Abstract
Objective
Loss of endothelium contributes to vein graft thrombosis and neointimal hyperplasia. We therefore sought to accelerate vein graft endothelialization and reduce vein graft thrombosis with human umbilical cord blood-derived endothelial cells (hCB-ECs).
Methods and Results
Under steady flow conditions in vitro, hCB-ECs adhered to smooth muscle cells (SMCs) 2.5–13 times more than ECs derived from peripheral blood (PB-ECs) or human aorta (HAECs) (p < 0.05). Compared with PB-ECs and HAECs, hCB-ECs had 1.4-fold more cell surface α5β1 integrin heterodimers/cell (p<0.05), and proliferated on fibronectin 4–10-fold more rapidly (p < 0.05). Therefore, we used hCB-ECs to enhance re-endothelialization of carotid interposition vein grafts implanted in NOD.CB17-Prkdcscid/J mice. Two weeks postoperatively, vein grafts from hCB-EC-treated mice demonstrated ~55% re-endothelialization and no luminal thrombosis. In contrast, vein grafts from sham-treated mice demonstrated luminal thrombosis in 75% of specimens (p < 0.05), and only ~14% re-endothelialization. In vein grafts from hCB-EC-treated mice, 33 ± 10% of the endothelium was of human origin, as judged by human MHC I expression.
Conclusions
The hCB-ECs adhere to SMCs under flow conditions in vitro, accelerate vein graft re-endothelialization in vivo, and prevent vein graft thrombosis. Thus, hCB-ECs offer novel therapeutic possibilities for vein graft disease.
Introduction
Saphenous vein graft failure from thrombosis occurs with remarkable frequency: ~3–12% within the first postoperative month1 and 28% within the first postoperative year.2 Vein graft thrombosis is generally attributed to alterations in the vessel wall and flow dynamics, vein graft endothelial damage associated with both vein harvest and vein graft distension by arterial pressure, and consequent compromise of anticoagulant vessel properties.1 Among these factors, the most practical target for therapy appears to be maintenance or restoration of the vein graft endothelium.
Re-endothelialization of vein grafts is a relatively slow process that involves proliferation and migration of both graft-intrinsic endothelial cells (ECs) and graft-extrinsic ECs,3 which may derive from adjacent arterial endothelium or from circulating endothelial progenitor cells (EPCs).4,5 Consequently, accelerating EC growth or increasing EC number with exogenous ECs may accelerate vein graft re-endothelialization. Exogenous ECs derived from peripheral and umbilical cord blood EPCs are relatively easy to obtain and possess substantial replicative capacity.6
Most studies that have examined adhesion of ECs to damaged vasculature have used ECs obtained from early-outgrowth cultures of EPCs, which have been found to be of a myeloid lineage and do not exhibit the anti-thrombotic properties of ECs.7–9 Few vascular repair experiments have examined ECs derived from late-outgrowth cultures of EPCs isolated from adult or umbilical cord blood. These late-outgrowth EPCs, or endothelial colony forming cells, exhibit characteristics only of ECs and not of monocytes.10,11 ECs obtained from late-outgrowth cultures of peripheral blood EPCs (PB-ECs) from healthy donors and patients with coronary artery disease are very similar to aortic ECs with regard to proliferation, viability, adhesion, and anti-thrombotic function.12 However, the clinical use of autologous PB-ECs would be limited to elective procedures because of the time required to isolate and expand them in culture. The suitability of PB-ECs for vein graft repair may be further limited because PB-ECs cannot be isolated from a substantial minority of patients with coronary artery disease (CAD).12 This limitation may be circumvented by using ECs derived from late-outgrowth cultures of human umbilical cord blood EPCs (hCB-ECs), which have demonstrated a variety of EC properties13 and can be cultured in advance so that sufficient numbers are available for emergency procedures. To facilitate non-autologous transplantation, hCB-ECs can be matched to donors with compatible major histocompatibility class (MHC) I proteins.14,15 As compared with human umbilical cord blood lineage-negative mononuclear cells we previously used to reduce vein graft neointimal hyperplasia,16 hCB-ECs would be expected to exhibit more uniformly EC function and lack monocytic function.
To assess the suitability of hCB-ECs for therapeutic re-endothelialization of vein grafts, we first compared the adhesion, integrin expression and growth of hCB-ECs with ECs derived from late-outgrowth cultures of adult human peripheral blood EPCs and with human aortic ECs in vitro. Subsequently, we used hCB-ECs in immunocompromised mice subjected to vein grafting.
Materials and Methods
Expanded methods are provided in the online Supplemental Material.
Cell Culture and Adhesion Experiments
We isolated and cultured hCB-ECs, PB-ECs, and HAECs as previously described, and characterized them with regard to expression of CD31, KLF-2, eNOS, and COX-2, as well as the lack of CD14 expression.12,13 Smooth muscle cells (SMCs) were prepared as previously described,17 and were plated at 80,000 cells/cm2 with SMC growth media on fibronectin-coated polystyrene. After seeding, SMCs were maintained in serum-free medium for seven days. One day prior to the experiment, the medium was replaced with serum-free medium containing extracellular matrix proteins (fibronectin, collagen I, and collagen III). To test adhesion, hCB-ECs, PB-ECs, or HAECs were superfused over SMCs in a parallel plate flow channel18 at a shear stress of 0.5 dyn/cm2. After 10 min, the number of adherent cells per cm2 was determined. In integrin-blocking studies, ECs were incubated with anti-integrin IgGs for 30 minutes, and then superfused over SMCs for 5 min. The net force imposed on the ECs during the dynamic adhesion experiments was determined by assuming that the ECs were spherical when initially adhered, as described in the online Expanded Methods.
Flow Cytometry
The expression of α5β1, αVβ3 and α2β1 integrins present on the plasma membrane of hCB-ECs, PB-ECs, and HAECs was determined by cell surface immunofluorescence and flow cytometry as previously described.13,19
EC Proliferation
ECs were seeded at sub-confluent density (25,000 cells/cm2) and, after 15 minutes of static incubation, were then placed inside a parallel plate flow chamber for 4 days. The number of cells present at days 0, 2, and 4 for static conditions and 0 and 4 days for flow conditions were used to find the approximate doubling time of the cells.
Vein Graft Surgery and hCB-EC Injections
All animal experiments conformed to protocols approved by the Duke Institutional Animal Care and Use Committee. Interposition vein grafting of the common carotid artery was performed as previously described,20 except that NOD.CB17-Prkdcscid/J (“SCID”) mice were used, to avoid immune-mediated rejection of the hCB-EC xenotransplantation. Before infusion into mice, hCB-ECs were trypsinized and rinsed with trypsin-neutralizing solutions. The hCB-ECs were then resuspended in Iscove’s Modified Dulbecco Medium (Invitrogen) at 6.7 × 106 cells per ml; 1 × 106 hCB-ECs (or a corresponding volume of cell-free medium) were injected at the time of surgery—33% directly into the vein graft lumen for 15 min before establishing vein graft blood flow, and 67% intravenously. Four days postoperatively, 1 × 106 hCB-ECs were again injected intravenously. Vein grafts were harvested 2 weeks postoperatively and placed in OCT medium and frozen at −80 °C prior to sectioning.
Histology
Serial vein graft sections were stained for CD31, which stains all ECs, human MHC I, and hematoxylin and eosin by routine methods.21,22 To quantitate vein graft endothelialization and hCB-EC-derived endothelialization, we used NIH ImageJ to measure the vein graft luminal perimeter and the percentage of this perimeter that stained for CD31 alone or CD31 and (on serial sections) human MHC I, respectively (n = 8 for both hCB-EC- and control-treated mice).
Data Analysis
Data are presented as means ± S.D. in the text and ± S.E. in the figures. Proportions were analyzed by Fisher’s exact test. Multiple group means were compared by one-way ANOVA with Tukey’s post-hoc test for multiple comparisons.
Results
hCB-ECs Demonstrate Superior Dynamic Adhesion and Proliferation
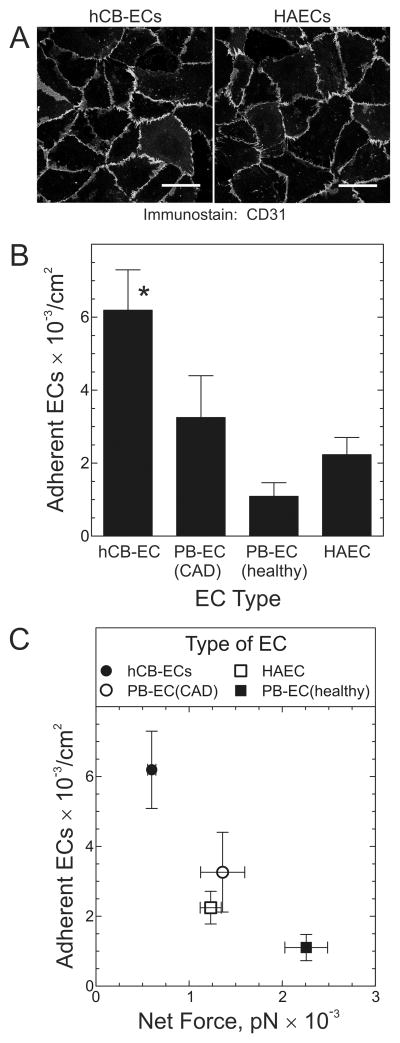
We previously found that hCB-ECs express the characteristic EC proteins vWF, CD31 and VE-cadherin, but not the monocyte marker CD14.13 Furthermore, we found that hCB-ECs responded to flow in a manner characteristic of vascular endothelium: by increasing NO release and up-regulating mRNA for KLF2, eNOS, COX2, and thrombomodulin.13 For the current study, we first compared hCB-ECs, PB-ECs, and HAECs with regard to CD31 and VE-cadherin expression, and found all EC types to be homogeneously equivalent (Figure 1A and data not shown).
Figure 1.
hCB-ECs demonstrate greater adhesion than HAECs or ECs derived from peripheral blood (PB-ECs). A, HAECs and hCB-ECs were immunostained for CD31 and imaged immunofluorescently. Shown are figures representative of ≥7 independent experiments. Scale bar = 50 μm. B, The indicated type of EC was suspended at 500,000 cells/ml and superfused for 10 min over quiescent SMCs to which was adsorbed exogenous fibronectin, collagen I and collagen III to augment adhesion (Supplemental Figure 1). The flow velocity used created shear stress of 0.5 dyn/cm2. The number of adherent ECs are plotted as the mean ± S.E. from 4 independent experiments. Compared with PB-EC(healthy) and HAEC: *, p < 0.05. C, The number of adherent ECs is plotted against the net fluid force acting on ECs exposed to 0.5 dyn/cm2. The net fluid force was calculated as described in Expanded Methods.
Because the goal of this study was to mitigate vein graft disease with exogenous ECs, we sought to test EC adhesion by using a model of the vein graft’s subendothelial surface exposed after the vein is over-distended by arterial pressure, when ECs and their basement membrane no longer cover the entire luminal surface of the vein graft.23 This subendothelial surface of the vein comprises substantially interstitial collagens I and III as well as fibronectin,24,25 in addition to basement membrane constituents like laminin and collagen IV.24 We used cultured, quiescent SMCs to model this subendothelial surface, and found by immunofluorescence that the endogenous SMC matrix includes fibronectin, laminin, and collagens I, III and IV (Supplemental Figure 1 and data not shown). To augment EC adhesion to SMC monolayers, we adsorbed exogenous fibronectin, collagen I, and collagen III to the SMCs (Supplemental Figure 2). Next, we compared dynamic adhesion among hCB-ECs, PB-ECs from healthy subjects, PB-ECs from CAD subjects, and HAECs, at a shear stress of 0.5 dyn/cm2. Following dynamic adhesion for 10 minutes, hCB-ECs adhered to an extent ~3 times greater than HAECs or PB-ECs from healthy adults (PB-ECs(healthy)) (Figure 1B).
One possible explanation for the superior adhesion of hCB-ECs derives from their size: the cross-sectional area of hCB-ECs was significantly smaller than that of other ECs: 239± 10 μm2 for hCB-ECs and 464± 46, 692± 40, and 428± 22 μm2 for PB-ECs(CAD), PB-ECs(healthy), and HAECs, respectively (p < 0.05). Consequently, the force exerted by flowing medium on hCB-ECs was only half that exerted on other ECs (see Equations 2a–2c in Supplemental Materials). Indeed, an inverse relationship between adhesion and net force per cell was observed (Figure 1C).
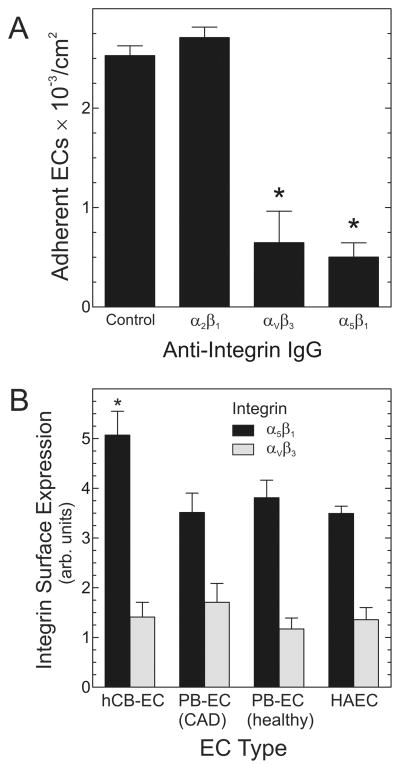
Another potentially important factor in determining EC adhesion under shear stress is EC integrin activity. By using IgG to block integrins α5β1, αVβ3, or α2β1, we found that dynamic adhesion of hCB-ECs to a SMC/matrix substrate was strongly dependent upon the α5β1 and αVβ3 integrins (Figure 2A). Using SMC monolayers in the absence of exogenous matrix proteins, we previously obtained concordant findings: α5β1 and αVβ3 integrins mediated static adhesion of hCB-ECs.13 To determine whether EC type-specific expression levels of α5β1 or/andαVβ3 integrins could explain why hCB-ECs adhere to the SMC/matrix substrate more avidly than other ECs (Figure 1B), we quantitated cell surface integrin expression on all of our EC types by flow cytometry. Cell surface expression of the α5β1 integrin was ~45% higher on hCB-ECs than on PB-ECs or HAECs (Figure 2B), and cell surface expression of αVβ3 was similar on all EC types (Figure 2B). Thus, because all EC types expressed similar αVβ3 integrin levels, the superior adhesion of hCB-ECs seems unlikely to derive from their αVβ3 integrin expression. Rather, the superior adhesion of hCB-ECs may derive in part from higher expression levels of cell surface α5β1 integrins. Of course, these experiments do not exclude the possibilities that superior hCB-EC dynamic adhesion also derives from additional integrins or hCB-EC-specific differences in integrin-promoted signaling.
Figure 2.
Adhesion of hCB-ECs to SMCs depends upon α5β1 and αVβ3 integrins. A, Adhesion assays with hCB-ECs were performed as in Figure 1, except that hCB-ECs were pre-incubated with the indicated control or integrin-blocking IgG’s. Adherent ECs were scored and plotted as in Figure 1, for 4 independent experiments. Compared with control IgG: *, p < 0.01. B, ECs of the indicated type were subjected to cell surface immunofluorescence and flow cytometry to quantitate expression of the indicated integrins. Plotted are the mean ± S.E. from 4 independent experiments. Compared with PB-EC(CAD), PB-EC(healthy), and HAEC: *, p < 0.05.
Adhesion of ECs to de-endothelialized vascular surfaces would optimally be followed by EC proliferation, to facilitate re-endothelialization. Accordingly, we tested the ability of hCB-ECs, PB-ECs and HAECs to proliferate under static and flow conditions on fibronectin-coated plastic. Although EC proliferation declined with shear stress, hCB-ECs demonstrated doubling times that were four to 13.7 times shorter than those of PB-ECs and HAECs under flow conditions (Table).
Table.
EC Proliferation as a Function of Shear Stress
| Shear Stress | EC Doubling Time (days, n ± S.E.) |
||
|---|---|---|---|
| hCB-EC | PB-EC(CAD) | HAEC | |
| None (static) | 1.7 ± 0.2† | 3.0 ± 0.7† | 3.0 ± 0.6† |
| 5 dyn/cm2 | 3.4 ± 1.6* | 15.6 ± 7.8 | 31.8 ± 5.5 |
| 15 dyn/cm2 | 6.0 ± 1.5* | 24.0 ± 9.5* | 82.4 ± 26.0 |
p < 0.01 compared with HAEC or/and PB-EC(CAD).
p < 0.05 compared with ECs exposed to shear stress.
hCB-ECs Re-endothelialize Vein Grafts and Eliminate Thrombosis
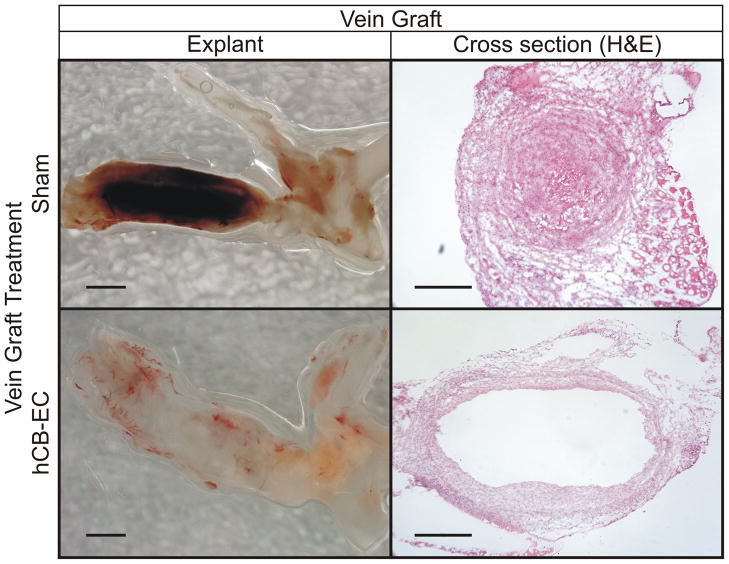
Because hCB-ECs demonstrated adhesion and proliferation that was superior to PB-ECs and HAECs, we tested the ability of hCB-ECs to re-endothelialize vein grafts. For this purpose, we performed interposition vein grafting to the common carotid artery with inferior venae cavae (IVCs) in NOD.CB17-Prkdcscid/J (“SCID”) mice, which we treated with cell-free or hCB-EC (xenograft) infusions. We harvested the vein grafts two weeks post-operatively, at a time when vein graft re-endothelialization is incomplete in C57BL/6 mice.20–22 Unlike C57BL/6 mice,16,20–22 NOD.CB17-Prkdcscid mice demonstrated a remarkable thrombotic diathesis: in control mice infused with just cell-free medium, 6 of 8 vein grafts thrombosed. In contrast, none of the 8 vein grafts implanted in hCB-EC-treated mice demonstrated thrombosis (p = 0.007). Thus, hCB-EC infusion prevented vein graft thrombosis. Representative vein graft explants demonstrate vein graft thrombosis, while cross section photomicrographs demonstrate analogous findings (Figure 3).
Figure 3.
Infusion of hCB-ECs prevents vein graft thrombosis. Mice subjected to vein grafting were treated without (top) or with (bottom) hCB-EC infusion, and vein grafts were harvested 2 weeks post-operatively en bloc with the aortic arch (on the right of each explant panel). Thrombus in the vein graft from a sham-treated mouse (dark red, top left; eosinophilic, top right) was present in 6 of 8 sham-treated mice. The translucency (and patency) of the vein graft from an hCB-EC-treated mouse was found in 8 of 8 hCB-EC-treated mice (scale bars = 200 mm).
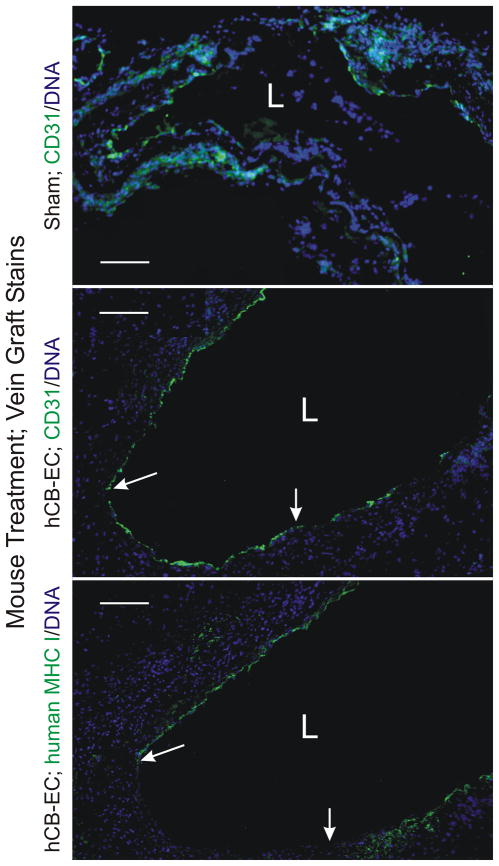
To test the hypothesis that hCB-ECs prevented vein graft thrombosis by accelerating vein graft re-endothelialization, we immunostained vein grafts for the EC marker CD31, using an IgG that recognizes both human and mouse proteins. Judging by the percentage of vein graft luminal surface that stained with CD31, we found endothelialization of vein grafts from hCB-EC-injected mice to be ~4-fold greater than that in control-injected mice with patent vein grafts (Figure 4): 54 ± 6% versus 14 ± 1% of the luminal surface was covered with CD31-staining cells in vein grafts from hCB-EC- and sham-treated mice, respectively.
Figure 4.
Infusion of hCB-ECs enhances vein graft endothelialization. Vein grafts from mice with the indicated treatment were harvested 2 wk post-operatively, and serial cross sections were stained for DNA (Hoechst 33342) and either CD31 (PECAM), with IgG that recognizes both human and mouse CD31 (top two panels), or human (but not mouse) MHC I (bottom panel). The lumen is designated with an “L.” Between the arrows lies intimal surface covered by CD31-positive cells that are negative for human MHC I. Shown are individual vein grafts representative of specimens from eight hCB-EC-treated and 2 sham-treated mice (all other sham-treated mice had thrombosed grafts). Serial sections stained with isotype control IgG yielded no green color (not shown). Scale bars = 100 mm (original magnification × 110).
To determine the contribution of injected hCB-ECs to vein graft re-endothelialization, we calculated the percentage of CD31-positive cells that stained for human MHC I antigen on serial sections of the vein grafts. Vein grafts from mice that were not injected with hCB-ECs demonstrated no staining for human MHC I (data not shown). In contrast, the endothelium in vein grafts from mice that were injected with hCB-ECs did stain for human MHC I, along 33 ± 6% of the endothelium present in the 2-wk-old vein grafts (Figure 4). Thus, injected hCB-ECs engrafted on the vein graft subendothelial surface, and contributed ~33% of the endothelial cells during its re-endothelialization.
Discussion
These studies demonstrate for the first time that hCB-ECs adhere to extracellular matrix or SMCs and proliferate under flow conditions not only in vitro but also in vein grafts, in vivo. Adhesive properties of the EPC-derived hCB-ECs in vitro proved superior to those of several lines of adult EPC-derived ECs and aortic ECs. Furthermore, the adhesion of hCB-ECs to vein grafts in vivo prevented vein graft thrombosis.
The greater adhesion of hCB-ECs compared with adult primary ECs is likely influenced by the higher expression of α5β1 integrins and the smaller relative size of hCB-ECs. The inverse relation between the force and the number of adherent ECs per cm2 (Figure 1C) suggests that the force acting on the cell strongly influences adhesion. EC size affects adhesion in two potentially competing ways. First, larger cells experience a greater fluid force and the net fluid force on the cell increases with the square of the cell radius (cf. equations (2a) – (2c) in Supplementary Methods). Second, the contact area (AC) between the EC and surface increases with EC radius and equals 2πhR, where h is the distance over which adhesion bonds can form and R is the cell radius. The contact area and receptor number are related to the force exerted by the bonds (Fb), which is approximately NRACfb = NR2πhRfb, where NR represents the number of receptors per unit cell surface area and fb is the force per bond.26 Note that the bond force increases linearly with cell radius whereas the fluid force increases with R2. Accounting for the effect of force and contact area on adhesion, increasing cell size should lead to reduced adhesion—as long as receptor expression levels are similar. The relative change in force per cell for two cell types of different sizes is NR2R2/NR1R1. Using Figure 2B to obtain the α5β1 integrin levels and measurements of the cell radius, this ratio between hCB-ECs and the other EC types is close to one for all cell types except for PB-ECs from healthy individuals (0.6 ± 0.2). Thus, the increased contact area for larger cells is offset by the higher receptor expression on hCB-ECs and the net fluid force acting on the cells is the dominant factor influencing adhesion of the different cell types.
Cell size also affects the likely retention of ECs in the microcirculation. The larger ECs derived from peripheral blood EPCs and HAECs are more likely to be limited in their ability to pass through capillaries than the smaller hCB-ECs. However, the hCB-ECs (radius 8.4 μm) are larger than monocytes (radius 6.1 μm) 27 and neutrophils (radius 4.1 μm).28 Thus, ECs injected into the circulation may be trapped predominantly in the microcirculation. Indeed, autologous EPC-derived ECs infused intravenously have been shown to be sequestered in the spleen.29 The most critical steps in promoting EC attachment to the vein graft, therefore, may occur during the static incubation with the vein graft and initial adhesion after injection.
By demonstrating the anti-thrombotic therapeutic efficacy of hCB-EC infusion, our vein graft studies exploited an unusual tendency to thrombosis in NOD.CB17-Prkdcscid/J mice. While thrombosis occurred in ~75% of our IVC carotid interposition isografts in NOD.CB17-Prkdcscid/J mice, thrombosis occurs in only ~2% of our IVC carotid interposition isografts in C57BL/6 mice, without or with the SCID mutation.16,20–22 The mechanisms by which hCB-EC infusion achieves anti-thrombotic efficacy in NOD.CB17-Prkdcscid/J mice remain to be determined. However, based on our current data, it seems likely that these mechanisms include a reduction in the area of subendothelial surface exposed to blood as well as the host of anti-thrombotic properties inherent in hCB-ECs.13 Previous studies have shown that thrombosis is inhibited in vitro by EPC-derived ECs.30,31 Furthermore, thrombosis of de-cellularized iliac arteries is inhibited in vivo when these arteries are pre-seeded in vitro with autologous EPC-derived ECs in a manner that achieves full re-endothelialization.32 Our results are consistent with these studies and demonstrate that infused hCB-ECs adhere to the vein graft luminal surface and inhibit thrombosis—even without complete coverage of the luminal surface. Inhibition of thrombosis in the context of incomplete re-endothelialization may rely in part on prostacyclin secreted by the hCB-ECs.30 Of course, the optimal hCB-EC dose and route of administration for preventing vein graft thrombosis remains to be established.
Adhesion of hBC-ECs to the vein grafts is likely facilitated by the reduction in shear stress attendant to the relatively large diameter of the vein grafts we used. Assuming unilateral common carotid blood flow of 0.28 ml/min33 and a common carotid artery radius of 0.15 mm,34 we can use Equation 3 in the Supplemental Material to infer that normal carotid shear stress would be ~52 dyn/cm2, which is slightly higher than other reports of 35 dyn/cm.35 However, if we assume that overall carotid flow and pressure are not altered by the vein graft, we can infer that shear stress in the vein graft is only ~1.7 dyn/cm2, because the radius of the IVC carotid interposition graft is ~0.47 mm post-operatively.20
Study Limitations
Because it was performed in a highly thrombogenic mouse model, our study may overestimate the true benefit of hCB-EC therapy for vein graft thrombosis in humans. Moreover, from a therapeutic perspective, using hCB-ECs to prevent human vein graft thrombosis would currently require matching donor hCB-ECs and the recipient for MHC antigens.15 When stimulated with interferon-γ or TNF, even late-outgrowth hCB-ECs have been shown to express significant levels of MHC I, MHC II and adhesion molecules, and to stimulate proliferation of allogeneic CD4+ T lymphocytes.15 Moreover, EC tubes formed in SCID mice by hCB-ECs are destroyed when these mice are injected with peripheral blood mononuclear cells that are allogeneic to the hCB-ECs.15 The high proliferative capacity of hCB-ECs14 may facilitate matching these ECs for MHC antigens to subjects undergoing vein grafting.
In a physiologic model of vein grafting,20 our hBC-EC re-endothelialization and anti-thrombotic data accord well with studies that demonstrate enhanced re-endothelialization of injured arteries with administration of PB-ECs.7–9 However, it seems likely that there would be a therapeutic advantage associated with the superior adhesive and proliferative properties of hCB-ECs. It remains to be determined whether in vivo comparisons of hCB-ECs with other EPC-derived ECs will support this hypothesis.
Supplementary Material
Acknowledgments
We would like to thank Mathew Angelos for his assistance with the design of the dynamic adhesion experiments.
Sources of Funding: This work was supported by NIH grants HL-44972 and HL-88825 (G.A.T.), HL077185 and HL073005 (N.J.F.), an American Heart Association Postdocotral Fellowship Grant (0815029E, M.A.B.), and by the Edna and Fred L. Mandel, Jr. Foundation.
Footnotes
Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Motwani JG, Topol EJ. Aortocoronary saphenous vein graft disease: pathogenesis, predisposition, and prevention. Circulation. 1998;97:916–931. doi: 10.1161/01.cir.97.9.916. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, Goyal A, Gibson M, Mack MJ, Gennevois D, Califf RM, Kouchoukos NT. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–2454. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, Freedman NJ, Brian L, Peppel K. Graft-extrinsic cells predominate in vein graft arterialization. Arterioscler Thromb Vasc Biol. 2004;24:470–476. doi: 10.1161/01.ATV.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]
- 4.Thompson MM, Budd JS, Eady SL, Underwood MJ, James RF, Bell PR. The effect of transluminal endothelial seeding on myointimal hyperplasia following angioplasty. Eur J Vasc Surg. 1994;8:423–434. doi: 10.1016/s0950-821x(05)80961-2. [DOI] [PubMed] [Google Scholar]
- 5.Wassmann S, Werner N, Czech T, Nickenig G. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ Res. 2006;99:e74–83. doi: 10.1161/01.RES.0000246095.90247.d4. [DOI] [PubMed] [Google Scholar]
- 6.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 7.Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108:2710–2715. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- 8.Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 9.Nowak G, Karrar A, Holmen C, Nava S, Uzunel M, Hultenby K, Sumitran-Holgersson S. Expression of vascular endothelial growth factor receptor-2 or Tie-2 on peripheral blood cells defines functionally competent cell populations capable of re-endothelialization. Circulation. 2004;110:3699–3707. doi: 10.1161/01.CIR.0000143626.16576.51. [DOI] [PubMed] [Google Scholar]
- 10.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gulati R, Jevremovic D, Peterson TE, Witt TA, Kleppe LS, Mueske CS, Lerman A, Vile RG, Simari RD. Autologous culture-modified mononuclear cells confer vascular protection after arterial injury. Circulation. 2003;108:1520–1526. doi: 10.1161/01.CIR.0000089084.48655.49. [DOI] [PubMed] [Google Scholar]
- 12.Stroncek JD, Grant BS, Brown MA, Povsic TJ, Truskey GA, Reichert WM. Comparison of endothelial cell phenotypic markers of late-outgrowth endothelial progenitor cells isolated from patients with coronary artery disease and healthy volunteers. Tissue Eng Part A. 2009;15:3473–3486. doi: 10.1089/ten.tea.2008.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown MA, Wallace CS, Angelos M, Truskey GA. Characterization of umbilical cord blood-derived late outgrowth endothelial progenitor cells exposed to laminar shear stress. Tissue Eng Part A. 2009;15:3575–3587. doi: 10.1089/ten.tea.2008.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang L, Yang R, Han ZC. Transplantation of umbilical cord blood-derived endothelial progenitor cells: a promising method of therapeutic revascularisation. Eur J Haematol. 2006;76:1–8. doi: 10.1111/j.1600-0609.2005.00579.x. [DOI] [PubMed] [Google Scholar]
- 15.Suarez Y, Shepherd BR, Rao DA, Pober JS. Alloimmunity to human endothelial cells derived from cord blood progenitors. J Immunol. 2007;179:7488–7496. doi: 10.4049/jimmunol.179.11.7488. [DOI] [PubMed] [Google Scholar]
- 16.Zhu S, Malhotra A, Zhang L, Deng S, Zhang T, Freedman NJ, Storms R, Peppel K, Goldschmidt-Clermont PJ, Dong C. Human umbilical cord blood endothelial progenitor cells decrease vein graft neointimal hyperplasia in SCID mice. Atherosclerosis. 2010 doi: 10.1016/j.atherosclerosis.2010.04.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace CS, Champion JC, Truskey GA. Adhesion and function of human endothelial cells co-cultured on smooth muscle cells. Ann Biomed Eng. 2007;35:375–386. doi: 10.1007/s10439-006-9230-5. [DOI] [PubMed] [Google Scholar]
- 18.Truskey GA, Proulx TL. Relationship between 3T3 cell spreading and the strength of adhesion on glass and silane surfaces. Biomaterials. 1993;14:243–254. doi: 10.1016/0142-9612(93)90114-h. [DOI] [PubMed] [Google Scholar]
- 19.Brown MA, Wallace CS, Anamelechi CC, Clermont E, Reichert WM, Truskey GA. The use of mild trypsinization conditions in the detachment of endothelial cells to promote subsequent endothelialization on synthetic surfaces. Biomaterials. 2007;28:3928–3935. doi: 10.1016/j.biomaterials.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Hagen PO, Kisslo J, Peppel K, Freedman NJ. Neointimal hyperplasia rapidly reaches steady state in a novel murine vein graft model. J Vasc Surg. 2002;36:824–832. [PubMed] [Google Scholar]
- 21.Zhang L, Peppel K, Brian L, Chien L, Freedman NJ. Vein graft neointimal hyperplasia is exacerbated by tumor necrosis factor receptor-1 signaling in graft-intrinsic cells. Arterioscler Thromb Vasc Biol. 2004;24:2277–2283. doi: 10.1161/01.ATV.0000147766.68987.0d. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Sivashanmugam P, Wu JH, Brian L, Exum ST, Freedman NJ, Peppel K. Tumor necrosis factor receptor-2 signaling attenuates vein graft neointima formation by promoting endothelial recovery. Arterioscler Thromb Vasc Biol. 2008;28:284–289. doi: 10.1161/ATVBAHA.107.151613. [DOI] [PubMed] [Google Scholar]
- 23.Cai X, Freedman NJ. New therapeutic possibilities for vein graft disease in the post- edifoligide era. Future Cardiology. 2006;2:493–501. doi: 10.2217/14796678.2.4.493. [DOI] [PubMed] [Google Scholar]
- 24.Mayne R. Collagenous proteins of blood vessels. Arteriosclerosis. 1986;6:585–593. doi: 10.1161/01.atv.6.6.585. [DOI] [PubMed] [Google Scholar]
- 25.Stenman S, Vaheri A. Distribution of a major connective tissue protein, fibronectin, in normal human tissues. J Exp Med. 1978;147:1054–1064. doi: 10.1084/jem.147.4.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Truskey GA, Yuan F, Katz DF. Transport Phenomena in Biological Systems. Upper Saddle River, New Jersey: Pearson Prentice Hall, Inc; 2004. [Google Scholar]
- 27.Territo MC, Cline MJ. Monocyte function in man. J Immunol. 1977;118:187–192. [PubMed] [Google Scholar]
- 28.Ting-Beall HP, Needham D, Hochmuth RM. Volume and osmotic properties of human neutrophils. Blood. 1993;81:2774–2780. [PubMed] [Google Scholar]
- 29.Werner N, Junk S, Laufs U, Link A, Walenta K, Bohm M, Nickenig G. Intravenous transfusion of endothelial progenitor cells reduces neointima formation after vascular injury. Circ Res. 2003;93:e17–24. doi: 10.1161/01.RES.0000083812.30141.74. [DOI] [PubMed] [Google Scholar]
- 30.Abou-Saleh H, Yacoub D, Theoret JF, Gillis MA, Neagoe PE, Labarthe B, Theroux P, Sirois MG, Tabrizian M, Thorin E, Merhi Y. Endothelial progenitor cells bind and inhibit platelet function and thrombus formation. Circulation. 2009;120:2230–2239. doi: 10.1161/CIRCULATIONAHA.109.894642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirota T, He H, Yasui H, Matsuda T. Human endothelial progenitor cell-seeded hybrid graft: proliferative and antithrombogenic potentials in vitro and fabrication processing. Tissue Eng. 2003;9:127–136. doi: 10.1089/107632703762687609. [DOI] [PubMed] [Google Scholar]
- 32.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbee RW, Perry BD, Re RN, Murgo JP. Microsphere and dilution techniques for the determination of blood flows and volumes in conscious mice. Am J Physiol. 1992;263:R728–733. doi: 10.1152/ajpregu.1992.263.3.R728. [DOI] [PubMed] [Google Scholar]
- 34.Kim J, Zhang Z, Peppel K, Wu JH, Zidar DA, Brian L, DeWire SM, Exum ST, Lefkowitz RJ, Freedman NJ. β-arrestins regulate atherosclerosis and neointimal hyperplasia by controlling smooth muscle cell proliferation and migration. Circ Res. 2008;103:70–79. doi: 10.1161/CIRCRESAHA.108.172338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castier Y, Brandes RP, Leseche G, Tedgui A, Lehoux S. p47phox-dependent NADPH oxidase regulates flow-induced vascular remodeling. Circ Res. 2005;97:533–540. doi: 10.1161/01.RES.0000181759.63239.21. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.