, normalizing expression of the gene of interest to that of GAPDH, with the average expression of wild-type cells treated with PGN plus poly(I:C) arbitrarily set to 100. PCR assays were performed in duplicate for each of the triplicate tissue culture samples.
Mice
All procedures involving animals were approved by the NorthShore University HealthSystem Animal Care and Use Committee and conformed to the Guide for Care and Use of Laboratory Animals (1996, National Academy of Sciences).
The following mouse strains were used: The CD1 outbred strain (Harlan), MYD88-deficient and TICAM1-deficient (both the kind gift of Professor Shizuo Akira), and MYD88/TICAM1 doubly-deficient (bred in house) and B6129/F2J wild-type controls (Jackson Laboratory, Bar Harbor, ME).
For pregnancy outcome experiments, CD1 mice in estrus were selected by the gross appearance of the vaginal epithelium and were impregnated naturally by CD1 males. Mating was confirmed by the presence of a vaginal plug. Intrauterine injections were performed on Day 14.5 of a 19- to 20-day gestation, as previously described [21]. Briefly, animals were anesthetized with 0.015 ml/g body weight of Avertin (2.5% tribromoethyl alcohol and 2.5% tert-amyl alcohol in PBS). A 1.5-cm midline incision was made in the lower abdomen. In the mouse, the uterus is a bicornuate structure in which the fetuses are arranged in a “beads-on-a-string” pattern. A 100-μl solution containing either PGN (0.015–0.15 mg/mouse), poly(I:C) (0.05–0.5 mg/mouse), both, or neither was injected into the midsection of the right uterine horn at a site between two adjacent fetuses, taking care not to inject individual fetal sacs. Surgical procedures lasted approximately 10 min. The abdomen was closed in two layers, with 4–0 polyglactin sutures at the peritoneum and wound clips at the skin.
Animals recovered in individual, clean cages in the animal facility. Twice-daily observations were made for both preterm delivery and maternal health status. Preterm delivery was defined as the finding of at least one fetus in the cage or in the lower vagina within 48 h of surgery. Because cannibalization of pups by mothers is common, a sudden drop in maternal girth was seen as presumptive evidence of delivery, even if no fetuses were found. In all cases, this was confirmed at necropsy. Necropsies were performed either after delivery or, at the latest, by 48 h after inoculation. The number of fetuses delivered or remaining in utero and the survival status of these retained fetuses (as determined by cardiac or vascular pulsations visible in the fetal bodies or membranes) were recorded.
Statistical Analysis
Differences in gene expression between groups were assessed with Student t-test or ANOVA (for comparison of multiple variables). Categorical variables (e.g., occurrence of preterm birth) were analyzed using Fisher exact test or contingency tables. Differences were considered to be statistically significant when P < 0.05.
RESULTS
Costimulation with TLR2 and TLR3 Agonists and Synergy in Cytokine and Chemokine Expression of MYD88-Dependent and MYD88-Independent Effectors
The mouse macrophage cell line RAW 264.7 was stimulated in vitro for 5 h with either medium only or the TLR2 ligand PGN (1 μg/ml), the TLR3 ligand poly(I:C) (10 μg/ml), or PGN plus poly(I:C) (Fig. 1). Costimulation of cells with both PGN and poly(I:C) resulted in synergistic expression of mRNA for IL1B, NOS2, CCL5 (RANTES), and TNF compared with either PGN or poly(I:C) alone. These transcripts were chosen because they are established markers of activation of TLR signaling pathways. Significantly, transcripts thought to be primarily controlled either via MYD88 (i.e., IL1B and NOS2), TICAM1 (CCL5), or both (TNF) were expressed in synergistic fashion. Both PGN and poly(I:C) induce the expression of TLR2 above background levels (P < 0.01). Thus, the mechanism of synergy between TLR2 and TLR3 may involve increased expression of TLR2. Tlr3 mRNA is induced by TLR3 activation but not by TLR2 activation.
FIG. 1.
Synergy between TLR2 and TLR3 activation. RAW 264.7 cells were stimulated with either PBS, PGN (1 μg/ml), poly(I:C) (10 μg/ml), or both PGN and poly(I:C) for 5 h. Real-time PCR was performed for IL1B, NOS2, CCL5 (RANTES), TNF, TLR2, and TLR3, normalized to the housekeeping gene GAPDH and with average expression for the combined (PGN plus poly(I:C)) group in each case set to 100. Error bars represent mean ± SD. P values were calculated for all four treatments simultaneously by ANOVA (n = 3 replicates/condition). Depicted is a representative example from among three repeat experiments.
Either TLR2 or TLR3 Engagement Can Prime Cells for Synergistic Activation Via the Alternate Ligand
We next tested whether synergy between TLR2 and TLR3 requires each of the respective ligands to be present simultaneously. Sequential stimulation was performed by incubating RAW 264.7 cells with either PGN or poly(I:C) for 5 h, followed by washing and incubation for an additional 5 h with the alternate ligand (Fig. 2). These conditions were directly compared with both 5 and 10 h of single- and double-ligand exposure. Sequential stimulation with either PGN followed by poly(I:C) or with poly(I:C) followed by PGN induced synergistic expression of the same mRNAs as seen with simultaneous stimulation, albeit at lower levels. Thus, either PGN or poly(I:C) can prime cells for synergistic activation under the influence of the alternate ligand without the need for simultaneous exposure.
FIG. 2.
Priming of cells for synergistic expression of cytokines and chemokines occurs with either PGN or poly(I:C). RAW 264.7 cells were treated in tissue culture with either PGN (1 μg/ml), poly(I:C) (10 μg/ml), or both PGN and poly(I:C) either simultaneously or sequentially for 5–10 h. For sequential incubations, cultures were incubated with the first reagent for 5 h, washed with PBS, and then incubated for an additional 5 h with the second reagent. Real-time PCR was performed using GAPDH as a control gene. The average expression of combined treatment with PGN plus poly(I:C) for 5 h was set to 100. Error bars represent mean ± SD. P values for the four treatment groups calculated by ANOVA are indicated separately for each set of culture conditions (n = 3 replicates/condition). Depicted is a representative example from among three repeat experiments.
A Requirement for Functional TLR Signaling Pathways for Synergistic Effects of PGN and Poly(I:C)
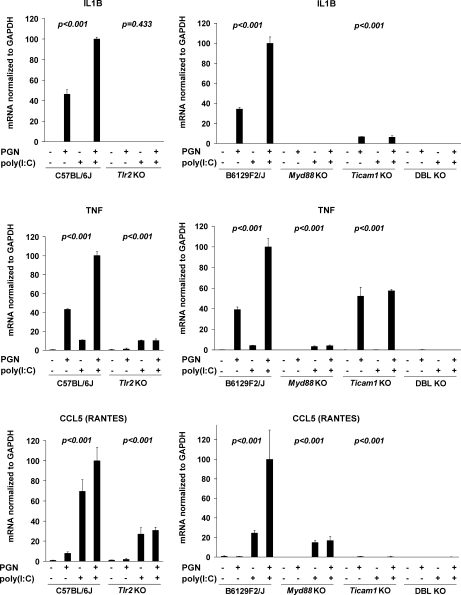
Peritoneal macrophages freshly obtained from control, TLR2-deficient, MYD88-deficient, TICAM1-deficient, or MYD88/TICAM1 doubly-deficient mice were incubated with PGN and/or poly(I:C) (Fig. 3). Absence of any of TLR2, MYD88, or TICAM1 abolishes synergistic expression of the inflammatory mediators IL1B, CCL5, and TNF. For these experiments, we analyzed one expression marker each primarily governed by the MYD88 pathway, the TICAM1 pathway, and both pathways (IL1B, CCL5, and TNF, respectively). The regulation of NOS2 parallels that of IL1B and, therefore, was omitted from this analysis.
FIG. 3.
Synergy between PGN and poly(I:C) requires functional TLR2, MYD88, and TICAM1. Primary mouse peritoneal macrophages extracted from TLR2-deficient (Tlr2KO) and wild-type (C57BL/6J) control mice and from MYD88-deficient (Myd88KO), TICAM1-deficient (Ticam1KO), doubly deficient (DBL KO), and wild-type (B6129F2/J) controls were stimulated with either PGN (1 μg/ml), poly(I:C) (10 μg/ml), or both for 5 h. Duplex RT-PCR was performed for IL1B, TNF, and CCL5 (RANTES), normalized to the housekeeping gene GAPDH. Means ± SD are shown for triplicate wells. Average expression for wild-type macrophages treated with combined PGN plus poly(I:C) was set to 100. P values for the four treatment groups calculated by ANOVA are indicated separately for each genotype. Absent P values indicate unmeasurable values. Depicted is a representative example from among three repeat experiments.
Although Il1b mRNA was primarily regulated via MYD88 and that of CCL5 primarily via TICAM1, the levels of each of these effector molecule mRNAs also seemed to be governed by the presence of the alternate pathway: The absence of TICAM1 diminished the effect of PGN on IL1B expression, and the absence of TLR2 diminished the effect of poly(I:C) on CCL5 expression. These observations suggest the existence of cross-talk between the MYD88-dependent and MYD88-independent pathways even with single TLR ligand exposure. In animals lacking both MYD88 and TICAM1, the tested transcripts were essentially nondetectable.
These data also confirm the specificity of PGN signaling through the TLR2 receptor and, conversely, show that the effects of poly(I:C) do not depend upon TLR2. The results suggest that cytokine upregulation was not caused by contamination of reagents with endotoxin (which, if present, would have been expected to increase cytokine/chemokine expression via TLR4 in animals mutated for TLR2, MYD88, or TICAM1).
Synergy in Preterm Delivery Because of Simultaneous Activation of TLR2 and TLR3
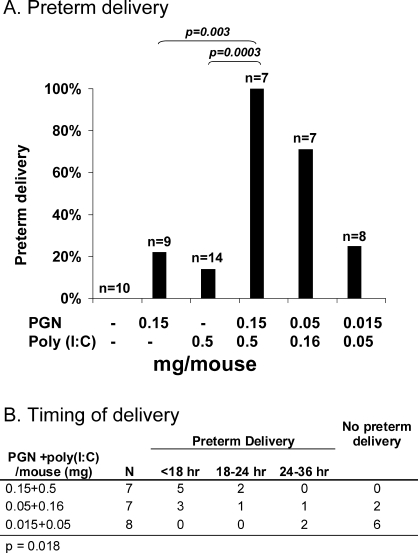
To test whether the in vitro synergistic relationship between TLR2 and TLR3 has an in vivo correlate, we injected the uteri of pregnant mice on Day 14.5 of a 19- to 20-day gestation with PGN, poly(I:C), both, or neither (Fig. 4A). Intrauterine injection of various agents in mice has been used by our group and others to study pathogen-induced preterm birth [5, 8, 22–24]. Previous findings include the demonstration of a dose-dependent effect of PGN and a limited effect of poly(I:C) on preterm delivery [8]. Sterile medium did not produce preterm birth in any of the animals tested. For this experiment, we selected a dose of PGN (0.15 mg/mouse) that induced preterm delivery in 22% of animals and a dose of poly(I:C) (0.5 mg/mouse) that induced preterm delivery in only 14% of animals. The effect of combined PGN/poly(I:C) administration was synergistic, inducing delivery in 100% of subjects. Rates of preterm delivery using combined treatment became comparable to those achieved with single-agent injections only after a 10-fold reduction in combined dosage. A dose-response relationship was found between the combined PGN/poly(I:C) stimulus and time to delivery (Fig. 4B). All pups were born dead.
FIG. 4.
Synergistic effect of intrauterine administration of PGN and poly(I:C) on the occurrence (A) and timing (B) of preterm delivery in Day 14.5 pregnant mice. Preterm delivery was defined as delivery of at least one pup within 48 h (all deliveries occurred in less than 36 h). Delivery data for PGN and poly(I:C) alone were presented previously as single points of dose-response curves [8]. P values are by Fisher exact test (A) and a 3 × 4 contingency table (B).
DISCUSSION
In the present paper, we report that combined stimulation with TLR2 and TLR3 agonists leads to synergistic expression of both TLR2-dependent and TLR3-dependent proinflammatory mediators. We present evidence that this synergy is mediated, in part, via induction of TLR2 by both TLR2 and TLR3 and that pretreatment with either a TLR2 ligand or a TLR3 ligand can prime cells for a synergistic response to the alternate ligand. We further show that this synergy is mediated by and dependent upon MYD88 and TICAM1, and that an interaction between the MYD88-dependent and MYD88-independent pathways influences cytokine/chemokine responses following exposure to even a single TLR ligand. Finally, we demonstrate in vivo one of the consequences of such synergy—namely, a 10-fold increase in susceptibility to preterm delivery in a mouse model.
Acute infection within the gestational compartment may be life-threatening for the pregnant female. Therefore, infection-induced preterm labor can be considered a strategy by which the host evacuates an infected body cavity, thus ensuring her own survival with retention of future reproductive potential. In this context, the existence of synergy in initiation of parturition may represent a “two-hit” trigger mechanism that minimizes responses to stimuli of limited biological significance (e.g., subclinical infection with a single organism) while providing an efficient amplification strategy for rapid activation of labor in response to multiple or more severe insults.
Given the observation that labor in general has certain elements characteristic of inflammatory processes [25], we further hypothesize that synergistic activation of the innate immune system may play a role in noninfectious causes of preterm labor. In such cases, abnormal expression of one of several known, endogenously produced TLR ligands might prime the organism for a synergized reaction to a second insult (e.g., subclinical infection) that otherwise would not be expected to impact upon a normal pregnancy. At least two such candidate endogenous TLR ligands have potential relevance to pregnancy—namely, low-molecular-weight hyaluronan (a component of cervical extracellular matrix degraded during cervical ripening) [26, 27] and surfactant protein A [28–30] (a protein produced by the maturing fetal lung that acts as a hormone signaling the onset of parturition in mice [30]).
The results demonstrating that TLR3 activation can participate in initiating parturition should prompt consideration of a possible role for viral infection in preterm birth. The evidence that viruses may cause preterm labor is scanty [31, 32]. However, the presence of viral pathogens within the gestational compartment likely is underestimated in clinical practice because of limitations in culturing and molecular methodologies. It is thus possible that viral infection plays a previously underappreciated role in culture-negative preterm labor.
Synergy between various pairs of TLRs has been reported previously [33–37], as has upregulation of TLR2 protein in response to a variety of TLR ligands, including those for TLR2, TLR3, TLR4, and TLR9 [34]. For example, the production of nitrites and NOS2 as well as expression of TNF protein in macrophages cultured in vitro was synergistic when stimulated by lipopolysaccharide and various ligands for TLR2 [35]. The mechanism for this latter phenomenon involved induction of TLR2 both by TLR4 and by TLR2 itself. In contrast, no such induction was seen in the expression of TLR4 by either TLR4 or TLR2 ligands. In human airway smooth muscle cells, poly(I:C) induced increased expression of TLR2, TLR3, and TLR4 mRNA, and PGN induced the expression of TLR2 mRNA [38]. Thus, there appears to be a positive feedback loop in which TLR2 in particular is either autoinduced or is inducible by other TLRs. Combined activation of the MYD88-dependent and MYD88-independent (TICAM1-dependent) pathways appears to produce synergy, whereas activation of the MYD88 pathway only (by either alternate TLRs or by sequential exposure to TLR ligands) produces tolerance [39, 40].
The specific interaction of TLR2 and TLR3 has been studied in murine bone marrow-derived dendritic cells [36]. TLR2 and TLR3 had a synergistic effect on production of the inflammatory cytokines TNF and IL6. Functional consequences of combined stimulation of dendritic cells as compared with treatment using either agent alone included enhanced production of interferon gamma by natural killer cells and proliferation of T cells. Varied effects on the expression of various proteins related to IL12 activity and function (i.e., synergy, no effect, or diminished effect) were found. Interestingly, TLR2 diminished the expression of the TLR3-induced, interferon-stimulated genes Mig/Cxcl9 (protein and mRNA), Ip10/Cxcl10, Ifit2 (Garg39) (mRNA), and Ifnb (mRNA but not protein). In our own data presented here and conducted in macrophages, the TLR3-dependent chemokine CCL5 mRNA was synergistically induced by combined TLR2 and TLR3 activation.
Despite decades of research, the incidence of preterm birth has not fallen in the United States and, indeed, has been rising in recent years. Currently, more than 12% of deliveries occur prematurely, with even higher proportions in African Americans. The perinatal mortality and both short- and long-term morbidity associated with preterm birth are very high, and the mechanisms underlying preterm labor remain largely unknown. A thorough understanding of these mechanisms may lead to effective interventions to either prevent or treat preterm labor. The present studies reinforce the growing body of evidence that TLR signaling is a key factor in this process.
Acknowledgment
Marci G. Adams assisted with statistical analysis.
Footnotes
Supported in part by NIH 1RO1HD41689 and March of Dimes 21-FY06-573.
REFERENCES
- Goldenberg RL.The management of preterm labor. Obstet Gynecol 2002; 100: 1020–1037. [DOI] [PubMed] [Google Scholar]
- Klein LL, Gibbs RS.Use of microbial cultures and antibiotics in the prevention of infection-associated preterm birth. Am J Obstet Gynecol 2004; 190: 1493–1502. [DOI] [PubMed] [Google Scholar]
- Goncalves LF, Chaiworapongsa T, Romero R.Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002; 8: 3–13. [DOI] [PubMed] [Google Scholar]
- Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD.Cytokines, prostaglandins and parturition—a review. Placenta 2003; 24(suppl A):S33–S46. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M.A new model for inflammation-induced preterm birth: the role of platelet-activating factor and toll-like receptor-4. Am J Pathol 2003; 163: 2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y.Lipoteichoic acid induces preterm delivery in mice. J Pharmacol Toxicol Methods 1998; 39: 147–154. [DOI] [PubMed] [Google Scholar]
- Wang H, Hirsch E.Bacterially-induced preterm labor and regulation of prostaglandin metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod 2003; 69: 1957–1963. [DOI] [PubMed] [Google Scholar]
- Ilievski V, Lu SJ, Hirsch E.Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod Sci 2007; 14: 315–320. [DOI] [PubMed] [Google Scholar]
- Krediet TG, Wiertsema SP, Vossers MJ, Hoeks SB, Fleer A, Ruven HJ, Rijkers GT.Toll-like receptor 2 polymorphism is associated with preterm birth. Pediatr Res 2007; 62: 474–476. [DOI] [PubMed] [Google Scholar]
- Hartel C, Finas D, Ahrens P, Kattner E, Schaible T, Muller D, Segerer H, Albrecht K, Moller J, Diedrich K, Gopel W.Polymorphisms of genes involved in innate immunity: association with preterm delivery. Mol Hum Reprod 2004; 10: 911–915. [DOI] [PubMed] [Google Scholar]
- Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, Tromp G, Espinoza J, Bujold E, Abrahams VM, Mor G.Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004; 191: 1346–1355. [DOI] [PubMed] [Google Scholar]
- Filipovich Y, Lu SJ, Akira S, Hirsch E.The adaptor protein MyD88 is essential for E. coli-induced preterm delivery in mice. Am J Obstet Gynecol 2009; 200: e91–e98. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S.Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity 1999; 11: 443–451. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA.Recognition of double-stranded RNA and activation of NF-kappaB by toll-like receptor 3. Nature 2001; 413: 732–738. [DOI] [PubMed] [Google Scholar]
- Takeda K, Akira S.TLR signaling pathways. Semin Immunol 2004; 16: 3–9. [DOI] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 1998; 282: 2085–2088. [DOI] [PubMed] [Google Scholar]
- Qureshi ST, Lariviere L, Leveque G, Clermont S, Moore KJ, Gros P, Malo D.Endotoxin-tolerant mice have mutations in toll-like receptor 4 (Tlr4). J Exp Med 1999; 189: 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy E, Zouain CS, Vanhoutte F, Fontaine J, Pavelka N, Thieblemont N, Willems F, Ricciardi-Castagnoli P, Goldman M, Capron M, Ryffel B, Trottein F.Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J Biol Chem 2005; 280: 277–283. [DOI] [PubMed] [Google Scholar]
- Trinchieri G, Sher A.Cooperation of toll-like receptor signals in innate immune defense. Nat Rev Immunol 2007; 7: 179–190. [DOI] [PubMed] [Google Scholar]
- Ding AH, Nathan CF.Trace levels of bacterial lipopolysaccharide prevent interferon-gamma or tumor necrosis factor-alpha from enhancing mouse peritoneal macrophage respiratory burst capacity. J Immunol 1987; 139: 1971–1977. [PubMed] [Google Scholar]
- Mussalli GM, Blanchard R, Brunnert SR, Hirsch E.Inflammatory cytokines in a murine model of infection-induced preterm labor: cause or effect? J Soc Gynecol Invest 1999; 6: 188–195. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Blanchard R, Mehta S.Differential fetal and maternal contributions to the cytokine milieu in a murine model of infection-induced preterm birth. Am J Obstet Gynecol 1999; 180(2 pt 1):429–434. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Filipovich Y, Mahendroo M.Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol 2006; 194: 1334–1340. [DOI] [PubMed] [Google Scholar]
- Hirsch E, Muhle R.Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biol Reprod 2002; 67: 1337–1341. [DOI] [PubMed] [Google Scholar]
- Dudley DJ, Collmer D, Mitchell MD, Trautman MS.Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Invest 1996; 3: 328–335. [PubMed] [Google Scholar]
- Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, et al. Regulation of lung injury and repair by toll-like receptors and hyaluronan. Nat Med 2005; 11: 1173–1179. [DOI] [PubMed] [Google Scholar]
- Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC.Oligosaccharides of hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med 2002; 195: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillot L, Balloy V, McCormack FX, Golenbock DT, Chignard M, Si-Tahar M.Cutting edge: the immunostimulatory activity of the lung surfactant protein-A involves toll-like receptor 4. J Immunol 2002; 168: 5989–5992. [DOI] [PubMed] [Google Scholar]
- Murakami S, Iwaki D, Mitsuzawa H, Sano H, Takahashi H, Voelker DR, Akino T, Kuroki Y.Surfactant protein A inhibits peptidoglycan-induced tumor necrosis factor-alpha secretion in U937 cells and alveolar macrophages by direct interaction with toll-like receptor 2. J Biol Chem 2002; 277: 6830–6837. [DOI] [PubMed] [Google Scholar]
- Condon JC, Jeyasuria P, Faust JM, Mendelson CR.Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A 2004; 101: 4978–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ZA, Benedetti J, Selke S, Ashley R, Watts DH, Corey L.Asymptomatic maternal shedding of herpes simplex virus at the onset of labor: relationship to preterm labor. Obstet Gynecol 1996; 87: 483–488. [DOI] [PubMed] [Google Scholar]
- Srinivas SK, Ma Y, Sammel MD, Chou D, McGrath C, Parry S, Elovitz MA.Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol 2006; 195: 797–802. [DOI] [PubMed] [Google Scholar]
- Gautier G, Humbert M, Deauvieau F, Scuiller M, Hiscott J, Bates EE, Trinchieri G, Caux C, Garrone P.A type I interferon autocrine-paracrine loop is involved in toll-like receptor-induced interleukin-12p70 secretion by dendritic cells. J Exp Med 2005; 201: 1435–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen N, Nonstad U, Khan N, Knetter CF, Akira S, Sundan A, Espevik T, Lien E.Lipopolysaccharide and double-stranded RNA upregulate toll-like receptor 2 independently of myeloid differentiation factor 88. J Biol Chem 2004; 279: 39727–39735. [DOI] [PubMed] [Google Scholar]
- Paul-Clark MJ, McMaster SK, Belcher E, Sorrentino R, Anandarajah J, Fleet M, Sriskandan S, Mitchell JA.Differential effects of gram-positive versus gram-negative bacteria on NOSII and TNFalpha in macrophages: role of TLRs in synergy between the two. Br J Pharmacol 2006; 148: 1067–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoutte F, Paget C, Breuilh L, Fontaine J, Vendeville C, Goriely S, Ryffel B, Faveeuw C, Trottein F.Toll-like receptor (TLR)2 and TLR3 synergy and cross-inhibition in murine myeloid dendritic cells. Immunol Lett 2008; 116: 86–94. [DOI] [PubMed] [Google Scholar]
- Whitmore MM, DeVeer MJ, Edling A, Oates RK, Simons B, Lindner D, Williams BR.Synergistic activation of innate immunity by double-stranded RNA and CpG DNA promotes enhanced antitumor activity. Cancer Res 2004; 64: 5850–5860. [DOI] [PubMed] [Google Scholar]
- Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, Issa R, Chung KF.Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol 2006; 118: 641–648. [DOI] [PubMed] [Google Scholar]
- Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J.MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol 2007; 178: 1164–1171. [DOI] [PubMed] [Google Scholar]
- Ouyang X, Negishi H, Takeda R, Fujita Y, Taniguchi T, Honda K.Cooperation between MyD88 and TRIF pathways in TLR synergy via IRF5 activation. Biochem Biophys Res Commun 2007; 354: 1045–1051. [DOI] [PubMed] [Google Scholar]