Abstract
Orthostatic hypotension affects patients with autonomic failure producing considerable disability because of pre-syncopal symptoms. Severely affected patients may have residual sympathetic tone that can be engaged to increase blood pressure with the alpha-2 adrenergic antagonist yohimbine. This medication activates sympathetic outflow centrally and unrestrains norepinephrine release from noradrenergic neurons. Alternatively, the acetylcholinesterase inhibitor, pyridostigmine, can increase sympathetic tone by improving ganglionic cholinergic neurotransmission. Our purpose was to compare these complementary approaches and test the hypothesis that the combination would lead to synergistic increases in blood pressure. We compared the effects of pyridostigmine 60 mg and yohimbine 5.4 mg in a single-blind, randomized, placebo-controlled, crossover fashion. In a subset of patients we tested the combination of pyridostigmine and yohimbine. Our primary outcome was the change in standing diastolic blood pressure 60 minutes after drug administration from baseline. We studied a total of 31 patients with confirmed severe autonomic failure. Yohimbine significantly improved standing diastolic blood pressure as compared to placebo (11±3 mm Hg 95%CI: 6 to 16, P <0.001). On the contrary, Pyridostigmine did not increase the standing diastolic blood pressure (0.6±3 mm Hg 95%CI: −5 to 5, P =0.823). Only yohimbine showed a significant improvement in presyncopal symptoms. Sixteen patients received the combination of pyridostigmine and yohimbine, but no evidence of synergistic pressor effect was found. Engaging residual sympathetic tone with yohimbine is a more effective approach to improve orthostatic hypotension as compared to pyridostigmine in patients with severe orthostatic hypotension.
Keywords: Pyridostigmine bromide, orthostatic hypotension, autonomic failure, autonomic drugs, dysautonomia
Introduction
Severe orthostatic hypotension and pre-syncopal symptoms are the main cause of disability among patients with autonomic failure1. The current recommended therapeutic approaches are limited to medications that increase sodium reabsorption and expand plasma volume such as fludrocortisones,2, 3 or vasoactive agents such as the alpha-1 adrenergic agonist, midodrine.4-7 Even though these interventions can effectively reduce symptoms related to orthostatic hypotension, in most cases they induce supine hypertension that further complicate their treatment.8, 9 In this context, previous studies have reported the beneficial effect of pyridostigmine as an alternative therapy for orthostatic hypotension.10, 11 Pyridostigmine inhibits the enzyme acetylcholinesterase and increases the transmission of impulses from cholinergic neurons across the synaptic cleft. Because the pre-ganglionic sympathetic neuron is cholinergic it is proposed to work as a facilitator of sympathetic ganglionic neurotransmission. The traffic to the autonomic ganglia is reduce while supine but maximally activated during upright posture.11 Therefore, this medication has the potential to increase vascular adrenergic tone when engaged by upright posture without worsening supine hypertension.12 Although this mechanism of action appears ideal, the initial enthusiasm was damped by a recent report of poor improvement in standing blood pressure.10 Alternatively, we have previously reported that yohimbine, an alpha-2 adrenergic receptor antagonist that enhances residual sympathetic tone, increases blood pressure in autonomic failure patients and can be used as possible therapeutic agent.13
In this study, we compared these two therapeutic approaches and tested the hypotheses that (1) both yohimbine and pyridostigmine would be superior than placebo and (2) yohimbine would be superior than pyridostigmine on hemodynamic and symptoms parameters while standing. We also explored whether the combination pyridostigmine with yohimbine would have any synergistic effect. We postulate that this effect could be particularly useful in patients severely affected with autonomic failure who may not benefit from the use of a single agent alone.
Methods
Subjects
A total of 31 patients with severe autonomic failure (9 with multiple system atrophy, MSA, 16 with pure autonomic failure, PAF, and 6 with Parkinson's Disease, PD) were recruited from referrals to the Autonomic Dysfunction Center at Vanderbilt University. Orthostatic hypotension was defined as at least a 20 mmHg decrease in systolic blood pressure or at least 10 mmHg of diastolic blood pressure within 3 minutes upon standing, according to the definition of the American Autonomic Society.14 Patients were excluded if they had secondary causes of autonomic failure (e.g., diabetes mellitus, amyloidosis). The study was approved by the Institutional Review Board at Vanderbilt University and all subjects gave written informed consent.
All the studies were conducted in the morning, 2.5 hours after meals to avoid any acute hemodynamic effects from eating, and in a post-void state. Patients were given a tablet of placebo, pyridostigmine 60 mg (Valeant Pharmaceuticals International, Aliso viejo,CA) or yohimbine 5.4 mg (Goldline, Ft. Lauderdale, Florida) in a randomized, single-blind, crossover fashion. In 16 patients we tested the combination of pyridostigmine 60 mg and yohimbine 5.4 mg.
The study was conducted with the patients seated on a chair with their feet on the floor. Blood pressure (BP) and heart rate (HR) were recorded every 5 minutes with an automated brachial blood pressure cuff (Dinamap; Critikon, Tampa, Florida), and digitally acquired into a custom designed database (Microsoft Access, Microsoft Corporation). Baseline parameters were measured for 30 minutes and orthostatic tolerance was tested by measuring BP and HR on standing. Blood pressure was measured for 60 minutes after drug administration. Assessment of orthostatic tolerance was repeated at the end of this period, as described above.
The change in standing diastolic blood pressure (ΔDBP) 60 minutes after drug administration from baseline was the primary outcome. Secondary outcomes include the orthostatic symptom score and the change in seated systolic/diastolic blood pressure 1 hour after drug administration. We selected diastolic blood pressure as the primary outcome because two previous studies10, 11 used diastolic blood pressure as their main endpoint when reporting a significant improvement in blood pressure with pyridostigmine.
Statistics
All data are presented as mean ± SEM. For our primary analysis, we used paired t-test to determine differences in the following comparisons (placebo versus yohimbine, placebo versus pyridostigmine and pyridostigmine versus yohimbine). We have used Bonferroni correction to adjust for multiple comparisons in the above 3 primary analyses. Our study had 80% power to detect an increase of 6 mm Hg in standing blood pressure with pyridostigmine with an SD of 12 as reported previously in similar studies.10
In sixteen patients, we performed exploratory analyses for the combination of pyridostigmine and yohimbine, we used paired t-test to determine differences in our primary outcome between each drug alone (placebo, yohimbine, pyridostigmine) and the combination. We assessed the carry-over effect by examining differences in the DBP at baseline before each intervention using one-way analysis of variance with repeated measures. Before and after comparison for the orthostatic symptoms score between treatments was tested using Wilcoxon signed-rank test. We also performed a subgroup analyses according to the level of the autonomic lesion. The treatment effect (drug-placebo) was compared in patients with central autonomic failure (Multiple System Atrophy) versus those with peripheral autonomic failure (Pure Autonomic Failure and Parkinson's Disease) using Wilcoxon rank-sum test. All tests were two-tailed, and a p-value of <0.05 was considered statistically significant. Analyses were performed with the SPSS statistical software (SPSS statistical software (SPSS version 17.0, SPSS, Inc, Chicago, IL, USA).
Results
Basal cardiovascular and autonomic function
A total of 31 patients were studied, 17 were female and the average age was 66±2 years. Patients with Parkinson's disease were approximately 10 years older than those with PAF and MSA, Table 1. All subjects had a substantial decrease in systolic and diastolic blood pressure upon standing (−67±6/−33±4 mmHg) without an adequate compensatory heart rate increase (16±2 bpm). As expected, patients with MSA have higher supine plasma norepinephrine (NE) as compared to patients with PAF. No significant differences were found between MSA and PD in plasma catecholamines. The results of the autonomic function tests are presented in Table 2. The decrease in systolic blood pressure during phase II of the Valsalva maneuver was exaggerated compared to responses in normal controls, and the systolic blood pressure overshoot during phase IV was absent. The Valsalva ratio was low, indicating inadequate compensatory changes of heart rate. The pressor responses to isometric handgrip exercise or pain stimulus (cold pressor test) were impaired. Sinus arrhythmia was markedly reduced. Hence, autonomic testing indicated severe sympathetic and parasympathetic involvement.
Table 1.
Subject Characteristics
| Diagnosis | Gender M/F |
BMI (Kg/m2) |
Age (years) |
Heart rate (bpm) |
Norepinephrine (pg/ml) |
||
|---|---|---|---|---|---|---|---|
| supine | upright | supine | upright | ||||
| MSA | 5/4 | 24±0.9 | 61±3.1 | 75±3.8 | 92±4.8 | 351±120.0 | 397±98.5 |
| PAF | 6/10 | 26±0.7 | 64±2.3 | 67±2.3 | 84±4.1 | 78±10.0 | 139±27.5 |
| PD | 3/3 | 27±2.1 | 70±3.3 | 79±7.2 | 91±10.3 | 287±138.9 | 491±279.5 |
MSA, multiple system atrophy; PAF, pure autonomic failure; PD, parkinson's disease.
Table 2.
Autonomic Function Tests and Orthostatic Stress
| Parameters (Unit) | All subjects |
Normals‡ |
|---|---|---|
| Orthostatic change in systolic blood pressure, mmHg | −67±5.7 | ≤ 20 |
| Orthostatic change in heart rate, beats per minute | 16±2.1 | ≤ 20 |
| *Sinus Arrhytmia ratio | 1.07±0.01 | 1.2±0.1 |
| Depressor response to Valsalva in phase II, mmHg | −58±4.7 | ≤ 20 |
|
†Blood pressure response to Valsalva phase IV, mmHg |
−31±4.1 | >20 |
| Valsalva ratio | 1.10±0.02 | 1.5±0.2 |
| Depressor response to hyperventilation, mmHg | −34±3.2 | −5±6.3 |
| Pressor response to cold pressor, mmHg | 7±1.7 | 24±13 |
| Pressor response to handgrip, mmHg | 1.7±2.4 | 16±6 |
Sinus arrhytmia ratio
A negative value for phase IV of the valsalva maneuver indicates that the blood pressure overshoot was absent in most patients.
Normal values are from the Autonomic Dysfunction Center Database at Vanderbilt University.
Pressor effect of drugs
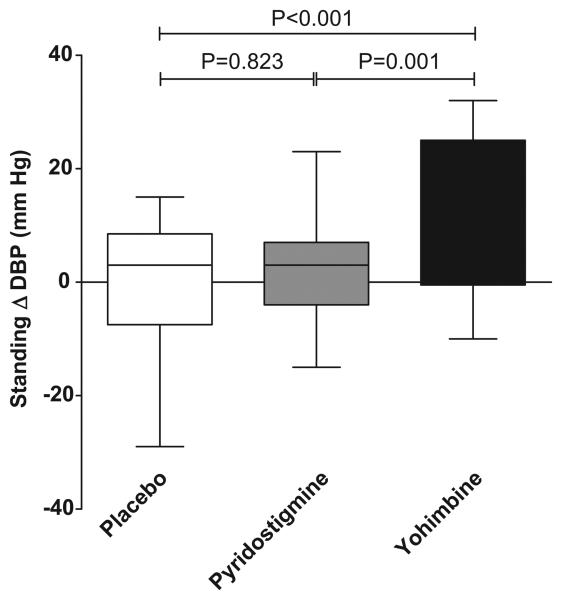
For our primary endpoint, we found that only yohimbine significantly increased standing diastolic blood pressure from baseline at 60 minutes after drug administration compared with placebo (ΔDBP 11±3 mm Hg 95%CI: 6 to 16, P <0.001, significant using Bonferroni correction 0.05/3 for 3 comparisons). We did not find a significant increase in standing DBP from baseline with pyridostigmine (ΔDBP 0.6±3 mm Hg 95%CI: −5 to 5, P =0.823). There was a significantly increased in standing DBP with yohimbine compared with pyridostigmine (ΔDBP 9±3 mm Hg 95%CI: 4 to 15, P=0.001, <0.05/3), Figure 1.
Figure 1.
Changes in standing diastolic blood pressure 60 minutes after drug administration, there was a statistical significant difference between yohimbine versus placebo and yohimbine versus pyridostigmine (P<0.001 and P=0.001, respectively). No significant difference was found between pyridostigmine versus placebo.
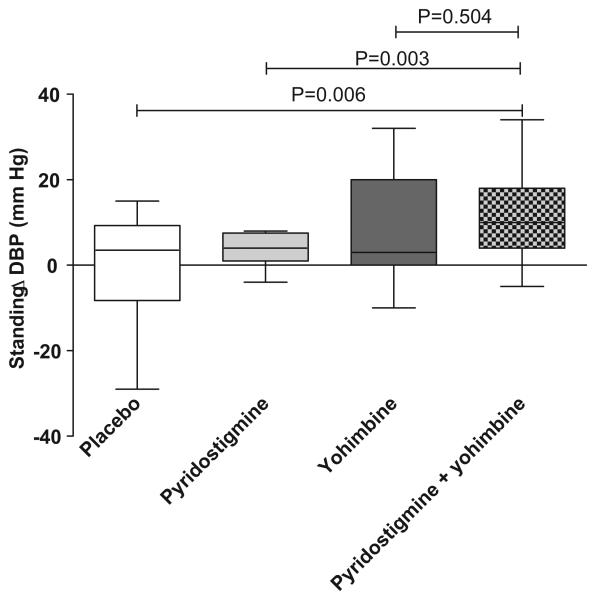
In our exploratory analyses with sixteen patients, the combination of pyridostigmine and yohimbine increased significantly standing diastolic blood pressure from baseline compared with placebo (ΔDBP 12±4 mm Hg 95%CI: 4 to 19, P=0.006) and pyridostigmine alone (ΔDBP 11±3 mm Hg 95%CI: 4 to 17, P=0.003). However the combination of pyridostigmine and yohimbine did not significantly increased standing diastolic blood pressure compared with yohimbine alone (ΔDBP 3±4 mm Hg 95%CI: −6 to 12, P=0.504). Therefore, the pressor effect of the combination is driven by yohimbine alone; we did not have a synergistic effect on standing blood pressure, Figure 2.
Figure 2.
Changes in standing diastolic blood pressure 60 minutes after drug administration from baseline in sixteen patients in whom we tried the combination yohimbine and pyridostigmine, there was a significant difference between the combination versus placebo (P=0.006) and the combination versus pyridostigmine alone (P=0.003). There was no statistical significant difference between the combination and yohimbine alone.
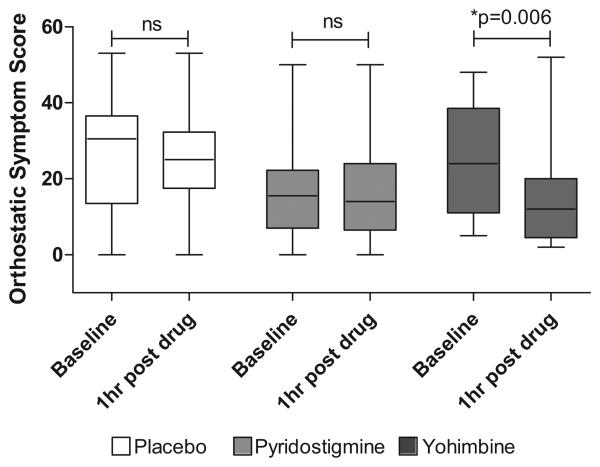
Regarding secondary endpoints, the magnitude of pre-syncopal symptoms significantly improved after yohimbine alone (p=0.006). Neither pyridostigmine nor placebo experienced a significant improvement in pre-syncopal symptoms, Figure 3. We found no differences in the standing diastolic blood pressure at baseline indicating that there was no carry-over effect.
Figure 3.
Orthostatic symptoms score at baseline and 1 hour post-drug administration. There was a significant decrease in pre-syncopal symptoms only after yohimbine administration.
Seated systolic and diastolic blood pressure increased significantly during yohimbine as compared to placebo (18±5.1/11±2.4 versus −1±4.2/1±2.7, P=0.007, P=0.011, respectively). As reported previously, pyridostigmine did not increase seated blood pressure significantly, indicating that without orthostatic stimuli pyridostigmine did not exert any pressor effect.
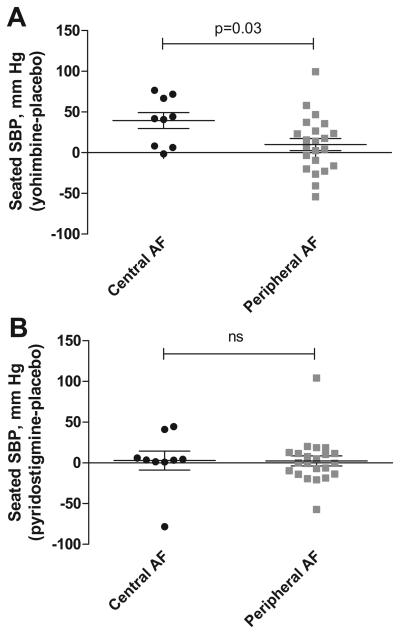
We analyzed the effect of seated systolic and diastolic blood pressure between patients with central versus those with peripheral autonomic failure. Patients with central autonomic failure had statistically significant increase in seated blood pressure with yohimbine as compared to those with peripheral autonomic failure. No significant differences in the effect of pyridostigmine between patients with central versus those with peripheral autonomic failure were found, Figure 4.
Figure 4.
Panel A showed the treatment effect of yohimbine in patients with central autonomic failure (multiple system atrophy) versus those with peripheral autonomic failure (Parkinson's Disease and Pure Autonomic Failure). Panel B showed the treatment effect of pyridostigmine between patients with central versus peripheral autonomic failure
Discussion
The main finding of this study is that targeting residual sympathetic tone with yohimbine, a central and peripheral adrenergic alpha-2 adrenergic antagonist improves standing diastolic blood pressure and pre-syncopal symptoms in patients with severe autonomic failure. On the contrary, the pressor effect of pyridostigmine on standing blood pressure was not different from placebo and was not sufficient to improve pre-syncopal symptoms in this group of patients. Furthermore, the combined use of yohimbine and pyridostigmine did not exert any synergistic effect on standing blood pressure.
Yohimbine increases norepinephrine release from sympathetic nerves by augmenting sympathetic nervous outflows and by interfering with inhibitory modulation of alpha-2 adrenoreceptors. This translates into a pressor effect that can be used as therapeutic agent for patients with autonomic failure.15, 16 The pressor effect of yohimbine depends on the presence of residual sympathetic tone. 15 Moreover, we found a statistically significant difference in the pressor effect to yohimbine depending on the level of the lesion. Patients with central autonomic failure (MSA) have intact efferent sympathetic nerves and had a greater pressor response than patients with peripheral autonomic failure (PAF, PD), who are affected by loss of efferent noradrenergic fibers. Previous studies have reported similar findings,17 in patients with peripheral autonomic failure, the pressor response to yohimbine has been shown to correlate with neuroimaging evidence for cardiac sympathetic denervation as indicated by low concentration of 6-[18F] fluorodopamine-derived radioactivity in the interventricular septal myocardium. Even in this later group, the response to yohimbine varied significantly supporting the hypothesis that the loss of autonomic function is incomplete in many patients with severe autonomic failure.18 On the other hand, some of the heterogeneity in response to yohimbine may be explained by inter-individual differences in bioavailability of yohimbine, differences in receptor sensitivity to released norepinephrine, or blockade of vascular α2-adrenoreceptors.19 Of note, yohimbine had no pressor effects in cases of autonomic failure due to dopamine-β-hydroxylase deficiency,20 presumably because there is no norepinephrine in the neurons of these patients.
Two previous studies have reported the beneficial effect of pyridostigmine as a treatment for orthostatic hypotension in patients with autonomic failure. The effect of pyridostigmine on standing diastolic blood pressure was first tested in an open label study11 and then in a double-blind, randomized 4-way crossover study.10 Pyridostigmine facilitates sympathetic ganglionic neurotransmission, which is cholinergic. The appeal of this pharmacological approach is that it will be silent under conditions of low sympathetic tone such as in supine posture, but will potentiate the sympathetic activation that occurs on standing. This drug has the potential, therefore, to improve orthostatic blood pressure without worsening supine hypertension. In disagreement with previous published studies, we found that not to be the case in our patients. The magnitude of the increase in standing blood pressure was not different from placebo and this explains the lack of improvement in pre-syncopal symptoms. Furthermore, no differences were found in the pressor effect of pyridostigmine between central versus peripheral autonomic failure. Our results would seem to challenge the concept that pyridostigmine is beneficial for the treatment of orthostatic hypotension in autonomic failure patients. It should be noted, however, that our cohorts of patients were more severely affected by autonomic impairment than those recruited in previous studies; the average decrease in systolic blood pressure reported by Singer et al., was ~44 mm Hg11 whereas in our population the average decrease was ~ 60 mm Hg. Thus, it is possible that a significant effect could be achieved in patient with greater autonomic “reserve” as would be expected by the proposed mechanism of action of pyridostigmine.
We hypothesized that the combination of pyridostigmine and yohimbine would result in synergistic pressor effect, given their distinct and complementary sites of action. This, however, was not the case. It is possible that residual sympathetic tone was maximally activated with yohimbine in these patients so that an additional effect of pyridostigmine was no apparent. It remains possible that a synergistic benefit of this combination could be apparent in patients with less severe autonomic failure.
Perspectives
This is the first study to compare the effect of yohimbine and pyridostigmine and the combination on blood pressure in patients with severe autonomic failure. Both drugs act by engaging residual sympathetic tone, producing a pressor effect in patients with autonomic failure. Our results support the concept that the loss of autonomic failure is incomplete in patients with autonomic failure suggesting that this mechanism is an important therapeutic target.
Acknowledgements
The authors would like to thank the personnel of Vanderbilt's General Clinic Research Center and the patients that participated in this research.
Sources of Funding
This work was supported in part by grants P01 HL56693, R01 NS055670, UL1-RR24975 from the National Center for Research Resources, National Institute of Health. C.S. is supported by grant KL2-RR024977 from the National Institute of Health.
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Shibao C, Gamboa A, Diedrich A, Biaggioni I. Management of hypertension in the setting of autonomic failure: a pathophysiological approach. Hypertension. 2005;45:469–476. doi: 10.1161/01.HYP.0000158835.94916.0c. [DOI] [PubMed] [Google Scholar]
- 2.Shear L. Orthostatic hypotension. Treatment with sodium chloride and sodium retaining steroid hormones. Arch Intern Med. 1968;122:467–471. doi: 10.1001/archinte.122.6.467. [DOI] [PubMed] [Google Scholar]
- 3.Frick MH. 9-alpha-fluorohydrocortisone in the treatment of postural hypotension. Acta Med Scand. 1966;179:293–299. doi: 10.1111/j.0954-6820.1966.tb05461.x. [DOI] [PubMed] [Google Scholar]
- 4.Jankovich J, Gilden JL, Hiner BC, Kaufmann H, Brown DC, Coghlan CH, Rubin M, Fouad-Tarazi FM. Neurogenic orthostatic hypotension: a double-blind, placebo-controlled study with Midodrine. Am J Med. 1993;95:38–48. doi: 10.1016/0002-9343(93)90230-m. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann H, Brannan T, Krakoff L, Yahr MD, Mandeli J. Treatment of orthostatic hypotension due to autonomic failure with a peripheral alpha-adrenergic agonist (midodrine) Neurology. 1988;38:951–956. doi: 10.1212/wnl.38.6.951. [DOI] [PubMed] [Google Scholar]
- 6.Wright RA, Kaufmann HC, Perera R, Opfer-Gehrking TL, McElligott MA, Sheng KN, Low PA. A double-blind, dose-response study of midodrine in neurogenic orthostatic hypotension. Neurology. 1998;51:120–124. doi: 10.1212/wnl.51.1.120. [DOI] [PubMed] [Google Scholar]
- 7.Freeman R. Treatment of orthostatic hypotension. Semin Neurol. 2003;23:435–442. doi: 10.1055/s-2004-817727. [DOI] [PubMed] [Google Scholar]
- 8.Vagaonescu TD, Saadia D, Tuhrim S, Phillips RA, Kaufmann H. Hypertensive cardiovascular damage in patients with primary autonomic failure. Lancet. 2000;26:725–726. doi: 10.1016/S0140-6736(99)05320-9. [DOI] [PubMed] [Google Scholar]
- 9.Chobanian AV, Volicer L, Tifft CP, Gavras H, Liang CS, Faxon D. Mineralocorticoid-induced hypertension in patients with orthostatic hypotension. N Engl J Med. 1979;301:68–73. doi: 10.1056/NEJM197907123010202. [DOI] [PubMed] [Google Scholar]
- 10.Singer W, Sandroni P, Opfer-Gehrking TL, Suarez GA, Klein CM, Hines S, O'Brien PC, Slezak J, Low PA. Pyridostigmine treatment trial in neurogenic orthostatic hypotension. Arch Neurol. 2006;63:513–518. doi: 10.1001/archneur.63.4.noc50340. [DOI] [PubMed] [Google Scholar]
- 11.Singer W, Opfer-Gehrking TL, McPhee BR, Hilz MJ, Bharucha AE, Low PA. Acetylcholinesterase inhibition: a novel approach in the treatment of neurogenic orthostatic hypotension. J Neurol Neurosurg Psychiatry. 2003;74:1294–1298. doi: 10.1136/jnnp.74.9.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Killian TJ, Robertson D, Biaggioni I, Haile V, Biscaia I, Robertson RM. Sympathetic nervous system function in man is enhanced by acetylcholinesterase inhibition. Circulation. 1990;82(suppl III):III–636. [Google Scholar]
- 13.Biaggioni I, Robertson RM, Robertson D. Manipulation of norepinephrine metabolism with yohimbine in the treatment of autonomic failure. J Clin Pharmacol. 1994;34:418–423. doi: 10.1002/j.1552-4604.1994.tb04981.x. [DOI] [PubMed] [Google Scholar]
- 14.Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy The Consensus Committee of the American Autonomic Society and the American Academy of Neurology. Neurol. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 15.Onrot J, Goldberg MR, Biaggioni I, Wiley R, Hollister AS, Robertson D. Oral yohimbine in human autonomic failure. Neurology. 1987;37:215–220. doi: 10.1212/wnl.37.2.215. [DOI] [PubMed] [Google Scholar]
- 16.Jordan J, Shannon JR, Biaggioni I, Norman R, Black BK, Robertson D. Contrasting actions of pressor agents in severe autonomic failure. American Journal of Medicine. 1998;105:116–124. doi: 10.1016/s0002-9343(98)00193-4. [DOI] [PubMed] [Google Scholar]
- 17.Sharabi Y, Eldadah B, Li ST, Dendi R, Pechnik S, Holmes C, Goldstein DS. Neuropharmacologic distinction of neurogenic orthostatic hypotension syndromes. Clin Neuropharmacol. 2006;29:97–105. doi: 10.1097/01.WNF.0000220822.80640.0D. [DOI] [PubMed] [Google Scholar]
- 18.Jordan J, Shannon JR, Black BK, Lance RH, Squillante MD, Costa F, Robertson D. N(N)-nicotinic blockade as an acute human model of autonomic failure. Hypertension. 1998;31:1178–1184. doi: 10.1161/01.hyp.31.5.1178. [DOI] [PubMed] [Google Scholar]
- 19.Goldberg MR, Robertson D. Evidence for the existence of vascular α2-adrenergic receptors in humans. Hypertension. 1984;6:551–556. doi: 10.1161/01.hyp.6.4.551. [DOI] [PubMed] [Google Scholar]
- 20.Robertson D, Goldberg MR, Onrot J, Hollister AS, Thompson JC, Wiley R, Robertson RM. Isolated failure of autonomic noradrenergic neurotransmission: evidence for impaired beta-hydroxylation of dopamine. N Engl J Med. 1986;314:1494–1497. doi: 10.1056/NEJM198606053142307. [DOI] [PubMed] [Google Scholar]