Abstract
The transcription factor Ets1 is normally expressed in the proliferative layer of stratified epithelium, but expression of Ets1 is significantly upregulated in squamous cell carcinomas. How elevated levels of Ets1 impact tumor initiation and progression is not well understood. To determine the biological consequences of overexpression of Ets1, we developed a transgenic mouse model that allows induction of Ets1 expression in keratinocytes of stratified epithelium in a regulatable fashion. Induction of Ets1 during embryonic development results in a dramatic alteration in epidermal structure and function by suppressing the expression of multiple stratum corneum constituents, while at the same time inducing expression of EGF ligands, AP1 transcription factors and matrix metalloproteases. Interestingly, expression of certain immune-related genes, including defensins, chemokines and cytokines was increased as well, suggesting a possible role for immune dysregulation in the promotion of squamous dysplasia. Experiments using cultured mouse keratinocytes indicate that Ets1 can induce expression of some of these mediators in a cell-intrinsic fashion. Collectively, our data reveal that elevated expression of Ets1 has a much broader array of pro-tumorigenic effects on epithelial cells than previously appreciated.
Keywords: Mmp, Chemokine, Differentiation, Keratinocyte, Transgenic, EGF ligand
Introduction
The stratified squamous epithelium of the skin is comprised of the inter-follicular epidermis (IFE), which covers most of the body surface, and interspersed hair follicles and sebaceous glands. Keratinocytes, the main cell type in the IFE, undergo a well-organized differentiation process to yield a water-impermeable, mechanically resistant cornified barrier that prevents both loss of internal fluids and entry of external substances and foreign bodies (Fuchs, 2007; Nagarajan et al., 2008; Proksch et al., 2008). During gestation, when a functional cornified layer has not yet developed, embryos are encased by periderm, a protective layer that acts as a transient barrier (M'Boneko and Merker, 1988). Stratified squamous epithelia of the hard palate and gingiva in the oral cavity undergo a differentiation process similar to that of IFE, leading to the production of a cornified epithelium (Presland and Jurevic, 2002; Squier and Kremer, 2001). In other areas of the oral mucosa, keratinocytes differentiate but do not cornify, hence producing a flexible, non-cornified layer capable of movements associated with speech, chewing and swallowing.
The barrier function of the skin depends on the precise differentiation of the IFE keratinocytes, and disruptions to this sequence can cause serious, even life-threatening disease (Segre, 2006). Early stages of keratinocyte differentiation involve the expression of large amounts of intermediate filament proteins called keratins (Fuchs, 2007; Nagarajan et al., 2008; Proksch et al., 2008). In late stages of stratified squamous epithelial differentiation, the expression of genes encoding keratin proteins is downregulated and the expression of genes encoding cornified envelope proteins (such as involucrin, filaggrin, loricrin, small proline-rich proteins and late cornified envelope proteins) is upregulated (Fuchs, 2007; Nagarajan et al., 2008; Proksch et al., 2008). These cornified envelope constituents are crosslinked by the action of transglutaminase enzymes to form a tough sac enclosing the keratin filaments (Eckert et al., 2005; Hitomi, 2005). Granular layer keratinocytes synthesize and secrete a number of specialized lipids that contribute to the water-impermeable barrier function of the skin (Kalinin et al., 2002). The final differentiation step involves programmed cell death of the keratinocytes to yield anucleate squames that compose the cornified layer.
Squamous cell carcinoma (SCC) is a malignant tumor that can arise in stratified squamous epithelia. SCC tumors exhibit aberrations in the normal differentiation pattern, which can include loss of cell polarity, hyperkeratosis, parakeratosis, acanthosis, increased mitotic figures, and crowded, pleomorphic and hyperchromatic nuclei (Arlette and Trotter, 2004; Bhawan, 2007; Cockerell, 2000). These changes to keratinocyte differentiation are caused by aberrant activation of oncogenes and inactivation of tumor suppressor genes. SCC lesions are also frequently associated with high-level expression of matrix metalloprotease (Mmp) enzymes, which are thought to contribute to tumor cell migration, invasion and proliferation (Ala-aho and Kahari, 2005; Kerkela and Saarialho-Kere, 2003; Westermarck and Kahari, 1999). Squamous cell tumors are often associated with a significant inflammatory response, which has been linked to tumor angiogenesis, proliferation and migration (Cataisson et al., 2009; Coppe et al., 2008; Coussens et al., 1999; Takahashi et al., 2010).
One of the oncogenes linked to the pathogenesis of SCC is the transcription factor Ets1, a downstream effector of the Ras–MAPK pathway. In normal tissues, Ets1 is expressed only in the basal layer of stratified squamous epithelium (Nagarajan et al., 2009). However, in a large fraction of SCC tumors, Ets1 is highly expressed within the malignant keratinocytes, and its levels directly correlate with the invasive and metastatic potential of the tumor (Bai et al., 2009; Endo et al., 2006; Keehn et al., 2004; Pande et al., 1999; Saeki et al., 2000; Vairaktaris et al., 2007). Tumors that express high levels of Ets1 typically also express high levels of several matrix metalloprotease (Mmp) genes (Dittmer, 2003; Hahne et al., 2008; Lincoln and Bove, 2005) and Ets1 has been shown to directly regulate expression of multiple Mmp genes (Baillat et al., 2006; Nagarajan et al., 2009; Ozaki et al., 2000; Reisdorff et al., 2002; Wei et al., 2009; Westermarck et al., 1997). Yet, it has been unclear whether the sole oncogenic role of Ets1 is upregulation of Mmp expression or whether this protein also promotes squamous cell carcinoma development by affecting other pathways. To address this question, we developed an inducible transgenic mouse model in which Ets1 can be induced in differentiating squamous keratinocytes (Nagarajan et al., 2009). Our studies indicate that induction of Ets1 in adult mice leads to severe dysplastic lesions accompanied by high levels of several Mmp enzymes, most notably Mmp13. To further explore the role of Ets1 in squamous epithelial homeostasis as well as carcinogenic transformation, we now report the immediate effects of Ets1 induction during embryonic development of mice. As described herein, our studies identify a number of key regulatory programs that are influenced by Ets1 and that might have causal implications in squamous carcinogenesis.
Results
Ectopic expression of Ets1 in differentiated layers of the epidermis leads to major epidermal defects
We have previously described a bi-transgenic (designated BT) mouse model system in which we can inducibly express the transcription factor Ets1 in a tightly regulated, tissue- and differentiation-specific pattern (Nagarajan et al., 2009). In this system, one transgenic line (the responder transgenic) contains an N-terminal hemagglutinin (HA)-tagged mouse Ets1 transgene, cloned downstream of the doxycycline-regulated pTight promoter. Another transgenic line (the driver transgenic), expresses the tetracycline-regulated transactivator protein (tTA) under the control of the human involucrin promoter and enhancer elements (Jaubert et al., 2004). The involucrin promoter is active in the upper spinous and granular layers of stratified squamous epithelium, including skin epidermis. BT offspring derived from crossing these two transgenic mouse lines express HA-tagged Ets1 in the upper spinous and granular layers of stratified squamous epithelial tissues in the absence of doxycycline (i.e. a Tet-off system). Induction of Ets1 in adult mice leads to severe dysplastic lesions of the skin, reminiscent of squamous cell carcinoma-in-situ (Nagarajan et al., 2009).
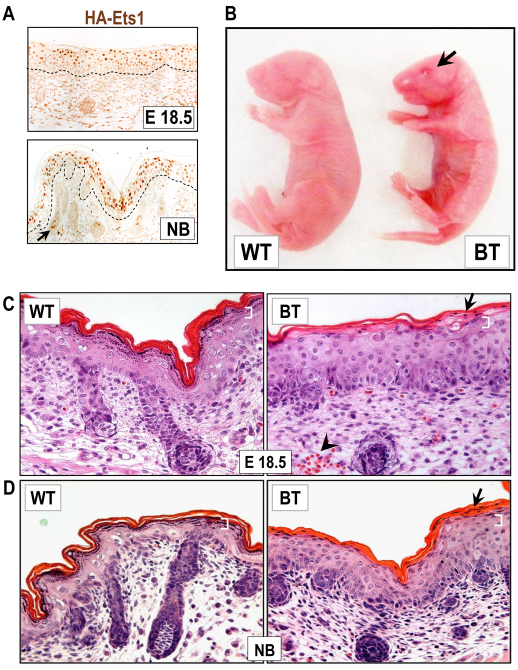
To further explore the effects of Ets1 induction on the epidermis, we withheld doxycycline supplementation during gestation. Under these conditions, the HA-Ets1 transgene is expected to be expressed when the involucrin promoter and enhancer elements become active during embryonic development (i.e. beginning at E15.5, and upregulated at E16.5 and later time points) (Jaubert et al., 2004). Indeed, at embryonic day E18.5 and in newborn mice (NB), we detected strong expression of the transgene using immunostaining (Fig. 1A). Overexpression of the Ets1 transgene was confirmed with western blotting of skin extracts from E18.5 day embryos (supplementary material Fig. S1). BT mice were born in the expected ratio and could be easily distinguished from their wild-type and single transgenic littermates because they were slightly smaller and had a shiny, taut and translucent skin (Fig. 1B). In addition, some BT embryos also demonstrated an eyes-open-at-birth phenotype. The BT animals did not survive long after birth, probably because their severe skin phenotype led to rapid dehydration.
Fig. 1.
Epidermal defects in mice overexpressing Ets1 in the suprabasal layers of epidermis. (A) Expression of the HA-Ets1 transgene in the skin of BT mice. Arrow indicates transgene expression in the inner root sheath of the hair follicle. (B) BT newborn mice are smaller, with an eyes-open-at-birth phenotype (arrow) and have a shiny, translucent skin. (C,D) Hematoxylin and eosin staining to identify epidermal alterations in E18.5 (C) and newborn (NB) (D) BT mice as compared with the wild-type. The BT epidermis is hyperplastic, has a reduced granular layer (white brackets) and is characterized by parakeratosis (arrows) and a compact stratum corneum. Arrowhead in C indicates the presence of a superficial dermal capillary.
To analyze the epidermal defects caused by Ets1 induction, we examined skin sections from E18.5 and newborn wild-type and BT mice (Fig. 1C,D). The BT epidermis was hyperplastic, as evidenced by the increased thickness. Cells in the epidermal basal layer appeared crowded, causing the cells to adopt a columnar appearance and suggesting increased cellular proliferation in this compartment. The epidermis contained a compact cornified layer of reduced thickness, while the granular layer was largely absent (hypogranulosis) and, instead, the bulk of the suprabasal compartment was occupied by spinous keratinocytes (acanthosis). In addition, there was retention of nuclei in the stratum corneum (parakeratosis) of the BT epidermis. These histological alterations indicate that there was aberrant terminal differentiation of BT keratinocytes. The dermal compartment appeared largely normal, except for the presence of increased numbers of superficial dermal capillaries.
Induction of Ets1 results in epidermal barrier insufficiency
To determine whether the epidermal alterations of the BT mice led to an impairment in barrier function, we performed a whole mount skin permeability assay (Hardman et al., 1998) at various stages of embryonic development (Fig. 2A). BT mouse embryos exhibited an impaired epidermal barrier function at both E18.5 and at birth. To determine whether there were structural defects in the stratum corneum, we compared the morphology of wild-type and BT cornified epidermocytes (Fig. 2B). Whereas the wild-type squames were intact and polygonal, the BT cornified envelopes were small and irregularly shaped. Less than 5% retained their polygonal shape, while the rest were grossly fragmented and disintegrating. This phenotype suggests that expression of Ets1 might interfere with the normal program of cornification in the epidermis, which is the probable cause of the epidermal barrier defect and perinatal lethality in these animals.
Fig. 2.
Epidermal permeability barrier deficiency in mice overexpressing Ets1. (A) Dye exclusion assay on E16.5, E18.5 and newborn (NB) mice. (B) Morphology of newborn wild-type (top) and BT (bottom) cornified envelopes; inset shows higher magnification of the boxed regions. The wild-type demonstrates intact, polygonal cornified envelopes, whereas the BT shows two intact and three fragmented cornified envelopes.
Expression of Ets1 in differentiating keratinocytes disrupts the epidermal proliferation–differentiation equilibrium
To further examine the epidermal differentiation defects, we compared the expression of keratinocyte marker proteins in newborn BT and wild-type skin. We have previously shown that in adult mice, induction of Ets1 leads to expansion of the domain that expresses early keratinocyte differentiation markers such as keratin 14 (K14) and keratin 10 (K10), while there is a concomitant loss of expression of late differentiation markers, such as loricrin (Nagarajan et al., 2009). We observed a similar pattern of altered differentiation in BT embryos in that K10 and K14 expression was increased, whereas loricrin and filaggrin expression was decreased (Fig. 3; supplementary material Fig. S2). The expression of involucrin was normal in BT newborn mice (Fig. 3C), but was significantly decreased in E18.5 embryos (supplementary material Fig. S2). The results of the immunostaining were confirmed with western blotting of skin protein extracts (supplementary material Fig. S1). Decreased expression of these late markers is consistent with the striking reduction of granular layer keratinocytes, which contain keratohyalin granules composed of these proteins. It is also consistent with a major defect in assembly of the cornified envelope. Defects in the differentiation of oral mucosa were also observed with increased expression of keratin 5 (K5), keratin (K6) and proliferating cell nuclear antigen (PCNA) and decreased expression of keratin 13 (K13) and loricrin (supplementary material Figs S3 and S4).
Fig. 3.
Ets1 overexpression in the suprabasal layer blocks keratinocyte terminal differentiation. Immunofluorescent staining of epidermal differentiation markers on dorsal skin of newborn BT and wild-type mice counterstained with β4 integrin to mark the basement membrane. The following markers were used to assess the differentiation pattern of wild-type and BT epidermis: (A) K14, (B) K10, (C) involucrin (INV), (D) filaggrin (FIL) and (E) loricrin (LOR).
Similar to previous observations in adult mice, the skin of E18.5 BT embryos expressed K6 (a marker normally absent from E18.5 IFE) in the suprabasal layers (Fig. 4A). Expression of K6 by BT epidermis might reflect a stressed or hyperproliferative condition in the late-stage gestation epidermis. Indeed, BT epidermis was highly proliferative, as demonstrated by the expression of Ki67, PCNA and the basal cell marker ΔNp63, all of which extended to three to four layers in the BT epidermis as compared with only the basal layer in the controls (Fig. 4B–D). Together, our studies indicate that Ets1 expression in differentiating keratinocytes inhibits terminal differentiation and cornification of the developing epidermis, while at the same time promoting epidermal proliferation.
Fig. 4.
Ets1 induction in the suprabasal layers results in keratinocyte activation and enhanced proliferation. Immunostaining for keratinocyte activation and proliferation markers on dorsal skin of BT newborn mice and littermate wild-type mice. Samples were counterstained with DAPI to detect nuclei or β4 integrin to mark the basement membrane. The following markers were used (A) K6, (B) ΔNp63, (C) Ki67 and (D) PCNA.
Ets1 induction does not affect periderm morphogenesis
The periderm is a transient intra-uterine barrier that protects the developing embryo before the definitive epidermal barrier is generated (M'Boneko and Merker, 1988). When epidermal cornification is complete, the periderm is shed. Because Ets1 expression blocks the cornification of stratifying keratinocytes resulting in a defective epidermal permeability barrier, the shedding of periderm in BT embryos might be delayed. Moreover, because periderm cells express involucrin (Akiyama et al., 1999), the Ets1 transgene might be expressed in BT periderm, potentially leading to alterations in its morphology, maturation or shedding. To investigate these possibilities, we examined periderm organization in BT embryos and their littermates by histological examination of E16.5 embryonic skin (Fig. 5A). At this stage, the developing epidermis is typically two to three cell layers thick and overlaid by the darkly staining periderm. The morphology of the periderm was similar in wild-type and BT embryos.
Fig. 5.
Periderm shedding is not affected by suprabasal expression of Ets1. Skin sections of E16.5 embryos stained with (A) hematoxylin and eosin (dashed lines mark the periderm–epidermal junction) or (B) K6, counterstained with DAPI (white dashed line represents the location of epidermal basement membrane, grey dashed line indicates the periderm–epidermal interface). (C,D) Same as above for E18.5 embryonic skin. The arrowheads mark the surface of epidermis.
Because no morphologic differences could be identified between wild-type and BT periderm, we examined whether the expression of the periderm marker protein K6 was altered in BT mice (Fig. 5B). Both the wild-type and the BT sections stained similarly for the expression of K6, indicating that the periderm is not affected at this stage. We then examined E18.5 embryos, when periderm shedding is usually complete. Upon histological examination, no periderm remnants were identified in either sample at this stage (Fig. 5C). Immunostaining for K6 revealed its localization in the inner root sheath (IRS) in the wild-type sample (Fig. 5D), whereas in the BT sample, K6 was expressed in the hyperplastic BT epidermal keratinocytes as well as in the IRS. Thus, periderm generation and shedding was not affected by ectopic expression of Ets1 in the suprabasal layers.
Ets1 induction does not affect the hair follicle morphogenesis program
Another morphogenetic program that coincides with the later stages of epidermal maturation is the development of the hair follicle. To investigate whether altered expression of Ets1 affects hair follicle development, we examined sections of E18.5 embryo and newborn skin. Histological examination revealed that hair follicles of wild-type and BT embryos were at an equivalent developmental stage, i.e. the elongated bulbous peg stage (Fig. 6A). The maturation of dermal papilla (dermal fibroblasts enclosed within the lower end of hair follicle) as marked by alkaline phosphatase staining was normal in wild-type and BT skin (Fig. 6B). Similar results were obtained with sections from newborn mice (Fig. 6C,D). The expression of P-cadherin, a marker for the hair follicle matrix and regions of outer root sheath and for the basal layer of inter-follicular epidermis, was similar in BT and control mice (Fig. 6E). We also examined earlier stages of hair follicle morphogenesis at E15.5 and E16.5. These early morphogenetic events of hair follicle development were also unaffected (supplementary material Fig. S5). Thus, the epidermo–mesenchymal interactions that induce and maintain hair follicles are not affected by Ets1 induction, despite significant alterations in the overall maturation of the epidermis.
Fig. 6.
Early hair follicle morphogenesis is similar in wild-type and BT embryos. Skin sections of E18.5 wild-type and BT embryos stained with (A) hematoxylin and eosin or (B) for alkaline phosphatase activity (Alk. Ph.) to mark the dermal papilla (counterstained with hematoxylin). (C) Newborn skin stained with hematoxylin and eosin. (D) Newborn skin stained for alkaline phosphatase and counterstained with DAPI. (E) Newborn skin stained for P-cadherin and counterstained with β4 integrin to mark the basement membrane.
Induction of Ets1 alters programs of keratinocyte differentiation at the mRNA level
To understand the molecular mechanisms by which Ets1 functions as a pro-proliferative and anti-differentiation signal in stratified squamous epithelia, we chose to investigate global gene expression profiles using microarray technology. For these studies, we harvested skin from wild-type and BT epidermis at day 18.5 of embryonic development. We chose to do these analyses at this stage of differentiation for the following reasons: first, the uterine environment is sterile, which eliminates any possibility of secondary changes in the skin owing to bacterial infection; and second, the short duration of induction of the Ets1 transgene (2–3 days) meant that the observed changes in gene expression were more likely to be owing to primary or direct effects of Ets1 on keratinocytes, rather than on global changes in the physiology of the animal.
Gene expression microarray experiments identified a large number of genes (~2200) that displayed statistically significant (P≤0.05) changes between wild-type and BT epidermis (supplementary material Table S1). Of those, there were ~470 genes displaying at least twofold upregulation and ~220 genes showing at least twofold downregulation. Expression patterns of selected genes are summarized in the heat map visualizations shown in Fig. 7. RT-PCR confirmation of changes in the expression of some of these genes is shown in supplementary material Fig. S6.
Fig. 7.
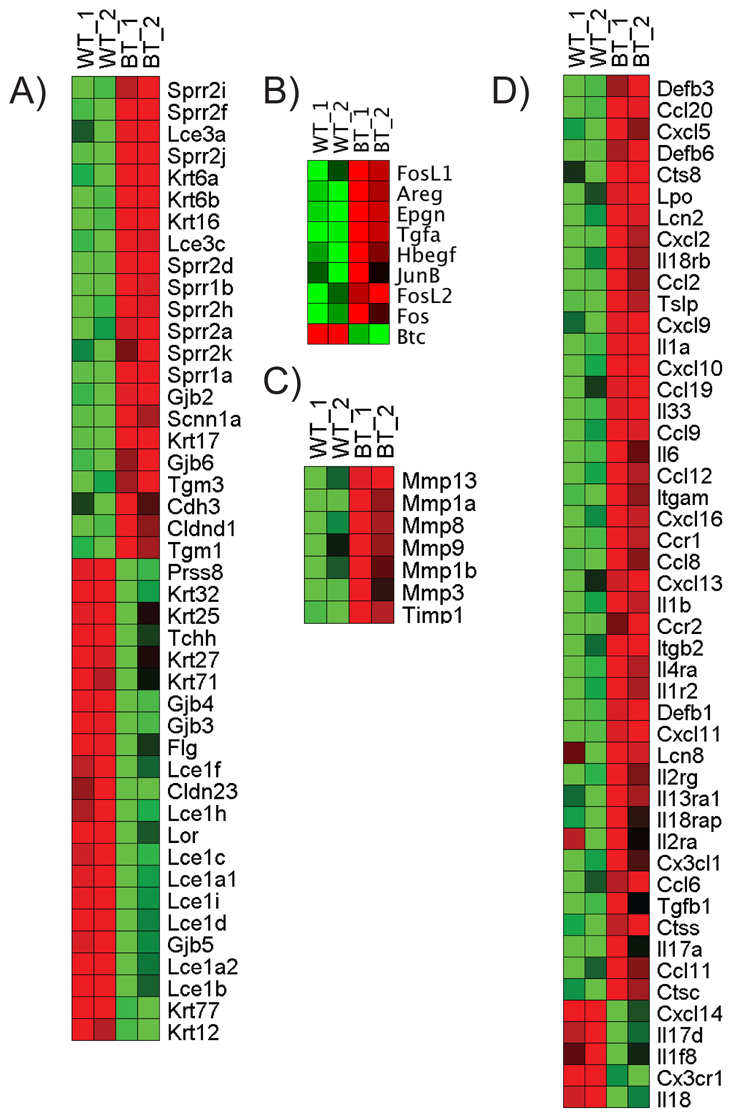
Induction of Ets1 leads to major alterations in the differentiation program of keratinocytes. Heatmap visualizations of changes in expression of genes involved in keratinocyte (A) structure, (B) proliferation, (C) motility and (D) immune function. Red indicates increased expression, whereas green indicates decreased expression.
Ets1 strikingly alters expression of genes encoding epidermal structural proteins
Examination of the data shows that induction of the Ets1 transgene dramatically inhibits the expression of many genes expressed in the cornified envelope (Fig. 7A) including Lor and Fil (encoding loricrin and filaggrin, respectively) as well as multiple group 1 late cornified envelope (Lce) genes (Lce1a1, Lce1a2, Lce1b, Lce1c, Lce1d, Lce1f, Lce1h and Lce1i). Interestingly, all of these genes are closely linked on mouse chromosome 3F1 and found within the epidermal differentiation complex (EDC) (Brown et al., 2007). However, the expression of certain other genes within the EDC, such as Ivl (encoding involucrin) and the S100 genes, was not changed. Furthermore, the expression of some genes in the EDC, including many of the small proline-rich protein genes (Sprr genes) and some group 3 Lce genes (Lce3a and Lce3c), was dramatically upregulated. The Tgm1 gene (encoding transglutaminase 1) is localized outside the EDC, but plays a major role in formation of the cornified envelope by crosslinking envelope proteins and lipids to form the structurally resistant cornified envelope of the keratinocyte (Eckert et al., 2005; Hitomi, 2005). Tgm1 demonstrated increased expression, possibly as a response to impaired barrier formation. Decreased expression of loricrin, filaggrin and several Lce genes is highly consistent with the phenotypic abnormalities of the skin and corneocytes described above.
In keeping with our immunostaining results (Fig. 4), we detected increased expression of Krt6 (the gene encoding K6) and its partner Krt16 (encoding keratin 16) in BT skin (Fig. 7A). There was also increased expression of Krt17 (encoding keratin 17), another marker of keratinocyte activation. BT skin demonstrated reduced expression of certain hair-associated genes including Tchh (trichohyalin) and Krt25, Krt27, Krt32 and Krt71, but increased expression of the hair-shaft-associated Tgm3 (transglutaminase 3) and Cdh3 (P-cadherin) genes.
Expression of several connexins (which are involved in gap junction formation) was also affected, with Gjb2 and Gjb6 (connexins 26 and 30) being upregulated in BT skin, while Gjb4, Gjb3 and Gjb5 (connexins 30.3, 31 and 31.1) were downregulated (Fig. 7A). Furthermore, the expression of the Prss8 gene [encoding channel activating protease 1 (CAP1) or prostasin] was also downregulated, although expression of the epithelial sodium channel α-subunit (which is activated by CAP1) was upregulated. Prss8 is required for normal skin barrier acquisition, and Prss8 knockout epidermis demonstrates an absence of functional tight junctions within the granular layer (Leyvraz et al., 2005). Thus, the structural integrity of both gap junctions and tight junctions might be affected in BT skin.
Induction of Ets1 affects expression of growth-associated genes and matrix metalloproteases
We detected upregulation in the expression of several epidermal growth factor receptor (EGF-R) ligands in BT skin, including Areg (encoding amphiregulin), Epgn (encoding epigen), Tgfa (encoding TGFα) and Hbegf (encoding heparin-binding EGF) (Fig. 7B). A fifth EGF receptor ligand, Btc (encoding betacellulin), was downregulated in BT skin, whereas two others, EGF (Egf) and epiregulin (Ereg), were unchanged. EGF-R ligands are important for keratinocyte proliferation; hence, increased expression of certain EGF-R ligands by BT keratinocytes might be the cause of the hyperproliferative effects of Ets1 on the epidermis.
EGF signaling triggered by its ligands is known to stimulate the expression of several AP1 proteins (Tomic-Canic et al., 1998) and we detected increased expression of several AP1 family genes in BT skin, including Fos, Fosl1, Fosl2 and Junb (Fig. 7B). Both Ets1 and AP1 proteins are known to be potent transactivators of Mmp genes. In keeping with this, we noted strong upregulation of several members of the matrix metalloprotease gene family (Fig. 7C), most prominently Mmp13. We have previously detected high Mmp13 expression in adult BT mice, in which Ets1 transgene expression was induced at weaning age (Nagarajan et al., 2009). The induction of these Mmp enzymes might lead to degradation of epidermal extracellular components such as adherens junctions or desmosomes, or dermal components such as collagen, and might contribute to increased keratinocyte motility.
Induction of Ets1 causes a sterile inflammatory response
Unexpectedly, microarray analysis detected altered expression of many genes involved in the epidermal immune response, including defensins, cytokines, chemokines and chemokine receptors (Fig. 7D). Because the skin was harvested from E18.5 embryos in a sterile uterine environment, it is highly unlikely that the upregulation of immune-response genes represents a secondary response to infection. Instead, it is likely that such genes are upregulated as a response to the failure in barrier formation, perhaps as a physiological response to potential infection at birth. Immunostaining confirmed that there was an infiltration of CD11b+ myeloid cells in the dermis and epidermis of BT skin (supplementary material Fig. S7). In summary, our gene expression analyses indicate that expression of Ets1 in the differentiating keratinocytes of the suprabasal layers of the epidermis leads to a block in expression of cornified envelope proteins, while at the same time promoting expression of genes involved in keratinocyte proliferation, motility and immune response.
Ets1 induces inflammation in keratinocytes in a cell-intrinsic fashion
The expression of the immune-related genes might be owing to their induction by Ets1 in keratinocytes. Alternatively, infiltrating inflammatory cells might be the major producers of these proteins. To determine whether Ets1 can directly induce expression of inflammatory genes in keratinocytes, we infected cultured mouse keratinocytes with a retrovirus encoding mouse Ets1, with a retrovirus encoding a DNA-binding-defective mutant of Ets1 or with a control empty retrovirus (Fig. 8A). Cells infected with the wild-type Ets1 retrovirus demonstrated strong upregulation of each of three chemokine genes tested, Ccl2, Ccl20 and Cxcl5 (Fig. 8B). By contrast, both the empty retrovirus as well as the DNA-binding-deficient Ets1 retrovirus failed to induce these genes. These results suggest that Ets1 binds to the regulatory segments for the chemokine genes and directly induces their expression. Indeed, transduction of a wild-type Ets1 retrovirus, but not the mutant Ets1 retrovirus, also induced the expression of two Mmp genes that are well-validated Ets1 targets (Mmp9 and Mmp13) (Fig. 8B).
Fig. 8.

Overexpression of Ets1 in cultured keratinocytes drives expression of Mmps and chemokines. (A) Retroviral constructs used to infect keratinocytes: MIGR1, control empty retrovirus; MIGR1-Ets1, retrovirus encoding mouse Ets1; MIGR1-R391D, retrovirus encoding the DNA-binding-defective mutant of Ets1. R391D represents a mutation in a key arginine residue of Ets1 required for DNA binding. This mutant version of Ets1 fails to bind DNA or regulate target genes. (B) RT-PCR analysis of Mmp and chemokine gene expression in retrovirally infected mouse keratinocytes. Ccl2, Ccl20 and Cxcl5 are the three chemokines tested; Mmp9 and Mmp13 are the two Mmps tested; Hprt is an internal control for RNA quantity and integrity.
Discussion
The Ets1 transcription factor is normally expressed within the proliferative basal layer of the epidermis (Nagarajan et al., 2009), but in many squamous cell cancers, Ets1 is overexpressed in malignant keratinocytes, where its expression is correlated with invasion and metastasis (Bai et al., 2009; Endo et al., 2006; Keehn et al., 2004; Pande et al., 1999; Saeki et al., 2000; Vairaktaris et al., 2007). In order to address the potential role of Ets1 in keratinocyte biology and in the induction of squamous cell tumors, we generated a mouse model in which we can inducibly express Ets1 in keratinocytes that have already begun their differentiation process. When Ets1 is induced in the epidermis in adult mice, severe dysplastic lesions develop, consistent with an important role for Ets1 in driving the pathological process (Nagarajan et al., 2009). In this report, we further explore the effects of Ets1 on skin development and investigate the mechanisms of Ets1 oncogenic action.
Induction of Ets1 in differentiating keratinocytes leads to a failure of cornified envelope formation
Ets1 causes dramatic alterations to the normal differentiation program of the epidermis, leading to a failure in barrier formation (as demonstrated by impaired dye exclusion), resulting in early postnatal lethality. The striking effects induced by Ets1 overexpression in differentiating keratinocytes appear to be limited mainly to IFE keratinocytes of the skin. The refractoriness to the effects of Ets1 in oral, hair follicle or periderm cells might reflect lower expression of the Ets1 transgene in these tissues. Alternatively, Ets1 might have different effects because a distinct set of developmental cues and signaling pathways mediate development and homeostasis in these tissues. The barrier defect in the skin is probably owing to aberrant formation of cornified envelopes, because Ets1 prevents proper expression of filaggrin, loricrin and group 1 late cornified envelope (Lce) genes. Expression of another major cornified envelope constituent, involucrin, was decreased in BT embryos at E18.5, but not at the newborn stage. This is most consistent with a delayed, but otherwise normal, involucrin expression.
Individual mutations in either loricrin (gene knockout) or filaggrin (in the spontaneous flaky tail mouse mutant) do not lead to major defects in the assembly of the cornified envelope (Koch et al., 2000; Presland et al., 2000). However, the downregulation of loricrin, filaggrin and Lce proteins together, as seen in Ets1 BT mice, appears to result in major alterations to the cornified envelope of the cell, as demonstrated by the irregular shapes and fragmentation of the squames extracted from newborn skin. It has been proposed that alterations to the barrier function of skin might make the epidermal cells more susceptible to environmental carcinogens that can gain easier access to the deeper layers of the skin in the absence of a proper barrier (Demehri et al., 2009). Thus the alteration to skin barrier function as seen in BT mice might represent one important mechanism by which Ets1 acts as an oncogene.
The expression of some of the small proline-rich proteins (Sprr) is strongly upregulated in BT epidermis along with expression of some group 3 Lce genes. Interestingly, one study has identified an Ets binding site in the promoter segment of the human SPRR1A gene, which was shown to bind the Ets factor ESE-1 (ELF3) (Sark et al., 1998). Potentially, other Ets factors, such as Ets1, could also bind to the same site and result in induction of the Sprr1a gene in BT mice. Group 3 Lce genes are normally expressed in internal stratified squamous epithelia (such as oral epithelia), but not in epidermis. Dramatic upregulation of one group 3 Lce gene (LCE3E) has been reported in human epidermal keratinocytes in response to the stress imposed by UV radiation (Jackson et al., 2005). Another study showed upregulation of human group 3 LCE genes in response to tape-stripping of the cornified layer, which temporarily impairs the local skin barrier function (de Cid et al., 2009). We detected significant upregulation of two group 3 Lce genes (Lce3a and Lce3c) in E18.5 BT skin, which might also reflect a response to epidermal stress or to impaired barrier function. Alternatively, exposure to the aqueous environment in the uterus might send signals to the epidermis that results in upregulation of genes normally expressed in internal epithelia.
In addition to the protein constituents of the cornified envelope, barrier function in the skin epidermis is also highly dependent on secretion of specialized lipids and their assembly into lipid lamellae (Houben et al., 2007; Segre, 2003). Examination of microarray data revealed that the expression of genes encoding enzymes involved in the synthesis and processing of lipids and those encoding the major protein substrates to which the lipids are attached was normal. The normal or elevated expression of these genes implies functional competence to assemble and crosslink the lipid envelope to the outer surface of the corneocytes.
Ets1 expression in differentiating keratinocytes induces expression of several genes involved in epidermal proliferation and motility
BT epidermis is thicker than wild-type epidermis and shows more Ki67 and PCNA staining. The increased proliferative response in the basal layer is most probably secondary to changes in the suprabasal layers, because the transgene is not expressed in the basal compartment. One possible mechanism by which induction of Ets1 might mediate this effect is through stimulation of the expression of EGF-R ligands such as amphiregulin, epigen, heparin-binding EGF and TGFα. These ligands are strongly mitogenic to keratinocytes (Pastore et al., 2008; Schneider et al., 2008), and a recent report has implicated TGFα as a major downstream mediator of the oncogenic effects of Ets1 in several different human tumor cell lines (Holterman et al., 2010). Furthermore, Ets1 was demonstrated to bind to the human TGFα gene promoter, indicating that TGFα is a direct transcriptional target of Ets1 (Holterman et al., 2010).
Ets1 affects the expression of several genes involved in regulating keratinocyte homeostasis. Importantly, expression of several genes encoding AP1 family members Fos, Fosl1 (Fra-1), Fosl2 (Fra-2) and JunB is significantly upregulated in BT epidermis. Expression of other AP1 genes such as Jun (c-jun), Jund (JunD) or Fosb (FosB) is not significantly changed. AP1 proteins form dimeric transcription factor complexes consisting of Jun homodimers and Fos-Jun heterodimers that regulate a variety of target genes. Interestingly, there are many examples of genes that are coordinately regulated by AP1 proteins and Ets family proteins. Indeed, adjacent AP1 and Ets motifs in promoters can form so-called Ras-responsive elements that mediate cellular responses to Ras–MAPK signaling (McCarthy et al., 1997; Westwick et al., 1994; Yang et al., 1996). Similar AP1 and Ets motifs have been detected within the promoters of Mmp genes, where they play a major role in regulating expression of these genes (Kapila et al., 2009; Nelson et al., 2006; Watabe et al., 1998). Several Mmp genes demonstrate dramatically increased expression in E18.5 BT skin, and we have previously demonstrated upregulation of many Mmp genes in epidermis of BT mice induced at an adult age (Nagarajan et al., 2009). This appears to be a direct effect of Ets1 on these genes because transduction of an Ets1-expressing retrovirus into a keratinocyte cell line could induce expression of Mmp9 and Mmp13, as shown in this study. We have previously shown by chromatin immunoprecipitation (ChIP) assay that Ets1 directly binds to the promoter of the Mmp13 gene (Nagarajan et al., 2009). Other laboratories have demonstrated that Ets1 also directly regulates the Mmp3 and Mmp9 genes (Baillat et al., 2006; Wei et al., 2009). Together, the Mmps induced by Ets1 expression function to degrade a wide array of extracellular protein substrates, possibly leading to decreased epidermal keratinocyte cohesion and increased keratinocyte proliferation and motility.
Ets1 induction results in an innate immune response in the skin
One of the most interesting findings of our study was the upregulation of many immunologically relevant target genes in BT epidermis. These include 16 different chemokines and seven different cytokines with diverse biological activities. We have recently reviewed the role of Ets1 in regulating cytokine and chemokine gene expression, and a great deal of evidence supports a role for Ets1 in directly regulating expression of these genes by binding promoter and/or enhancer elements (Russell and Garrett-Sinha, 2010). Expression of the chemokine molecules probably helps to recruit immune cells into the skin and, indeed, expression of Itgam and Itgb2 (which encode the integrins that together constitute the myeloid-specific Mac-1 receptor) is strongly increased in BT skin. We demonstrate that CD11b+ (Itgam+) myeloid cells are found in high numbers in BT skin. By contrast, we do not detect increased expression of B or T cell genes in microarray analysis and preliminary immunostaining experiments did not identify CD3+ T cells or CD19+ B cells in the skin of E18.5 BT mice (data not shown). This contrasts with the situation found in involucrin, envoplakin and periplakin triple-deficient mice, which also have a severe barrier defect, but exhibit recruitment of CD4+ T cells into the skin (Sevilla et al., 2007).
As with the Mmp genes, Ets1 probably serves as a direct regulator of chemokine gene expression. Transduction of an Ets1-expressing retrovirus into mouse keratinocytes induced strong expression of the chemokines Ccl2, Ccl20 and Cxcl5 in a cell-intrinsic fashion. Previous ChIP studies have confirmed that the Ccl2 gene (encoding monocyte chemoattractant protein 1, MCP-1) is a direct target of Etsl (Aoki et al., 2010; Zhan et al., 2005). Furthermore, the Ccl20 gene is a known target of another Ets factor, ESE-1 (Elf3) (Kwon et al., 2003). Very interestingly, recent studies have strongly linked inflammatory reactions to cancer initiation and progression. Expression of chemokines and cytokines by tumor cells is thought to recruit myeloid cells that secrete further cytokines and chemokines to stimulate tumor cell growth, survival and invasion and to promote angiogenesis (Lazennec and Richmond, 2010).
In addition to chemokines and cytokines, the expression of β-defensins and lysozyme enzymes is strongly upregulated in BT skin. Increased expression of chemokines, cytokines, defensins and lysozyme and the infiltration of myeloid cells cannot be owing to skin infection because we analyzed E18.5 embryos harvested from the sterile environment of the uterus. However, several studies point to induction of an innate immune response in keratinocytes in response to wounding or to an impaired barrier formation (Aberg et al., 2008; Zaja-Milatovic and Richmond, 2008). Thus, the most likely explanation for the induction of these inflammatory responses is a keratinocyte response to the impaired barrier function of the skin in BT animals.
Potential involvement of Blimp1 in the Ets1 BT phenotype
We have previously demonstrated that Ets1 binds to and inhibits the function of the transcription factor Blimp1 (John et al., 2008). Blimp1 is highly expressed within differentiating keratinocytes in the granular layer of the epidermis. One study has demonstrated that Blimp1 plays an important role in regulating terminal differentiation of epidermal keratinocytes (Magnusdottir et al., 2007), although another study failed to detect alterations in the IFE of Blimp1 conditional knockout mice (Horsley et al., 2006). Blimp1 is thought to regulate the differentiation of granular layer keratinocytes, and in its absence numerous genes involved in epidermal terminal differentiation are altered in their expression. Interestingly, some of these genes [such as late cornified envelope genes (Lce1d, Lce1f, Lce1h and Lce1i), small proline-rich protein genes (Sprr1a), EGF ligands (Epgn, Areg and Btc) and inflammatory mediators (Il1a and Cxcl16)] show similar patterns of upregulation or downregulation in Blimp1 conditional knockout epidermis and Ets1 BT epidermis. Thus, Ets1-dependent suppression of Blimp1 activity might contribute to its ability to block terminal differentiation of keratinocytes, a possibility that remains to be explored in the future. However, the effects of overexpressing Ets1 in the epidermis are much more striking than the effects of deleting Blimp1. Indeed, Ets1 BT mice die at birth from severe barrier defects, whereas Blimp1 conditional knockout mice do not. Together, these results imply that the effects of Ets1 on skin epidermis extend beyond its ability to block Blimp1 activity and probably involve Ets1-dependent activation of target genes such as those encoding Mmps and chemokines.
Conclusion
Ets1 regulates a variety of transcriptional programs to alter the differentiation, proliferation, migratory and inflammatory properties of skin keratinocytes. Many of the effects of Ets1 are probably owing to its direct binding to target sequences in the promoters or enhancers of genes such as those encoding Mmps and chemokines. It is also possible that Ets1 functions by additional mechanisms as well. For instance, Ets1 might indirectly regulate the expression of some target genes by inducing other transcription factors that can turn these genes on or off.
Our results have major implications for how we view the role of Ets1 in squamous cell tumors. Previous research correlated Ets1 expression with increased invasion and metastasis in squamous cell tumors, which has been linked to the ability of Ets1 to upregulate Mmp genes. Our results suggest that in addition to this important function, Ets1 also serves to keep keratinocytes in an undifferentiated or partially differentiated state and to promote their proliferation. Furthermore, Ets1 induces an innate immune response in the skin. Given the recent data linking immune activation with promotion of squamous cell carcinogenesis (Coussens and Werb, 2002; Vicari and Caux, 2002), this pathway might also be important in neoplastic or pre-neoplastic lesions expressing Ets1. On the whole, Ets1 exhibits multiple activities predicted to enhance the malignant properties of keratinocytes, suggesting that its function in tumor progression might be more complex than previously appreciated.
Materials and Methods
Antibodies
The following primary antibodies were used for western blotting, immunofluorescence and/or immunohistochemistry: rat monoclonal anti-HA (clone 3F10, Roche Applied Sciences), rabbit polyclonal anti-Ets1 (N-276 or C-20, Santa Cruz Biotechnology), mouse monoclonal anti-GAPDH (clone 6C5, Chemicon International), rabbit polyclonal anti-Ki67 (NCL-Ki67p, Novocastra), rat monoclonal anti-β4 integrin (CD104, clone 346-11A, BD Biosciences), rat monoclonal anti-CD11b (M1/70, BD Biosciences), mouse monoclonal anti-E-cadherin (DECMA1, Santa Cruz Biotechnology), mouse monoclonal anti-P-cadherin (56/P-cadherin, BD Biosciences) and mouse monoclonal anti-PCNA (clone PC10, Dakocytomation). A rabbit polyclonal anti-ΔNp63 antibody (RR-14, developed in the laboratory of S.S.) was used for detecting p63 (Romano et al., 2006). In addition, several polyclonal rabbit antibodies specific for keratinocyte marker proteins (K5, K6, K10, K14, involucrin, filaggrin and loricrin) were generous gifts from Julie Segre (National Human Genome Research Institute, NIH, Bethesda, MD).
Animals
The generation of BT mice carrying the involucrin-tTA transgene and the doxycycline-inducible Ets1 transgenes has previously been described (Nagarajan et al., 2009). Single transgenic mice on a diet lacking doxycycline were mated and checked for copulatory plugs. Newborn mice and embryos at various gestational ages were harvested for analysis.
Histology
For histological analyses, skin samples from various regions of the body were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned for staining with hematoxylin and eosin. For alkaline phosphatase staining, frozen sections of dorsal skin from BT and wild-type mice were air-dried and then stained with the Vector Red kit (Vector Labs) followed by counterstaining with DAPI or hematoxylin.
Whole mount dye penetration assay and extraction of cornified envelopes
To identify skin barrier defects, newborn mice and embryos obtained from timed pregnancies were dehydrated by incubating in methanol, rehydrated briefly in water, stained with 0.1% toluidine blue, rinsed quickly in water and then photographed immediately. To isolate cornified envelopes from skin, the newborn epidermis was separated from the dermis by incubating in Dispase II or 0.1 M EDTA. The epidermis was then heated in an extraction buffer containing 0.1 M Tris-HCl (pH 8.5), 2% SDS, 20 mM DTT and 5 mM EDTA for 10 minutes at 95°C, and the cornified envelopes collected by centrifugation.
Western blots
Whole tissue lysates were prepared from BT and control mouse dorsal skins. Membranes from western blots were incubated with rat anti-HA, anti-Ets1, anti-GAPDH, anti-K6, anti-filaggrin or anti-loricrin and developed with horseradish peroxidase-conjugated anti-rat, anti-rabbit or anti-mouse secondary antibody. Specific bands were detected by chemiluminescence.
Indirect immunofluorescence
For immunohistochemistry, 4-μm thick skin sections were deparaffinized and subjected to antigen retrieval by heating in sodium citrate buffer in a microwave oven. The sections were then stained with specific primary antibodies (anti-HA, anti-Ets1 or anti-Ki67) using the Vectastain ABC kit (Vector Labs) using diaminobenzidine as the enzyme substrate. For immunofluorescence, 5-μm thick OCT-embedded fresh frozen sections were fixed in methanol. After blocking, the sections were incubated with primary antibodies and then incubated with fluorescent (Alexa Fluor 488, Alexa Fluor 568 or FITC) secondary antibodies (anti-rabbit IgG, anti-rat IgG or anti-mouse IgG). Sections were counterstained with DAPI, mounted in 80% glycerol and photographed with an Axiophot Zeiss microscope.
Gene expression microarray analysis
Total RNA was extracted from E18.5 wild-type and BT embryos using TRIzol (Invitrogen, Carlsbad, CA) and then purified using the RNeasy Mini kit (Qiagen, Valencia, CA). Purified total RNA was analyzed on an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) to determine the quantity and RNA integrity. RNA was subjected to one round of amplification and labeling to obtain biotinylated cRNA for hybridization to Affymetrix GeneChip Mouse Genome 430 2.0 Arrays. Two independent sets of biological replicates of each tested condition (wild-type and BT epidermis) were used for analysis.
Scanned microarray images were imported into GeneChip Operating Software (GCOS, Affymetrix, Santa Clara, CA) and checked for quality. Data was then normalized using the MAS5.0 algorithm in the Affy package of Bioconductor in the R statistical computing environment. Probe sets, whose expression intensities were close to background noise across the majority of samples, were filtered out to yield ~10,000 genes with significant expression. Comparison of BT and wild-type populations identified ~2200 of these genes whose expression was statistically different (P≤0.05) between samples.
Quantitative RT-PCR analysis
cDNA was prepared from the same RNA samples that were used in microarray analysis using SuperScript II reverse transcriptase kit (Invitrogen). Primer sets for PCR amplification are given in supplementary material Table S2. PCR cycling was performed using the following conditions: denaturation at 94°C for 4 minutes, 30 cycles of amplification with denaturation at 94°C for 20 seconds, annealing at 55°C for 30 seconds, extension at 72°C for 30 seconds, and final extension at 72°C for 10 minutes.
Retroviral transductions and RT-PCR assays
Retroviruses in the MIGR1 vector were produced as described before (John et al., 2008) using the Platinum-E packaging cell line. Retroviral supernatants were used to infect a mouse keratinocyte cell line (mK cells) (Sinha and Fuchs, 2001). At least 50% of infected cells were GFP-positive, indicating retroviral transduction. At 48 hours post-infection, cells were harvested and RNA was prepared. RNA was reverse transcribed into cDNA and semi-quantitative PCR was performed to analyze the expression of genes of interest.
Supplementary Material
Acknowledgments
These studies were supported by research grants from the American Cancer Society (Grant #0705201DDC) and the National Institutes of Dental and Craniofacial Research (Grant #DE016944). We thank Kirsten Smalley and Irene Kulik for technical assistance, Steven Gill for help with the microarray analyses and Julie Segre for providing transgenic mice carrying the tetracycline-transactivator under the control of the involucrin regulatory elements.
Footnotes
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/123/20/3566/DC1
References
- Aberg K. M., Man M. Q., Gallo R. L., Ganz T., Crumrine D., Brown B. E., Choi E. H., Kim D. K., Schroder J. M., Feingold K. R., et al. (2008). Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J. Invest. Dermatol. 128, 917-925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama M., Smith L. T., Yoneda K., Holbrook K. A., Hohl D., Shimizu H. (1999). Periderm cells form cornified cell envelope in their regression process during human epidermal development. J. Invest. Dermatol. 112, 903-909 [DOI] [PubMed] [Google Scholar]
- Ala-aho R., Kahari V. M. (2005). Collagenases in cancer. Biochimie 87, 273-286 [DOI] [PubMed] [Google Scholar]
- Aoki T., Kataoka H., Nishimura M., Ishibashi R., Morishita R., Miyamoto S. (2010). Ets-1 promotes the progression of cerebral aneurysm by inducing the expression of MCP-1 in vascular smooth muscle cells. Gene Ther. Epub ahead of print [DOI] [PubMed] [Google Scholar]
- Arlette J. P., Trotter M. J. (2004). Squamous cell carcinoma in situ of the skin: history, presentation, biology and treatment. Austr. J. Dermatol. 45, 1-9; quiz 10 [DOI] [PubMed] [Google Scholar]
- Bai W., Wang L., Ji W., Gao H. (2009). Expression profiling of supraglottic carcinoma: PTEN and thrombospondin 2 are associated with inhibition of lymphatic metastasis. Acta Otolaryngol. 129, 569-574 [DOI] [PubMed] [Google Scholar]
- Baillat D., Leprivier G., Regnier D., Vintonenko N., Begue A., Stehelin D., Aumercier M. (2006). Stromelysin-1 expression is activated in vivo by Ets-1 through palindromic head-to-head Ets binding sites present in the promoter. Oncogene 25, 5764-5776 [DOI] [PubMed] [Google Scholar]
- Bhawan J. (2007). Squamous cell carcinoma in situ in skin: what does it mean? J. Cutan. Pathol. 34, 953-955 [DOI] [PubMed] [Google Scholar]
- Brown S. J., Tilli C. M., Jackson B., Avilion A. A., MacLeod M. C., Maltais L. J., Lovering R. C., Byrne C. (2007). Rodent Lce gene clusters; new nomenclature, gene organization, and divergence of human and rodent genes. J. Invest. Dermatol. 127, 1782-1786 [DOI] [PubMed] [Google Scholar]
- Cataisson C., Ohman R., Patel G., Pearson A., Tsien M., Jay S., Wright L., Hennings H., Yuspa S. H. (2009). Inducible cutaneous inflammation reveals a protumorigenic role for keratinocyte CXCR2 in skin carcinogenesis. Cancer Res. 69, 319-328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockerell C. J. (2000). Histopathology of incipient intraepidermal squamous cell carcinoma (“actinic keratosis”). J. Am. Acad. Dermatol. 42, 11-17 [DOI] [PubMed] [Google Scholar]
- Coppe J. P., Patil C. K., Rodier F., Sun Y., Munoz D. P., Goldstein J., Nelson P. S., Desprez P. Y., Campisi J. (2008). Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 6, 2853-2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L. M., Werb Z. (2002). Inflammation and cancer. Nature 420, 860-867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens L. M., Raymond W. W., Bergers G., Laig-Webster M., Behrendtsen O., Werb Z., Caughey G. H., Hanahan D. (1999). Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev. 13, 1382-1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cid R., Riveira-Munoz E., Zeeuwen P. L., Robarge J., Liao W., Dannhauser E. N., Giardina E., Stuart P. E., Nair R., Helms C., et al. (2009). Deletion of the late cornified envelope LCE3B and LCE3C genes as a susceptibility factor for psoriasis. Nat. Genet. 41, 211-215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demehri S., Turkoz A., Kopan R. (2009). Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell 16, 55-66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer J. (2003). The biology of the Ets1 proto-oncogene. Mol. Cancer 2, 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert R. L., Sturniolo M. T., Broome A. M., Ruse M., Rorke E. A. (2005). Transglutaminase function in epidermis. J. Invest. Dermatol. 124, 481-492 [DOI] [PubMed] [Google Scholar]
- Endo K., Shirai A., Furukawa M., Yoshizaki T. (2006). Prognostic value of cell motility activation factors in patients with tongue squamous cell carcinoma. Hum. Pathol. 37, 1111-1116 [DOI] [PubMed] [Google Scholar]
- Fuchs E. (2007). Scratching the surface of skin development. Nature 445, 834-842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne J. C., Okuducu A. F., Sahin A., Fafeur V., Kiriakidis S., Wernert N. (2008). The transcription factor ETS-1: its role in tumour development and strategies for its inhibition. Mini Rev. Med. Chem. 8, 1095-1105 [DOI] [PubMed] [Google Scholar]
- Hardman M. J., Sisi P., Banbury D. N., Byrne C. (1998). Patterned acquisition of skin barrier function during development. Development 125, 1541-1552 [DOI] [PubMed] [Google Scholar]
- Hitomi K. (2005). Transglutaminases in skin epidermis. Eur. J. Dermatol. 15, 313-319 [PubMed] [Google Scholar]
- Holterman C. E., Franovic A., Payette J., Lee S. (2010). ETS-1 oncogenic activity mediated by transforming growth factor alpha. Cancer Res. 70, 730-740 [DOI] [PubMed] [Google Scholar]
- Horsley V., O'Carroll D., Tooze R., Ohinata Y., Saitou M., Obukhanych T., Nussenzweig M., Tarakhovsky A., Fuchs E. (2006). Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126, 597-609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houben E., De Paepe K., Rogiers V. (2007). A keratinocyte's course of life. Skin Pharmacol. Physiol. 20, 122-132 [DOI] [PubMed] [Google Scholar]
- Jackson B., Tilli C. M., Hardman M. J., Avilion A. A., MacLeod M. C., Ashcroft G. S., Byrne C. (2005). Late cornified envelope family in differentiating epithelia-response to calcium and ultraviolet irradiation. J. Invest. Dermatol. 124, 1062-1070 [DOI] [PubMed] [Google Scholar]
- Jaubert J., Patel S., Cheng J., Segre J. A. (2004). Tetracycline-regulated transactivators driven by the involucrin promoter to achieve epidermal conditional gene expression. J. Invest. Dermatol. 123, 313-318 [DOI] [PubMed] [Google Scholar]
- John S. A., Clements J. L., Russell L. M., Garrett-Sinha L. A. (2008). Ets-1 regulates plasma cell differentiation by interfering with the activity of the transcription factor Blimp-1. J. Biol. Chem. 283, 951-962 [DOI] [PubMed] [Google Scholar]
- Kalinin A. E., Kajava A. V., Steinert P. M. (2002). Epithelial barrier function: assembly and structural features of the cornified cell envelope. BioEssays 24, 789-800 [DOI] [PubMed] [Google Scholar]
- Kapila S., Xie Y., Wang W. (2009). Induction of MMP-1 (collagenase-1) by relaxin in fibrocartilaginous cells requires both the AP-1 and PEA-3 promoter sites. Orthod. Craniofac. Res. 12, 178-186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keehn C. A., Smoller B. R., Morgan M. B. (2004). Ets-1 immunohistochemical expression in non-melanoma skin carcinoma. J. Cutan. Pathol. 31, 8-13 [DOI] [PubMed] [Google Scholar]
- Kerkela E., Saarialho-Kere U. (2003). Matrix metalloproteinases in tumor progression: focus on basal and squamous cell skin cancer. Exp. Dermatol. 12, 109-125 [DOI] [PubMed] [Google Scholar]
- Koch P. J., de Viragh P. A., Scharer E., Bundman D., Longley M. A., Bickenbach J., Kawachi Y., Suga Y., Zhou Z., Huber M., et al. (2000). Lessons from loricrin-deficient mice: compensatory mechanisms maintaining skin barrier function in the absence of a major cornified envelope protein. J. Cell Biol. 151, 389-400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon J. H., Keates S., Simeonidis S., Grall F., Libermann T. A., Keates A. C. (2003). ESE-1, an enterocyte-specific Ets transcription factor, regulates MIP-3alpha gene expression in Caco-2 human colonic epithelial cells. J. Biol. Chem. 278, 875-884 [DOI] [PubMed] [Google Scholar]
- Lazennec G., Richmond A. (2010). Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol. Med. 16, 133-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyvraz C., Charles R. P., Rubera I., Guitard M., Rotman S., Breiden B., Sandhoff K., Hummler E. (2005). The epidermal barrier function is dependent on the serine protease CAP1/Prss8. J. Cell Biol. 170, 487-496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln D. W., 2nd, Bove K. (2005). The transcription factor Ets-1 in breast cancer. Front. Biosci. 10, 506-511 [DOI] [PubMed] [Google Scholar]
- M'Boneko V., Merker H. J. (1988). Development and morphology of the periderm of mouse embryos (days 9-12 of gestation). Acta Anat. (Basel) 133, 325-336 [DOI] [PubMed] [Google Scholar]
- Magnusdottir E., Kalachikov S., Mizukoshi K., Savitsky D., Ishida-Yamamoto A., Panteleyev A. A., Calame K. (2007). Epidermal terminal differentiation depends on B lymphocyte-induced maturation protein-1. Proc. Natl. Acad. Sci. USA 104, 14988-14993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S. A., Chen D., Yang B. S., Garcia Ramirez J. J., Cherwinski H., Chen X. R., Klagsbrun M., Hauser C. A., Ostrowski M. C., McMahon M. (1997). Rapid phosphorylation of Ets-2 accompanies mitogen-activated protein kinase activation and the induction of heparin-binding epidermal growth factor gene expression by oncogenic Raf-1. Mol. Cell. Biol. 17, 2401-2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagarajan P., Romano R. A., Sinha S. (2008). Transcriptional control of the differentiation program of interfollicular epidermal keratinocytes. Crit. Rev. Eukaryot. Gene Expr. 18, 57-79 [DOI] [PubMed] [Google Scholar]
- Nagarajan P., Parikh N., Garrett-Sinha L. A., Sinha S. (2009). Ets1 induces dysplastic changes when expressed in terminally-differentiating squamous epidermal cells. PLoS ONE 4, e4179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson K. K., Subbaram S., Connor K. M., Dasgupta J., Ha X. F., Meng T. C., Tonks N. K., Melendez J. A. (2006). Redox-dependent matrix metalloproteinase-1 expression is regulated by JNK through Ets and AP-1 promoter motifs. J. Biol. Chem. 281, 14100-14110 [DOI] [PubMed] [Google Scholar]
- Ozaki I., Mizuta T., Zhao G., Yotsumoto H., Hara T., Kajihara S., Hisatomi A., Sakai T., Yamamoto K. (2000). Involvement of the Ets-1 gene in overexpression of matrilysin in human hepatocellular carcinoma. Cancer Res. 60, 6519-6525 [PubMed] [Google Scholar]
- Pande P., Mathur M., Shukla N. K., Ralhan R. (1999). Ets-1: a plausible marker of invasive potential and lymph node metastasis in human oral squamous cell carcinomas. J. Pathol. 189, 40-45 [DOI] [PubMed] [Google Scholar]
- Pastore S., Mascia F., Mariani V., Girolomoni G. (2008). The epidermal growth factor receptor system in skin repair and inflammation. J. Invest. Dermatol. 128, 1365-1374 [DOI] [PubMed] [Google Scholar]
- Presland R. B., Jurevic R. J. (2002). Making sense of the epithelial barrier: what molecular biology and genetics tell us about the functions of oral mucosal and epidermal tissues. J. Dent. Educ. 66, 564-574 [PubMed] [Google Scholar]
- Presland R. B., Boggess D., Lewis S. P., Hull C., Fleckman P., Sundberg J. P. (2000). Loss of normal profilaggrin and filaggrin in flaky tail (ft/ft) mice: an animal model for the filaggrin-deficient skin disease ichthyosis vulgaris. J. Invest. Dermatol. 115, 1072-1081 [DOI] [PubMed] [Google Scholar]
- Proksch E., Brandner J. M., Jensen J. M. (2008). The skin: an indispensable barrier. Exp. Dermatol. 17, 1063-1072 [DOI] [PubMed] [Google Scholar]
- Reisdorff J., En-Nia A., Stefanidis I., Floege J., Lovett D. H., Mertens P. R. (2002). Transcription factor Ets-1 regulates gelatinase a gene expression in mesangial cells. J. Am. Soc. Nephrol. 13, 1568-1578 [DOI] [PubMed] [Google Scholar]
- Romano R. A., Birkaya B., Sinha S. (2006). Defining the regulatory elements in the proximal promoter of DeltaNp63 in keratinocytes: Potential roles for Sp1/Sp3, NF-Y, and p63. J. Invest. Dermatol. 126, 1469-1479 [DOI] [PubMed] [Google Scholar]
- Russell L. M., Garrett-Sinha L. A. (2010). Transcription factor Ets-1 in cytokine and chemokine gene regulation. Cytokine 51, 217-226 [DOI] [PubMed] [Google Scholar]
- Saeki H., Kuwano H., Kawaguchi H., Ohno S., Sugimachi K. (2000). Expression of ets-1 transcription factor is correlated with penetrating tumor progression in patients with squamous cell carcinoma of the esophagus. Cancer 89, 1670-1676 [DOI] [PubMed] [Google Scholar]
- Sark M. W., Fischer D. F., de Meijer E., van de Putte P., Backendorf C. (1998). AP-1 and ets transcription factors regulate the expression of the human SPRR1A keratinocyte terminal differentiation marker. J. Biol. Chem. 273, 24683-24692 [DOI] [PubMed] [Google Scholar]
- Schneider M. R., Werner S., Paus R., Wolf E. (2008). Beyond wavy hairs: the epidermal growth factor receptor and its ligands in skin biology and pathology. Am. J. Pathol. 173, 14-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segre J. (2003). Complex redundancy to build a simple epidermal permeability barrier. Curr. Opin. Cell Biol. 15, 776-782 [DOI] [PubMed] [Google Scholar]
- Segre J. A. (2006). Epidermal barrier formation and recovery in skin disorders. J. Clin. Invest. 116, 1150-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevilla L. M., Nachat R., Groot K. R., Klement J. F., Uitto J., Djian P., Maatta A., Watt F. M. (2007). Mice deficient in involucrin, envoplakin, and periplakin have a defective epidermal barrier. J. Cell Biol. 179, 1599-1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha S., Fuchs E. (2001). Identification and dissection of an enhancer controlling epithelial gene expression in skin. Proc. Natl. Acad. Sci. USA 98, 2455-2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squier C. A., Kremer M. J. (2001). Biology of oral mucosa and esophagus. J. Natl. Cancer Inst. Monogr. 2001, 7-15 [DOI] [PubMed] [Google Scholar]
- Takahashi H., Ogata H., Nishigaki R., Broide D. H., Karin M. (2010). Tobacco smoke promotes lung tumorigenesis by triggering IKKbeta- and JNK1-dependent inflammation. Cancer Cell 17, 89-97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomic-Canic M., Komine M., Freedberg I. M., Blumenberg M. (1998). Epidermal signal transduction and transcription factor activation in activated keratinocytes. J. Dermatol. Sci. 17, 167-181 [DOI] [PubMed] [Google Scholar]
- Vairaktaris E., Papageorgiou G., Derka S., Moulavassili P., Nkenke E., Kessler P., Vassiliou S., Papakosta V., Spyridonidou S., Vylliotis A., et al. (2007). Expression of ets-1 is not affected by N-ras or H-ras during oral oncogenesis. J. Cancer Res. Clin. Oncol. 133, 227-233 [DOI] [PubMed] [Google Scholar]
- Vicari A. P., Caux C. (2002). Chemokines in cancer. Cytokine Growth Factor Rev. 13, 143-154 [DOI] [PubMed] [Google Scholar]
- Watabe T., Yoshida K., Shindoh M., Kaya M., Fujikawa K., Sato H., Seiki M., Ishii S., Fujinaga K. (1998). The Ets-1 and Ets-2 transcription factors activate the promoters for invasion-associated urokinase and collagenase genes in response to epidermal growth factor. Int. J. Cancer 77, 128-137 [DOI] [PubMed] [Google Scholar]
- Wei G., Srinivasan R., Cantemir-Stone C. Z., Sharma S. M., Santhanam R., Weinstein M., Muthusamy N., Man A. K., Oshima R. G., Leone G., et al. (2009). Ets1 and Ets2 are required for endothelial cell survival during embryonic angiogenesis. Blood 114, 1123-1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermarck J., Kahari V. M. (1999). Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 13, 781-792 [PubMed] [Google Scholar]
- Westermarck J., Seth A., Kahari V. M. (1997). Differential regulation of interstitial collagenase (MMP-1) gene expression by ETS transcription factors. Oncogene 14, 2651-2660 [DOI] [PubMed] [Google Scholar]
- Westwick J. K., Cox A. D., Der C. J., Cobb M. H., Hibi M., Karin M., Brenner D. A. (1994). Oncogenic Ras activates c-Jun via a separate pathway from the activation of extracellular signal-regulated kinases. Proc. Natl. Acad. Sci. USA 91, 6030-6034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B. S., Hauser C. A., Henkel G., Colman M. S., Van Beveren C., Stacey K. J., Hume D. A., Maki R. A., Ostrowski M. C. (1996). Ras-mediated phosphorylation of a conserved threonine residue enhances the transactivation activities of c-Ets1 and c-Ets2. Mol. Cell. Biol. 16, 538-547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S., Richmond A. (2008). CXC chemokines and their receptors: a case for a significant biological role in cutaneous wound healing. Histol. Histopathol. 23, 1399-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Brown C., Maynard E., Anshelevich A., Ni W., Ho I. C., Oettgen P. (2005). Ets-1 is a critical regulator of Ang II-mediated vascular inflammation and remodeling. J. Clin. Invest. 115, 2508-2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.