Abstract
Throughout the reproductive lifespan of most male mammals, sperm production is constant because of the regulated differentiation of spermatogonia. Retinoic acid (RA) and a downstream target, Stra8, are required for complete spermatogenesis. To examine the role of RA in initiating spermatogonial differentiation, a transgenic mouse model expressing beta-galactosidase under the control of an RA response element was used. Cells in the neonatal testis undergoing active RA signaling were visualized by beta-galactosidase activity, the relationship between RA and differentiation determined, and the role of RA-degrading enzymes in regulating RA demonstrated. Beta-galactosidase activity was found to be predominantly associated with differentiating, premeiotic germ cells and to be distributed nonuniformly throughout the seminiferous tubules. Additionally, beta-galactosidase activity in premeiotic germ cells colocalized with STRA8 protein and was induced in germ cells with exogenous RA treatment. The RA-degrading enzyme, CYP26B1, was found to have germ cell localization and nonuniform distribution between tubules via immunohistochemistry. Treatment with a CYP26 enzyme inhibitor resulted in an increased number of germ cells with both beta-galactosidase activity and STRA8 protein and an increase in the expression of genes associated with differentiation and reduced expression of a gene associated with undifferentiated germ cells. These results show the action of RA in a subset of spermatogonia leads to nonuniform initiation of differentiation throughout the neonatal testis, potentially mediated through the action of CYP26 enzymes. Thus, the presence of RA is a likely driving factor in the initiation of spermatogonial differentiation and may result in asynchronous spermatogenesis.
Keywords: CYP26, gametogenesis, germ cell differentiation, retinoic acid, spermatogenesis, spermatogonia, testis
The uneven distribution of retinoic acid in the neonatal testis leads to the asynchronous initiation of differentiation in the undifferentiated germ cell population, possibly leading to asynchronous spermatogenesis.
INTRODUCTION
Spermatogenesis is a highly regulated process of cellular differentiation, resulting in the production of mature sperm from spermatogonial stem cells (SSCs). Throughout the reproductive lifespan of most male mammals, the production of sperm is constant because of the regulated differentiation of spermatogonia, derived from SSCs [1]. In the adult, SSCs undergo a series of mitotic divisions, producing sequentially As, Apr, and Aal, all of which are termed undifferentiated spermatogonia. Aal spermatogonia commit to final differentiation when they transition from undifferentiated to differentiating spermatogonia, the earliest of which are termed A1 [2]. In the adult, the site of the Aal to A1 transition varies along the length of the seminiferous tubule over time. The temporal and spatial initiation of this transition along the longitudinal axis of the tubule gives rise to the apparent “stages of the cycle of the seminiferous epithelium” [3] and continuous asynchronous spermatogenesis along the length of the tubule. It is unclear when or how spermatogenesis is initiated asynchronously; however, the uneven localization of markers associated with meiosis [4] shortly after birth implies uneven initiation of differentiation likely occurs at or shortly after birth as well.
The neonatal testis contains two distinct populations of undifferentiated germ cells: the gonocytes, present from the day of birth until approximately 6 days postpartum (dpp), and the undifferentiated spermatogonia, derived from a subpopulation of gonocytes that transform into undifferentiated spermatogonia between 3 and 6 dpp [5, 6]. Despite containing two distinct populations of undifferentiated germ cells, both populations eventually give rise to the same population of differentiating spermatogonia (A1 and on). Differentiating spermatogonia will then undergo another series of mitotic divisions, eventually initiating meiosis to form the spermatocyte population [7].
One potential regulator of the Aal to A1 transition is retinoic acid (RA), which is known to induce differentiation in a number of cell populations, including germ cell-derived embryonal carcinoma cells [8]. Additionally, it is known that vitamin A, the precursor of RA, is required for spermatogenesis because extended absence of vitamin A (vitamin A deficiency, termed VAD) in both the rat and mouse results in testes containing only somatic cells and undifferentiated A spermatogonia [9]. With administration of a bolus of vitamin A to VAD animals, spermatogenesis is reinitiated synchronously by releasing the block on the Aal to A1 transition of spermatogonia [10]. A role for RA in germ cell differentiation has also been demonstrated in culture, because RA treatment of cultured neonatal testis increases the expression of genes associated with differentiating spermatogonia, such as kit oncogene (Kit), and stimulated by retinoic acid gene 8 (Stra8) [11]. STRA8 appears to be particularly important in germ cell maturation, because animals lacking Stra8 are infertile because of an inability to undergo meiosis [12, 13]. Additionally, STRA8 protein is observed in spermatogonia in a nonuniform pattern between and within tubules starting as early as 2 days after birth [4]. Although evidence from STRA8 knockout animals implies STRA8 is required exclusively for meiosis, histological evaluation of STRA8 demonstrates a consistent and strong association of STRA8 and the newly differentiating spermatogonial population [11]. These observations suggest STRA8 likely has a broader role in germ cell differentiation than is normally appreciated; however, evidence to support this role has been incomplete.
In spite of the fact that RA has clearly been implicated in regulating meiotic initiation in both neonatal and adult germ cells, no histological data have linked RA to the initial steps of differentiation, and very little is known about the mechanisms driving RA availability in undifferentiated spermatogonia. Examination of the RA-responsive and sex-specific onset of meiosis in the embryonic gonad has demonstrated a role for the RA-degrading enzymes: cytochrome p450 hydrolase, family 26 members (CYP26s), specifically Cyp26b1 [14, 15]. An analogous role in temporal and spatial regulation of the onset of differentiation in the neonatal testis is possible.
In this work a transgenic mouse model expressing β-galactosidase under the control of an RA response element (official symbol Tg(RARE-Hspa1b/lacZ)12Jrt, hereafter referred to as RARE-hsplacZ) [16] was used to demonstrate germ cells are responsive to both endogenous and exogenous RA. Additionally, germ cells undergoing active RA signaling are immunopositive for markers indicative of differentiation, implying they have undergone the undifferentiated (Aal) to differentiating (A1) transition. Last, the RA-degrading enzyme, cytochrome p450 hydrolase family 26, member B1 (CYP26B1), has been localized to germ cells. Inhibition of CYP26 enzyme activity in the neonatal testis results in a greater number of germ cells undergoing differentiation. This report is the first to conclusively establish the cell population undergoing active RA signaling in the neonatal testis, fully define the role of RA in initiating spermatogonial differentiation, and establish the role of RA degradation enzymes in regulating RA availability.
MATERIALS AND METHODS
Animal Treatment, Tissue Collection, Fixation, and Staining
All animal experiments were approved by the Washington State University Animal Care and Use Committee and were in accordance with the standards set by the National Institutes of Health. Testes of RARE-hsplacZ animals at 0, 3, 5, and 10 dpp (n = 3) were collected and fixed in 4% paraformaldehyde (PFA) with or without 0.25% glutaraldehyde from 2 to 5 h. Fixed tissue was washed and stained in bromo-chloro-indolyl-galactopyranoside (X-gal) as previously described [17], dehydrated, and paraffin embedded. For treated animals, all treatments were completed with a minimum of triplicate animals. Vehicle (dimethyl sulfoxide) or 50 μg of all-trans RA (atRA; Sigma-Aldrich, St. Louis, MO) in vehicle was injected subcutaneously at a single site. Testes were collected 24 h later and fixed with 4% PFA.
Organ Cultures
RARE-hsplacZ testes (2 dpp) were collected into sterile PBS, detunicated, and cut into four equal pieces under sterile conditions. Four pieces from a total of two testes were placed into the grove of an embryonic gonad culture agar mold (as described previously [18]) presoaked in Dulbecco minimal Eagle medium (DMEM) containing 10% fetal calf serum (Gibco) and 100 mg/ml ampicillin with either vehicle (dimethyl sulfoxide), 0.7 μM atRA, or 1 μM R115866 (a kind gift from Johnson & Johnson Pharmaceuticals). Testes were incubated for 24 h and then collected into 4% PFA with 0.25% glutaraldehyde and processed for histology or flash frozen on dry ice for RNA isolation.
Histology and Immunohistochemistry
Four-micrometer sections of X-gal-stained RARE-hsplacZ testes were used for all histological or immunohistochemical (IHC) procedures, with the exception of CYP26B1, which utilized Bouin fixed B6/129 neonatal testis. For β-galactosidase activity analysis, sections were hematoxylin counterstained, and cells were considered β-galactosidase positive if they contained two or more foci of β-galactosidase activity or definitive, diffuse cytoplasmic staining. For IHC procedures, the following antibodies were used with the species-appropriate biotinylated secondary (1:500; Chemicon): POU5F1 (rabbit anti-OCT3/4 at 1:200; Abcam), GATA4 (goat anti-GATA-4 at 1:2000; Santa Cruz Biotechnology), GFRA1 (goat anti-GFRα1 at 1:100; R&D Systems), SYCP3 (rabbit anti-SCP-3 at 1:500; Santa Cruz Biotechnology), STRA8 (rabbit anti-STRA8 at 1:1000 [Griswold] with trypsin [1 mg/ml] digestion for 7 min at 37°C in addition to antigen retrieval), and CYP26B1 (goat anti-CYP26B1 at 1:500 [MyBioSource] with no antigen retrieval). In all cases, 0.01 M sodium citrate heat-mediated antigen retrieval was used, unless otherwise noted. Visualization used streptavidin-horseradish peroxidase and diaminobenzidine (Invitrogen). In all cases, quantification was performed on a minimum of 100 tubule cross-sections per biological replicate on at least two planes separated by a minimum of 50 μm for whole testis and 50 cross-sections from a minimum of three separate pieces of testis for cultured testis. All IHC procedures and quantifications were performed with biological triplicate samples and technical duplicates.
RNA Isolation and Real-Time RT-PCR
Total RNA was extracted using a PicoPure RNA Isolation Kit (Molecular Devices, Sunnyvale, CA) and quantified in an ND-1000 Spectrometer (Thermo Scientific, Wilmington, DE). For real-time RT-PCR, forward and reverse primers (Supplemental Table S1; all Supplemental Data are available at www.biolreprod.org) were designed using Primer Express 2.0 (Applied Biosystems, Foster City, CA). The iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) and 200 ng of sample RNA were used for cDNA production. Real-time RT-PCR used a 7500 Fast Real-Time PCR System and Fast SYBR GREEN Mastermix (Applied Biosystems). Individual real-time RT-PCR sample reactions were analyzed in triplicate, and all treatments or ages were analyzed with biological triplicates. Relative fold change was calculated using the ddCT method as described previously [19] with ribosomal protein S2 (Rps2) as the endogenous control and vehicle-treated samples as biological controls.
Statistical Analyses
For all analyses, data were analyzed using a Student t-test for all pairs computed by JMP 7.0.1 (SAS Institute Inc.).
RESULTS
β-Galactosidase Activity in the RARE-hsplacZ Neonatal Testis
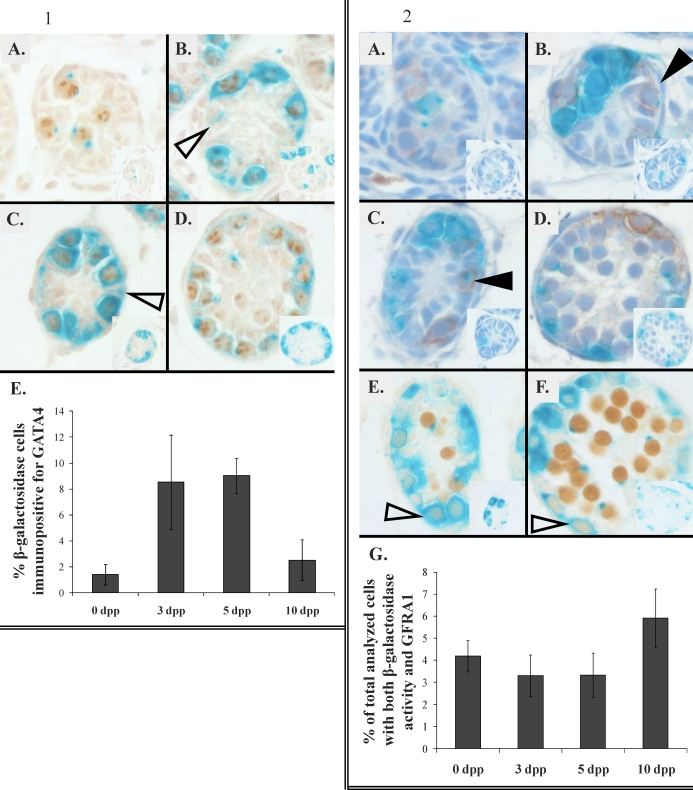
Colocalization of β-galactosidase activity with the germ cell marker POU5F1 [20] and the Sertoli cell marker GATA4 [21] was analyzed in neonatal RARE-hsplacZ testes at 0, 3, 5, and 10 dpp. Based on morphological analysis and colocalization of POU5F1 with β-galactosidase activity at all ages, the majority of cells with β-galactosidase activity appeared to be germ cells (Fig. 1, A–D); however, some β-galactosidase activity not associated with a clear POU5F1 signal was observed. Because this POU5F1-negative, β-galactosidase-positive cell population had morphological markers indicative of Sertoli cells (columnar appearance and nuclear shape), the percentage of β-galactosidase-positive Sertoli cells was determined using IHC detection of the nuclear Sertoli cell marker, GATA4. Quantification of β-galactosidase and GATA4 colocalization showed that at no age were more than 8% of the β-galactosidase-positive cells associated with a GATA4-positive nucleus (Fig. 1E).
PLATE I. Figures 1 and 2.
FIG. 1. Immunohistochemical localization of POU5F1 (brown) in RARE-hsplacZ testes stained for β-galactosidase activity (aqua): 0 dpp (A), 3 dpp (B), 5 dpp (C), and 10 dpp (D). Arrows indicate putative β-galactosidase-positive Sertoli cells. Original magnification ×400. Insets show secondary antibody only, scaled to 30%. E) Frequency of β-galactosidase-positive, GATA4-immunopositive cells by age (n = 3). Error bars represent SD.
FIG. 2. Immunohistochemical localization of GFRA1 or SYCP3 (brown) in RARE-hsplacZ testes stained for β-galactosidase activity (aqua). A–D) GFRA1 in RARE-hsplacZ testes at 0 dpp (A), 3 dpp (B), 5 dpp (C), and 10 dpp (D). Original magnification ×400, scaled to 110%. Closed arrowheads indicate weakly GFRA1- and β-galactosidase-positive cells. Insets show secondary antibody only, scaled to 40%. E and F) SYCP3 in RARE-hsplacZ testes at (E) 5 dpp and (F) 10 dpp. Open arrowheads indicate weakly SYCP3-immunopositive, β-galactosidase-positive cells. Insets show secondary antibody only, scaled to 30%. G) Frequency of β-galactosidase-positive, GFRA1-immunopositive cells by age (n = 3). Total number of analyzed cells represents the number of all cells scored as either β-galactosidase positive, GFRA1 positive, or positive for both. Error bars represent SD.
β-Galactosidase Activity in Differentiating Germ Cells
In order to further clarify which populations of germ cells were positive for β-galactosidase, the association of β-galactosidase activity with GFRA1, a marker of undifferentiated germ cells [22], and SYCP3, a marker of meiotic germ cells, was determined. β-Galactosidase activity was rarely associated with GFRA1-positive cells at all time points examined (Fig. 2, A–D). At 5 dpp, rare but strongly SYCP3-positive germ cells were observed in RARE-hsplacZ mice (Fig. 2E). These strongly SYCP3-positive germ cells were never associated with β-galactosidase activity. Strongly SYCP3-positive cells were observed at a greatly increased frequency in 10-dpp RARE-hsplacZ testes; however, as in the earlier age, strongly SYCP3-positive cells never contained β-galactosidase activity (Fig. 2F). At both ages, weakly SYCP3-positive cells were occasionally positive for β-galactosidase activity.
β-Galactosidase Activity Colocalization with STRA8
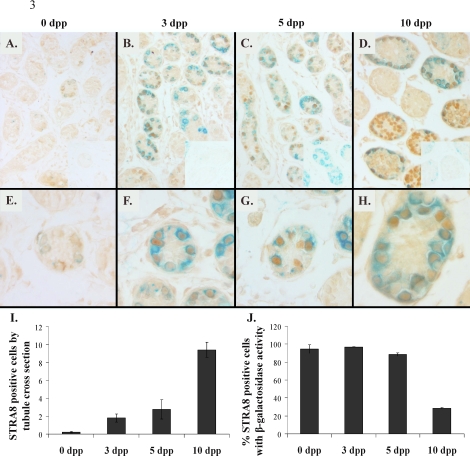
The localization of STRA8 was examined in RARE-hsplacZ neonatal testis stained for β-galactosidase activity (Fig. 3, A–H). Few STRA8-positive cells were observed in 0-dpp testes, where nearly all STRA8-positive cells contained β-galactosidase activity. A greater number of STRA8-positive cells were observed at 3 and 5 dpp and, as was the case at 0 dpp, nearly all STRA8-positive cells also contained β-galactosidase activity. The number of STRA8-positive germ cells dramatically increased from 5 to 10 dpp (Fig. 3I), coinciding with the onset of meiosis. However, the colocalization of STRA8 and β-galactosidase activity decreased during this time frame (Fig. 3J), presumably because of the increasing number of STRA8-positive meiotic germ cells not containing β-galactosidase activity. Germ cells with β-galactosidase activity but no STRA8 immunoreactivity were observed at all time points.
FIG. 3.
Immunohistochemical localization of STRA8 (brown) in RARE-hsplacZ testes stained for β-galactosidase activity (aqua) by age. A–D) Magnification ×200, scaled to 57%. Insets show secondary antibody only, scaled to 50%. E–H) Magnification ×400, scaled to 74%. I) STRA8-immunopositive cells per tubule by age (n = 3). J) Frequency of STRA8-positive cells containing β-galactosidase activity by age (n = 3). Error bars represent SD.
β-Galactosidase Activity Variation in the Neonatal Testis
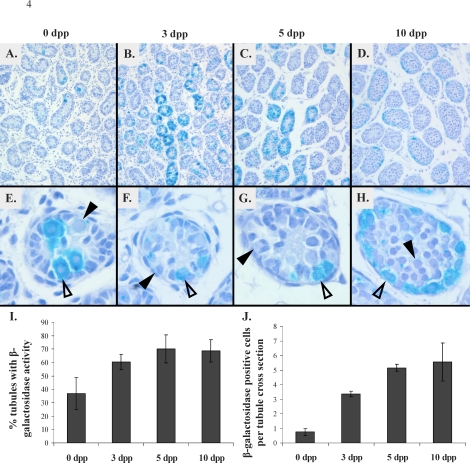
Whole 0-, 3-, 5-, and 10-dpp RARE-hsplacZ testes stained for β-galactosidase activity were sectioned and counterstained to determine whether β-galactosidase activity was consistent throughout the neonatal testis. Low-magnification images of these testes clearly demonstrated that β-galactosidase activity varies from tubule to tubule (Fig. 4, A–D). Closer examination of individual tubules demonstrated that β-galactosidase activity varies from cell to cell within a given tubule as well (Fig. 4, E–H). Temporally, β-galactosidase activity was relatively rare at 0 dpp, with the percentage of tubules containing strong or moderate β-galactosidase activity increasing to a maximum at 5 dpp (Fig. 4I). However, the frequency of β-galactosidase-positive cells per tubule generally increased with increasing age (Fig. 4J).
FIG. 4.
Distribution of β-galactosidase activity (aqua) in RARE-hsplacZ testes by age. Tissue counterstained with hematoxylin (blue). A–D) Magnification ×100, scaled to 70%. E–H) Magnification ×400, 10 dpp scaled to 75%. Open arrowheads show β-galactosidase-positive germ cells; closed arrowheads, β-galactosidase-negative germ cells. I and J) Frequency of β-galactosidase-positive tubules (I) and cells per tubule (J) by age. Error bars represent SD.
β-Galactosidase Activity and RA Response
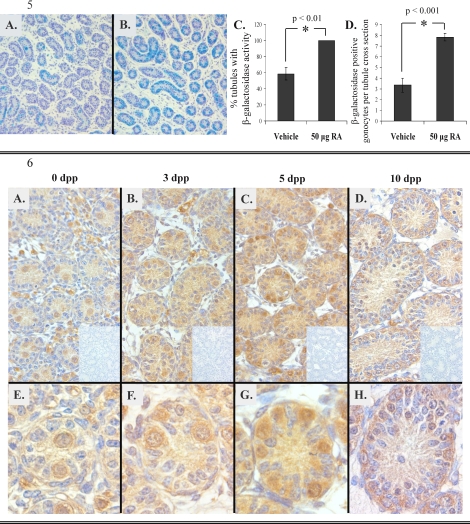
RARE-hsplacZ pups (2 dpp) were treated with 50 μg of atRA, and β-galactosidase activity was determined 24 hours later (Fig. 5, A and B). Treatment with exogenous RA resulted in a significant increase in the number of tubules containing β-galactosidase-positive cells as well as the number of β-galactosidase-positive cells per tubule (Fig. 5, C and D). After exposure to exogenous RA, greater than 90% of the germ cells in the transgenic neonatal testis contained β-galactosidase activity, as determined by colocalization of POU5F1 with β-galactosidase activity (Supplemental Fig. S1). Additionally, exogenous RA treatment resulted in an induction of Stra8 as determined by real-time RT-PCR. (Supplemental Fig. S2).
PLATE II. Figures 5 and 6.
FIG. 5. Induction of β-galactosidase with exogenous RA treatment. Aqua represents β-galactosidase activity. A and B) Three days postpartum RARE-hsplacZ testes from animals treated at 2 dpp with either vehicle (A) or 50 μg of atRA (B). Tissue counterstained with hemotoxylin (blue). Original magnification ×100, scaled to 62%. C and D) Frequency of tubules (C) or cells positive for β-galactosidase activity (D) with or without exogenous RA treatment (n = 3). Error bars represent SD. Asterisks represent significance (P < 0.05); reported values represent calculated P values for each comparison.
FIG. 6. Immunohistochemical localization of CYP26B1 (brown) counterstained with hematoxylin (blue) in neonatal testis. A–D) Magnification ×200, scaled to 75%. E–H) Magnification ×400, unscaled with the exception of 10 dpp, scaled to 73%. Age is indicated. Insets show secondary antibody only, original magnification ×200, scaled to 20%.
The RA-Degrading Enzyme, CYP26B1, Distribution in the Neonatal Testis
Immunohistochemical detection of CYP26B1 demonstrated enzyme in all cell types of the testis, with the majority of CYP26B1 associated with germ cells at all ages examined (Fig. 6). In Sertoli cells and germ cells, signal intensity was not uniform from tubule to tubule and from cell to cell within a tubule. However, at all ages examined, the greatest intensity of signal was observed in germ cells.
β-Galactosidase Activity and STRA8 Protein after Treatment with RA or Inhibition of CYP26 Enzymes
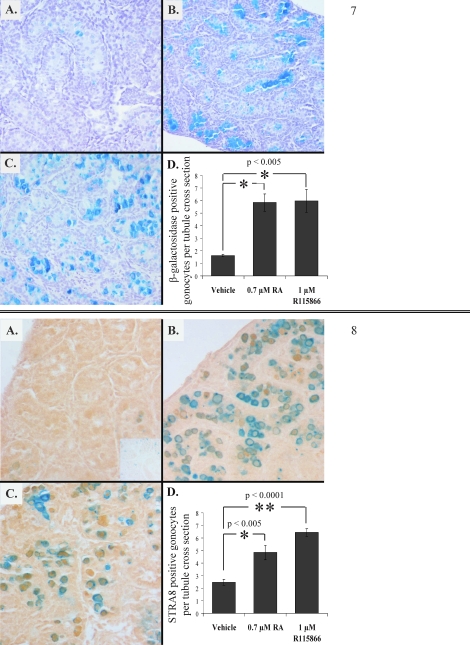
RARE-hsplacZ testes (2 dpp) were cultured for 24 h in the presence of RA or an inhibitor specific to the CYP26 enzymes, R115866. Cultured RARE-hsplacZ testes were stained for β-galactosidase activity, and the number of β-galactosidase-positive germ cells was determined (Fig. 7). Treatment of cultured testes with RA or R115866 resulted in an increase in the number of β-galactosidase-positive germ cells. The expression of two differentiation markers, Stra8 and Kit, and a marker associated with undifferentiated germ cells, Pou5f1, was also examined in cultured testes treated with RA or R115866 (Supplemental Fig. S3). Both Stra8 and Kit expression was observed to increase in the presence of either RA or R115866, whereas Pou5f1 expression decreased. Immunohistochemical localization of STRA8 in cultured 2-dpp RARE-hsplacZ testes (Fig. 8) showed that treatment with RA or inhibition of CYP26 enzymes resulted in an increased number of STRA8-positive germ cells.
PLATE III. Figures 7 and 8.
FIG. 7. β-Galactosidase activity in 2-dpp RARE-hsplacZ testes cultured for 24 h with vehicle alone (A), 0.7 μM atRA (B), or 1 μM R115866 (C). Original magnification ×100. D) Frequency of β-galactosidase-positive cells per tubule by treatment (n ≥ 3). Error bars represent SD. Asterisks represent significance (P < 0.05), and reported values represent calculated P values for each comparison.
FIG. 8. Immunohistochemical localization of STRA8 (brown) in 2-dpp RARE-hsplacZ testes cultured for 24 hours with vehicle alone (A), 0.7 μM atRA (B), or 1 μM R115866 (C). Original magnification ×200, scaled to 77%. Inset: secondary antibody only scaled to 30%. D) Frequency of STRA8 immunopositive cells by treatment (n ≥ 3). Error bars represent SD. Asterisks represent significance (P < 0.05) and reported values represent calculated P values for each comparison.
DISCUSSION
The current study used a transgenic mouse line, RARE-hsplacZ, expressing β-galactosidase under the control of an RA response element to demonstrate β-galactosidase activity is associated predominantly with differentiating, premeiotic germ cells in the neonatal testis. Additionally, in these animals β-galactosidase activity shows near perfect colocalization with STRA8 and occurs in a nonuniform manner throughout development. When cultured neonatal RARE-hsplacZ testes are treated with either RA or inhibitors specific to CYP26 enzymes; β-galactosidase activity, STRA8, and the expression of Stra8, Kit, and Pou5f1 can be altered. Like β-galactosidase activity and STRA8, CYP26B1 is observed in a nonuniform manner throughout neonatal testis development.
Retinoic acid signaling requires three main components: the ligand (RA), the ligand-binding receptor or receptors, and necessary cofactors for gene induction. Thus, the observed nonuniform β-galactosidase activity in neonatal RARE-hsplacZ testes may be a function of ligand (RA) availability or the presence/localization of receptors or cofactors necessary for transgene expression. The latter option can be more succinctly defined as the ability, or lack thereof, to respond to RA. Previous work has demonstrated the presence of several RA and retinoid receptors in early germ cells of the neonatal testis [23, 24], implying these cells have the necessary signaling components to respond to RA. Addition of ligand in the form of exogenous RA treatment resulted in a significant increase in the percentage of tubules and number of gonocytes with β-galactosidase activity. More than 90% of the germ cells displayed β-galactosidase activity 24 h after treatment, demonstrating most germ cells present at 2 dpp have the ability to respond to RA if it is available. Thus, β-galactosidase activity in the RARE-hsplacZ neonatal testis appears to be an accurate measure of active RA signaling and is probably a measure of available RA. Germ cells in meiotic prophase (preleptotenes) were rarely observed to have β-galactosidase activity. It is known that RARα is required for the normal transition from preleptotene to leptotene spermatocytes [25], implying RA is important for the normal function of preleptotene spermatocytes. The lack of β-galactosidase activity in preleptotene spermatocytes may be due to the fact that the preleptotene spermatocytes observed in this study are at a developmental stage that does not require RA or they lack the necessary signaling components to induce β-galactosidase.
Near perfect colocalization of STRA8 with β-galactosidase activity in differentiating spermatogonia was observed from the day of birth onward. However, β-galactosidase activity without STRA8 protein was commonly observed throughout development. This is notable because the role of STRA8 in spermatogonial differentiation has been unclear. Germ cells in animals lacking STRA8 appear to initiate the Aal to A1 transition as they reach the preleptotene spermatocyte stage of differentiation [13]; however, ample evidence has demonstrated STRA8 protein is also associated with differentiating spermatogonia [4, 11]. Evidence from VAD models and this work indicates RA is required for the Aal to A1 transition [10]. So although RA is required to initiate Stra8 expression, the overall role of RA in spermatogonial differentiation is broader and not entirely dependent on STRA8 protein.
Previous in vitro work has demonstrated the ability of RA to increase expression of known differentiation markers in isolated gonocytes [11]. A role for RA in germ cell differentiation was further validated in this work with the demonstration that treatment of cultured neonatal RARE-hsplacZ testes with RA resulted in increased expression of known differentiation markers (Stra8 and Kit); a reduction in the expression of Pou5f1, associated with undifferentiated germ cells; and an increased number of germ cells with β-galactosidase activity and STRA8 protein. Thus, RA must be a facilitating but limiting factor in the differentiation of germ cells.
Metabolically, the availability of RA is a function of the balance between RA production and RA degradation. The CYP26 family of cytochrome p450 hydrolases is the predominant RA-degrading enzyme [26], and the role of CYP26 enzymes, specifically CYP26B1, in initiating meiosis in the embryonic male and female gonad has been conclusively demonstrated [14, 15]. However, an understanding of the role for CYP26 enzymes in the initiation of differentiation in the neonatal testis has been elusive, in part because of conflicting evidence regarding the localization of CYP26 enzymes. The Cyp26b1 message has been localized to tubular, somatic cells using in silico subtractive array analysis [27], and CYP26B1 protein has been observed in peritubular myoid cells in developing testis [28]. Localization of CYP26B1 to germ cells throughout neonatal development in this report and the observation that culture of neonatal testes with an inhibitor specific to the CYP26 enzymes produces results similar to or even greater than that observed with RA treatment suggest CYP26 enzyme activity is capable of modulating RA availability in the neonatal testis. As yet, the exact cell population mediating this effect remains unclear, because CYP26B1 enzyme is observed at a low level in most cells of the testis, particularly Sertoli cells. However, ablation of CYP26B1 in Sertoli cells does not completely abolish sperm production [29], as would be expected if CYP26B1 activity in Sertoli cells was required to regulate differentiation in undifferentiated spermatogonia. Regardless of the exact cellular source of CYP26 enzyme activity, the increase in the number of cells undergoing active RA signaling and differentiation when treated with CYP26 inhibitors is further support for the hypothesis that, in at least a portion of cells, RA is a limiting factor possibly due to CYP26 enzyme activity.
The most notable observation regarding β-galactosidase activity in the RARE-hsplacZ testis as a reasonable measure of available RA is its nonuniform distribution. One likely role for nonuniform RA distribution would be nonuniform induction of differentiation, possibly mediated by STRA8. The observations in this work regarding the distribution and role of RA in the Aal to A1 transition are highly suggestive that asynchronous spermatogenesis is initiated in the neonate around the time of birth. These results are consistent with decades of morphological evaluations concluding that germ cell differentiation in the neonate must be described by defining the most advanced germ cell present at a given age [5], presumably because of significant tubule to tubule variation in germ cell composition.
The undifferentiated (Aal) to differentiating (A1) spermatogonial transition, organized both temporally and spatially, along the length of the seminiferous tubule is the driving factor behind asynchronous spermatogenesis. Thus, the triggers resulting in that differentiation step are of considerable importance for our understanding of mammalian spermatogenesis. The observations reported in this work suggest that RA is capable of inducing spermatogonial differentiation in cultured neonatal testes, appears to be limiting, and has nonuniform distribution throughout development, and its availability may be regulated by CYP26 enzymes. Taken as a whole, these results implicate RA availability in nonuniform initiation of differentiation in neonatal germ cells, possibly driving the onset of asynchronous spermatogenesis.
Acknowledgments
The authors would like to thank Debra Mitchell and Ryan Evanoff for technical assistance, the J. Rossant lab for kindly providing RARE-hsplacZ mice, J&J Research for the kind gift of R115866, and our funding sources.
Footnotes
Supported by National Institutes of Health grant HD10808.
REFERENCES
- Aponte PM, van Bragt MP, de Rooij DG, van Pelt AM.Spermatogonial stem cells: characteristics and experimental possibilities. APMIS 2005; 113: 727–742. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD.All you wanted to know about spermatogonia but were afraid to ask. J Androl 2000; 21: 776–798. [PubMed] [Google Scholar]
- Russell L, Ettlin R, Sinha Hikim A, Clegg E.Histological and Histopathological Evaluation of the Testis. Clearwater, FL: Cache River Press; 1990.
- Zhou Q, Nie R, Li Y, Friel P, Mitchell D, Hess RA, Small C, Griswold MD.Expression of stimulated by retinoic acid gene 8 (Stra8) in spermatogenic cells induced by retinoic acid: an in vivo study in vitamin A-sufficient postnatal murine testes. Biol Reprod 2008; 79: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellve AR, Cavicchia JC, Millette CF, O'Brien DA, Bhatnagar YM, Dym M.Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol 1977; 74: 68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel BR, Amarose AP, Hacket EM.Calendar of gametogenic development in the prepuberal male mouse. Science 1961; 134: 832–833. [DOI] [PubMed] [Google Scholar]
- de Rooij DG.Proliferation and differentiation of spermatogonial stem cells. Reproduction 2001; 121: 347–354. [DOI] [PubMed] [Google Scholar]
- Soprano DR, Teets BW, Soprano KJ.Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitam Horm 2007; 75: 69–95. [DOI] [PubMed] [Google Scholar]
- van Pelt AM, de Rooij DG.Synchronization of the seminiferous epithelium after vitamin A replacement in vitamin A-deficient mice. Biol Reprod 1990; 43: 363–367. [DOI] [PubMed] [Google Scholar]
- Griswold MD, Bishop PD, Kim KH, Ping R, Siiteri JE, Morales C.Function of vitamin A in normal and synchronized seminiferous tubules. Ann N Y Acad Sci 1989; 564: 154–172. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Li Y, Nie R, Friel P, Mitchell D, Evanoff RM, Pouchnik D, Banasik B, McCarrey JR, Small C, Griswold MD.Expression of stimulated by retinoic acid gene 8 (Stra8) and maturation of murine gonocytes and spermatogonia induced by retinoic acid in vitro. Biol Reprod 2008; 78: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG, Page DC.In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 2006; 38: 1430–1434. [DOI] [PubMed] [Google Scholar]
- Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM, Page DC.Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 2008; 105: 14976–14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubova J, Menke DB, Zhou Q, Capel B, Griswold MD, Page DC.Retinoic acid regulates sex-specific timing of meiotic initiation in mice. Proc Natl Acad Sci U S A 2006; 103: 2474–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles J, Knight D, Smith C, Wilhelm D, Richman J, Mamiya S, Yashiro K, Chawengsaksophak K, Wilson MJ, Rossant J, Hamada H, Koopman P.Retinoid signaling determines germ cell fate in mice. Science 2006; 312: 596–600. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguere V.Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev 1991; 5: 1333–1344. [DOI] [PubMed] [Google Scholar]
- McLean DJ, Russell LD, Griswold MD.Biological activity and enrichment of spermatogonial stem cells in vitamin A-deficient and hyperthermia-exposed testes from mice based on colonization following germ cell transplantation. Biol Reprod 2002; 66: 1374–1379. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B.Male-specific cell migration into the developing gonad. Curr Biol 1997; 7: 958–968. [DOI] [PubMed] [Google Scholar]
- Snyder EM, Small CL, Li Y, Griswold MD.Regulation of gene expression by estrogen and testosterone in the proximal mouse reproductive tract. Biol Reprod 2009; 81: 707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce M, Wang X, Wolgemuth DJ, Scholer H.Differential expression of the Oct-4 transcription factor during mouse germ cell differentiation. Mech Dev 1998; 71: 89–98. [DOI] [PubMed] [Google Scholar]
- Viger RS, Mertineit C, Trasler JM, Nemer M.Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Mullerian inhibiting substance promoter. Development 1998; 125: 2665–2675. [DOI] [PubMed] [Google Scholar]
- Hofmann MC, Braydich-Stolle L, Dym M.Isolation of male germ-line stem cells; influence of GDNF. Dev Biol 2005; 279: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour JM, Kim KH.Cellular and subcellular localization of six retinoid receptors in rat testis during postnatal development: identification of potential heterodimeric receptors. Biol Reprod 1999; 61: 1300–1308. [DOI] [PubMed] [Google Scholar]
- Boulogne B, Levacher C, Durand P, Habert R.Retinoic acid receptors and retinoid X receptors in the rat testis during fetal and postnatal development: immunolocalization and implication in the control of the number of gonocytes. Biol Reprod 1999; 61: 1548–1557. [DOI] [PubMed] [Google Scholar]
- Chung SS, Sung W, Wang X, Wolgemuth DJ.Retinoic acid receptor alpha is required for synchronization of spermatogenic cycles and its absence results in progressive breakdown of the spermatogenic process. Dev Dyn 2004; 230: 754–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JA, Guo YD, Baetz K, Beckett-Jones B, Bonasoro J, Hsu KE, Dilworth FJ, Jones G, Petkovich M.Identification of the retinoic acid-inducible all-trans-retinoic acid 4-hydroxylase. J Biol Chem 1996; 271: 29922–29927. [DOI] [PubMed] [Google Scholar]
- O'Shaughnessy PJ, Abel M, Charlton HM, Hu B, Johnston H, Baker PJ.Altered expression of genes involved in regulation of vitamin A metabolism, solute transportation, and cytoskeletal function in the androgen-insensitive tfm mouse testis. Endocrinology 2007; 148: 2914–2924. [DOI] [PubMed] [Google Scholar]
- Wu JW, Wang RY, Guo QS, Xu C.Expression of the retinoic acid-metabolizing enzymes RALDH2 and CYP26b1 during mouse postnatal testis development. Asian J Androl 2008; 10: 569–576. [DOI] [PubMed] [Google Scholar]
- Li H, MacLean G, Cameron D, Clagett-Dame M, Petkovich M.Cyp26b1 expression in murine Sertoli cells is required to maintain male germ cells in an undifferentiated state during embryogenesis. PLoS One 2009; 4: e7501 [DOI] [PMC free article] [PubMed] [Google Scholar]