Abstract
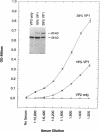
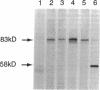
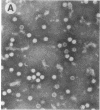
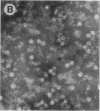
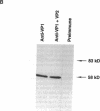
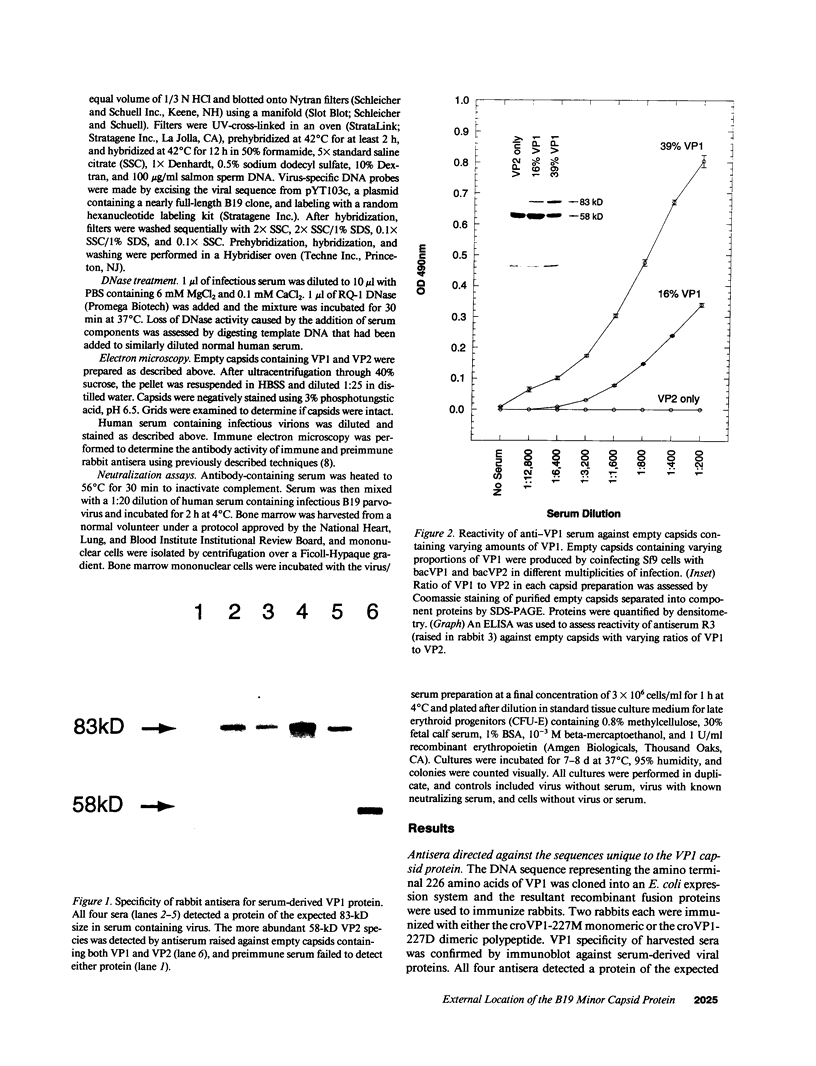
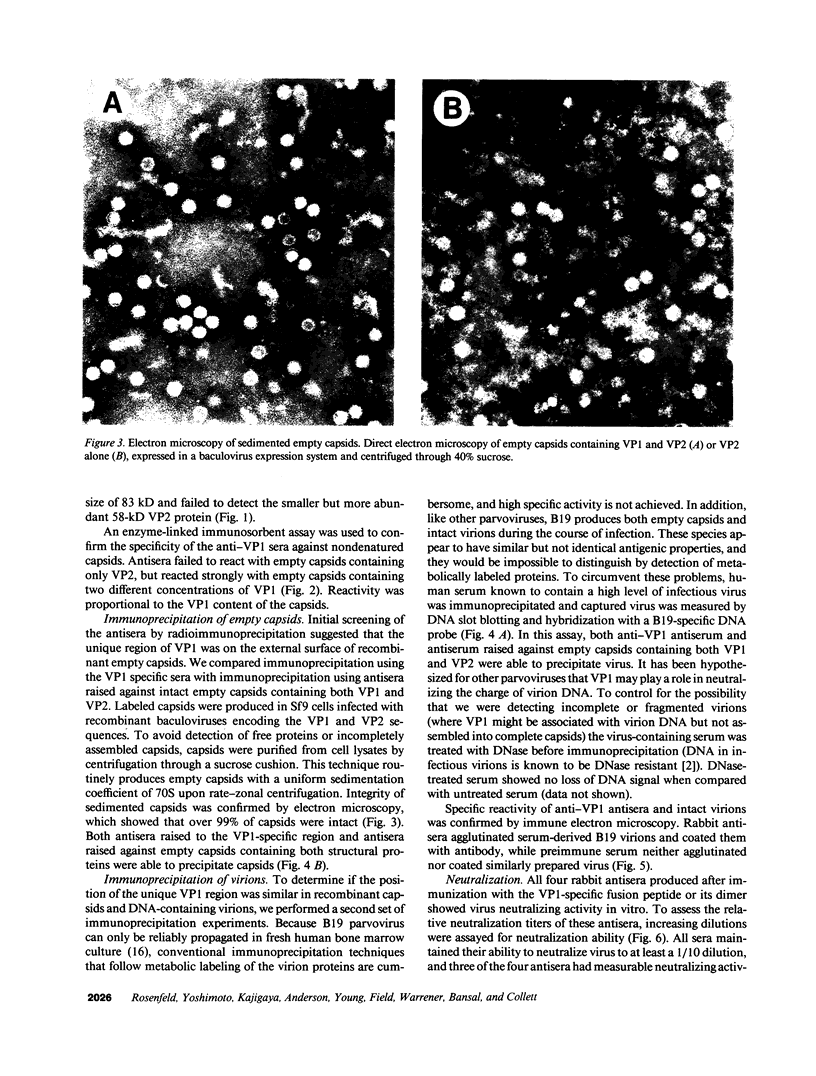
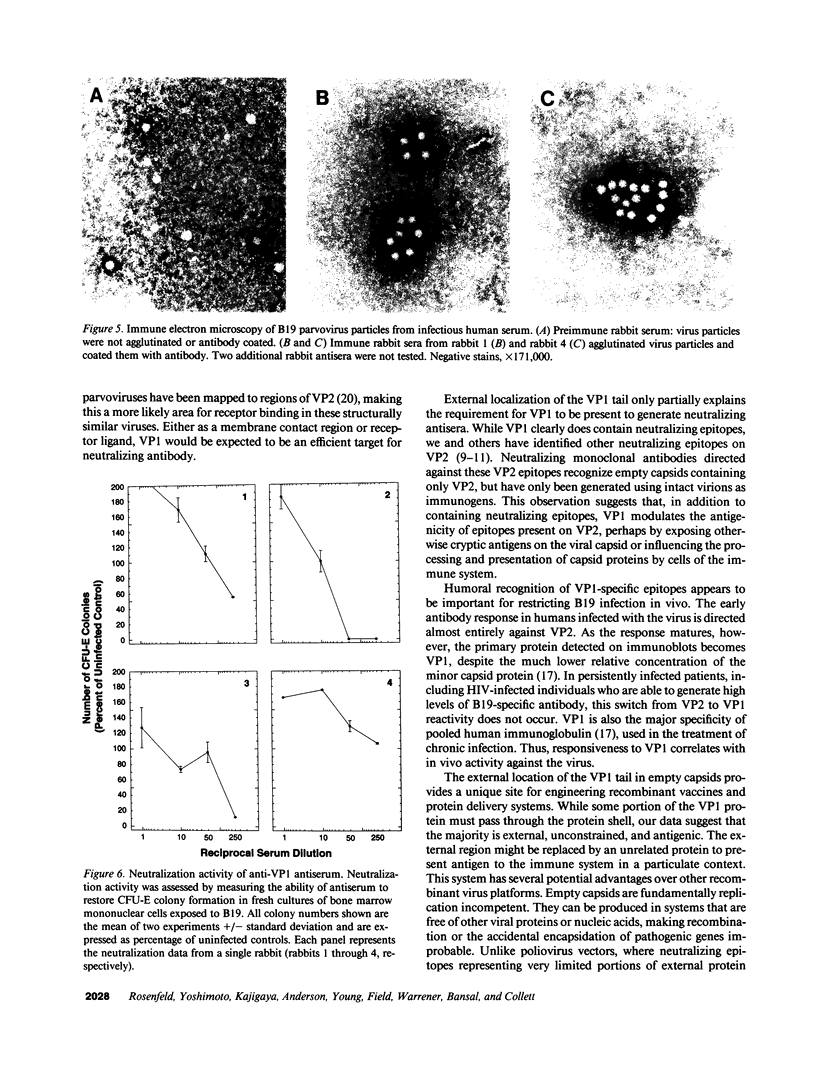
Capsids of the B19 parvovirus are composed of major (VP2; 58 kD) and minor (VP1; 83 kD) structural proteins. These proteins are identical except for a unique 226 amino acid region at the amino terminus of VP1. Previous immunization studies with recombinant empty capsids have demonstrated that the presence of VP1 was required to elicit virus-neutralizing antibody activity. However, to date, neutralizing epitopes have been identified only on VP2. Crystallographic studies of a related parvovirus (canine parvovirus) suggested the unique amino-terminal portion of VP1 assumed an internal position within the viral capsid. To determine the position of VP1 in both empty capsids and virions, we expressed a fusion protein containing the unique region of VP1. Antisera raised to this protein recognized recombinant empty capsids containing VP1 and VP2, but not those containing VP2 alone, in an enzyme-linked immunosorbent assay. The antisera immunoprecipitated both recombinant empty capsids and human plasma-derived virions, and agglutinated the latter as shown by immune electron microscopy. The sera contained potent neutralizing activity for virus infectivity in vitro. These data indicate that a portion of the amino terminus of VP1 is located on the virion surface, and that this region contains intrinsic neutralizing determinants. The external location of the VP1-specific tail may provide a site for engineered heterologous epitope presentation in novel recombinant vaccines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. J., Hurwitz E. S. Human parvovirus B19 and pregnancy. Clin Perinatol. 1988 Jun;15(2):273–286. [PubMed] [Google Scholar]
- Burke K. L., Evans D. J., Jenkins O., Meredith J., D'Souza E. D., Almond J. W. A cassette vector for the construction of antigen chimaeras of poliovirus. J Gen Virol. 1989 Sep;70(Pt 9):2475–2479. doi: 10.1099/0022-1317-70-9-2475. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Larson R., Belzer S. K., Retzel E. Proteins encoded by bovine viral diarrhea virus: the genomic organization of a pestivirus. Virology. 1988 Jul;165(1):200–208. doi: 10.1016/0042-6822(88)90673-3. [DOI] [PubMed] [Google Scholar]
- Evans D. J., McKeating J., Meredith J. M., Burke K. L., Katrak K., John A., Ferguson M., Minor P. D., Weiss R. A., Almond J. W. An engineered poliovirus chimaera elicits broadly reactive HIV-1 neutralizing antibodies. Nature. 1989 Jun 1;339(6223):385-8, 340. doi: 10.1038/339385a0. [DOI] [PubMed] [Google Scholar]
- Frickhofen N., Abkowitz J. L., Safford M., Berry J. M., Antunez-de-Mayolo J., Astrow A., Cohen R., Halperin I., King L., Mintzer D. Persistent B19 parvovirus infection in patients infected with human immunodeficiency virus type 1 (HIV-1): a treatable cause of anemia in AIDS. Ann Intern Med. 1990 Dec 15;113(12):926–933. doi: 10.7326/0003-4819-113-12-926. [DOI] [PubMed] [Google Scholar]
- Fricks C. E., Hogle J. M. Cell-induced conformational change in poliovirus: externalization of the amino terminus of VP1 is responsible for liposome binding. J Virol. 1990 May;64(5):1934–1945. doi: 10.1128/jvi.64.5.1934-1945.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajigaya S., Fujii H., Field A., Anderson S., Rosenfeld S., Anderson L. J., Shimada T., Young N. S. Self-assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4646–4650. doi: 10.1073/pnas.88.11.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan K., Collett M. S. Use of bacterial expression cloning to define the amino acid sequences of antigenic determinants on the G2 glycoprotein of Rift Valley fever virus. J Virol. 1986 May;58(2):263–270. doi: 10.1128/jvi.58.2.263-270.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman G. J., Cohen B. J., Field A. M., Oseas R., Blaese R. M., Young N. S. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J Clin Invest. 1989 Oct;84(4):1114–1123. doi: 10.1172/JCI114274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Productive infection by B19 parvovirus of human erythroid bone marrow cells in vitro. Blood. 1987 Aug;70(2):384–391. [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science. 1986 Aug 22;233(4766):883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- Parrish C. R. Mapping specific functions in the capsid structure of canine parvovirus and feline panleukopenia virus using infectious plasmid clones. Virology. 1991 Jul;183(1):195–205. doi: 10.1016/0042-6822(91)90132-u. [DOI] [PubMed] [Google Scholar]
- Rayment F. B., Crosdale E., Morris D. J., Pattison J. R., Talbot P., Clare J. J. The production of human parvovirus capsid proteins in Escherichia coli and their potential as diagnostic antigens. J Gen Virol. 1990 Nov;71(Pt 11):2665–2672. doi: 10.1099/0022-1317-71-11-2665. [DOI] [PubMed] [Google Scholar]
- Sato H., Hirata J., Furukawa M., Kuroda N., Shiraki H., Maeda Y., Okochi K. Identification of the region including the epitope for a monoclonal antibody which can neutralize human parvovirus B19. J Virol. 1991 Apr;65(4):1667–1672. doi: 10.1128/jvi.65.4.1667-1672.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Hirata J., Kuroda N., Shiraki H., Maeda Y., Okochi K. Identification and mapping of neutralizing epitopes of human parvovirus B19 by using human antibodies. J Virol. 1991 Oct;65(10):5485–5490. doi: 10.1128/jvi.65.10.5485-5490.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverdlov E. D., Tsarev S. A., Markova S. V., Rostapshov V. M., Azhikina T. L., Chernov I. P., Gorbalenya A. E., Kolesnikova M. S., Romanova L. I., Teterina N. L. Insertion of short hepatitis virus A amino acid sequences into poliovirus antigenic determinants results in viable progeny. FEBS Lett. 1989 Nov 6;257(2):354–356. doi: 10.1016/0014-5793(89)81570-4. [DOI] [PubMed] [Google Scholar]
- Tsao J., Chapman M. S., Agbandje M., Keller W., Smith K., Wu H., Luo M., Smith T. J., Rossmann M. G., Compans R. W. The three-dimensional structure of canine parvovirus and its functional implications. Science. 1991 Mar 22;251(5000):1456–1464. doi: 10.1126/science.2006420. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K., Rosenfeld S., Frickhofen N., Kennedy D., Hills R., Kajigaya S., Young N. S. A second neutralizing epitope of B19 parvovirus implicates the spike region in the immune response. J Virol. 1991 Dec;65(12):7056–7060. doi: 10.1128/jvi.65.12.7056-7060.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N. Hematologic and hematopoietic consequences of B19 parvovirus infection. Semin Hematol. 1988 Apr;25(2):159–172. [PubMed] [Google Scholar]