Abstract
Endothelin-1 (ET-1) is a potent vasoactive peptide implicated in the pathogenesis of hypertension and renal disease. The aim of the current study was to test the hypotheses that ET-1 increases albumin permeability of glomeruli isolated from normal rats and that chronic ET-1 infusion will increase glomerular permeability and inflammation independent of blood pressure. Glomerular permeability to albumin (Palb) was determined from the change in glomerular volume induced by exposing isolated glomeruli to oncotic gradients. Incubation of glomeruli taken from normal rats with ET-1 at a concentration that did not produce direct glomerular contraction (1 nM) significantly increased Palb, reaching a maximum after 4 hrs. Chronic ET-1 infusion for 2 weeks in Sprague-Dawley rats significantly increased Palb and nephrin excretion rate; effects that were attenuated in rats given an ETA receptor antagonist (ABT-627, 5 mg/kg/day). Urinary protein and albumin excretion and mean arterial pressure (telemetry) were not changed by ET-1 infusion. Acute incubation of glomeruli isolated from ET-1-infused rats with the selective ETA antagonist significantly reduced Palb; an effect not observed with acute treatment with a selective ETB antagonist. Chronic ET-1 infusion increased glomerular and plasma sICAM-1 (soluble inter-cellular adhesion molecule-1) and MCP-1 (monocyte chemoattractant protein-1) and elevated the number of macrophages and lymphocytes in renal cortices (ED-1 and CD3 positive staining, respectively). These effects were all attenuated in rats given an ETA selective antagonist. These data support the hypothesis that ET-1 directly increases glomerular permeability to albumin and renal inflammation via ETA receptor activation independent of changes in arterial pressure.
Keywords: intercellular adhesion molecule, monocyte chemotactic protein, macrophage, kidney, rat
Introduction
At a physiological level, endothelin (ET)-1 plays an important role in the control of fluid-volume balance and blood pressure. Specifically, ET-1 promotes diuresis and natriuresis within the collecting duct and action through ETB receptors.1 Systemically, ETB receptors clear ET-1 from the circulation and protect against ETA receptor dependent vasoconstriction, cell proliferation, matrix accumulation, and inflammation.2 Recent clinical studies have suggested that ETA antagonists may be a useful therapeutic approach for proteinuric renal disease,3 but the precise mechanism of action is not known. ETA receptors are responsible for a wide range of effects in the kidney including vasoconstriction of renal cortical vessels, mesangial cell contraction and proliferation, stimulation of extracellular matrix production and inflammation.4 The ET system has been implicated in a variety of renal diseases including chronic proteinuric disorders such as diabetes, hypertension and glomerulonephritis. Over-expression of ET-1 in the kidney causes renal inflammation and fibrosis.5 This role is supported by the finding that ETA or ETA/B antagonists attenuate development and progression of renal disease in models of these disorders.
Proteinuria and albuminuria represent early signs of glomerular injury, and their presence predicts not only an elevated risk for nephropathy, but also cardiovascular disease in general.6 The mechanistic pathways of albuminuria in chronic kidney disease (CKD) have not been resolved. A recent phase III clinical trial in patients with diabetic nephropathy demonstrated that avosentan, a modestly selective ETA antagonist, decreased urinary albumin excretion rate after 12 weeks of treatment.7 This benefit occurred even though nearly all of the subjects in this trial were receiving treatment with angiotensin receptor blockers and angiotensin converting enzyme inhibitors, indicating an independent anti-proteinuric effect of ET receptor antagonism. However, the main adverse effect of the new class of drugs was peripheral edema especially at high dosages of avosentan. Little is known about the specific mechanisms of ET-1 action in CKD, and many of the beneficial effects of ET antagonists have not been distinguished from their blood pressure lowering effect.
Chemokines such as MCP-1 and sICAM-1 are important in attracting inflammatory cells and in their attachment to the endothelium,8 thus facilitating the early process of macrophage and lymphocyte infiltration into the kidney. MCP-1 may contribute to the development of diabetic nephropathy by facilitating the formation of tubulo-interstitial lesions through macrophage recruitment and activation.9 Increased ICAM-1 expression along with increased leukocyte trafficking has been described in experimental models of nephropathy.10 ET-1 has been demonstrated to possess a chemoattractant role for renal and blood macrophages and lymphocytes in an ETA receptor-dependent manner.11, 12
The current study was designed to determine the direct actions of ET-1 in promoting changes in glomerular permeability and renal inflammation. We hypothesized that chronic ET-1 infusion would stimulate ETA receptors to increase expression of inflammatory mediators with subsequent infiltration of inflammatory cells into the kidney. We also proposed that ET-1 has a direct effect on glomeruli to increase permeability to albumin.
Methods
Intravenous infusion of ET-1
All surgical and experimental procedures were performed according to the guidelines for the care and use of animals established by the Medical College of Georgia, and was approved by the Institutional Animal Care and Use Committee. Male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN), 225–250 g, were housed under conditions of constant temperature and humidity and exposed to a 12:12-h light-dark cycle. ET-1 (2 pmol/kg/min; American Peptide Inc., CA) or saline vehicle (0.9% NaCl) was infused intravenously for 14 days via an osmotic mini-pump (model 2ML2; Alza Scientific, Palo Atlo, CA) connected to a catheter (PE-50) placed in the jugular vein, implanted under sodium pentobarbital (50 mg/kg, i.p.) anesthesia. Three groups of rats were studied (n= 5–9): (1) saline infusion (2) ET-1 infusion, and (3) ET-1 infusion plus ABT-627 (5 mg/kg/day, Abbott Laboratories, Abbott Park, IL) in the drinking water. An additional sub-set of rats was also given ABT-627 in the drinking water without any ET-1 infusion (n=4). ABT-627 is a selective ETA receptor antagonist at this dose in vivo; the dosing was identical to many previous studies.12 Blood pressure was measured by telemetry as described previously.13 Treatment with ABT-627 was initiated on the same day as ET-1 infusion. During the final two days of infusion (days 13 and 14), rats were placed in metabolic cages in order to collect urine for determination of the excretion rates of protein, albumin and nephrin.
Isolation of glomeruli
Details of glomeruli isolation are available in the online data supplement (please see http://hyper.ahajournals.org). Briefly, glomeruli were isolated by gradual sieving as described previously.14 Glomeruli were homogenized and total protein was determined in the supernatant using the Bradford assay.15
Measurement of glomerular albumin permeability (Palb)
Please see http://hyper.ahajournals.org for details of Palb measurement. In brief, the rationale for the determination of albumin permeability has been described in detail previously,16 Palb is measured via calculating the volume change created by the oncotic pressure induced by switching the surrounded medium from 5% into 1% BSA. This volume change is mathematically translated into Palb.
Immunohistochemical Analysis
Details of the immunohistochemical methods are available in the online data supplement (http://hyper.ahajournals.org). Briefly, harvested renal tissue from each group of rats was prepared and evaluated for CD68 and CD3 positive immunoreactivity for analyses of macrophages and T cells, respectively.
Plasma and urinary analyses
Commercially available kits for rat sICAM-1 and ET-1 (Quantikine sICAM-1 and QuantiGlo ET-1 Immunoassay respectively, R&D Systems, Minneapolis, MN USA) and MCP-1 (RayBioTech. Inc., Norcross, GA USA) were used for accurate determination of these two cytokines in plasma and glomerular homogenates (1 µg of total protein loaded). Nephrin concentration was determined in urine via ELISA kit (Exocell Inc., Philadelphia, PA). Urinary protein concentrations were determined using the Bradford colorimetric method (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer’s instructions. Urinary concentrations of albumin were measured using enzyme immunoassay kits from Cayman Chemical (Cayman Chemical, Ann Arbor, MI).
Transmission electron microscopy
Details of the transmission electron microscopy (TEM) in this study are available in the online data supplement (http://hyper.ahajournals.org). Briefly, renal cortical tissue from each group of rats was prepared and 4–5 sections were analyzed. Ten glomeruli per section were evaluated.
Statistical analysis
All data are presented as mean ± SEM. Data was compared using unpaired Student’s t-test or one-way ANOVA. Differences were considered statistically significant with P<0.05. Analyses were performed using GraphPad Prism Version 5.0 software (GraphPad Software Inc, La Jolla, CA).
Results
Acute effects of ET-1 on isolated glomeruli
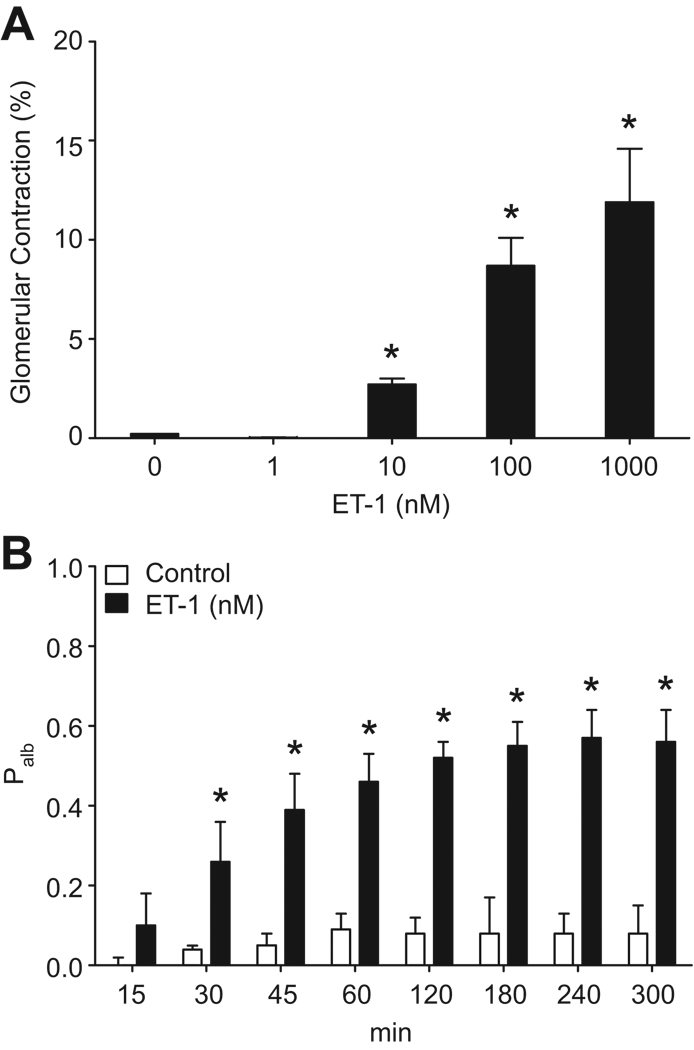
The addition of ET-1 to the 5% BSA incubation media elicited a significant concentration-dependent contraction of glomeruli isolated from normal rats observed within 5 min. The lowest concentration of ET-1 that elicited identifiable contraction (2.7±0.3%) was 10 nM (Figure 1A). No glomerular contraction was observed when 1 nM ET-1 was added for 5 min. Since the calculation of glomerular permeability relies on glomerular volume differences upon switching the media from 5% to 1% BSA, we chose a dose of 1 nM to study the effects of ET-1 on glomerular permeability in order to preclude volume changes due to contractile effects of ET-1 affecting our results.
Figure 1.
Concentration-response data showing contraction of glomeruli during incubation with different concentrations of ET-1 (A). n=3 rats at each concentration, 10–15 glomeruli/rat. *P<0.05 versus 0 nM ET-1. Time course of the effect of 1 nM ET-1 on Palb in isolated glomeruli (B). n=3–5 rats, 10–15 glomeruli/rat. *P<0.05 versus control.
Incubation of glomeruli with ET-1 (1 nM) caused a time-dependent increase of Palb. As shown in Figure 1B, there was a slight increase in Palb after 15 min (0.10 ± 0.08), compared with controls (0 nM ET-1; 0 ± 0.02), but the increase in Palb was not statistically significant until 30 min of incubation (0.26 ± 0.10) and was sustained at 4 h (0.57 ± 0.07).
Palb in chronic ET-1-infused rats
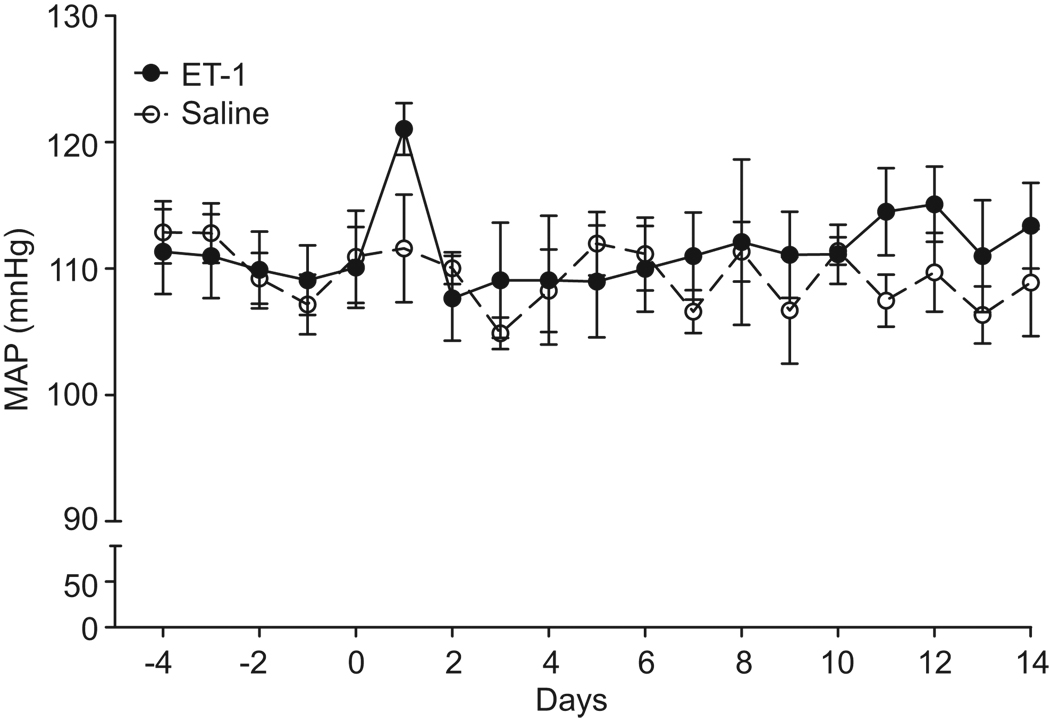
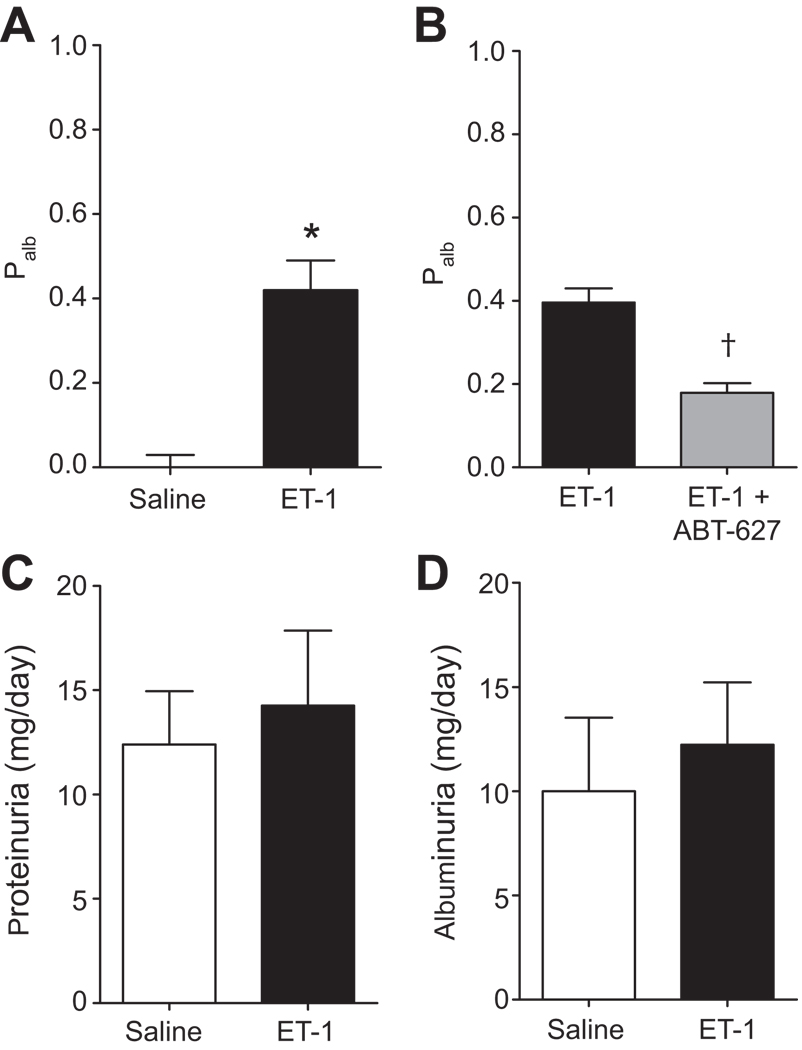
ET-1 infusion (2 pmol/kg/min; i.v.) for 2 weeks did not affect the MAP (measured continuously by telemetry; Figure 2). Palb was significantly increased in glomeruli isolated from ET-1-infused rats (P<0.05, Fig. 3A). However, there was no effect on protein or albumin excretion (Figure 3C and 3D respectively). Separate groups of rats were infused with ET-1 and given either normal drinking water or the ETA receptor antagonist, ABT-627, in the drinking water for 2 weeks. As depicted in Figure 3B, chronic ABT-627 treatment significantly attenuated the elevation of Palb in glomeruli isolated from ET-1-infused rats (0.18 ± 0.02 versus 0.4 ± 0.03; P<0.05). Glomeruli from non-ET-1-infused rats given ABT-627 maintained normal Palb (0.03 ± 0.04, n=4).
Figure 2.
Effect of 2-week i.v. infusion of saline (n=4) or ET-1 (n=9) via osmotic mini-pump on 24-hr average mean arterial pressure (telemetry). Infusion commenced at day 0.
Figure 3.
Effect of 2-week i.v. infusion of saline (n=5) or ET-1 (n=7) via osmotic mini-pump on glomerular permeability to albumin Palb (A). Effect of chronic ET-1 with (n=5) or without (n=5) the ETA selective antagonist, ABT-627 (5 mg/kg/day in drinking water) on glomerular Palb (B). *P<0.05 versus control. Effect of ET-1 infusion on protein (C) and albumin (D) excretion in saline and ET-1 infused rats. n = 4–9 rats/group.
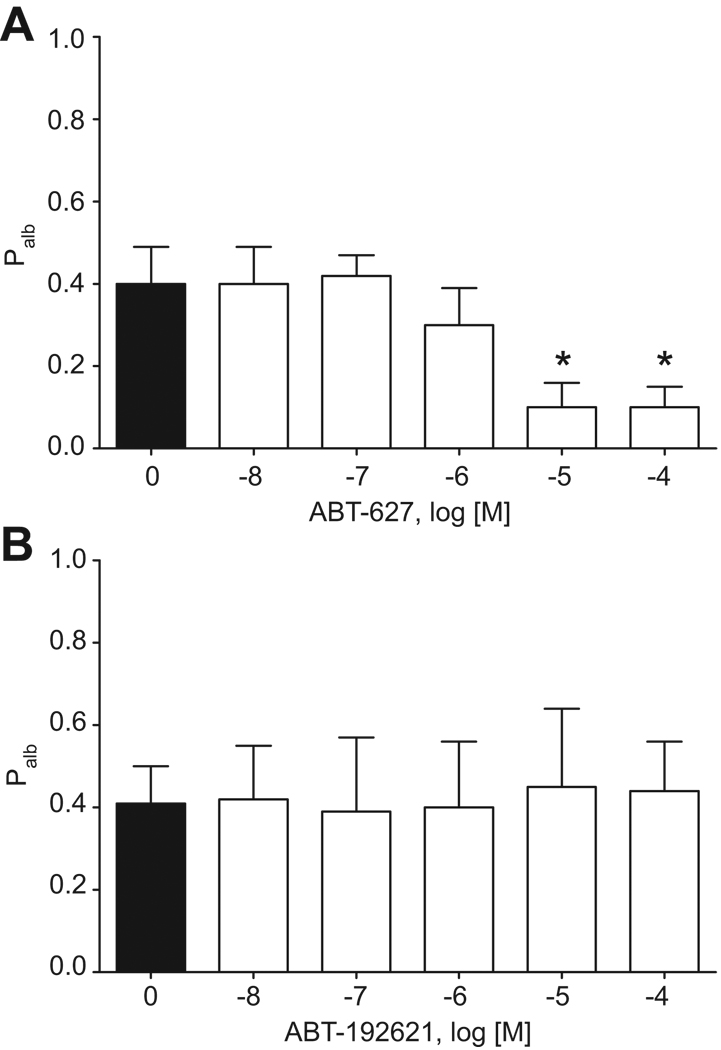
Incubation of glomeruli isolated from ET-1-infused rats with ABT-627 in vitro for 15 min at 37°C also significantly reduced the elevated Palb (Figure 4A). However, in vitro incubation of glomeruli from ET-1-infused rats with A-192612, a selective ETB antagonist, did not produce any significant changes in Palb (Figure 4B).
Figure 4.
Ex vivo effect of ABT-627, a selective ETA receptor antagonist (A) or A-192621, a selective ETB receptor antagonist (B) on Palb of glomeruli isolated from 2-week ET-1-infused rats. n=4 rats, 10–15 glomeruli/rat. *P<0.05 versus ET-1 alone.
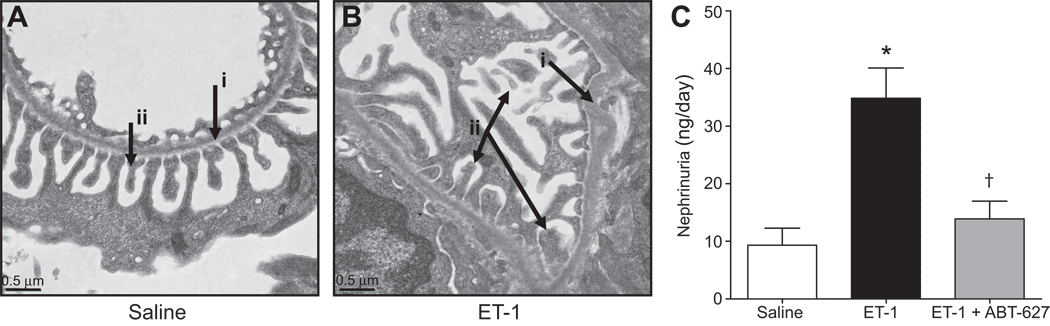
Representative transmission electron micrographs of kidneys showing glomerular structures from saline- and ET-1-infused rat glomeruli are depicted in Figures 5A and 5B, respectively. ET-1 infusion appeared to cause some detachment of podocytes and foot process effacement in glomerular tufts relative to saline-infused control rats, suggesting that ET-1 mediates podocyte injury and increases glomerular permeability. We also observed that ET-1 infusion increases nephrin shedding from the glomeruli into the urine. Nephrin is a filtration slit protein expressed in glomerular epithelial cells (podocytes)17 and acts as a size- and charge-selective filtration barrier.18 Shedding of nephrin into the tubular fluid and urine is considered a sign of reduced glomerular permeability and thus glomerular injury. Nephrin excretion was significantly increased in ET-1-infused rats compared to saline-infused control rats (P<0.05; Figure 5C). Chronic treatment of ET-1-infused rats with ABT-627 significantly decreased urinary nephrin excretion (Figure 5C).
Figure 5.
Transmission electron microscopy of the glomerular filtration barrier in saline (n=3) (A) or ET-1 infused rats (n=3) (B). Arrows indicate glomerular basement membrane (i) and podocytes (ii). Nephrin excretion in rats receiving saline (n=6), ET-1 (n=9), or ET-1 plus ABT-627 (n=5) for 2 weeks (C). *P<0.05 versus saline and †P<0.05 versus ET-1 alone.
Consistent with efficient clearance of ET-1 from the circulation, plasma ET-1 levels were not significantly changed by chronic ET-1 infusion with or without treatment with ABT-627; 1.06 ± 0.17 pg/ml (n=6) in saline-infused rats, 0.88 ± 0.25 pg/ml (n=7) in ET-1 rats, and 1.42 ± 0.51 pg/ml (n=5) in the ET-1 + ABT-627 group. Furthermore, there was no change in 24-hr ET-1 excretion; 0.28 ± 0.03 pg/day (n=6) in the saline treated group, 0.24 ± 0.02 pg/day (n=9) in the ET-1 infused group, and 0.27 ± 0.02 pg/day (n=5) in the ET-1+ABT-627 group.
ET-1 induced inflammation
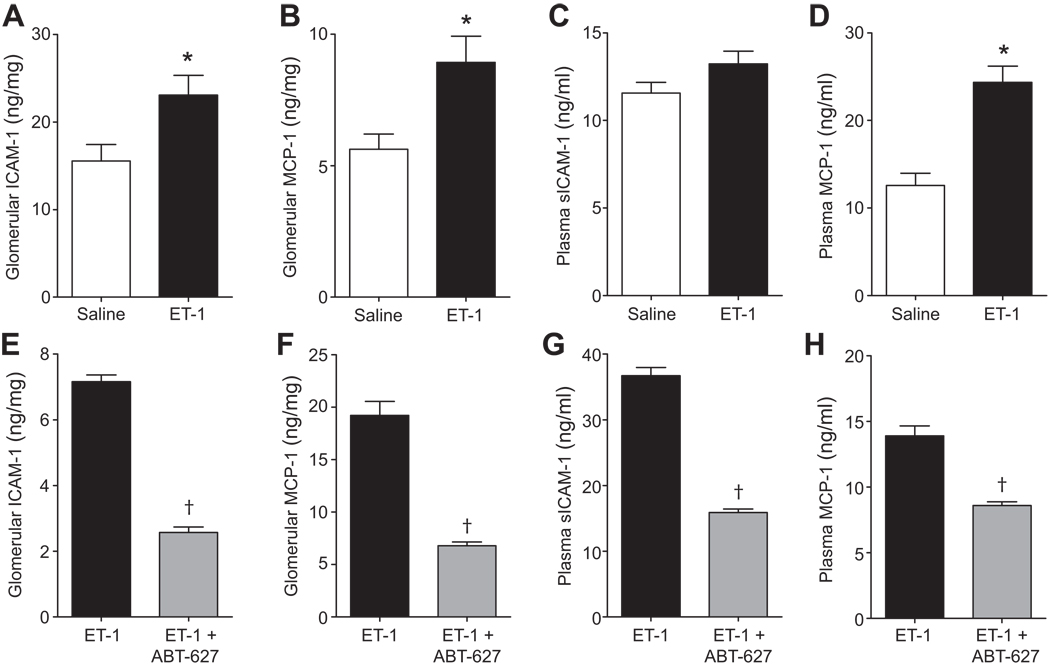
ET-1 infusion elevated glomerular sICAM-1 and MCP-1, and plasma MCP-1 (Figure 6A, 6B and 6D), but had no significant effect on plasma sICAM-1 concentration (Figure 6C). Treatment with ABT-627 significantly decreased glomerular and plasma sICAM-1 and MCP-1 concentrations (Figures 6E–H).
Figure 6.
Effect of ET-1 infusion (A–D) with or without concurrent treatment with ABT-627 (E–H) on glomerular and plasma sICAM-1 and MCP-1 concentrations. n=5–9 per group. *P<0.05 versus saline and †P<0.05 versus ET-1 alone.
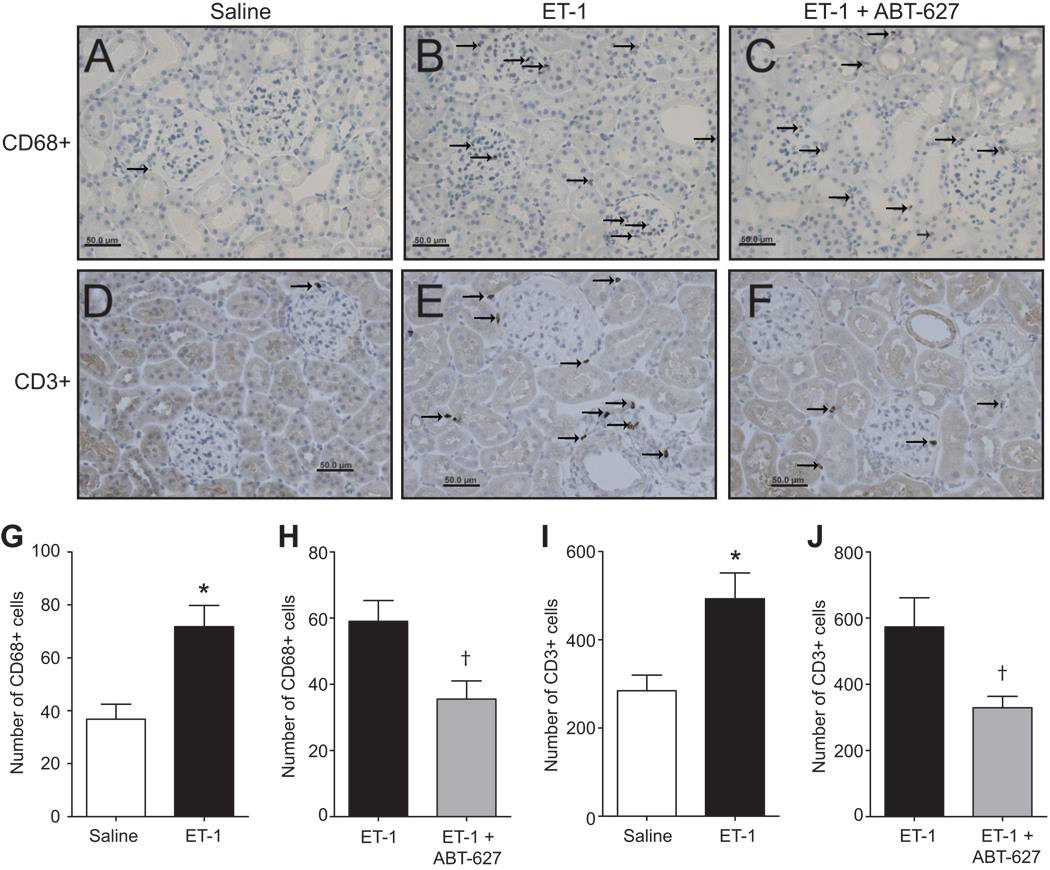
Figure 7 depicts immunohistochemical analysis of renal cortical tissue for monocyte/macrophage and T cells at 14 days after saline infusion, ET-1 infusion or ET-1 infusion plus ABT-627 treatment. The density of cells expressing the macrophage-specific CD68 antigen and T cell-specific CD3 antigen in the renal cortex was very low in saline-infused rats (Figure 7A and 7D) and markedly increased in ET-1-infused rats (Figure 7-B and 7-E). ABT-627 treatment reduced the number of both CD68 and CD3-positive cells in ET-1-infused rats (Figure 7C and 7F). Finally, there were no significant differences in kidney weight between saline and ET-1 infused animals (1.23 ± 0.03 and 1.22 ± 0.02 g, respectively).
Figure 7.
Representative immunostaining images of kidney cortical sections isolated from rats treated with saline (A,D), ET-1 (B,E), or ET-1 plus ABT-627 (C,F) for 2 weeks. Sections were stained for CD68-positive cells (monocytes/macrophages) (A–C) or CD3-positive cells (T cells) (D–F). Numbers of CD68-positive cells are represented in panels G and H with CD3 positive cell counts in panels I and J (indicated by arrows). *P<0.05 versus saline and †P<0.05 versus ET-1 alone. n=4–5 rats/group.
Discussion
Since the discovery of ET-1, the mechanisms by which ET-1 contributes to CKD have been slow in being resolved. In various animal models of hypertension, ET-1 has been shown to exert pro-inflammatory, pro-mitogenic and pro-fibrotic actions in the kidney, which may be at least partially attributable to the hypertensive effects of ET-1. In the present study, we demonstrated that ET-1 induces systemic and local glomerular inflammation as well as increases in glomerular permeability independent of effects on blood pressure.
In the present study, we showed in vitro that concentrations of ET-1 above 1 nM produced glomerular contraction in a dose-dependent manner. The contractile effect of ET-1 is potentially attributable to mesangial cell contraction as reported by Simonson et al.19 At lower concentrations, we observed a fairly rapid increase in Palb after only 30-min and a further increase during the 5-hr incubation period. The normal podocyte has a highly organized cytoskeleton with microfilaments including actin, α-actinin-4, and myosin that are also in association with focal adhesion-related proteins such as α3β1 integrins.20 Together, these results have led us to hypothesize that disruption of structural organization by ET-1 may play a role in alterations of normal podocyte function leading to increased glomerular permeability.
Consistent with our previous study,13 we found that infusion of ET-1 at 2 pmol/kg/min i.v. for 2 weeks had no effect on MAP. In a similar dose range (1–5 pmol/kg/min), others have also reported no effect of chronic ET-1 infusion on arterial pressure in rats.21, 22 However, Sedeek et al.23 and Yao et al.24 reported that chronic ET-1 infusion elevates arterial pressure in rats on a normal salt diet. Therefore, we took care to choose a dose of ET-1 that would allow us to examine the effects of ET-1 independent of hypertension, and used telemetry to verify the lack of an effect on arterial pressure. Dao et al. reported pressure independent actions of ET-1 in this model by infusing ET-1 at 1 and 5 pmol/kg/min for 28 days and observing hypertrophy and small artery hyperplasia, respectively.22 Therefore, it appears that variations of local ET-1 concentrations are sufficient to produce blood pressure-independent cellular responses in vivo.
Ours is not the first study to report blood pressure-independent effects of ET-1 on renal pathology. Hocher et al. demonstrated that transgenic over-expression of the human ET-1 gene in mice resulted in glomerulosclerosis and interstitial fibrosis without the presence of systemic hypertension.25 In the same animal model, Hocher et al. showed that ET-1 induced recruitment of inflammatory cells into the kidney, which contributed progressively to the fibrosis independent of blood pressure.5 In this study, blood pressure was similar in 3-month-old ET-1 transgenic mice and their corresponding littermates. However, ET-1 transgenic mice exhibit chronic renal inflammation characterized by infiltration of CD4+ T cells and macrophages. Such findings suggest that an activated renal ET system could be a blood pressure-independent risk factor for the progression of renal fibrosis to end-stage renal disease. However, we cannot rule out the possibility of alterations of intraglomerular capillary pressure during chronic ET-1 elevation. Moreover, our data indicate that a non-pressor 2-week infusion of ET-1 does not induce proteinuria or albuminuria in normal Sprague-Dawley rats. These data are in agreement with findings in ET-2 transgenic rats and human ET-1 transgenic mice that display glomerulosclerosis with the absence of proteinuria.25, 26 The absence of albuminuria suggests that the changes in permeability determined in isolated glomeruli are not sufficient to translate into measurable albuminuria. Recent studies have renewed interest in the role of proximal tubular uptake of albumin in protecting against albuminuria,27 and changes in Palb on the order of magnitude that we observed in the chronic ET-1 model, 0.4, are much less than those observed in rats displaying overt proteinuria associated with hyperglycemia, >0.8.28 Several studies have established that an increase in glomerular permeability typically occurs prior to the development of overt proteinuria.29–32 Thus, it remains possible that ET-1 infusion for longer periods than 2 weeks may result in significant albuminuria and/or proteinuria.
ETA receptor antagonism using ABT-627 attenuated Palb in glomeruli isolated from ET-1-infused rats even when glomeruli were treated ex vivo for only 15 min, confirming the role of endogenous ET-1 and ETA receptor activity within the glomerulus influencing permeability to albumin. It is unlikely that changes in expression (mRNA or protein) of glomerular filtration barrier molecules (i.e., nephrin, CD2AP, podocin) can explain the rapid effect of ETA receptor blockade on Palb. Rather, ETA receptor-mediated cytoskeletal rearrangement is a more likely mechanism of reduced Palb. This hypothesis is supported by previous studies by Morigi et al. showing that F-actin redistribution and gap formation occurred when exogenous ET-1 was added to cultured podocytes.20 Intervention with LU-302146, a selective ETA receptor antagonist, prevented F-actin redistribution and decreased intercellular gap formation induced by shigatoxin-2. Moreover, the same group has reported that inhibition of Rho kinases, which are crucial for the formation of stress fibers, resulted in a significant inhibition of F-actin rearrangement in response to ET-1.20 Treatment of non-ET-1 infused rats with ABT-627 had no effect on Palb as one might expect since Palb was no different from zero in these rats.
Our data further support a specific role for the ETA receptor in control of Palb since ETB receptor antagonism did not alter the increase in Palb induced by ET-1. This is consistent with relatively low expression of ETB receptors in glomeruli compared to ETA receptors.33 It is important to note, however, that this does not exclude a role for the ETB receptor in modulating the progression of glomerular injury and renal disease. Studies by Tazawa et al., and Pfab et al., have demonstrated that rats lacking a functional ETB receptor develop more severe renal dysfunction, proteinuria, and renal injury in response to sub-total nephrectomy34 and streptozotocin-induced hyperglycemia.35 In these studies, the rat lacking functional ETB receptors also developed disease-induced elevations in blood pressure that could account for the increased proteinuria. These findings taken together with our current results suggest that the protective role of ETB receptors is through hemodynamic mechanisms rather than direct ETB-dependent effects on permeability, which we were unable to observe in the permeability experiments.
We observed that chronic ETA receptor activation increased the excretion of nephrin into the urine, again suggesting that ETA receptors influence podocyte function and Palb. Collino et al. identified a mechanism of nephrin loss that may account for the enhanced glomerular permeability in pre-eclampsia.36 These investigators provided evidence for a factor(s) present in serum from pre-eclamptic patients that trigger production of ET-1 from glomerular endothelial cells, and that ET-1 in turn may induce shedding of nephrin from the surface of podocytes. They went on to conclude that ET-1 activates the podocyte cytoskeleton and modifies surface expression of nephrin, thus depleting it from the plasma membrane and excretion into the urine. Others have shown that up-regulation of ET-1 production by podocytes is induced by protein overload, resulting in cytoskeletal changes associated with foot-process effacement, a hallmark of chronic glomerular disease.37 ET-1 released by podocytes thus may contribute to glomerular barrier dysfunction by direct effects on podocytes themselves. Since ET-1 also increases reactive oxygen species,13 ET-1 may elevate glomerular permeability in chronic settings via superoxide-mediated enhancement of gelatinase synthesis by mesangial cells, reduction of de novo synthesis of proteoglycans and degradation of the glomerular basement membrane.38
We also observed that infusion of ET-1 for 2 weeks significantly increased glomerular sICAM-1, MCP-1, and plasma MCP-1 levels independent of hypertension or loss of glycemic control (euglycemic clamp test, unpublished observations). These data agree with previous work published by Amiri and colleagues39 who reported that transgenic mice that over-express human preproendothelin-1 in endothelial cells exhibit various vascular inflammatory responses including macrophage infiltration, transcription factor activation (AP-1 and NF-κB) and increases in VCAM-1 expression independent of blood pressure elevation. These data suggest that ET-1 directly triggers the initial phase of renal inflammation through up-regulation of systemic MCP-1 and local glomerular ICAM-1 and MCP-1. These inflammatory molecules are known to participate in macrophage infiltration into the kidney.8 Future studies are needed to explore the mechanism of ET-1-induced glomerular ICAM-1 and MCP-1 up-regulation. The pro-inflammatory effects of ET-1 were also blocked by ABT-627 consistent with the pro-inflammatory effects of ET-1 being ETA receptor mediated. We have previously observed that ETA blockade in intact rats has no effect on indices of inflammation.12 One challenge will be to determine whether the inflammatory effects of ET-1 contribute to changes in glomerular permeability, and furthermore, proteinuria.
Perspectives
The use of ETA selective and ETA/B receptor antagonists are under consideration for treatment of proteinuric renal disease,7, 40 but we have little knowledge of how they may confer benefit. We observed that ETA receptor activation results in over-expression of circulating and glomerular inflammatory mediators as well as changes in glomerular permeability to albumin independent of blood pressure. Our results provide mechanistic support for the use of ETA selective blockade in CKD, but thus far, the clinical data indicate frequent fluid retention problems of uncertain origin. Future studies are needed to specifically investigate receptor subtype specific actions in the human kidney.
Supplementary Material
Acknowledgments
We thank Dr. Ahmed Elmarakby (Medical College of Georgia) and Dr. Jan Williams (University of Mississippi) for their useful advice on how to isolate glomeruli and measure Palb. We would also like to thank Hiram Ocasio for his assistance with telemetry measurements, Janet Hobbs for expert technical assistance in the histology studies.
Sources of funding
This work was supported by grants from the National Heart Lung and Blood Institute (HL69999 and HL64776) and an American Heart Association pre-doctoral fellowship for M.A.S. A grant from the Egyptian government to M.A.S. also supported this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Clavell AL, Stingo AJ, Margulies KB, Brandt RR, Burnett JC., Jr Role of endothelin receptor subtypes in the in vivo regulation of renal function. Am J Physiol Renal Physiol. 1995;268:F455–F460. doi: 10.1152/ajprenal.1995.268.3.F455. [DOI] [PubMed] [Google Scholar]
- 2.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- 3.Hocher B, Schwarz A, Reinbacher D, Jacobi J, Lun A, Priem F, Bauer C, Neumayer HH, Raschack M. Effects of endothelin receptor antagonists on the progression of diabetic nephropathy. Nephron. 2001;87:161–169. doi: 10.1159/000045906. [DOI] [PubMed] [Google Scholar]
- 4.Kohan DE. Endothelins in the normal and diseased kidney. Am J Kidney Dis. 1997;29:2–26. doi: 10.1016/s0272-6386(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 5.Hocher B, Schwarz A, Slowinski T, Bachmann S, Pfeilschifter J, Neumayer HH, Bauer C. In vivo interaction of nitric oxide and endothelin. J Hypertens. 2004;22:111–119. doi: 10.1097/00004872-200401000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Perkins BA, Ficociello LH, Ostrander BE, Silva KH, Weinberg J, Warram JH, Krolewski AS. Microalbuminuria and the risk for early progressive renal function decline in type 1 diabetes. J Am Soc Nephrol. 2007;18:1353–1361. doi: 10.1681/ASN.2006080872. [DOI] [PubMed] [Google Scholar]
- 7.Wenzel RR, Littke T, Kuranoff S, Jurgens C, Bruck H, Ritz E, Philipp T, Mitchell A. Avosentan reduces albumin excretion in diabetics with macroalbuminuria. J Am Soc Nephrol. 2009;20:655–664. doi: 10.1681/ASN.2008050482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wenzel U, Schneider A, Valente AJ, Abboud HE, Thaiss F, Helmchen UM, Stahl RA. Monocyte chemoattractant protein-1 mediates monocyte/macrophage influx in anti-thymocyte antibody-induced glomerulonephritis. Kidney Int. 1997;51:770–776. doi: 10.1038/ki.1997.108. [DOI] [PubMed] [Google Scholar]
- 9.Chow FY, Nikolic-Paterson DJ, Ozols E, Atkins RC, Rollin BJ, Tesch GH. Monocyte chemoattractant protein-1 promotes the development of diabetic renal injury in streptozotocin-treated mice. Kidney Int. 2006;69:73–80. doi: 10.1038/sj.ki.5000014. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto H, Shikata K, Hirata K, Akiyama K, Matsuda M, Kushiro M, Shikata Y, Miyatake N, Miyasaka M, Makino H. Increased expression of intercellular adhesion molecule-1 (ICAM-1) in diabetic rat glomeruli: Glomerular hyperfiltration is a potential mechanism of ICAM-1 upregulation. Diabetes. 1997;46:2075–2081. doi: 10.2337/diab.46.12.2075. [DOI] [PubMed] [Google Scholar]
- 11.Ishizawa K, Yoshizumi M, Tsuchiya K, Houchi H, Minakuchi K, Izawa Y, Kanematsu Y, Kagami S, Hirose M, Tamaki T. Dual effects of endothelin-1 (1–31): Induction of mesangial cell migration and facilitation of monocyte recruitment through monocyte chemoattractant protein-1 production by mesangial cells. Hypertens Res. 2004;27:433–440. doi: 10.1291/hypres.27.433. [DOI] [PubMed] [Google Scholar]
- 12.Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol. 2007;18:143–154. doi: 10.1681/ASN.2006030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elmarakby AA, Loomis ED, Pollock JS, Pollock DM. NADPH oxidase inhibition attenuates oxidative stress but not hypertension produced by chronic ET-1. Hypertension. 2005;45:283–287. doi: 10.1161/01.HYP.0000153051.56460.6a. [DOI] [PubMed] [Google Scholar]
- 14.Misra RP. Isolation of glomeruli from mammalian kidneys by graded sieving. Am J Clin Pathol. 1972;58:135–139. doi: 10.1093/ajcp/58.2.135. [DOI] [PubMed] [Google Scholar]
- 15.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 16.Savin VJ, Sharma R, Lovell HB, Welling DJ. Measurement of albumin reflection coefficient with isolated rat glomeruli. J Am Soc Nephrol. 1992;3:1260–1269. doi: 10.1681/ASN.V361260. [DOI] [PubMed] [Google Scholar]
- 17.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc Natl Acad Sci U S A. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg P, Verma R, Nihalani D, Johnstone DB, Holzman LB. Neph1 cooperates with nephrin to transduce a signal that induces actin polymerization. Mol Cell Biol. 2007;27:8698–8712. doi: 10.1128/MCB.00948-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonson MS, Dunn MJ. Endothelin-1 stimulates contraction of rat glomerular mesangial cells and potentiates beta-adrenergic-mediated cyclic adenosine monophosphate accumulation. J Clin Invest. 1990;85:790–797. doi: 10.1172/JCI114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morigi M, Buelli S, Zanchi C, Longaretti L, Macconi D, Benigni A, Moioli D, Remuzzi G, Zoja C. Shigatoxin-induced endothelin-1 expression in cultured podocytes autocrinally mediates actin remodeling. Am J Pathol. 2006;169:1965–1975. doi: 10.2353/ajpath.2006.051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mortensen LH, Fink GD. Captopril prevents chronic hypertension produced by infusion of endothelin-1 in rats. Hypertension. 1992;19:676–680. doi: 10.1161/01.hyp.19.6.676. [DOI] [PubMed] [Google Scholar]
- 22.Dao HH, Bouvet C, Moreau S, Beaucage P, Lariviere R, Servant MJ, de Champlain J, Moreau P. Endothelin is a dose-dependent trophic factor and a mitogen in small arteries in vivo. Cardiovasc Res. 2006;71:61–68. doi: 10.1016/j.cardiores.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 23.Sedeek MH, Llinas MT, Drummond H, Fortepiani L, Abram SR, Alexander BT, Reckelhoff JF, Granger JP. Role of reactive oxygen species in endothelin-induced hypertension. Hypertension. 2003;42:806–810. doi: 10.1161/01.HYP.0000084372.91932.BA. [DOI] [PubMed] [Google Scholar]
- 24.Yao L, Kobori H, Rahman M, Seth DM, Shokoji T, Fan Y, Zhang GX, Kimura S, Abe Y, Nishiyama A. Olmesartan improves endothelin-induced hypertension and oxidative stress in rats. Hypertens Res. 2004;27:493–500. doi: 10.1291/hypres.27.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hocher B, Thone-Reineke C, Rohmeiss P, Schmager F, Slowinski T, Burst V, Siegmund F, Quertermous T, Bauer C, Neumayer HH, Schleuning WD, Theuring F. Endothelin-1 transgenic mice develop glomerulosclerosis, interstitial fibrosis, and renal cysts but not hypertension. J Clin Invest. 1997;99:1380–1389. doi: 10.1172/JCI119297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hocher B, Liefeldt L, Thone-Reineke C, Orzechowski HD, Distler A, Bauer C, Paul M. Characterization of the renal phenotype of transgenic rats expressing the human endothelin-2 gene. Hypertension. 1996;28:196–201. doi: 10.1161/01.hyp.28.2.196. [DOI] [PubMed] [Google Scholar]
- 27.Tucker BJ, Blantz RC. Determinants of proximal tubular reabsorption as mechanisms of glomerulotubular balance. Am J Physiol. 1978;235:F142–F150. doi: 10.1152/ajprenal.1978.235.2.F142. [DOI] [PubMed] [Google Scholar]
- 28.Fabris B, Candido R, Carraro M, Fior F, Artero M, Zennaro C, Cattin MR, Fiorotto A, Bortoletto M, Millevoi C, Bardelli M, Faccini L, Carretta R. Modulation of incipient glomerular lesions in experimental diabetic nephropathy by hypotensive and subhypotensive dosages of an ace inhibitor. Diabetes. 2001;50:2619–2624. doi: 10.2337/diabetes.50.11.2619. [DOI] [PubMed] [Google Scholar]
- 29.Bjorn SF, Bangstad HJ, Hanssen KF, Nyberg G, Walker JD, Viberti GC, Osterby R. Glomerular epithelial foot processes and filtration slits in IDDM patients. Diabetologia. 1995;38:1197–1204. doi: 10.1007/BF00422369. [DOI] [PubMed] [Google Scholar]
- 30.Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G. Nephrin expression is reduced in human diabetic nephropathy: Evidence for a distinct role for glycated albumin and angiotensin ii. Diabetes. 2003;52:1023–1030. doi: 10.2337/diabetes.52.4.1023. [DOI] [PubMed] [Google Scholar]
- 31.Melnick GF, Ladoulis CT, Cavallo T. Decreased anionic groups and increased permeability precedes deposition of immune complexes in the glomerular capillary wall. Am J Pathol. 1981;105:114–120. [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma M, Sharma R, Reddy SR, McCarthy ET, Savin VJ. Proteinuria after injection of human focal segmental glomerulosclerosis factor. Transplantation. 2002;73:366–372. doi: 10.1097/00007890-200202150-00009. [DOI] [PubMed] [Google Scholar]
- 33.Karet FE, Kuc RE, Davenport AP. Novel ligands BQ123 and BQ3020 characterize endothelin receptor subtypes ETA and ETB in human kidney. Kidney Int. 1993;44:36–42. doi: 10.1038/ki.1993.210. [DOI] [PubMed] [Google Scholar]
- 34.Tazawa N, Okada Y, Nakata M, Izumoto H, Takasu M, Takaoka M, Gariepy CE, Yanagisawa M, Matsumura Y. Exaggerated vascular and renal pathology in endothelin-B-receptor-deficient rats with subtotal nephrectomy. J Cardiovasc Pharmacol. 2004;44 Suppl 1:S467–S470. doi: 10.1097/01.fjc.0000166316.45882.94. [DOI] [PubMed] [Google Scholar]
- 35.Pfab T, Thone-Reineke C, Theilig F, Lange I, Witt H, Maser-Gluth C, Bader M, Stasch JP, Ruiz P, Bachmann S, Yanagisawa M, Hocher B. Diabetic endothelin B receptor-deficient rats develop severe hypertension and progressive renal failure. J Am Soc Nephrol. 2006;17:1082–1089. doi: 10.1681/ASN.2005080833. [DOI] [PubMed] [Google Scholar]
- 36.Collino F, Bussolati B, Gerbaudo E, Marozio L, Pelissetto S, Benedetto C, Camussi G. Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol. 2008;294:F1185–F1194. doi: 10.1152/ajprenal.00442.2007. [DOI] [PubMed] [Google Scholar]
- 37.Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G, Benigni A. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: Implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma R, Suzuki K, Nagase H, Savin VJ. Matrix metalloproteinase (stromelysin-1) increases the albumin permeability of isolated rat glomeruli. J Lab Clin Med. 1996;128:297–303. doi: 10.1016/s0022-2143(96)90031-1. [DOI] [PubMed] [Google Scholar]
- 39.Amiri F, Paradis P, Reudelhuber TL, Schiffrin EL. Vascular inflammation in absence of blood pressure elevation in transgenic murine model overexpressing endothelin-1 in endothelial cells. J Hypertens. 2008;26:1102–1109. doi: 10.1097/HJH.0b013e3282fc2184. [DOI] [PubMed] [Google Scholar]
- 40.Dhaun N, Macintyre IM, Melville V, Lilitkarntakul P, Johnston NR, Goddard J, Webb DJ. Blood pressure-independent reduction in proteinuria and arterial stiffness after acute endothelin-a receptor antagonism in chronic kidney disease. Hypertension. 2009;54:113–119. doi: 10.1161/HYPERTENSIONAHA.109.132670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.