Abstract
Background
To date, there is a wealth of literature describing the deleterious effects of active alcoholism on cognitive function. There has also been, more recently, a growing body of literature investigating the extent of cognitive recovery that can or may occur with abstinence. However, there is still a dearth of published findings on cognitive functioning in very long-term abstinence alcoholics, especially in the elderly population.
Methods
The current study examines 91 elderly abstinent alcoholics (EAA) (49 men and 42 women) with an average age of 67.3 years, abstinent for an average of 14.8 years (range 0.5 to 45 years), and age and gender comparable light/non-drinking controls. The EAA group was broken down into three sub-groups, individuals who attained abstinence before the age of 50, between the ages 50 and 60, and after the age of 60. Attention, verbal fluency, abstraction/cognitive flexibility, psychomotor, immediate memory, delayed memory, reaction time, spatial processing, and auditory working memory were assessed.
Results
Overall, the three EAA groups performed comparably to controls on all of the assessments of cognitive function. In fact, only the abstinent before age 50 group performed worse than controls, and this was only in the domain of auditory working memory.
Conclusions
Our data clearly show that it’s possible for elderly alcoholics with long-term abstinence to attain essentially normal cognitive functioning, even for those individuals who drank relatively late into life. These results don’t imply, however, that all individuals with long-term abstinence will attain normal cognition. It’s possible that selective survivorship may play a part in these findings (e.g. cognitively healthier alcoholics may be more likely to live into their sixties, seventies, or eighties).
Keywords: alcoholism, long-term abstinence, cognition, neuropsychology, aging
Introduction
The deleterious effects of chronic alcoholism on cognitive functioning have been well documented for over a century beginning as early as the 1880s with Wernicke and Korsakoff (Korsakoff, 1887; Wernicke, 1881). In the 1980’s, a study by Finlayson and colleagues reported that among patients receiving treatment for alcohol-related disorders, up to 23% had dementia of some type (Finlayson et al., 1988). More recent studies have continued to investigate the harmful effects of chronic alcohol abuse on brain structure and function (Brun and Andersson, 2001; Butterworth, 1995; Diamond and Messing, 1994; Emsley et al., 1996; Harper and Matsumoto, 2005; Heap et al., 2002; Kim et al., 2002; McMurtray et al., 2006; Mochizuki et al., 2005; Ratti et al., 1999; Saxton et al., 2000; Schmidt et al., 2005; Smith and Atkinson, 1995; Victor, 1994).
In more recent years, there has also been a shifting of focus towards examining the extent of cognitive recovery that can occur with sustained abstinence. There are a number of studies reporting the persistence of cognitive deficits in alcoholics with relatively short-term abstinence (Block et al., 2002; Di Sclafani et al., 1995; Fama et al., 2004; Fein et al., 1990; Moriyama et al., 2006; Munro et al., 2000; Sullivan et al., 2002; Sullivan et al., 2000b; Tedstone and Coyle, 2004; Zinn et al., 2004), especially in executive function, memory, and spatial processing. However, there is encouraging evidence from studies examining alcoholics with slightly longer abstinence durations that suggests that recovery or improvement in these domains can occur (Bates et al., 2005; Munro et al., 2000; Oscar-Berman et al., 2004; Rosenbloom et al., 2004; Sullivan et al., 2000a).
Despite research efforts in studying cognitive recovery in abstinent alcoholics, there is a scarcity of data on long-term abstinence, with most studies focusing on treatment samples and 3–12 month follow-up after treatment. The lack of research on cognitive functioning in long-term abstinence is even more pronounced in the elderly population. To our knowledge, there hasn’t been a single study published on the cognitive functioning of elderly alcoholics with very long-term abstinence. Studies in the elderly are particularly important because age has been consistently implicated as a major factor modulating the effects of alcohol abuse on brain structure and function (Fama et al., 2004; Goldman et al., 1983; Oscar-Berman et al., 2004). In fact, age is one of the strongest variables modulating the effects of chronic alcohol abuse on brain structure and function.
We recently published a manuscript examining cognitive performance in long-term abstinent (mean abstinence duration 6.7 years), middle-aged (mean age 46.8 years old) alcoholics (Fein et al., 2006). The abstinent alcoholics performed comparably to controls in all areas of cognitive functioning, except for a minor deficit in spatial processing. This current manuscript investigates whether or not elderly long-term abstinent alcoholics demonstrate impaired cognitive functioning when compared to age and gender comparable controls. Furthermore, this manuscript examines whether the aging brain is more vulnerable to the effects of heavy drinking on cognitive function by comparing abstinent elderly alcoholics who stopped drinking before age 50, those who stopped drinking between age 50 and 60, and those who stopped drinking after age 60.
Materials and Methods
Participants
A total of 143 participants were recruited from the San Francisco Bay Area community by postings at AA meetings, mailings, newspaper advertisements, a local Internet site, and participant referrals. The study consisted of two groups, elderly abstinent alcoholics (EAA) and age and gender comparable light/non-drinking normal controls (NC). The EAA group (n = 91) contained 49 men and 42 women, ranging from 58 to 85 years of age (mean = 67.3 years), abstinent from 6 months to 45 years (mean = 14.8 years). The EAA group was broken down into three sub-groups: 1) individuals who attained sobriety from alcohol before the age of 50 (EAA1); 2) individuals who attained sobriety between the ages of 50 and 60 (EAA2); and 3) individuals who attained sobriety after the age of 60 (EAA3). The inclusion criteria for the EAA groups were: 1) met lifetime DSM-IV (American Psychiatric Association, 2000) criteria for alcohol dependence 2) had a lifetime drinking average of at least 100 standard drinks per month for men, and 80 standard drinks per month for women, and 3) were abstinent for at least 6 months. A standard drink was defined as 12 oz. beer, 5 oz. wine, or 1.5 oz. liquor. The control group consisted of 22 men and 30 women, ranging in age from 60 to 85 years of age (mean = 68.8 years). The inclusion criteria for the NC group was a lifetime drinking average of less than 30 standard drinks per month, with no periods of drinking more than 60 drinks per month.
Exclusion criteria for both groups were: 1) lifetime or current diagnosis of schizophrenia or schizophreniform disorder (c-DIS) (Robins et al., 1998), 2) history of drug abuse or dependence (other than nicotine or caffeine), 3) significant history of head trauma or cranial surgery, 4) history of significant neurological disease, 5) history of diabetes, stroke, or hypertension that required medical intervention, 6) laboratory evidence of hepatic disease, or 7) clinical evidence of Wernicke-Korsakoff syndrome.
Procedures
All participants were fully informed of the study’s procedures and aims, and signed a consent form prior to their participation. Participants completed four sessions that lasted between an hour and a half and three hours, and included clinical, neuropsychological, electrophysiological, and neuroimaging assessments. Normal controls were asked to abstain from consuming alcohol for at least 24 hours prior to any lab visit. A Breathalyzer (Intoximeters, Inc., St. Louis, MO) test was administered to all participants. A 0.000 alcohol concentration was required of all participants in all sessions. Subjects were compensated for time and travel expenses upon completion of each session. Participants who completed the entire study were also given a completion bonus.
General Assessment
All participants participated in the following assessments: 1) psychiatric diagnoses and symptom counts were gathered using the c-DIS (Robins et al., 1998), 2) participants were interviewed on their drug and alcohol use using the lifetime drinking history methodology (Skinner and Allen, 1982; Skinner and Sheu, 1982; Sobell and Sobell, 1990; Sobell et al., 1988), 3) medical histories were reviewed, 4) blood was drawn to test liver functions, and 5) the Family Drinking Questionnaire was administered based on the methodology of Mann et al. (Mann et al., 1985; Stoltenberg et al., 1998). The Family Drinking Questionnaire asked participants to rate the members of their family as being alcohol abstainers, alcohol users with no problem, or problem drinkers. Family History Density (FHD) was defined as the proportion of 1st degree relatives that were problem drinkers.
Neuropsychological Assessment
The neuropsychological assessments were administered in one session. The battery began with the administration of the following individual tests: Rey-Osterrieth Complex Figure (copy, immediate, and 20 minute delayed) (Osterrieth, 1944), Trail Making Test A and B (Reitan and Wolfson, 1985), Symbol Digit Modalities Test (written administration only) (Smith, 1968), American version of the Nelson Adult Reading Test (AMNART) (Grober and Sliwinski, 1991), Short Category Test (booklet format) (Wetzel and Boll, 1987), Controlled Oral Word Association Test (COWAT) (Benton and Hamsher, 1983), Paced Auditory Serial Addition Test (PASAT) (Gronwall, 1977), Block Design (WAIS-R) (Wechsler, 1981), Stroop Color and Word Test (Golden, 1978), Fregly Ataxia Battery (Fregly et al., 1973), and the Simulated Gambling Task (Bechara et al., 1994).
After a 15 minute break, the participant completed the MicroCog (MC) Assessment of Cognitive Functioning (standard version) (Powell et al., 1993). The MicroCog is a computer-administered and -scored test that assesses important neurocognitive function in adults. MicroCog was designed to be sensitive to detecting cognitive impairment across a wide range, and takes into account levels of premorbid intellectual functioning by providing age- and education-level adjusted norms.
Normative scores derived from a nationally representative sample of adults are available for each test, either from the creators or distributors of the tests. Z-scores for the neuropsychological domains and measures were computed based on standardized norms adjusted for age [Stroop (Golden, 1978), Short Categories (Wetzel and Boll, 1987), PASAT (Stuss et al., 1988), Block Design (Wechsler, 1997), and Rey (Denman, 1987)], years of education [AMNART (Schwartz and Saffran, 1987)], age and years of education [Symbol Digit Modalities (Smith, 1982), MicroCog (Powell et al., 1993)], and age, gender, and years of education [Trails A and B (Heaton et al., 1991), COWAT (Ruff et al., 1996)]. The Stroop, Symbol Digit Modalities, and the MicroCog test norms are not specific to gender, since gender did not significantly affect scores in the normative samples (Golden, 1978; Powell et al., 1993; Smith, 1982). The AMNART is used as a measure of premorbid intelligence (Grober and Sliwinski, 1991). The AMNART did not have age norms because the test was designed to be resistant to the effects of normal aging and most neurodegenerative diseases. Additionally, Grober et al (Grober and Sliwinski, 1991) have reported that gender does not influence AMNART scores.
The final NP battery consisted of the following 9 domains, and their component tests: (1) Attention (Stroop Color, MC Numbers Forward, MC Numbers Reversed, MC Alphabet, MC Word List 1) (2) Verbal Ability (COWAT, AMNART), (3) Abstraction/Cognitive Flexibility (Short Categories, Stroop interference score, Trail Making Test B, MC Analogies, MC Object Match A), (4) Psychomotor (Trails A, Symbol Digit), (5) Immediate Memory (MC Story immediate recall, Rey immediate recall, MC Word List 2), (6) Delayed Memory (MC Story delayed recall, Rey delayed recall), (7) Reaction Time (MC Timers simple and cued), (8) Spatial Processing (MC Tic Tac, MC Clocks, Block Design), and (9) Auditory Working Memory (PASAT at delays of 2.4, 2.0, 1.6, and 1.2 seconds).
Global Clinical Impairment Score (GCIS)
Each domain’s average Z-score was converted to a global clinical impairment score (GCIS). A clinical impairment score of 0, or no impairment, was assigned to domain Z-scores falling above the 15th percentile, a score of 1, or moderately impaired, was assigned to domain Z-scores falling at or below the 15th and above the 5th percentile, and a score of 2, or severely impaired, was assigned to domain Z-scores falling at or below the 5th percentile. The cutoff points for the clinical impairment scores were designed to make the GCIS sensitive to clinically relevant impairment. The domain clinical impairment scores (0, 1, or 2) were then summed across domains to yield the GCIS, with greater GCIS scores indicating more severe impairment.
Statistical Analysis
The data were analyzed using the Statistical Package for the Social Sciences (SPSS Inc., 2004). First, a Multivariate Analysis of Variance examining the domain z-scores was carried out using the General Linear Models procedure. To control for multiple comparisons, individual domain z-scores were examined only if the multivariate tests were significant. Associations of demographic and alcohol use measures with the cognitive measures were examined using Spearman’s correlations. Some of the controls were used with more than one of the EAA groups so that the comparisons were between an EAA group and age and gender comparable controls.
Results
Demographic and Alcohol Use of the three EAA groups
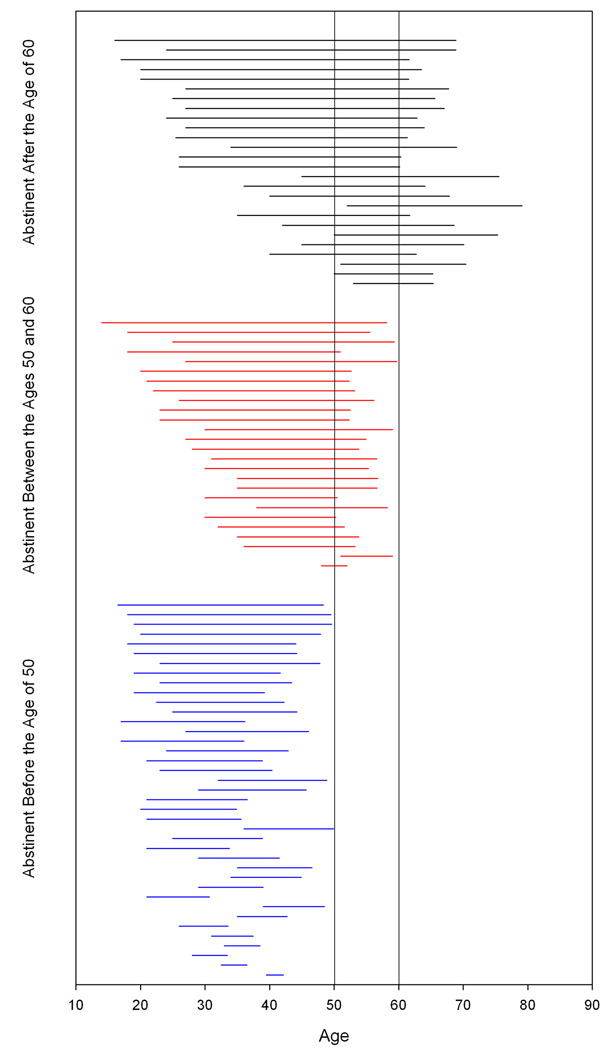
All of the abstinent alcohol groups had similar levels of education and AMNART scores (a measure of pre-morbid intelligence). However, the EAA3 group was significantly older than the other two abstinent alcohol groups. The EAA1 group had a higher proportion of first-degree relatives who were “problem drinkers” than either the EAA2 or EAA3 groups, suggesting a greater genetic loading for alcoholism. The groups also differed on specific measures of their prior alcohol use. Although all three groups had their first drinks at 17 to 19 years of age, the EAA1 group began drinking heavily a younger age (25.3 ± 6.6 years) than the EAA2 (29.0 ± 8.6 years) and EAA3 groups (34.5 ± 11.5 years). Figure 1 illustrates the differences between the groups in their periods of heavy drinking. Furthermore, the EAA3 group had the lowest lifetime drinking dose. The three groups all had similar durations and doses during their peak alcohol use. Table 1 summarizes the demographic and alcohol use difference between the groups.
Figure 1.
In Figure 1 the horizontal lines begin at the onset of heavy drinking and end at the ages at which abstinence was achieved for each member of the abstinent alcoholic groups.
Table 1.
Characteristics of the Elderly Abstinent Alcoholic Groups
| Alcoholics who achieved Abstinence: | Effect Size (%) | ||||||
|---|---|---|---|---|---|---|---|
| Variables | Before 50 | Between 50–60 | After 60 | ||||
| N = 39 | N = 26 | N = 26 | Before 50 vs Between 50–60 |
Between 50–60 vs After 60 |
Before 50 vs After 60 |
||
| Age (years) | 65.56 ± 5.00 | 66.62 ± 5.76 | 70.68 ± 6.56 | 1.5 | 9.0* | 16.3*** | |
| a Family History Density_1 | 0.43 ± 0.32 | 0.23 ± 0.20 | 0.26 ± 0.22 | 9.4** | 1.1 | 5.3 | |
| b Family History Density_2 | 0.19 ± 0.13 | 0.10 ± 0.13 | 0.12 ± 0.15 | 9.1* | 0.0 | 7.0* | |
| Years Education | 15.37 ± 2.61 | 16.42 ± 3.29 | 15.48 ± 2.28 | 3.7 | 5.1 | 0.1 | |
| AMNART | 1.31 ± 0.32 | 1.35 ± 0.51 | 1.35 ± 0.34 | 1.4 | 1.0 | 0.0 | |
|
| |||||||
| Alcohol Use Variables | |||||||
|
| |||||||
| Lifetime Alcohol Use (std. drinks) | 47,354.7 ± 27,605.2 | 82,645 ± 56,482.5 | 65,493.2 ± 30,830.1 | 11.7c | 2.2 | 6.9 c | |
| Age Started Drinking | 17.77 ± 4.09 | 17.35 ± 3.63 | 18.56 ± 3.31 | 0.4 | 5.4 | 2.2 | |
| Age at First Heavy Use | 25.33 ± 6.61 | 28.96 ± 8.64 | 34.46 ± 11.50 | 7.5* | 5.1 | 19.9*** | |
| Duration of Active Drinking (years) |
23.28 ± 6.27 | 36.71 ± 5.13 | 44.44 ± 7.80 | 57.8 c | 24.8c | 69.8c | |
| Average Lifetime Drinking Dose (std. drinks/month) |
169.33 ± 98.29 | 201.54 ± 181.97 | 126.21 ± 62.60 | 0.3 | 5.2 | 6.2* | |
| Alcohol Peak Use (std. drinks) | 25,065.5 ± 23,567.5 | 45,805.3 ± 57,548.1 | 31,667.7 ± 24,962.4 | 3.5 | 2.1 | 0.3 | |
| Duration of Peak Drinking (months) |
84.95 ± 81.32 | 128.35 ± 104.86 | 148.34 ± 146.00 | 3.4 | 0.2 | 3.8 | |
| Peak Drinking Dose (std. drinks/month) |
367.81 ± 265.12 | 348.00 ± 218.36 | 265.14 ± 151.84 | 0.5 | 3.1 | 4.4 | |
| Age At Sobriety | 41.62 ± 5.33 | 54.82 ± 2.95 | 66.50 ± 4.90 | 66.7c | 67.2c | 84.5c | |
| Years Abstinent | 23.94 ± 6.99 | 11.80 ± 6.93 | 4.18 ± 5.26 | 40.4c | 29.4c | 68.6c | |
Measures are reported mean ± standard deviation. Effect is significant:
p ≤ 0.05;
p ≤ 0.01;
p ≤ 0.001
Total number of problem drinking 1st degree relatives divided by the total number of 1st degree relatives.
Total number of problem drinking 2nd degree relatives divided by the total number 2nd degree relatives.
Statistical comparisons on variables directly influenced by or related to the age at which the individuals achieved abstinence are not appropriate since this was associated with the group selection criteria.
NP Performance of the three EAA groups
Multivariate analyses comparing the three EAA groups with each other revealed significant group differences (Wilks λ18,142 = 0.679, p < 0.05). Examining the individual domains showed that the differences were primarily in delayed memory (F2,84 = 6.15, p = 0.003) and spatial processing (F2,84 = 3.80, p < 0.03) with the EAA3 performing the best and the EAA1 group performing the worst on both domains. Multivariate analyses did not reveal any significant gender or group by gender effects. However, data uncorrected for multiple comparison s revealed one group by gender interaction difference on the assessment of verbal ability (F2,84 = 3.16, p < 0.05).
NP Performance of individuals who achieved sobriety before the age of 50
Multivariate tests revealed a difference between the EAA1 group and their controls (Wilks λ9,58 = 0.653, p = 0.002). However, the only individual neuropsychological domain that differed between the groups was auditory working memory (F1,69 = 7.86, p = 0.007), with the abstinent alcoholic group performing poorer than controls. No gender or group by gender differences were observed (see Tables 2a and 2b).
Table 2.
| Table 2a. Demographics and Neuropsychological Measures – Alcoholics (Abstinent Before Age 50) vs Controls | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Sober Before Age 50 | Controls | Effect Size (%) | ||||
| Male (n=16) |
Female (n=23) |
Male (n=16) |
Female (n=23) |
Group | Gender | Group × Gender | |
| Demographics | |||||||
| Age (years) | 66.40 ± 5.49 | 64.98 ± 4.67 | 66.86 ± 3.98 | 65.75 ± 4.56 | 0.4 | 1.8 | 0.0 |
| Years Education | 14.59 ± 2.76 | 15.91 ± 2.41 | 17.19 ± 2.17 | 16.13 ± 1.69 | 9.0** | 0.1 | 6.6* |
|
| |||||||
| Neuropsychological Measures | |||||||
| Average z-score | 0.21 ± 0.43 | 0.21 ± 0.37 | 0.31 ± 0.42 | 0.26 ± 0.40 | 0.8 | 0.1 | 0.1 |
| Attention | 0.16 ± 0.62 | 0.01 ± 0.34 | −0.18 ± 0.70 | 0.04 ± 0.54 | 2.9 | 0.3 | 3.5 |
| Auditory Working Memory | −0.75 ± 1.24 | −0.35 ± 0.67 | 0.17 ± 0.60 | −0.16 ± 0.68 | 10.6** | 0.0 | 4.9 |
| Verbal | 1.19 ± 0.41 | 1.03 ± 0.53 | 0.90 ± 0.76 | 0.95 ± 0.53 | 2.8 | 0.0 | 1.2 |
| Abstraction/Cognitive Flexibility | 0.06 ± 0.68 | −0.06 ± 0.48 | 0.29 ± 0.65 | 0.14 ± 0.39 | 1.8 | 1.4 | 0.0 |
| Psychomotor | −0.04 ± 0.82 | −0.02 ± 0.72 | −0.06 ± 0.73 | −0.10 ± 0.69 | 1.3 | 0.1 | 0.0 |
| Immediate Memory | 0.49 ± 0.54 | 0.52 ± 0.66 | 0.51 ± 0.55 | 0.61 ± 0.56 | 0.0 | 0.4 | 0.0 |
| Delayed Memory | 0.19 ± 0.66 | 0.30 ± 0.72 | 0.26 ± 0.75 | 0.37 ± 0.79 | 0.0 | 0.9 | 0.4 |
| Reaction Time | 0.38 ± 0.65 | 0.45 ± 0.41 | 0.45 ± 0.50 | 0.24 ± 0.58 | 0.4 | 0.3 | 1.7 |
| Spatial Processing | 0.22 ± 0.59 | 0.03 ± 0.42 | 0.43 ± 0.43 | 0.24 ± 0.55 | 2.6 | 3.9 | 0.3 |
| AMNART | 1.34 ± 0.34 | 1.29 ± 0.32 | 1.35 ± 0.51 | 1.21 ± 0.43 | 0.2 | 1.4 | 0.3 |
| Global Clinical Impairment Score | 1.13 ± 1.71 | 0.30 ± 0.47 | 0.38 ± 1.02 | 0.35 ± 0.47 | 2.7 | 3.9 | 3.4 |
| Table 2b. Neuropsychological Domains and Individual Tests (Raw Scores) – EAA1 vs Controls | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Alcoholics Abstinent Before 50 |
Controls | Effect Size (%) | ||||
| Male (n=16) |
Female (n=23) |
Male (n=16) |
Female (n=23) |
Group | Gender | Group by Gender |
|
|
Neuropsychological Domains (Raw scores) | |||||||
| Abstraction/Cognitive Flexibility | |||||||
| MicroCog Analogies | 12.5 ± 3.3 | 11.0 ± 2.5 | 13.0 ± 2.8 | 11.4 ± 3.0 | 0.7 | 6.8* | 0.0 |
| MicroCog Object Match A | 10.4 ± 3.3 | 10.2 ± 2.4 | 10.9 ± 2.3 | 10.8 ± 2.4 | 1.0 | 0.1 | 0.0 |
| Short Categories | 40.2 ± 11.1 | 43.2 ± 10.8 | 35.6 ± 17.3 | 36.5 ± 13.7 | 4.4 | 0.6 | 0.2 |
| Stroop-Interference | 29.1 ± 8.4 | 38.0 ± 10.1 | 35.8 ± 12.0 | 37.4 ± 7.6 | 2.5 | 7.1* | 3.5 |
| Trails B | 82.4 ± 37.2 | 81.9 ± 35.2 | 88.3 ± 32.6 | 74.9 ± 25.5 | 0.0 | 1.1 | 1.0 |
| Attention | |||||||
| MicroCog Alphabet | 11.4 ± 0.5 | 11.4 ± 0.5 | 11.5 ± 0.6 | 11.5 ± 0.5 | 0.5 | 0.1 | 0.0 |
| MicroCog Numbers Forward | 11.5 ± 2.9 | 10.1 ± 2.2 | 8.7 ± 3.6 | 9.8 ± 3.3 | 6.6* | 0.1 | 4.1 |
| MicroCog Numbers Reversed | 9.8 ± 4.1 | 8.7 ± 2.5 | 7.9 ± 3.8 | 8.9 ± 2.5 | 1.3 | 0.0 | 2.3 |
| MicroCog Wordlist 1 | 11.1 ± 2.6 | 10.9 ± 2.1 | 10.7 ± 3.2 | 11.1 ± 2.4 | 0.0 | 0.0 | 0.5 |
| Stroop-Color | 64.6 ± 8.4 | 68.5 ± 11.8 | 61.7 ± 15.6 | 68.8 ± 11.0 | 0.3 | 5.1* | 0.5 |
| Auditory Working Memory | |||||||
| PASAT 2.4 (seconds delay) | 28.9 ± 15.3 | 36.6 ± 9.7 | 42.8 ± 11.6 | 38.4 ± 11.1 | 10.0** | 0.4 | 6.2* |
| PASAT 2.0 (seconds delay) | 30.5 ± 14.5 | 33.3 ± 13.6 | 38.2 ± 10.2 | 33.6 ± 11.0 | 2.6 | 0.1 | 2.2 |
| PASAT 1.6 (seconds delay) | 26.8 ± 14.1 | 26.3 ± 12.5 | 33.8 ± 8.6 | 29.4 ± 10.4 | 4.5 | 1.2 | 0.7 |
| PASAT 1.2 (seconds delay) | 19.7 ± 11.1 | 19.3 ± 8.6 | 24.7 ± 5.2 | 22.1 ± 7.4 | 5.4 | 0.8 | 0.4 |
| Immediate Memory | |||||||
| MicroCog Story Recall | 9.1 ± 1.4 | 9.3 ± 2.0 | 9.3 ± 1.4 | 9.3 ± 1.6 | 0.2 | 0.2 | 0.0 |
| MicroCog Wordlist 2 | 13.8 ± 3.2 | 14.2 ± 3.6 | 14.1 ± 1.9 | 14.3 ± 3.5 | 0.1 | 0.2 | 0.1 |
| Rey-Immediate Recall | 39.1 ± 7.3 | 38.1 ± 13.1 | 38.4 ± 13.3 | 41.1 ± 12.1 | 0.2 | 0.1 | 0.6 |
| Delayed Memory | |||||||
| MicroCog Story-Delayed Recall | 10.0 ± 3.0 | 10.3 ± 2.6 | 9.9 ± 2.9 | 10.6 ± 3.6 | 0.0 | 0.7 | 0.1 |
| Rey-Delayed Recall | 37.1 ± 7.2 | 38.2 ± 13.4 | 38.8 ± 12.8 | 40.0 ± 10.7 | 0.6 | 0.3 | 0.0 |
| Psychomotor | |||||||
| Symbol Digit Modalities | 43.1 ± 7.8 | 47.6 ± 10.6 | 45.5 ± 5.5 | 47.3 ± 8.7 | 0.4 | 3.3 | 0.6 |
| Trails A | 35.9 ± 9.4 | 36.3 ± 11.4 | 37.6 ± 14.5 | 38.3 ± 12.1 | 0.6 | 0.1 | 0.0 |
| Reaction Time | |||||||
| MicroCog Cued Timers | 10.9 ± 2.4 | 11.7 ± 1.7 | 11.4 ± 1.8 | 11.1 ± 1.9 | 0.0 | 0.3 | 1.7 |
| MicroCog Simple Timers | 11.3 ± 1.9 | 11.1 ± 1.3 | 11.3 ± 1.5 | 10.3 ± 1.9 | 1.3 | 3.6 | 1.1 |
| Spatial Processing | |||||||
| Block Design | 35.5 ± 9.0 | 31.7 ± 8.9 | 40.4 ± 9.8 | 34.9 ± 10.2 | 4.4 | 5.8* | 0.2 |
| MicroCog Clocks | 12.3 ± 2.0 | 12.6 ± 1.0 | 12.6 ± 1.2 | 12.3 ± 1.7 | 0.0 | 0.0 | 1.5 |
| MicroCog Tic Tac | 8.6 ± 2.5 | 7.7 ± 2.2 | 8.6 ± 2.1 | 9.0 ± 2.1 | 2.1 | 0.3 | 1.7 |
| Verbal | |||||||
| AMNART | 36.6 ± 4.5 | 34.7 ± 4.6 | 34.9 ± 7.6 | 33.3 ± 6.7 | 1.7 | 2.1 | 0.0 |
| COWAT | 43.4 ± 9.9 | 41.6 ± 10.0 | 39.4 ± 11.3 | 40.8 ± 9.3 | 1.4 | 0.0 | 0.6 |
Measures are reported mean ± standard deviation. Effect is significant:
= p ≤ 0.05;
= p ≤ 0.01
NP performance of individuals who achieved sobriety between the ages of 50 and 60
The multivariate test did not reveal any group differences (Wilks λ9,37 = 0.742, p = 0.211), gender differences, or group by gender interactions. Although not controlled for multiple comparisons, the EAA2 group performed better than controls in the areas of attention (F1,48 = 10.867, p = 0.002) and verbal ability (F1,48 = 4.70, p < 0.04), men performed better than women in auditory working memory (F1,48 = 5.11, p < 0.03), and group by gender differences were present in verbal ability (F1,48 = 4.63, p < 0.04) (see Tables 3a and 3b).
Table 3.
| Table 3a. Demographics and Neuropsychological Measures – Alcoholics (Abstinent Between the Ages of 50–60) vs Controls | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Abstinent Between 50 – 60 | Controls | Effect Size (%) | ||||
| Male (n=16) |
Female (n=10) |
Male (n=16) |
Female (n=10) |
Group | Gender | Group × Gender | |
| Demographics | |||||||
| Age (years) | 65.38 ± 4.84 | 68.62 ± 6.78 | 66.40 ± 3.15 | 68.18 ± 6.56 | 0.1 | 5.6 | 0.5 |
| Years Education | 16.50 ± 3.14 | 16.3 ± 3.68 | 17.19 ± 2.17 | 16.20 ± 1.48 | 0.3 | 1.2 | 0.5 |
|
| |||||||
| Neuropsychological Measures | |||||||
| Average z-score | 0.36 ± 0.49 | 0.40± 0.32 | 0.27 ± 0.40 | 0.03 ± 0.36 | 8.7* | 1.5 | 2.9 |
| Attention | 0.17 ± 0.47 | 0.42 ± 0.54 | −0.27 ± 0.71 | −0.24 ± 0.58 | 19.2** | 1.8 | 0.6 |
| Auditory Working Memory | −0.07 ± 0.77 | −0.25 ± 0.90 | 0.10 ± 0.60 | −0.85 ± 1.24 | 1.7 | 10.2* | 5.0 |
| Verbal | 0.84 ± 0.59 | 1.36 ± 0.44 | 0.84 ± 0.76 | 0.52 ± 0.42 | 9.5* | 1.3 | 9.3* |
| Abstraction/Cognitive Flexibility | 0.15 ± 0.77 | 0.33 ± 0.45 | 0.28 ± 0.60 | −0.03 ± 0.40 | 1.0 | 0.1 | 3.5 |
| Psychomotor | −0.06 ± 0.75 | −0.05 ± 0.48 | −0.13 ± 0.76 | −0.26 ± 0.63 | 2.5 | 0.0 | 0.0 |
| Immediate Memory | 0.78 ± 0.46 | 0.54 ± 0.90 | 0.45 ± 0.53 | 0.44 ± 0.38 | 3.4 | 1.7 | 0.5 |
| Delayed Memory | 0.58 ± 0.69 | 0.38 ± 0.96 | 0.28 ± 0.74 | 0.17 ± 0.73 | 3.2 | 2.4 | 0.1 |
| Reaction Time | 0.40 ± 0.68 | 0.35 ± 0.48 | 0.46 ± 0.49 | 0.12 ± 0.50 | 1.0 | 2.8 | 1.6 |
| Spatial Processing | 0.42 ± 0.66 | 0.48 ± 0.45 | 0.37 ± 0.31 | 0.27 ± 0.55 | 2.5 | 0.3 | 1.4 |
| AMNART | 1.20 ± 0.56 | 1.62 ± 0.28 | 1.34 ± 0.50 | 1.03 ± 0.44 | 5.2 | 0.4 | 13.1** |
| Global Clinical Impairment Score | 0.50 ± 1.15 | 0.60 ± 1.07 | 0.50 ± 1.10 | 0.60 ± 0.97 | 0.0 | 0.2 | 0.0 |
| Table 3b. Neuropsychological Domains and Individual Tests (Raw Scores) – EAA2 vs Controls | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Alcoholics Abstinent Between 50 – 60 |
Controls | Effect Size (%) | ||||
| Male (n=16) |
Female (n=10) |
Male (n=16) |
Female (n=10) |
Group | Gender | Group by Gender |
|
|
Neuropsychological Domains (Raw scores) | |||||||
| Abstraction/Cognitive Flexibility | |||||||
| MicroCog Analogies | 11.4 ± 3.2 | 12.7 ± 3.1 | 13.1 ± 3.1 | 10.8 ± 3.2 | 0.0 | 0.6 | 7.7* |
| MicroCog Object Match A | 10.3 ± 2.8 | 11.3 ± 1.4 | 11.0 ± 2.1 | 9.9 ± 2.9 | 0.6 | 0.0 | 4.9 |
| Short Categories | 36.7 ± 13.7 | 35.1 ± 16.0 | 37.5 ± 17.2 | 37.8 ± 13.0 | 0.3 | 0.0 | 0.1 |
| Stroop-Interference | 33.3 ± 7.8 | 40.2 ± 6.9 | 36.3 ± 11.0 | 35.6 ± 9.8 | 0.2 | 2.8 | 4.3 |
| Trails B | 71.9 ± 31.7 | 77.2 ± 25.9 | 85.6 ± 31.3 | 90.2 ± 30.8 | 4.7 | 0.7 | 0.0 |
| Attention | |||||||
| MicroCog Alphabet | 11.3 ± 0.5 | 11.7 ± 0.7 | 11.5 ± 0.6 | 11.6 ± 0.5 | 0.1 | 4.4 | 1.6 |
| MicroCog Numbers Forward | 10.9 ± 3.0 | 12.5 ± 3.9 | 7.9 ± 3.4 | 8.5 ± 4.0 | 20.7*** | 2.3 | 0.5 |
| MicroCog Numbers Reversed | 11.1 ± 3.4 | 9.1 ± 3.5 | 7.9 ± 4.2 | 7.7 ± 3.0 | 9.3* | 2.2 | 1.5 |
| MicroCog Wordlist 1 | 11.1 ± 3.1 | 12.5 ± 0.7 | 10.5 ± 3.2 | 10.2 ± 3.2 | 6.3 | 0.9 | 2.2 |
| Stroop-Color | 64.4 ± 8.9 | 73.8 ± 8.2 | 61.7 ± 14.9 | 62.8 ± 12.0 | 8.2* | 4.9 | 3.1 |
| Auditory Working Memory | |||||||
| PASAT 2.4 (seconds delay) | 38.9 ± 15.2 | 38.5 ± 15.2 | 41.2 ± 11.6 | 25.6 ± 13.7 | 4.1 | 8.9* | 8.0* |
| PASAT 2.0 (seconds delay) | 38.3 ± 10.5 | 37.9 ± 7.3 | 36.9 ± 10.2 | 24.4 ± 14.9 | 10.7* | 8.2 | 7.3 |
| PASAT 1.6 (seconds delay) | 32.9 ± 9.6 | 29.8 ± 7.6 | 32.8 ± 7.7 | 23.9 ± 15.3 | 2.3 | 8.4 | 2.0 |
| PASAT 1.2 (seconds delay) | 22.7 ± 10.3 | 23.5 ± 7.8 | 24.6 ± 5.1 | 17.8 ± 11.3 | 1.2 | 3.0 | 4.6 |
| Immediate Memory | |||||||
| MicroCog Story Recall | 8.9 ± 1.5 | 9.3 ± 1.6 | 9.1 ± 1.3 | 8.2 ± 1.7 | 2.6 | 0.7 | 4.1 |
| MicroCog Wordlist 2 | 15.1 ± 1.3 | 13.9 ± 4.3 | 13.8 ± 1.9 | 14.1 ± 1.6 | 1.2 | 0.9 | 2.3 |
| Rey-Immediate Recall | 45.7 ± 11.5 | 38.8 ± 14.3 | 38.6 ± 12.4 | 38.8 ± 11.6 | 2.1 | 1.8 | 2.1 |
| Delayed Memory | |||||||
| MicroCog Story-Delayed Recall | 10.6 ± 2.6 | 10.6 ± 4.2 | 10.1 ± 3.1 | 9.1 ± 3.5 | 2.3 | 0.7 | 0.6 |
| Rey-Delayed Recall | 44.9 ± 11.7 | 38.7 ± 13.5 | 39.0 ± 11.9 | 38.7 ± 7.0 | 1.7 | 2.1 | 1.7 |
| Psychomotor | |||||||
| Symbol Digit Modalities | 46.6 ± 7.8 | 49.4 ± 6.6 | 43.6 ± 6.5 | 45.3 ± 7.6 | 5.8 | 2.5 | 0.2 |
| Trails A | 34.4 ± 9.3 | 42.8 ± 11.9 | 36.2 ± 12.9 | 43.0 ± 15.1 | 0.2 | 9.0* | 0.1 |
| Reaction Time | |||||||
| MicroCog Cued Timers | 12.0 ± 1.7 | 11.2 ± 2.2 | 11.3 ± 1.9 | 11.1 ± 2.0 | 1.0 | 2.0 | 0.5 |
| MicroCog Simple Timers | 10.5 ± 2.7 | 11.0 ± 1.4 | 11.4 ± 1.4 | 9.7 ± 1.1 | 0.2 | 2.9 | 9.0* |
| Spatial Processing | |||||||
| Block Design | 41.2 ± 13.2 | 38.8 ± 10.9 | 38.8 ± 9.9 | 39.0 ± 11.1 | 0.2 | 0.2 | 0.3 |
| MicroCog Clocks | 12.0 ± 2.5 | 13.1 ± 1.3 | 12.6 ± 1.2 | 11.5 ± 1.4 | 2.2 | 0.0 | 8.9* |
| MicroCog Tic Tac | 9.4 ± 2.1 | 8.7 ± 1.3 | 8.6 ± 1.6 | 8.6 ± 2.1 | 1.6 | 0.8 | 1.0 |
| Verbal | |||||||
| AMNART | 32.9 ± 8.1 | 40.6 ± 4.1 | 34.8 ± 7.5 | 30.2 ± 6.9 | 8.4* | 1.2 | 16.3** |
| COWAT | 38.6 ± 11.7 | 47.6 ± 15.1 | 38.1 ± 11.3 | 33.7 ± 6.4 | 9.1* | 1.0 | 7.9* |
Measures are reported mean ± standard deviation. Effect is significant:
p ≤ 0.05;
p ≤ 0.01;
= p ≤ 0.001
NP Performance of individuals who achieved sobriety after the age of 60
Multivariate tests did not reveal group, (Wilks λ9,36 = 0.688, p = 0.099) gender, or a group by gender interactions. However, uncorrected for multiple comparisons, the EAA3 group performed better than the control sample on the assessments of immediate memory (F1,47 = 6.68, p < 0.02), delayed memory (F1,47 = 4.55, p < 0.04), and reaction time (F1,47 = 6.42, p < 0.02), with no areas in which the EAA3 group performed worse than controls. A group by gender interaction was observed in the assessment of spatial processing (F1,47 = 4.37, p < 0.05) (see Tables 4a and 4b).
Table 4.
| Table 4a. Demographics and Neuropsychological Measures – Alcoholics (Abstinent After Age 60) vs Controls | |||||||
|---|---|---|---|---|---|---|---|
| Variables | Abstinent After 60 | Controls | Effect Size (%) | ||||
| Male (n=17) |
Female (n=9) |
Male (n=17) |
Female (n=9) |
Group | Gender | Group × Gender | |
| Demographics | |||||||
| Age (years) | 70.25 ± 4.84 | 71.51 ± 9.30 | 69.79 ± 4.54 | 71.36 ± 9.46 | 0.1 | 1.1 | 0.0 |
| Years Education | 16.18 ± 2.19 | 14.00 ± 1.77 | 16.76 ± 2.54 | 15.67 ± 1.73 | 6.0 | 11.8* | 1.4 |
|
| |||||||
| Neuropsychological Measures | |||||||
| Average z-score | 0.44 ± 0.37 | 0.34 ± 0.49 | 0.33 ± 0.37 | 0.12 ± 0.43 | 3.8 | 3.3 | 0.4 |
| Attention | 0.31 ± 0.49 | 0.13 ± 0.57 | −0.05 ± 0.70 | −0.03 ± 0.49 | 6.8 | 0.0 | 0.1 |
| Auditory Working Memory | −0.33 ± 0.76 | −0.16 ± 0.77 | −0.07 ± 0.57 | −0.38 ± 0.50 | 0.0 | 0.3 | 3.0 |
| Verbal | 0.95 ± 0.45 | 0.94 ± 0.65 | 0.82 ± 0.70 | 0.84 ± 0.30 | 2.3 | 0.3 | 0.1 |
| Abstraction/Cognitive Flexibility | 0.30 ± 0.57 | 0.03 ± 0.86 | 0.36 ± 0.60 | 0.26 ± 0.47 | 0.0 | 0.1 | 0.2 |
| Psychomotor | −0.04 ± 0.69 | −0.26 ± 0.61 | −0.06 ± 0.77 | −0.62 ± 0.80 | 6.0 | 2.3 | 2.3 |
| Immediate Memory | 0.67 ± 0.32 | 0.70 ± 0.54 | 0.49 ± 0.50 | 0.23 ± 0.63 | 13.2* | 0.2 | 5.4 |
| Delayed Memory | 0.90 ± 0.78 | 0.72 ± 1.18 | 1.52 ± 0.80 | 0.41 ± 0.95 | 9.4* | 0.1 | 2.1 |
| Reaction Time | 0.72 ± 0.30 | 0.60 ± 0.24 | 0.39 ± 0.48 | 0.36 ± 0.57 | 12.7* | 0.1 | 0.1 |
| Spatial Processing | 0.44 ± 0.57 | 0.36 ± 0.68 | 0.48 ± 0.54 | 0.04 ± 0.56 | 6.4 | 1.1 | 9.0* |
| AMNART | 1.39 ± 0.32 | 1.27 ± 0.38 | 1.26 ± 0.50 | 1.17 ± 0.37 | 2.1 | 1.8 | 0.0 |
| Global Clinical Impairment Score | 0.35 ± 0.70 | 0.33 ± 0.71 | 0.41 ± 1.06 | 0.67 ± 1.12 | 1.1 | 0.4 | 0.6 |
| Table 4b. Neuropsychological Domains and Individual Tests (Raw Scores) – EAA3 vs Controls | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Alcoholics Abstinent After 60 |
Controls | Effect Size (%) | ||||
| Male (n=17) |
Female (n=9) |
Male (n=17) |
Female (n=9) |
Group | Gender | Group by Gender |
|
|
Neuropsychological Domains (Raw scores) | |||||||
| Abstraction/Cognitive Flexibility | |||||||
| MicroCog Analogies | 13.1 ± 3.0 | 12.1 ± 3.6 | 13.4 ± 3.2 | 12.2 ± 3.6 | 0.1 | 2.9 | 0.0 |
| MicroCog Object Match A | 10.8 ± 2.4 | 10.1 ± 2.9 | 11.7 ± 2.0 | 12.5 ± 1.5 | 11.6* | 0.0 | 3.0 |
| Short Categories | 34.0 ± 13.3 | 39.0 ± 17.9 | 38.8 ± 15.3 | 37.4 ± 16.9 | 0.3 | 0.3 | 1.0 |
| Stroop-Interference | 29.5 ± 8.0 | 34.4 ± 7.8 | 33.8 ± 10.4 | 34.3 ± 6.1 | 1.5 | 2.4 | 1.5 |
| Trails B | 81.6 ± 28.1 | 87.4 ± 27.7 | 84.8 ± 26.2 | 88.3 ± 23.6 | 0.1 | 0.7 | 0.0 |
| Attention | |||||||
| MicroCog Alphabet | 12.1 ± 0.2 | 10.3 ± 3.7 | 11.8 ± 0.5 | 10.6 ± 3.3 | 0.0 | 11.6* | 0.3 |
| MicroCog Numbers Forward | 11.9 ± 3.5 | 10.4 ± 2.3 | 8.9 ± 3.8 | 9.4 ± 4.8 | 6.7 | 0.3 | 1.8 |
| MicroCog Numbers Reversed | 10.7 ± 2.9 | 10.7 ± 4.7 | 9.2 ± 3.3 | 9.9 ± 4.6 | 2.3 | 0.2 | 0.3 |
| MicroCog Wordlist 1 | 11.3 ± 1.8 | 11.1 ± 2.8 | 11.0 ± 3.0 | 11.8 ± 2.1 | 0.1 | 0.4 | 0.9 |
| Stroop-Color | 61.7 ± 8.1 | 69.8 ± 7.5 | 59.6 ± 12.2 | 61.6 ± 8.9 | 6.4 | 6.1 | 2.4 |
| Auditory Working Memory | |||||||
| PASAT 2.4 (seconds delay) | 37.8 ± 13.1 | 37.3 ± 11.1 | 38.9 ± 10.9 | 34.1 ± 10.5 | 0.2 | 1.2 | 0.8 |
| PASAT 2.0 (seconds delay) | 32.1 ± 9.0 | 35.7 ± 12.9 | 34.3 ± 9.7 | 30.6 ± 8.2 | 0.6 | 0.0 | 3.4 |
| PASAT 1.6 (seconds delay) | 27.1 ± 12.7 | 28.6 ± 15.8 | 30.7 ± 9.5 | 25.8 ± 8.8 | 0.0 | 0.5 | 1.8 |
| PASAT 1.2 (seconds delay) | 17.3 ± 11.6 | 21.0 ± 10.7 | 22.9 ± 7.9 | 20.1 ± 3.7 | 1.3 | 0.0 | 2.5 |
| Immediate Memory | |||||||
| MicroCog Story Recall | 9.1 ± 1.6 | 9.7 ± 1.6 | 9.0 ± 1.3 | 8.4 ± 1.2 | 5.0 | 0.0 | 3.5 |
| MicroCog Wordlist 2 | 14.2 ± 1.9 | 15.0 ± 1.7 | 13.6 ± 2.9 | 13.4 ± 5.1 | 2.9 | 0.3 | 0.7 |
| Rey-Immediate Recall | 43.1 ± 10.4 | 36.4 ± 11.3 | 39.5 ± 12.8 | 30.8 ± 8.3 | 4.1 | 10.5* | 0.2 |
| Delayed Memory | |||||||
| MicroCog Story-Delayed Recall | 12.4 ± 3.5 | 12.7 ± 5.1 | 10.9 ± 3.3 | 11.1 ± 4.2 | 3.5 | 0.1 | 0.0 |
| Rey-Delayed Recall | 43.9 ± 10.4 | 33.9 ± 12.1 | 39.9 ± 12.8 | 32.9 ± 9.4 | 1.2 | 12.2** | 0.4 |
| Psychomotor | |||||||
| Symbol Digit Modalities | 43.9 ± 7.1 | 43.2 ± 8.4 | 43.6 ± 6.3 | 40.2 ± 10.2 | 1.1 | 1.7 | 0.8 |
| Trails A | 37.7 ± 11.7 | 42.2 ± 9.7 | 37.4 ± 12.5 | 48.1 ± 21.2 | 1.0 | 7.0 | 1.3 |
| Reaction Time | |||||||
| MicroCog Cued Timers | 12.6 ± 1.0 | 11.9 ± 1.2 | 11.2 ± 1.9 | 11.8 ± 1.3 | 6.1 | 0.0 | 5.3 |
| MicroCog Simple Timers | 11.7 ± 1.6 | 11.7 ± 0.5 | 11.2 ± 1.4 | 10.3 ± 2.3 | 8.9* | 1.7 | 1.7 |
| Spatial Processing | |||||||
| Block Design | 35.8 ± 7.9 | 34.9 ± 11.4 | 38.5 ± 9.2 | 29.1 ± 7.0 | 0.8 | 7.6* | 5.2 |
| MicroCog Clocks | 12.9 ± 1.2 | 13.0 ± 1.3 | 12.6 ± 1.5 | 12.3 ± 2.6 | 2.0 | 0.1 | 0.3 |
| MicroCog Tic Tac | 9.1 ± 2.7 | 8.1 ± 1.8 | 9.0 ± 2.0 | 8.1 ± 1.8 | 0.0 | 4.0 | 0.0 |
| Verbal | |||||||
| AMNART | 36.3 ± 4.6 | 35.7 ± 5.6 | 33.7 ± 7.5 | 32.8 ± 6.5 | 4.6 | 0.4 | 0.0 |
| COWAT | 38.9 ± 9.0 | 39.8 ± 13.4 | 37.9 ± 9.0 | 38.2 ± 4.5 | 0.4 | 0.1 | 0.0 |
Measures are reported mean ± standard deviation. Effect is significant:
= p ≤ 0.05;
= p ≤ 0.01;
Correlations between Demographic data, Neuropsychological Tests, and Alcohol Use Variables
Spearman’s correlational analyses revealed that delayed memory was the only neuropsychological domain that was associated with age in both the abstinent alcoholic and control groups (r = 0.28, p = 0.001). Years of education were positively associated with auditory working memory (r = 0.23, p = 0.008), verbal ability (r = 0.22, p = 0.008), abstraction/cognitive flexibility (r = 0.22, p = 0.009), as well as the average z-score (r = 0.19, p < 0.03). Within the EAA samples, alcohol lifetime duration was the only alcohol use variable associated with the neuropsychological measures, specifically, attention (r = 0.23, p < 0.03), abstraction/cognitive flexibility (r = 0.24, p < 0.03), delayed memory (r = 0.25, p < 0.02), reaction time (r = 0.21, p < 0.05), spatial processing (r = 0.41, p < 0.001), and the overall average z-score (r = 0.26, p < 0.02).
Discussion
This study examined cognitive function in three groups of elderly abstinent alcoholics; those who attained abstinence before the age of 50 (EAA1), between the ages of 50 and 60 (EAA2), or after the age of 60 (EAA3), all compared to age and gender comparable light/non-drinking controls. The controls and the abstinent alcoholics all had similar AMNART scores (a measure of pre-morbid intelligence), indicating that any findings were not a function of group differences in pre-morbid intellectual abilities. The only abstinent alcoholic group to perform significantly worse than their control sample was the EAA1 group, and this was only on the assessment of auditory working memory. However, given that the EAA1 group had significantly fewer years of education than their controls, and that auditory working memory was associated with years of education (r = 0.23, p = 0.008), this finding should be interpreted with caution.
Somewhat surprisingly, the abstinent alcoholics from the EAA2 and EAA3 group performed better than controls on a number of domains; however, those comparisons were uncorrected for multiple comparisons. The EAA2 group performed better than controls on the assessments of attention, verbal ability, and the EAA3 group performed better than controls on the immediate memory, delayed memory, and reaction time assessments.
One possible explanation for these findings, as well as the findings regarding the association between alcohol lifetime duration and some of the neuropsychological measures, is selective survivorship. Heavy alcohol consumption has been shown to negatively impact life expectancy both directly and indirectly (Goldacre et al., 2004; Jarque-Lopez et al., 2001; McDonnell and Maynard, 1985; Ojesjo et al., 1998; Poldrugo et al., 1993; Rehm et al., 2006; Sher, 2005; Wojtyniak et al., 2005). Furthermore the CDC reported that in 2001, there were approximately 75,000 deaths attributable to either excessive or risky drinking in the U.S., making alcohol the third leading actual cause of death (Centers for Disease Control, 2004). Given the negative impact of alcoholism on life expectancy, selective survivorship increases the likelihood that cognitively healthier alcoholics are more likely to survive into their sixties, seventies, or eighties.
Another interesting result was the alcohol use differences seen between the abstinent alcoholics groups. The individuals who attained abstinence before the age of 50 met criteria for heavy drinking at a younger age than either the EAA2 or EAA3 group (25.3 years old vs. 29.0 years old and 34.5 years old respectively), and on average drank more than the EAA3 group (169 drinks/months vs. 126 drinks/month). Furthermore, the EAA1 group had significantly higher proportions of relatives who were “problem drinkers” than either the EAA2 or EAA3 group. The combination of a later onset of heavy drinking and a relatively low family history density for alcoholism indicates that the EAA2 and EAA3 groups are late-onset alcoholics, in comparison to the EAA1 group, which has the characteristics of early-onset alcoholics. Interestingly, the alcohol use and family history of the EAA1 participants are highly similar to the participants from our recently published study on 35–55 year old long-term abstinent alcoholics (Fein et al., 2006). Despite these differences in drinking history among the EAA groups, all of the EAA groups had their first alcoholic drink at a similar age, and had comparable peak alcohol use (# of drinks during peak use periods, durations of peak use, and peak use drinking dose).
Our results clearly show that it is possible for elderly alcoholics with long-term abstinence to attain essentially normal cognitive functioning, even if they have drank during their fifties or sixties. These findings argue against the hypothesis that aging brain is more vulnerable to the effects of alcohol. However, we note that it’s also possible that the aging brain is indeed more vulnerable, but that cognitive deficits resulting from chronic alcohol abuse tend to resolve with significant abstinence. These results combined with the findings from our previous paper (Fein et al., 2006) strongly indicate that many of the cognitive deficits associated with alcoholism can resolve with long-term abstinence. These results are incredibly encouraging, especially given the wealth of literature citing the deleterious effects of alcohol abuse on cognition. Furthermore, they indicate that even cognitive function in the aging brain has the capacity to rebound and recover from the harmful effects of alcohol abuse.
Acknowledgments
This work was supported by Grants AA11311 (GF) and AA13659 (GF), both from the National Institute of Alcoholism and Alcohol Abuse.
References
- Bates ME, Voelbel GT, Buckman JF, Labouvie EW, Barry D. Short-term neuropsychological recovery in clients with substance use disorders. Alcohol Clin Exp Res. 2005;29(3):367–377. doi: 10.1097/01.alc.0000156131.88125.2a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50(1–3):7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K. Multilingual aphasia examination. Iowa City: AJA Associates; 1983. [Google Scholar]
- Block RI, Erwin WJ, Ghoneim MM. Chronic drug use and cognitive impairments. Pharmacol Biochem Behav. 2002;73(3):491–504. doi: 10.1016/s0091-3057(02)00816-x. [DOI] [PubMed] [Google Scholar]
- Brun A, Andersson J. Frontal dysfunction and frontal cortical synapse loss in alcoholism--the main cause of alcohol dementia? Dement Geriatr Cogn Disord. 2001;12(4):289–294. doi: 10.1159/000051271. [DOI] [PubMed] [Google Scholar]
- Butterworth RF. Pathophysiology of alcoholic brain damage: synergistic effects of ethanol, thiamine deficiency and alcoholic liver disease. Metab Brain Dis. 1995;10(1):1–8. doi: 10.1007/BF01991777. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. Alcohol-attributal deaths and years of potential life lost--United States, 2001. MMWR Morb Mortal Wkly Rep. 2004;53(37):866–870. [PubMed] [Google Scholar]
- Denman SB. Denman Neuropsychology Memory Scale. Charleston, SC: SB Denman; 1987. [Google Scholar]
- Di Sclafani V, Ezekiel F, Meyerhoff DJ, MacKay S, Dillon WP, Weiner MW, Fein G. Brain atrophy and cognitive function in older abstinent alcoholic men. Alcohol Clin Exp Res. 1995;19(5):1121–1126. doi: 10.1111/j.1530-0277.1995.tb01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond I, Messing RO. Neurologic effects of alcoholism. West J Med. 1994;161(3):279–287. [PMC free article] [PubMed] [Google Scholar]
- Emsley R, Smith R, Roberts M, Kapnias S, Pieters H, Maritz S. Magnetic resonance imaging in alcoholic Korsakoff’s syndrome: evidence for an association with alcoholic dementia. Alcohol Alcohol. 1996;31(5):479–486. doi: 10.1093/oxfordjournals.alcalc.a008182. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and age. Alcohol Clin Exp Res. 2004;28(11):1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152(5):531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006;30(9):1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson RE, Hurt RD, Davis LJ, Jr, Morse RM. Alcoholism in elderly persons: a study of the psychiatric and psychosocial features of 216 inpatients. Mayo Clin Proc. 1988;63(8):761–768. doi: 10.1016/s0025-6196(12)62355-6. [DOI] [PubMed] [Google Scholar]
- Fregly AR, Smith MJ, Graybiel A. Revised normative standards of performance of men on a quantitative ataxia test battery. Acta Otolaryngol. 1973;75(1):10–16. doi: 10.3109/00016487309139631. [DOI] [PubMed] [Google Scholar]
- Goldacre MJ, Duncan M, Griffith M, Cook-Mozaffari P. Alcohol as a certified cause of death in a 'middle England' population 1979–1999: database study. J Public Health (Oxf) 2004;26(4):343–346. doi: 10.1093/pubmed/fdh183. [DOI] [PubMed] [Google Scholar]
- Golden CJ. Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Los Angeles, CA: Western Psychological Services; 1978. [Google Scholar]
- Goldman MS, Williams DL, Klisz DK. Recoverability of psychological functioning following alcohol abuse: prolonged visual-spatial dysfunction in older alcoholics. J Consult Clin Psychol. 1983;51(3):370–378. doi: 10.1037//0022-006x.51.3.370. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. J Clin Exp Neuropsychol. 1991;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Gronwall DM. Paced auditory serial-addition task: a measure of recovery from concussion. Percept Mot Skills. 1977;44(2):367–373. doi: 10.2466/pms.1977.44.2.367. [DOI] [PubMed] [Google Scholar]
- Harper C, Matsumoto I. Ethanol and brain damage. Curr Opin Pharmacol. 2005;5(1):73–78. doi: 10.1016/j.coph.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Heap LC, Pratt OE, Ward RJ, Waller S, Thomson AD, Shaw GK, Peters TJ. Individual susceptibility to Wernicke-Korsakoff syndrome and alcoholism-induced cognitive deficit: impaired thiamine utilization found in alcoholics and alcohol abusers. Psychiatr Genet. 2002;12(4):217–224. doi: 10.1097/00041444-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Maththews CG. Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, FL: Psychological Assessment Resources, Inc; 1991. [Google Scholar]
- Jarque-Lopez A, Gonzalez-Reimers E, Rodriguez-Moreno F, Santolaria-Fernandez F, Lopez-Lirola A, Ros-Vilamajo R, Espinosa-Villarreal JG, Martinez-Riera A. Prevalence and mortality of heavy drinkers in a general medical hospital unit. Alcohol Alcohol. 2001;36(4):335–338. doi: 10.1093/alcalc/36.4.335. [DOI] [PubMed] [Google Scholar]
- Kim JM, Shin IS, Stewart R, Yoon JS. Alcoholism in older Korean men: prevalence, aetiology, and comorbidity with cognitive impairment and dementia in urban and rural communities. Int J Geriatr Psychiatry. 2002;17(9):821–827. doi: 10.1002/gps.687. [DOI] [PubMed] [Google Scholar]
- Korsakoff S. Disturbance of psychic activity in alcoholic paralysis. Vestn Klin Psichiat Neurol. 1887;4:1–102. [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug Alcohol Depend. 1985;15(1–2):61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- McDonnell R, Maynard A. Estimation of life years lost from alcohol-related premature death. Alcohol Alcohol. 1985;20(4):435–443. [PubMed] [Google Scholar]
- McMurtray A, Clark DG, Christine D, Mendez MF. Early-onset dementia: frequency and causes compared to late-onset dementia. Dement Geriatr Cogn Disord. 2006;21(2):59–64. doi: 10.1159/000089546. [DOI] [PubMed] [Google Scholar]
- Mochizuki H, Masaki T, Matsushita S, Ugawa Y, Kamakura K, Arai H, Motoyoshi K, Higuchi S. Cognitive impairment and diffuse white matter atrophy in alcoholics. Clin Neurophysiol. 2005;116(1):223–228. doi: 10.1016/j.clinph.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Moriyama Y, Muramatsu T, Kato M, Mimura M, Kashima H. Family history of alcoholism and cognitive recovery in subacute withdrawal. Psychiatry Clin Neurosci. 2006;60(1):85–89. doi: 10.1111/j.1440-1819.2006.01464.x. [DOI] [PubMed] [Google Scholar]
- Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcohol Clin Exp Res. 2000;24(10):1510–1516. [PubMed] [Google Scholar]
- Ojesjo L, Hagnell O, Otterbeck L. Mortality in alcoholism among men in the Lundby Community Cohort, Sweden: a forty-year follow-up. J Stud Alcohol. 1998;59(2):140–145. doi: 10.15288/jsa.1998.59.140. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Kirkley SM, Gansler DA, Couture A. Comparisons of Korsakoff and non-Korsakoff alcoholics on neuropsychological tests of prefrontal brain functioning. Alcohol Clin Exp Res. 2004;28(4):667–675. doi: 10.1097/01.alc.0000122761.09179.b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieth PA. Le test de copie d'une figure complex. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- Poldrugo F, Chick JD, Moore N, Walburg JA. Mortality studies in the long-term evaluation of treatment of alcoholics. Alcohol Alcohol. 1993 Suppl 2:151–155. [PubMed] [Google Scholar]
- Powell DH, Kaplan EF, Whitla D, Weinstraub S, Catlin R, Funkenstein HH. MicroCog Assessment of Cognitive Functioning. San Antonio, TX: The Psychological Corporation; 1993. [Google Scholar]
- Ratti MT, Soragna D, Sibilla L, Giardini A, Albergati A, Savoldi F, Bo P. Cognitive impairment and cerebral atrophy in "heavy drinkers". Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(2):243–258. doi: 10.1016/s0278-5846(98)00103-1. [DOI] [PubMed] [Google Scholar]
- Rehm J, Patra J, Popova S. Alcohol-attributable mortality and potential years of life lost in Canada 2001: implications for prevention and policy. Addiction. 2006;101(3):373–384. doi: 10.1111/j.1360-0443.2005.01338.x. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- Robins LN, Cottler L, Buckholz K, Compton W. The Diagnostic Interview Schedule for DSM-IV. St. Louis, MO: Washington University School of Medicine; 1998. [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18(3):589–597. doi: 10.1037/0894-4105.18.3.589. [DOI] [PubMed] [Google Scholar]
- Ruff RM, Light RH, Parker SB, Levin HS. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- Saxton J, Munro CA, Butters MA, Schramke C, McNeil MA. Alcohol, dementia, and Alzheimer's disease: comparison of neuropsychological profiles. J Geriatr Psychiatry Neurol. 2000;13(3):141–149. doi: 10.1177/089198870001300308. [DOI] [PubMed] [Google Scholar]
- Schmidt KS, Gallo JL, Ferri C, Giovannetti T, Sestito N, Libon DJ, Schmidt PS. The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord. 2005;20(5):286–291. doi: 10.1159/000088306. [DOI] [PubMed] [Google Scholar]
- Schwartz M, Saffran E. The American-NART: Replication and extension of the British findings on the persistence of the word pronunciation skills in patients with dementia. Philadelphia, PA: 1987. [Google Scholar]
- Sher L. Alcohol use and suicide rates. Med Hypotheses. 2005;65(6):1010–1012. doi: 10.1016/j.mehy.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psychol. 1982;91(3):199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. J Stud Alcohol. 1982;43(11):1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith A. The symbol digit modalities test: A neuropsychological test of learning and other cerebral disorders. In: Helmuth J, editor. Learning Disorders. Seattle, WA: Special Child Publications; 1968. pp. 83–91. [Google Scholar]
- Smith A. Symbol Digit Modalities Test. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- Smith DM, Atkinson RM. Alcoholism and dementia. Int J Addict. 1995;30(13–14):1843–1869. doi: 10.3109/10826089509071058. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Self-reports issues in alcohol abuse: State of the art and future directions. Behaviorial Assessment. 1990;12:77–90. [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers' self-reports of drinking and life events that occurred in the distant past. J Stud Alcohol. 1988;49(3):225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- SPSS Inc. SPSS 13.0 for Windows. Chicago IL: SPSS Inc; 2004. 13.0 ed. [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93(10):1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Stethem LL, Pelchat G. Three tests of attention and rapid information processing: an extension. The Clinical Neuropsychologist. 1988;2:246–250. [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16(1):74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology. 2000a;14(2):178–188. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000b;24(5):611–621. [PubMed] [Google Scholar]
- Tedstone D, Coyle K. Cognitive impairments in sober alcoholics: performance on selective and divided attention tasks. Drug Alcohol Depend. 2004;75(3):277–286. doi: 10.1016/j.drugalcdep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Victor M. Alcoholic dementia. Can J Neurol Sci. 1994;21(2):88–99. doi: 10.1017/s031716710004899x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale -- Third Edition: Administration and Scoring Manual. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wernicke C. Lehrbuch der Gehirnkrankheiten fur Aerzte und Studirende. Vol. 2. Kassel U. Berlin: Theodor Fisher; 1881. pp. 229–242. [Google Scholar]
- Wetzel L, Boll T. Short Category Test, Booklet Format. Los Angeles, CA: Western Psychological Services; 1987. [Google Scholar]
- Wojtyniak B, Moskalewicz J, Stokwiszewski J, Rabczenko D. Gender-specific mortality associated with alcohol consumption in Poland in transition. Addiction. 2005;100(12):1779–1789. doi: 10.1111/j.1360-0443.2005.01247.x. [DOI] [PubMed] [Google Scholar]
- Zinn S, Stein R, Swartzwelder HS. Executive functioning early in abstinence from alcohol. Alcohol Clin Exp Res. 2004;28(9):1338–1346. doi: 10.1097/01.alc.0000139814.81811.62. [DOI] [PubMed] [Google Scholar]