Abstract
Intrauterine growth restriction (IUGR) is observed in conditions with limitations in uterine space (e.g., uterine anomalies and multifetal gestations). IUGR is associated with reduced fetal weight, organ growth, and a spectrum of adult-onset diseases. To examine the interaction of uterine anomalies and multifetal gestations, we developed a surgical uterine space restriction model with a unilateral uterine horn ligation before breeding (unilateral surgery). Placentas and fetuses were studied on Gestational Day (GD) 120 and GD 130 (term = 147 days). Unilateral surgery decreased placentome numbers in singleton and twin pregnancies (25% and 50%, respectively) but not unilateral triplets. Unilateral surgery decreased total placentome weight in twin pregnancies (decreased 24%). Fetuses categorized as uterine space restricted (unilateral twin and both groups of triplets) had 51% fewer placentomes per fetus and a 31% reduction in placentomal weight per fetus compared to the nonrestricted group (control singleton, unilateral singleton, and control twin). By GD 130, uterine space-restricted fetuses exhibited decreased weight, smaller crown-rump, abdominal girth, and thoracic girth as well as decreased fetal heart, kidney, liver, spleen, and thymus weights. Lung and brain weights were unaffected, demonstrating asymmetric IUGR. At GD 130, placental efficiency (fetal weight per total placentomal weight) was elevated in uterine space-restricted fetuses. However, fetal arterial creatinine, blood urea nitrogen, and cholesterol were elevated, suggesting insufficient placental clearance. Maternal-to-fetal glucose and triglycerides ratios were elevated in the uterine space-restricted pregnancies, suggesting placental nutrient transport insufficiency. This model allows for examination of interactive effects of uterine space restriction-induced IUGR on placental adaptation and fetal organ growth.
Keywords: cotyledon, fetal development, IUGR, placenta, placentome, pregnancy
Surgically induced uterine anomalies and multifetal gestation create uterine space restriction, resulting in fetal IUGR and altered placental development.
INTRODUCTION
Uterine anomalies are observed in 4.3% of women in the United States and are associated with both a reduction in fertility and an increased incidence of asymmetric intrauterine growth restriction (IUGR) [1]. The incidence of asymmetric IUGR is further compounded by multifetal pregnancies with a 65% to 85% greater instance of IUGR compared to singleton human pregnancies, resulting in dramatically increased morbidity to the fetus [2, 3]. Furthermore, both uterine anomalies (e.g., uterine bicornuate, unicornuate, or didelphys) and multifetal gestations are associated with decreased uterine space- and growth-restricted fetuses [4–12].
Uterine anomalies [4–6, 13, 14] and multifetal gestations [7–9] are two independent clinical factors associated with IUGR because of limiting uterine space, which in turn is associated with uteroplacental insufficiency. Current practices in assisted reproductive technologies have greatly improved conception and pregnancy rates in women with uterine anomalies [15]. Furthermore, these techniques also increase the incidence of multifetal pregnancies, further reducing uterine space. However, the interactive effects of these two conditions are not well characterized except in clinical case reports [1, 4–6, 14–18]. Additional biological and clinical significance are derived from studies demonstrating that IUGR is associated with a predisposition to a multitude of adult-onset diseases, including coronary heart disease, diabetes, dislipidaemia, and impaired neural development [12, 19, 20].
Thus, the aim of this study was to develop an ovine surgical model of uterine space restriction that investigates the interactive effects of uterine anomalies and multifetal gestations on fetal and placental growth. This model allows for the quantitative examination of IUGR relative to placental adaptation and fetal growth during development.
MATERIALS AND METHODS
Animals
Ewes of mixed Western breeds (n = 45) were obtained from the University of Wisconsin–Madison Arlington farm facility. Ewes were group housed throughout gestation and fed identical diets that consisted of a mixture of hay and corn silage that met the NRC requirement for all stages of gestation. Animal protocols were approved by University of Wisconsin–Madison Research Animal Care and Use Committee of the School of Medicine and Public Health and the College of Agriculture and Life Sciences.
Surgical Procedures
General surgical procedures [21–23] and the surgical model [24–28] were modified from those previously described. In brief, nonpregnant multiparous ewes were assigned to two treatment groups: unilateral (n = 21) and control (n = 24). Unilateral ewes were subjected to uterine surgical remodeling. Sheep were administered atropine (0.02 mg/kg, i.m.; Sigma Aldrich) and antibiotics (400 000 units of penicillin G benzathine and gentamicin sulfate; IVX Animal Health) and anesthetized with ketamine (16 mg/kg, i.m.; IVX Pharmaceuticals). The jugular vein was cannulated (Tygon tubing; 0.7-mm outer diameter and 0.4-mm inner diameter) for intravenous administration of ketamine (100 mg/ml) in 0.9% saline and 5% dextrose with supplemental sodium pentobarbital (50 mg/ml; Sigma Aldrich) as needed for additional anesthesia. A midventral laparotomy was performed to access the uterus. In the unilateral group, a single uterine horn was completely disconnected, including separation of all intercornual connections using an electrocautery, and one horn was double ligated with silk ligature and then transected (Fig. 1). Other than intercornual vascular connections, all the other uterine and ovarian vasculature was unaltered by this surgery. Furthermore, a subset of unilateral ewes had a single oviductal ligation (n = 8), ipsilateral to the horn ligated, whereas the remaining ewes (n = 13) did not. Similarly, control ewes either had a single oviductal ligation (n = 12) or had no surgery (n = 12). Single oviductal ligations were performed in an effort to achieve reduced fetal numbers. Statistical analyses demonstrated that oviductal ligation did not affect placental or fetal parameters (P > 0.05). After surgery, ewes were given flunixin meglumine (75 mg i.m.; IVX Pharmaceuticals) analgesia and given access to food and water ad libitum.
FIG. 1.
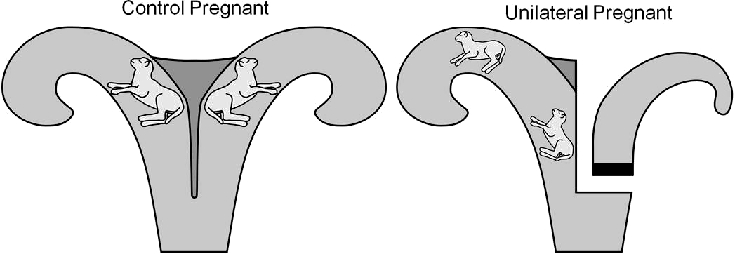
Diagram of the ovine surgical model of uterine space restriction. The figure shows an example of a control pregnant twin (left) and a unilateral pregnant surgical uterine anomaly (right) of a uterus containing two IUGR fetuses.
Reproductive Synchronization Protocol
At least 2 mo after surgery, a progesterone-controlled internal drug release (CIDR; 0.3 g; Pfizer) was placed in the vagina of these nonpregnant ewes. After at least 6 days, an i.m. injection of prostaglandin F2α (15 mg; Pfizer) was administered. Between Days 10 and 12, the CIDR was removed, and 500 IU equine chorionic gonadotropin (Sioux Biochemical Inc.) was injected i.m. Ewes were bred to a fertile ram, and pregnancy was confirmed by ultrasonography by gestational day (GD) 60.
Fetal Measurements, Tissue Collection, and Placentome Classification
Nonsurvival surgery was performed on either GD 120 (120.5 ± 0.4; n = 22) or GD 130 (129.8 ± 0.5; n = 23; term = 147 days) under a surgical plane of anesthesia with pentobarbital (50 mg/ml). Maternal arterial blood was collected via a catheter (Tygon tubing; 0.7-mm outer diameter and 0.4-mm inner diameter) inserted into the superficial saphenous branch of the femoral artery. Using a needle and heparinized syringe, umbilical vein and artery samples were simultaneously collected from each fetus prior to being removed from the uterus and euthanized. Blood was centrifuged (Beckman GPR Centrifuge) for 10 min at 3000 rpm (3600 × g) at 4°C, and plasma was aspirated and stored at −80°C. Plasma samples were further analyzed for clinical chemistry and nutritional components. Fetal growth was assessed by measuring fetal weight, crown-rump length, abdominal girth, thoracic girth, head length (tip of snout to crown of head), and head width (distance between ears). Fetal heart, kidney, liver, spleen, thymus, brain, and lung were collected and weighed. In addition, fetal body mass index (BMI) was calculated as fetal weight (kg)/crown-rump length (m2), and ponderal index (PI) was calculated as fetal weight (g)/crown-rump length (m3). A, B, C, and D placentomes were dissected from the uterus, classified according to the protocol of Vatnick and colleagues [29], individually counted, and weighed.
Fetuses were evaluated as either control or unilateral and by the number of fetuses as follows: control singletons (C-1; n = 9 fetuses), control twins (C-2, n = 14), control triplets (C-3, n = 21), control quadruplets (C-4, n = 4), unilateral singletons (U-1; n = 13), unilateral twins (U-2, n = 12), and unilateral triplets (U-3, n = 6). Placental data for GD 120 and GD 130 were combined because the number of placentomes and placental weight remain unchanged after GD 90 [30], and in this experiment total placentome number (P > 0.6) and total placentome weight (P > 0.3) were similar between GD groups.
Analysis of Placental Efficiency and Plasma Chemistry
As is traditional, placental efficiency was calculated as fetal weight (g)/placentome weight (g). Creatinine, blood urea nitrogen (BUN), and cholesterol were measured in the umbilical arterial plasma samples. Glucose and triglycerides were measured in the maternal arterial plasma and umbilical venous plasma samples. These data were obtained as part of a Chem 20 panel run in the Clinical Chemistry Laboratory at the Meriter Laboratories, Madison, WI (Cobas Integra 800; Roche Pharmaceuticals).
Statistical Analysis
Statistical analyses were performed using a one- or two-way analysis of variance followed by a Student-Newman-Keuls post hoc test using SigmaStat 1.0 software (Jandel Corporation). Data are presented as means ± SEM; P < 0.05 was considered significant.
RESULTS
Placental Changes with Uterine Space Restriction
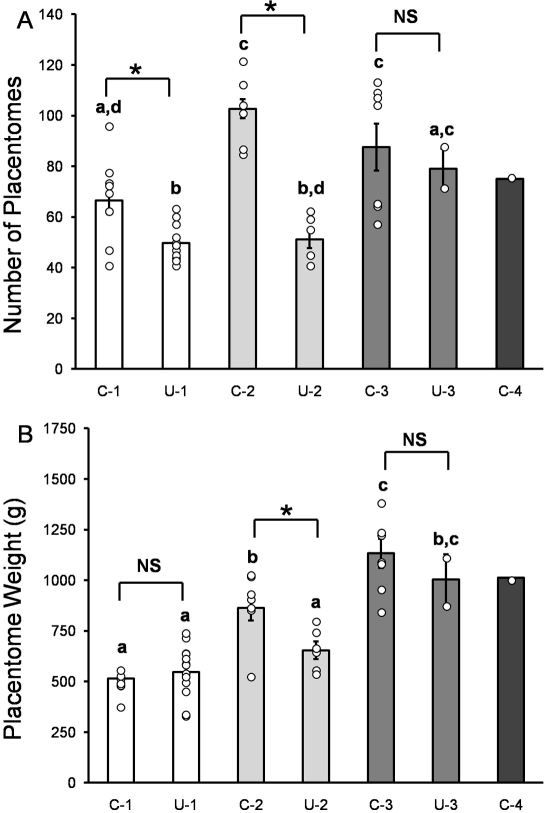
A significant interaction between the factors “surgical uterine anomaly” and “multifetal gestation” was observed. Compared to singleton controls, twin and triplet control pregnancies had a 55% and a 32% increase in the total placentome number per ewe, respectively (Fig. 2A). Unilateral surgery intervention decreased the total number of placentomes in singleton and twin pregnancies (Fig. 2A). Singleton unilateral pregnancies had 25% fewer total placentomes per ewe compared to controls, and twin unilateral pregnancies had 50% fewer total placentomes per ewe. For triplet pregnancies, there was no difference in total placentome number per ewe between control and unilateral sheep, demonstrating overall placental adaptation. Compared to singletons, total placentome weight was increased by 68% and 120% in twin and triplet control pregnancies, respectively, but only by a 31% increase compared to twins (Fig. 2B). Unilateral surgery decreased total placentome weight by 24%, in twin pregnancies only, whereas singleton and triplet pregnancies had no difference in total placentome weight (Fig. 2B).
FIG. 2.
Placental measurements per ewe in control and unilateral pregnant sheep. A) total number of placentomes per ewe and (B) total placental weight per ewe in control singletons (C-1), unilateral singletons (U-1), control twins (C-2), unilateral twins (U-2), control triplets (C-3), unilateral triplets (U-3), and control quadruplets (C-4). Bars indicate means ± SEM. Individual data points are shown by circles. Means that do not share a common superscript letter are different (P < 0.05). * Indicates significance between key comparisons. NS = not significant.
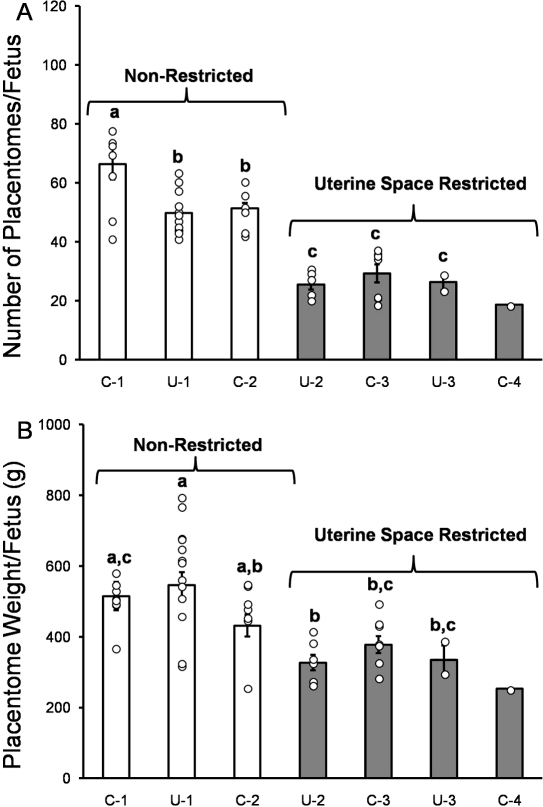
Based on the interactive effects of the uterine anomaly surgical intervention and the number of fetuses per pregnancy, we utilized the dependent placental factors, namely, the number of placentomes and the total placentomal weight per fetus, as indices of potential nutrient delivery to the fetus. We then categorized the fetuses as nonrestricted (control and unilateral singletons and control twins) and uterine space restricted (unilateral twins, control and unilateral triplets, and control quadruplets) (Fig. 3, A and B). Unilateral twins, all triplets and quadruplet pregnancies, had 51% fewer placentomes per fetus (54 ± 2 vs. 27 ± 2, respectively) and a 31% reduction in placentomal weight per fetus (515 ± 20 vs. 345 ± 16 g, respectively) compared to all singletons and control twins (Fig. 3, A and B; P < 0.0001).
FIG. 3.
Placental measurements per fetus in control and unilateral pregnant sheep. A) total number of placentomes per fetus and (B) total placentomal weight per fetus in control singletons (C-1), unilateral singletons (U-1), control twins (C-2), unilateral twins (U-2), control triplets (C-3), unilateral triplets (U-3), and control quadruplets (C-4). Bars indicate means ± SEM. Individual data points are shown by circles. Means that do not share a common superscript letter are different (P < 0.05). Based on these results, pregnancies were categorized as either nonrestricted (C-1, U-1, and C-2) or uterine space restricted (U-2, C-3, U-3, and C-4).
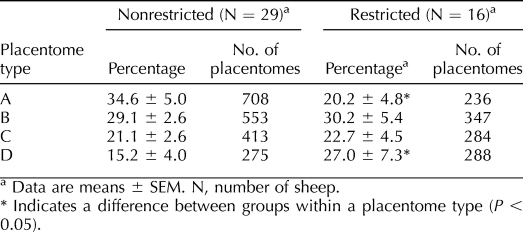
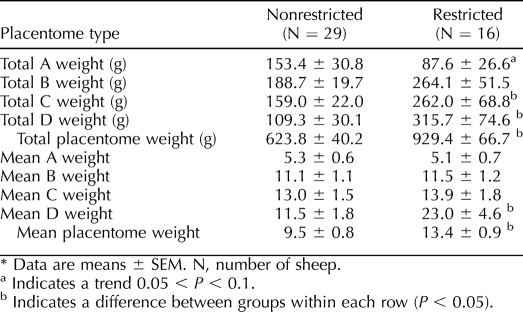
Uterine space restriction altered the percentage of A and D placentomes. Compared to nonrestricted pregnancies, there were 42% fewer A and 78% more D placentomes in the uterine space-restricted group (Table 1). No difference was observed in the percentage of B and C placentomes between groups. Space restriction also showed a tendency (P = 0.08) to reduce total A placentome weight by 42% (also in Table 2). Further, there was a 65% increase in total C placentome weight and a 189% increase in total D weight in uterine space-restricted pregnancies. Total placentome weight was elevated by 40% in uterine space-restricted pregnancies. We also calculated mean placentome weight by dividing total placentome weight by the number of placentomes. Within each type, only D placentomes were heavier, increasing by 100%. Therefore, on average, each placentome was 41% larger in the uterine space-restricted group (Table 2).
TABLE 1.
Percentage of A, B, C, and D placentomes in nonrestricted and uterine space-restricted ewes.
TABLE 2.
Total placentome weight and mean placentome weight in nonrestricted and uterine space-restricted ewes.*
Fetal Changes with Uterine Space Restriction
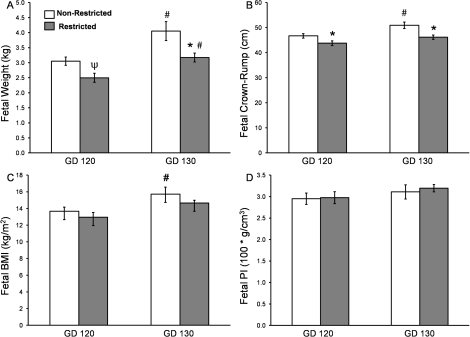
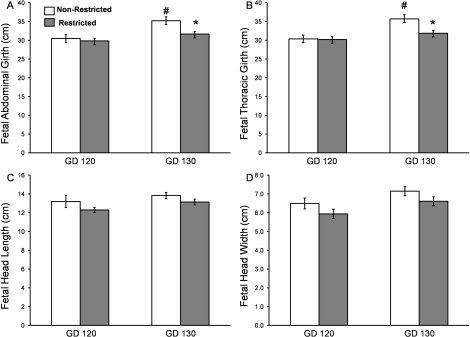
Compared to the nonrestricted group, uterine space-restricted fetuses were 22% lighter in weight at GD 130 and had a 6% and 9% shorter crown-rump length at GD 120 and GD 130, respectively (Fig. 4, A and B). In contrast, there was no difference in BMI or PI between nonrestricted and restricted fetuses at either GD (Fig. 4C). Fetal abdominal and thoracic girth were unchanged between groups at GD 120 (Fig. 5, A and B), but by GD 130, the uterine space-restricted fetuses had a 10% smaller abdominal and 9% smaller thoracic girth when compared with nonrestricted fetuses (Fig. 5, A and B). Brain sparing was observed in the uterine space-restricted fetuses, as fetal head length, head width, and brain weights were similar between groups at either GD 120 or GD 130 (Fig. 5, C and D; see also Table 3).
FIG. 4.
Fetal morphometric measurements in nonrestricted and uterine space-restricted pregnancies. A) fetal weight, (B) fetal crown-rump, (C) fetal body mass index (BMI), and (D) ponderal index (PI) at GD 120 and GD 130. Bars indicate means ± SEM. * Indicates a decrease in the restricted group vs. the nonrestricted group within a GD (P < 0.05); Ψ indicates a trend (0.05 < P < 0.1); # indicates an increase by GD (P < 0.05).
FIG. 5.
Fetal morphometric measurements in nonrestricted and uterine space-restricted pregnancies. A) fetal abdominal girth, (B) fetal thoracic girth, (C) fetal head length, and (D) fetal head width at GD 120 and GD 130. Bars indicate means ± SEM. * Indicates a decrease in the restricted group vs. the nonrestricted group within a GD (P < 0.05). # Indicates an increase by GD (P < 0.05).
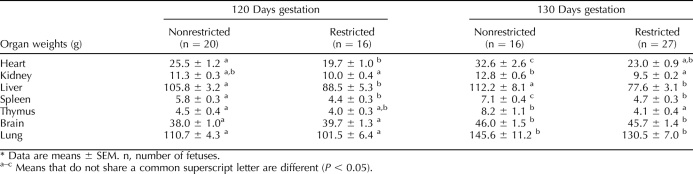
TABLE 3.
Fetal organ weights in nonrestricted and uterine space-restricted fetuses at 120 and 130 days of gestation.*
Uterine space restriction decreased heart, liver, and spleen weights at GD 120 and by GD 130 were 29%, 31%, and 34% lighter in restricted fetuses, respectively (Table 3). Kidney and thymus weights in the restricted group were not different at GD 120; however, by GD 130, they were 26% and 50% lighter, indicating a lack of continual growth. Fetal brain and lung weights were not different between groups at either GD (Table 3), demonstrating brain sparing and asymmetric IUGR. When weights were examined in proportion to fetal body weight (standardized fetal weight), there were no differences in heart, kidney, liver, and lung weights per fetal body weight between groups at either GD (data not shown). In contrast, compared to nonrestricted fetuses, uterine space-restricted spleen and thymus weights per fetal weight were not different at GD 120; however, by GD 130, they were 21% and 36% lighter (data not shown). Uterine space-restricted brain weights per fetal weight were 22% and 19% larger at GD 120 and GD 130, respectively (data not shown).
Placental Efficiency and Blood Chemistry Changes with Uterine Space Restriction
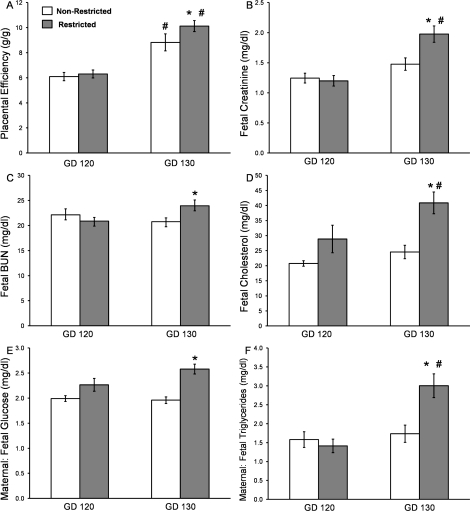
Classically calculated placental efficiency is the ratio of fetal weight (g) per placental weight (g). According to this definition, placentas showed similar efficiency at GD 120, and by this definition, uterine space-restricted placentas were 15% more efficient by GD 130 (Fig. 6A). We also evaluated fetal levels of creatinine, BUN, and cholesterol (Fig. 6, B–D). At GD 120, uterine space restriction did not alter umbilical artery plasma creatinine, BUN, or cholesterol levels. However, at GD 130, uterine space-restricted fetuses had 34% higher umbilical arterial creatinine, 15% higher BUN, and 67% higher cholesterol. The maternal artery-to-umbilical vein ratio of glucose and triglycerides (Fig. 6, E and F) were unchanged at GD 120, whereas the ratios were increased 32% and 73% in GD 130 space-restricted fetuses.
FIG. 6.
Placental efficiency and blood chemistry changes in nonrestricted and uterine space-restricted pregnancies. A) Placental efficiency measured as fetal body weight per total placentomal weight (g/g). Blood chemistry in umbilical artery for (B) creatinine, (C) blood urea nitrogen (BUN), and (D) cholesterol and the ratios of umbilical vein to maternal artery for (E) glucose and (F) triglycerides at GD 120 and GD 130. Bars indicate means ± SEM. * Indicates a decrease in the restricted group vs. the nonrestricted group within a GD (P < 0.05). # Indicates an increase by GD (P < 0.05).
DISCUSSION
This study provides the first in-depth examination of the interactions between multifetal gestations and a surgically created uterine anomaly model on placental adaptations and IUGR, giving us the ability to quantify specific placental adaptations that function to modulate fetal growth and development. The placenta adapted by increasing the total number of placentomes formed, increasing individual placentomal weights through greater conversion from A to D morphological types, and increasing C and D total placentomal weights. Multifetal pregnancies with uterine anomaly induced fetal growth arrest and asymmetric IUGR once the ability of the placenta to adapt was surpassed. These fetal growth deficits were associated with alterations in functional placental efficiency and adaptations that are vitally important for maintaining a viable albeit compromised pregnancy.
Several earlier ovine models of uterine space reduction were developed by reducing the endometrial surface area and thus the potential placental attachment sites. These models have reduced the number of placentomes by either carunclectomy prior to breeding [31, 32], by hemihysterectomy, [33] or, in a more analogous fashion to the current model, by performing unilateral uterine horn ligations either before [34] or 5 days after breeding [35, 36]. In the unilateral ligation and hemihysterectomy models, placentome number was reduced by 14% [33], 23% [34], and 47% [36]. Although some of these studies had twin pregnancies [36], they did not stratify their multifetal data or, as in the current study, further stress the maternal-fetal system with triplets. Herein, we surgically separated the intercornual connections and isolated one uterine horn to more closely mimic the anatomy of human uterine anomalies (uterine bicornuate, unicornuate, or didelphys) in which the septum is devoid of vascular connections. The first report using this specific local ovine uterine horn isolation model was by Moor and Rowson [28]. They showed local early ovine embryo maintenance of corpus luteum function, which was subsequently shown to transpire via a local venoarterial pathway [27, 37]. However, the current study is the first to use this surgical model to evaluate uteroplacental function and fetal development in late ovine gestation.
Numerous studies have described a direct relationship between total placental size (i.e., placentome number and placentome weight) and morphology with nutrient transport ability and fetal growth [32, 38–42]. In the present study, control multifetal pregnancies increased overall placentome number from 66 ± 5 placentomes in singletons to 103 ± 4 in twins and 88 ± 8 in triplet pregnancies. These results are similar to other multifetal gestation studies with 20%–28% more placentomes reported [39, 43, 44]. This was likely due to placentomes formed in the uterine horns proximal to the uterotubal junction, with caruncles normally not activated in singleton pregnancies; these types of placentomes are thought to compensate for placental insufficiency [45]. In the current study, unilateral surgery decreased the total number of placentomes by 25% in singleton and 50% in twin pregnancies but not in triplets. This may be explained by the activation of these uterotubal placentomes in triplets but not singletons or twins. The overall decrease in number of placentomes in singleton and twin pregnancies was due to surgically reduced potential for placentation without the complete adaptation of uterotubal placentomes. This is consistent with the other unilateral models with 14%–46% fewer placentomes [33, 34, 36]. Regardless of the number of caruncles removed in any surgical model, the number of placentomes in the pregnancy is partly preserved, indicating placental adaptation.
After initial placental attachment, the number of placentomes do not change [30]. Therefore, increased placental weight (i.e., placental hypertrophy) and changes in morphology are two important ways in which the placenta can adapt to the increased fetal requirements seen throughout pregnancy. As fetal number increased, a dramatic increase in total placentomal weight was observed. Specifically, compared to singletons, the twins and triplets had a 68% and 120% increase in total placentomal weight, respectively. This was similar to a study by Vonnahme and coworkers [9] in which increasing fetal number from singletons to twins or triplets increased placental weight by 26%–52%, respectively, confirming the original observations of Barcroft [46], who compared singletons to twins. In contrast, others have reported little or no change in overall placentomal weight with increases in fetal number [43, 44]. Unilateral uterine surgery as a model for uterine anatomical anomalies did not alter total placentome weight in singleton or triplet but reduced total placentome weight in twin pregnancies. Data from other surgical uterine space restriction models are not in agreement with this study or each other. Caton et al. [36] and Ott et al. [34] found no change in total placentome weight, whereas Cefalo et al. [33] found a 23% increase in placentome weight. Differences among these studies are likely due to the lack of separation of singleton and twin data. Indeed, if we were to combine singletons and twins in the current study, we also would find no difference in total placentomal weight.
In the current study, pregnancies were classified into nonrestricted or uterine space-restricted based on the clear demarcation in the number of placentomes and placental weight per fetus (Fig. 3, A and B). Specifically, we defined the groups as nonrestricted and uterine space-restricted due to a 51% reduction in total placentome number and a 31% reduction in total placentome weight per fetus. These finding agree with those of Robinson et al. [31], who showed in the carunculectomy model that at least a 40% reduction in placentome number was necessary to produce IUGR. In the present model, we found that uterine space-restricted pregnancies had fewer A-type and more D-type placentomes and heavier C and D placentomes. This change in placental morphology and weight may be due to placental compensation (i.e., hypertrophy) described earlier. Current literature suggests that an increased percentage of C and D placentomes is an adaptation in an effort to increase nutrient delivery [32, 38, 42]. However, a functional difference between placentome types at the level of vascularity has recently been contested [47] and should be part of the focus in subsequent experiments with this particular model.
We also studied the effects of uterine space restriction on fetal morphometric parameters. IUGR in humans results in greater morbidity and mortality [48, 49]. Additionally, the Barker hypothesis proposes that IUGR is responsible for multiple adult-onset diseases due to permanent metabolic reprogramming [19, 48]. Two classifications of IUGR have been defined: symmetric and asymmetric. Symmetric, or proportionate, IUGR is associated with genetic abnormalities or first-trimester infections [20]. Asymmetric IUGR, however, is more common and is associated with placental insufficiency and asymmetric fetal linear growth, decreased fetal body and organ weight, and brain sparing [20]. In the present study, we observed complete fetal growth arrest between GD 120 and GD 130 such that asymmetric IUGR was clearly evident in uterine space-restricted fetuses by GD 130 with some signs manifesting as early as GD 120. This asymmetric growth is consistent with a disruption in the normal late gestation exponential growth [50].
As in humans, fetuses from uterine space-restricted pregnancies had lower body weight, crown-rump, abdominal girth, and thoracic girth. Restricted fetuses also had lighter hearts, kidneys, livers, spleens, and thymuses. Brain weights were similar between groups at GD 120 and GD 130 likely due to a redistribution of blood flow known as brain sparing, often seen in asymmetric IUGR [48, 51]. Lungs were an exception and were grown normally, consistent with humans, as fetal lung development is regulated by gestational age and cortisol levels but not by nutrient delivery [52–54]. Taken together, these findings are consistent with in utero predictors of human IUGR due to reduced fetal nutrient delivery and placental insufficiency [20].
In the current study, we first utilized the ratio of fetal weight to total placentome weight as an index of placental efficiency [55–60] and found increased placental efficiency in the uterine space-restricted ewes at 130 days. However, this traditional index of placental efficiency utilizes only placental weight and does not utilize true functional indices of placental insufficiency, such as placental clearance and/or delivery of nutrients and metabolites. In agreement with human IUGR, we observed at 130 days increased fetal creatinine [61], BUN [61], cholesterol [62], and ratios of maternal to fetal glucose and triglyceride levels [20, 63, 64], observations that were attributed to decreased placental ability to deliver nutrients and clear fetal metabolites. Some of these measures not only may have a causal relationship with IUGR but also could be the consequence of IUGR and/or dysfunctional fetal metabolism. However, best estimates of placental efficiency will require measurements of both uterine and placental blood flows and subsequent calculation of nutrient delivery rates and fetal nutrient consumption. Collectively, these observations demonstrate functional uteroplacental maladaptations in response to maternal uterine space restriction.
In conclusion, the present study provides a quantitative evaluation of two interacting factors, multifetal gestations and surgically created uterine anomaly, on placental adaptations and IUGR. While uterine anomalies might not always cause fetal IUGR, when it is combined with multifetal gestations, the severity of the detrimental effects of uterine space restriction are greatly compounded. The placenta appears to adapt to reductions in uterine space by increasing placentome numbers, by increasing individual placentomal weights, and through conversion from A to D anatomical types. Even as placental adaptations occur, fetal growth arrest is seen, giving rise to asymmetric IUGR. Thus, changes in placental efficiency with consequent reduction in fetal growth are fundamentally central to preserve a viable albeit compromised fetus. In short, this study provides a consistent model of IUGR that can be used to study numerous physiological and pathophysiologic processes involved in fetal and placental development.
Acknowledgments
Manuscript preparation assistance was provided by Sheikh Omar Jobe, Timothy Morschauser, Mayra Pastore, and Mary Sun. Graphical representation of the model was provided by Robert Gordon of the Department of Pediatrics. Technical support was provided by the Magness laboratory (Jason Austin, Kreg Grindle, Gladys Lopez, and Terry Phernetton), the Kling laboratory (Sharon Blohowiak and Jason Habeck), and Todd Taylor at the University of Wisconsin Arlington Farm Facility. This work is in partial fulfillment of the master's of science degree for the Endocrinology and Reproductive Physiology Training Program.
Footnotes
Supported by the University of Wisconsin Department of Pediatrics and NIH grants HL49210, HL87144, and HD38843 (R.R.M.).
REFERENCES
- Grimbizis GF, Camus M, Tarlatzis BC, Bontis JN, Devroey P.Clinical implications of uterine malformations and hysteroscopic treatment results. Hum Reprod Update 2001; 7: 161–174. [DOI] [PubMed] [Google Scholar]
- Kiely JL, Brett KM, Yu S, Rowley D.Low birth weight and intrauterine growth retardation. Wilcox LS, Marks JS.From Data To Action: CDC's Public Health Surveillance For Women, Infants, And Children. Washington, DC: US Department of Health and Human Services; 1994: 185–202.
- Romo A, Carceller R, Tobajas J.Intrauterine growth retardation (IUGR): epidemiology and etiology. Pediatr Endocrinol Rev 2009; 6(suppl 3):332–336. [PubMed] [Google Scholar]
- Reichman D, Laufer MR, Robinson BK.Pregnancy outcomes in unicornuate uteri: a review. Fertil Steril 2009; 91: 1886–1894. [DOI] [PubMed] [Google Scholar]
- Cohen A, Chhibber G.Obstetric complications of congenital anomalies of the paramesonephric ducts. Semin Reprod Endocrinol 1986; 4: 59–65. [Google Scholar]
- Akar ME, Bayar D, Yildiz S, Ozel M, Yilmaz Z.Reproductive outcome of women with unicornuate uterus. Aust N Z J Obstet Gynaecol 2005; 45: 148–150. [DOI] [PubMed] [Google Scholar]
- Cleary-Goldman J, D'Alton ME.Growth abnormalities and multiple gestations. Semin Perinatol 2008; 32: 206–212. [DOI] [PubMed] [Google Scholar]
- Gootwine E, Spencer TE, Bazer FW.Litter-Size-Dependent Intrauterine Growth Restriction in Sheep. New York:Cambridge University Press;2007. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Evoniuk J, Johnson ML, Borowicz PP, Luther JS, Pant D, Redmer DA, Reynolds LP, Grazul-Bilska AT.Placental vascularity and growth factor expression in singleton, twin, and triplet pregnancies in the sheep. Endocrine 2008; 33: 53–61. [DOI] [PubMed] [Google Scholar]
- Luke B.Reducing fetal deaths in multiple births: optimal birthweights and gestational ages for infants of twin and triplet births. Acta Genet Med Gemellol (Roma) 1996; 45: 333–348. [DOI] [PubMed] [Google Scholar]
- Kiely JL.The epidemiology of perinatal mortality in multiple births. Bull N Y Acad Med 1990; 66: 618–637. [PMC free article] [PubMed] [Google Scholar]
- de Boo HA, Harding JE.The developmental origins of adult disease (Barker) hypothesis. Aust N Z J Obstet Gynaecol 2006; 46: 4–14. [DOI] [PubMed] [Google Scholar]
- Hendrix N, Berghella V.Non-placental causes of intrauterine growth restriction. Semin Perinatol 2008; 32: 161–165. [DOI] [PubMed] [Google Scholar]
- Gagnon AL, Galerneau F, Williams K.Twin pregnancy with one fetus in a rudimentary horn: a case report of a surviving twin. Br J Obstet Gynaecol 1998; 105: 1326–1328. [DOI] [PubMed] [Google Scholar]
- Monteagudo A, Strok I, Greenidge S, Timor-Tritsch IE.Quadruplet pregnancy: two sets of twins, each occupying a horn of a septate (complete) uterus. J Ultrasound Med 2004; 23: 1107–1111;.quiz 1112–1113. [DOI] [PubMed] [Google Scholar]
- Green LK, Harris RE.Uterine anomalies: frequency of diagnosis and associated obstetric complications. Obstet Gynecol 1976; 47: 427–429. [PubMed] [Google Scholar]
- Kekkonen R, Nuutila M, Laatikainen T.Twin pregnancy with a fetus in each half of a uterus didelphys. Acta Obstet Gynecol Scand 1991; 70: 373–374. [DOI] [PubMed] [Google Scholar]
- Narlawar RS, Chavhan GB, Bhatgadde VL, Shah JR.Twin gestation in one horn of a bicornuate uterus. J Clin Ultrasound 2003; 31: 167–169. [DOI] [PubMed] [Google Scholar]
- Barker DJP.Mothers, Babies, and Health Later in Life. Edinburgh:Churchill Livingstone;1998. [Google Scholar]
- Anthony RV, Scheaffer AN, Wright CD, Regnault TR.Ruminant models of prenatal growth restriction. Reprod Suppl 2003; 61: 183–194. [PubMed] [Google Scholar]
- Magness RR, Phernetton TM, Zheng J.Systemic and uterine blood flow distribution during prolonged infusion of 17beta-estradiol. Am J Physiol 1998; 275: H731–H743. [DOI] [PubMed] [Google Scholar]
- Magness RR, Phernetton TM, Gibson TC, Chen DB.Uterine blood flow responses to ICI 182 780 in ovariectomized oestradiol-17beta-treated, intact follicular and pregnant sheep. J Physiol 2005; 565: 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson TC, Phernetton TM, Wiltbank MC, Magness RR.Development and use of an ovarian synchronization model to study the effects of endogenous estrogen and nitric oxide on uterine blood flow during ovarian cycles in sheep. Biol Reprod 2004; 70: 1886–1894. [DOI] [PubMed] [Google Scholar]
- Magness RR, Huie JM, Hoyer GL, Huecksteadt TP, Reynolds LP, Seperich GJ, Whysong G, Weems CW.Effect of chronic ipsilateral or contralateral intrauterine infusion of prostaglandin E2 (PGE2) on luteal function of unilaterally ovariectomized ewes. Prostaglandins Med 1981; 6: 389–401. [DOI] [PubMed] [Google Scholar]
- Huie JM, Magness RR, Reynolds LP, Hoyer G, Huecksteadt T, Colcord M, Stalcup B, Whysong GL, Weems CW.Effect of chronic ipsilateral or contralateral intrauterine infusion of prostaglandin E1 (PGE1) on luteal function of unilaterally ovariectomized ewes. Prostaglandins 1981; 21: 945–955. [DOI] [PubMed] [Google Scholar]
- Ginther OJ.Effect of unilateral hysterectomy and separation or ligation of uterine horns on luteolytic action of intrauterine device in sheep. Am J Vet Res 1970; 31: 2127–2130. [PubMed] [Google Scholar]
- Ginther OJ.Internal regulation of physiological processes through local venoarterial pathways: a review. J Anim Sci 1974; 39: 550–564. [DOI] [PubMed] [Google Scholar]
- Moor RM, Rowson LE.Local maintenance of the corpus luteum in sheep with embryos transferred to various isolated portions of the uterus. J Reprod Fertil 1966; 12: 539–550. [DOI] [PubMed] [Google Scholar]
- Vatnick I, Schoknecht PA, Darrigrand R, Bell AW.Growth and metabolism of the placenta after unilateral fetectomy in twin pregnant ewes. J Dev Physiol 1991; 15: 351–356. [PubMed] [Google Scholar]
- Ehrhardt RA, Bell AW.Growth and metabolism of the ovine placenta during mid-gestation. Placenta 1995; 16: 727–741. [DOI] [PubMed] [Google Scholar]
- Robinson JS, Kingston EJ, Jones CT, Thorburn GD.Studies on experimental growth retardation in sheep: the effect of removal of a endometrial caruncles on fetal size and metabolism. J Dev Physiol 1979; 1: 379–398. [PubMed] [Google Scholar]
- Alexander G.Studies on the placenta of the sheep (Ovis aries L.): effect of surgical reduction in the number of caruncles. J Reprod Fertil 1964; 7: 307–322. [DOI] [PubMed] [Google Scholar]
- Cefalo RC, Simkovich JW, Abel F, Hellegers AE, Chez RA.Effect of potential placental surface area reduction on fetal growth. Am J Obstet Gynecol 1977; 129: 434–439. [DOI] [PubMed] [Google Scholar]
- Ott TL, Wiley AA, Bartol FF.Effects of stage of gestation and uterine ligation on ovine placentome development and glycosaminoglycans. J Anim Sci 1997; 75: 1053–1062. [DOI] [PubMed] [Google Scholar]
- Bazer FW, Roberts RM, Basha SM, Zavy MT, Caton D, Barron DH.Method for obtaining ovine uterine secretions from unilaterally pregnant ewes. J Anim Sci 1979; 49: 1522–1527. [DOI] [PubMed] [Google Scholar]
- Caton D, Bazer FW, Kalra PS, Moffatt RJ.Adaptations to reduction of endometrial surface available for placental development in sheep. J Reprod Fertil 1984; 72: 357–364. [DOI] [PubMed] [Google Scholar]
- Moor RM, Booth WD, Rowson LE.Effect of hysterectomy on the life-span of corpora lutea induced artificially in progesterone-treated ewes. J Reprod Fertil 1966; 12: 385–387. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Ward JW, Wooding FP, Forhead AJ, Constancia M.Programming placental nutrient transport capacity. J Physiol 2006; 572: 5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur R.Morphometry of the placental exchange area. Adv Anat Embryol Cell Biol 1977; 53: 3–65. [DOI] [PubMed] [Google Scholar]
- Mellor DJ.Nutritional and placental determinants of foetal growth rate in sheep and consequences for the newborn lamb. Br Vet J 1983; 139: 307–324. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Caton JS, Redmer DA, Grazul-Bilska AT, Vonnahme KA, Borowicz PP, Luther JS, Wallace JM, Wu G, Spencer TE.Evidence for altered placental blood flow and vascularity in compromised pregnancies. J Physiol 2006; 572: 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G.Studies on the placenta of the sheep (Ovis aries L.): placental size. J Reprod Fertil 1964; 7: 289–305. [DOI] [PubMed] [Google Scholar]
- Dwyer CM, Calvert SK, Farish M, Donbavand J, Pickup HE.Breed, litter and parity effects on placental weight and placentome number, and consequences for the neonatal behaviour of the lamb. Theriogenology 2005; 63: 1092–1110. [DOI] [PubMed] [Google Scholar]
- Grazul-Bilska AT, Pant D, Luther JS, Borowicz PP, Navanukraw C, Caton JS, Ward MA, Redmer DA, Reynolds LP.Pregnancy rates and gravid uterine parameters in single, twin and triplet pregnancies in naturally bred ewes and ewes after transfer of in vitro produced embryos. Anim Reprod Sci 2006; 92: 268–283. [DOI] [PubMed] [Google Scholar]
- Bjorkman N.Morphological and histochemical studies on the bovine placenta. Acta Anat (Basel) 1954; 22: 1–91. [PubMed] [Google Scholar]
- Barcroft SJ.Researches on Pre-Natal Life. Oxford:Blackwell Scientific Publications;1946. [Google Scholar]
- Vonnahme KA, Arndt WJ, Johnson ML, Borowicz PP, Reynolds LP.Effect of morphology on placentome size, vascularity, and vasoreactivity in late pregnant sheep. Biol Reprod 2008; 79: 976–982. [DOI] [PubMed] [Google Scholar]
- McMillen IC, Adams MB, Ross JT, Coulter CL, Simonetta G, Owens JA, Robinson JS, Edwards LJ.Fetal growth restriction: adaptations and consequences. Reproduction 2001; 122: 195–204. [DOI] [PubMed] [Google Scholar]
- Newton ER, Kennedy JL, Jr, Louis F, Cetrulo CL, Sbarra A, Feingold M.Obstetric diagnosis and perinatal mortality. Am J Perinatol 1987; 4: 300–304. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Wallace JM, Reynolds LP.Effect of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domest Anim Endocrinol 2004; 27: 199–217. [DOI] [PubMed] [Google Scholar]
- Morrison JL.Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol 2008; 35: 730–743. [DOI] [PubMed] [Google Scholar]
- Newnham JP, Ross MG.Early Life Origins of Human Health and Disease. Basel:S. Karger;2009. [Google Scholar]
- Lipsett J, Tamblyn M, Madigan K, Roberts P, Cool JC, Runciman SI, McMillen IC, Robinson J, Owens JA.Restricted fetal growth and lung development: a morphometric analysis of pulmonary structure. Pediatr Pulmonol 2006; 41: 1138–1145. [DOI] [PubMed] [Google Scholar]
- Duncan KR, Issa B, Moore R, Baker PN, Johnson IR, Gowland PA.A comparison of fetal organ measurements by echo-planar magnetic resonance imaging and ultrasound. BJOG 2005; 112: 43–49. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Biensen NJ, Ford SP.Novel insight into the control of litter size in pigs, using placental efficiency as a selection tool. J Anim Sci 1999; 77: 1654–1658. [DOI] [PubMed] [Google Scholar]
- Angiolini E, Fowden A, Coan P, Sandovici I, Smith P, Dean W, Burton G, Tycko B, Reik W, Sibley C, Constancia M.Regulation of placental efficiency for nutrient transport by imprinted genes. Placenta 2006; 27(suppl A):S98–S102. [DOI] [PubMed] [Google Scholar]
- Biensen NJ, Wilson ME, Ford SP.The impacts of uterine environment and fetal genotype on conceptus size and placental vascularity during late gestation in pigs. J Anim Sci 1999; 77: 954–959. [DOI] [PubMed] [Google Scholar]
- Biensen NJ, Wilson ME, Ford SP.The impact of either a Meishan or Yorkshire uterus on Meishan or Yorkshire fetal and placental development to days 70, 90, and 110 of gestation. J Anim Sci 1998; 76: 2169–2176. [DOI] [PubMed] [Google Scholar]
- Mesa H, Safranski TJ, Johnson RK, Lamberson WR.Correlated response in placental efficiency in swine selected for an index of components of lifter size. J Anim Sci 2003; 81: 74–79. [DOI] [PubMed] [Google Scholar]
- Mesa H, Safranski TJ, Fischer KA, Cammack KM, Lamberson WR.Selection for placental efficiency in swine: genetic parameters and trends. J Anim Sci 2005; 83: 983–991. [DOI] [PubMed] [Google Scholar]
- Arad I, Bar-Oz B, Peleg O.Early plasma creatinine values in discordant twins. Twin Res 2001; 4: 215–218. [DOI] [PubMed] [Google Scholar]
- Alvino G, Cozzi V, Radaelli T, Ortega H, Herrera E, Cetin I.Maternal and fetal fatty acid profile in normal and intrauterine growth restriction pregnancies with and without preeclampsia. Pediatr Res 2008; 64: 615–620. [DOI] [PubMed] [Google Scholar]
- Ergaz Z, Avgil M, Ornoy A.Intrauterine growth restriction-etiology and consequences: what do we know about the human situation and experimental animal models? Reprod Toxicol 2005; 20: 301–322. [DOI] [PubMed] [Google Scholar]
- Marconi AM, Paolini C, Buscaglia M, Zerbe G, Battaglia FC, Pardi G.The impact of gestational age and fetal growth on the maternal-fetal glucose concentration difference. Obstet Gynecol 1996; 87: 937–942. [DOI] [PubMed] [Google Scholar]