Abstract
Objective
Circulating angiogenic cells (CACs), also termed endothelial progenitor cells, play an integral role in vascular repair and are functionally impaired in coronary artery disease (CAD). The role of nitric oxide (NO) in CAC function is poorly understood. We hypothesized that CAC migration toward angiogenic signals is modulated by both NO synthase (NOS) expression and functional response to NO.
Methods and Results
Similar to endothelial cells, CAC chemotaxis to VEGF was blocked by inhibition of NOS, phosphoinositide-3 kinase, or guanylyl cyclase, or by treatment with an NO scavenger. Addition of a NO donor (SNAP) and the NOS-substrate L-arginine increased random cell migration (chemokinesis) and enhanced VEGF-dependent chemotaxis. Healthy CACs expressed eNOS, but eNOS was not detected in CAD patient CACs. Both chemokinesis and chemotaxis to VEGF of patient CACs were decreased compared to healthy CACs, but were restored to healthy values by SNAP. In parallel, CAD patients exhibited lower flow-mediated vasodilation and plasma NO source nitrite than young healthy subjects, indicating endothelial dysfunction with reduced NO bioavailability.
Conclusions
NOS activity is required for CAC chemotaxis. In CAD patients, impairment of NOS expression and NO bioavailability, rather than response to NO, may contribute to CAC dysfunction and limit their regenerative capacity.
Keywords: circulating angiogenic cells, endothelial progenitor cells, nitric oxide, coronary artery disease, migration
NO is an important signaling molecule in vascular biology.1 Physiologically, many integral functions of the vascular endothelium are modulated by eNOS-derived NO, including the inhibition of platelet and leukocyte adhesion, and smooth muscle relaxation, and proliferation. Newer literature shows that NO does not only act in paracrine manner but may also exert systemic effects via reversible formation of more stable storage forms, including nitrite and nitoso-adducts. The disruption of this pathway in endothelial cells is associated with chronic vascular disease.2 Risk factors appear to selectively damage the vascular endothelium leading to a dysfunctional maladaptive endothelial phenotype.3,4 Studies suggest that endothelial NOS activity, expression and circulating NO storage forms in blood are progressively decreased with cardiovascular risk factors including aging, hypertension, hypercholesterolemia, diabetes, and smoking and cigarette smoke exposure.2,5–7 Over time, chronic endothelial dysfunction leads to intimal hyperplasia and enhanced plaque formation in predisposed areas of the vascular tree. Notably, the functional capacity of the vascular endothelium not only depends on the degree of damage, but also on the presence and status of repair systems including circulating angiogenic cells (CACs).8
Vascular repair involves not only local migration and proliferation of mature endothelial cells but also angiogenic cells that circulate in blood and the recruitment of the latter cells to sites of injury. Literature from the last 10 years suggests that circulating pro-angiogenic blood cells can enhance angiogenesis and the replacement of vascular endothelium.8–10 These cells were initially termed endothelial progenitor cells (EPCs) because of their phenotypical similarities with mature endothelial cells including KDR, eNOS, and PECAM (CD31), but also with stem cells (CD34 and CD133) and myeloid cells (CD14 and CD45). Newer literature suggests that these early outgrowth angiogenic cells temporarily aid endothelial repair, rather than develop into mature endothelial cells, in contrast to late outgrowth endothelial colony forming cells that can form endothelial tubes and monolayers.11,12 Therefore, these cells are herein referred to as “CACs” rather than “early EPCs.” Clinical and experimental studies show that the reparative and therapeutic potency of CACs is determined by their functional status, which, in turn, is characterized by migratory capacity towards chemotactic signals such as vascular endothelial growth factor (VEGF).13,14
Several studies suggest that cardiovascular disease may not only be caused by endothelial damage, but also may cause or be caused by CAC dysfunction. The number and or function of these cells is reduced with aging,6 hypertension, diabetes,15 smoking,16 and environmental smoke exposure.17 CAC dysfunction was shown to limit the therapeutic potency of these cells when transplanted.13 It is conceivable that the functional capacities of CACs in patients may also be affected by the patho-mechanisms that impair endothelial cell dysfunction, including decreased NO production and bioavailability, further facilitating vascular disease progression.18 The role of NO activity in fundamental functional CAC capacities is not well studied. We hypothesized that CAC migration is modulated by NO and that CAC dysfunction in coronary artery disease (CAD) patients is a result of reduced NO bioavailability in blood or decreased NOS expression.
We first characterized the chemotactic response of CACs and compared the results to human umbilical vein endothelial cells (HUVECs) serving as a standard endothelial cell system. We then studied the effect of the NO donor SNAP on migration of CACs and HUVECs. Lastly, we measured eNOS expression and migratory responses in ex vivo differentiated CACs isolated from CAD patients, who were shown to suffer from endothelial dysfunction with impaired NO bioavailablity, and compared them with young healthy volunteers.
Methods
Study subjects
(See Supplement Table I for characteristics) CACs were isolated from 10 young healthy subjects without cardiovascular risk factors (hypertension, diabetes mellitus, smoking, and hypercholesterolemia, which are associated with impaired number and function of CACs), and with normal endothelial function (as measured by flow-mediated dilation of the brachial artery of greater than 6%).2,6,19 We also isolated CACs from 10 patients with angiographically documented CAD as defined by >70% stenosis of at least one coronary artery on optimal medical therapy according to current secondary prevention guidelines 20 and endothelial dysfunction with FMD <5%. The characterization of CACs, including mechanistic experiments, was performed in CACs isolated from the healthy subjects. The protocol was approved by the UCSF Committee on Human Research and volunteers gave written informed consent.
Cell culture and characterization of blood-derived CACs
CACs were differentiated ex vivo from peripheral blood mononuclear cells (MNC) as previously described (see online supplement for more detailed characterization protocols).11,21 CACs were isolated from MNCs as adherent cells on fibronectin coated dishes after 7 days. Culture was preceeded by 1 day preplating to remove platelets and shedded endothelial cells. eNOS protein was quantitated in cell lysates of CACs at day 7 and VEGF in cell medium of adherent and non-adherent cells using commercially available ELISA-kits following the manufacturer’s protocol (Quantikine, R&D Systems). Marker expression CD45, CXCR4, CD31, KDR, CD11b, CD14, CD3, CD34, CD133 of day 7 cells was determined by flow cytometry.
Pooled HUVECs were purchased from Cambrex (Walkersville, MD), cultured in EBM-2 (supplemented with Singlequots 5% FBS) and used no later than passage 3.
Chemotaxis and chemokinesis assay
Cell migration was quantified by a transwell chemotaxis assay using a modified Boyden chamber.13,22,23 Migration of both CACs and HUVECs was measured as follows: Cells (2×104) were plated in EBM-2 medium (0.5% BSA, without other supplements containing 63 mg/l L-arginine) in the upper of two chambers divided by a membrane with 8 µm pores (Corning Transwell). We tested the chemotactic properties of the following chemoattractants in only the lower chamber: vascular endothelial growth factor (VEGF, Sigma), stromal cell-derived factor (SDF-1α; Sigma), and pleiotrophin (PTN, Sigma) at 10–500 ng/mL, monocyte chemoattractant protein-1 (MCP-1, Sigma), sphingosine-1-phosphate (S1P, Sigma), and interleukin-6 (IL6, Sigma) at 10–100 ng/ml. The following were added to both the upper and lower chamber: NOS substrate L-arginine (100 µmol/l), NOS inhibitor L-NNA (100 µmol/l), NO scavenger PTIO (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, 100 µmol/l), guanylyl cyclase inhibitor ODQ (1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one, 100 µmol/l), the PI3 kinase inhibitor Wortmannin (WM; 100 nmol/l), and NO-donor S-nitroso-N-acetylpenicillamine (SNAP, Sigma) at 1 nmol/l–10 µmol/l.The number of migrated cells was determined on 5 random 100× optical fields per membrane. To distinguish chemokinetic from chemotactic properties of VEGF and SNAP, both substances were added to upper and lower chambers in a checkerboard fashion.
Cell proliferation and apopotosis assays
BrdU incorporation assays were performed in 96-well dishes following the manufacturer’s protocol (Cell Proliferation BrdU Assay, Roche). Apoptosis assays were performed with FACS essentially as described in the manufacturer’s (Guava, Hayward, CA) protocol. cGMP levels were measured in 105 cells at baseline unstimulated conditions and after 30 incubation with SNAP at 1 µmol/L over 30 min using an ELISA kit following the manufacturer’s protocol (GE Healthcare).
Flow-mediated dilation (FMD)
Endothelium-dependent dilation of the brachial artery (BA) was measured by ultrasound (Sonosite Micromax, Bothell, WA) in combination with an automated analysis system (Brachial Analyzer, Medical Imaging Applications, Iowa City, IA) as described (see supplement for details).17
Plasma nitrite level
The plasma nitrite levels, representing a sensitive read-out of NOS activity, was measured as recently described using gas-phase chemiluminescence (see supplement for details).24
Statistical analyses
Data are presented as mean ± standard error of the mean. Group differences were calculated with repeated measurements ANOVA and consecutive post hoc test. P-values of less than 0.05 were regarded as significant. Correlations were Pearson’s r. All experiments were performed in triplicate.
Results
CAC characterization
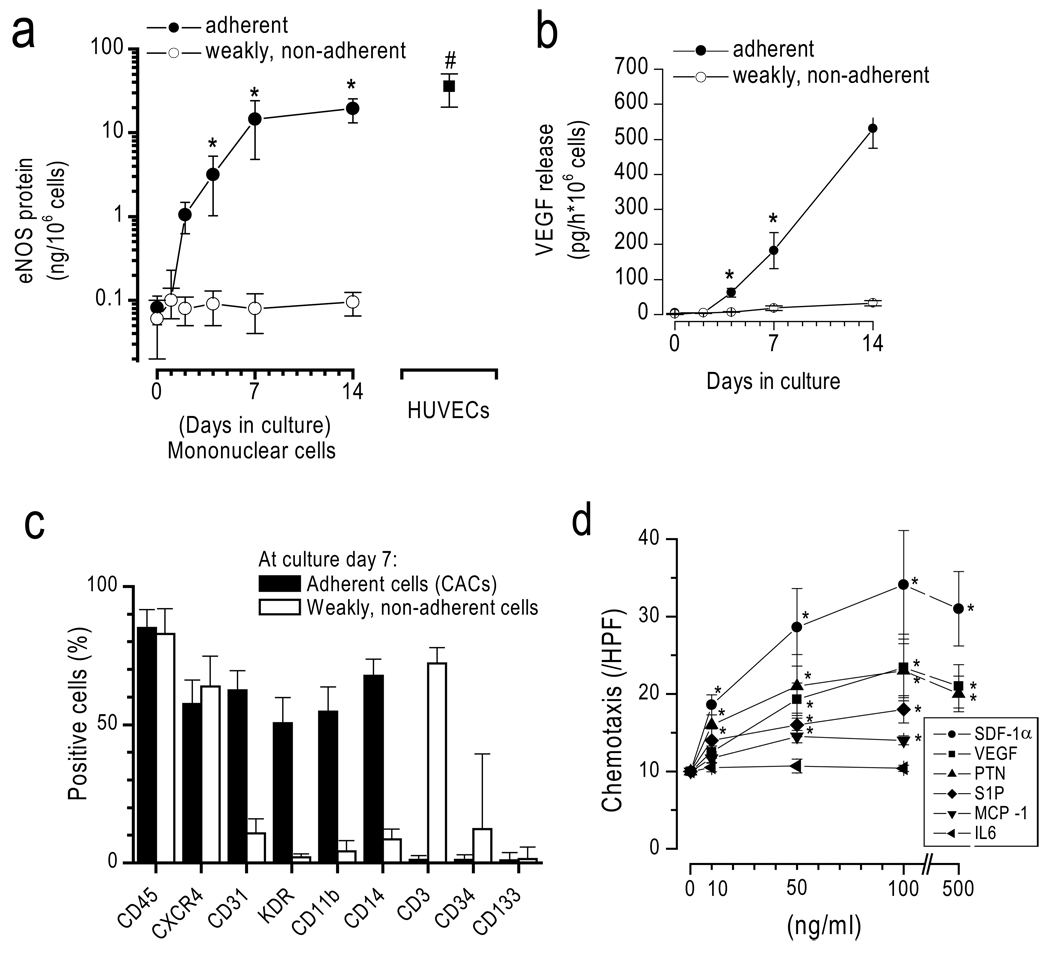
During culture, MNCs gave rise to adherent cells progressively expressing eNOS and releasing VEGF (Figure 1). At day 7, the majority of adherent cells expressed markers consistent with the early pro-angiogenic hematopoietic EPC type as described in the literature, whereas non-adherent cells, which were not further studied herein, were mainly consistent with lymphocytes.11,25,26 CACs migrated dose-dependently to a number of chemokines including VEGF, SDF-1α, PTN23, S1P, and MCP-1, but not to IL-6 at the concentrations tested (Figure 1d). Of the investigated chemokines, SDF-1α exerted the strongest migratory response (SDF-1α >VEGF=PTN> MCP-1= S1P).
Figure 1. Characterization of CACs.
Adherence-selected mononuclear cells (a) progressively express eNOS, (b) release VEGF, and (c) express hematopoietic, monocytic, and endothelial markers. (d) Day 7 CACs exhibit chemotaxis toward VEGF, PTN, SDF-1α, MCP-1, and S1P, but not towards IL-6. *p<0.05 vs control.
Mechanisms of CAC chemotaxis: Similarity with endothelial cells (HUVECs)
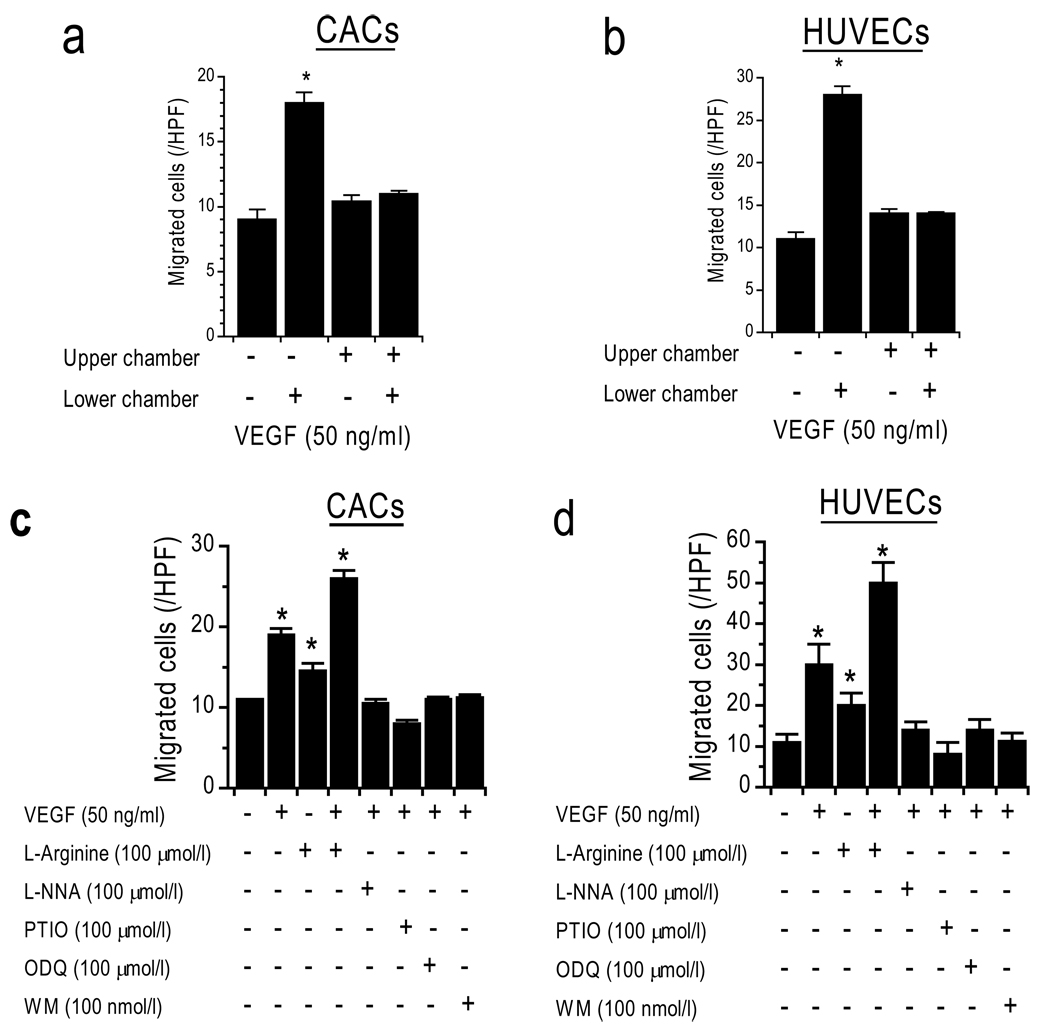
Further experiments showed that random cell movement in the presence of VEGF (50 ng/ml) in the upper and lower chamber (that is, no gradient) did not lead to significantly more cells on the lower side of the membrane as compared to the negative control lacking VEGF (Figure 2a,b). This confirms that VEGF does not merely induce a significant chemokinetic response, but stimulates specific chemotactic responses in CACs and HUVECs 23.
Figure 2. Mechanisms of CAC chemotaxis are similar to those of HUVECs.
VEGF is a chemotactic stimulus for (a) CACs and (b) HUVECs. Chemotaxis to VEGF is inhibited by L-NNA (NOS-inhibitor), PTIO (NO-scavenger), ODQ (guanylyl-cyclase-inhibitor), and WM (PI3K-inhibitor), and is increased by L-arginine (NOS-substrate). *p<0.05 vs. control. (c–d: VEGF was only added to lower chamber; L-NNA, ODQ, PTIO, WM, and L-arginine to upper and lower chambers)
In order to gain mechanistic insight into chemotaxis of CACs, we performed inhibitor studies to attempt to block the VEGF-induced chemotaxis (Figure 2c,d). Chemotaxis toward VEGF at 50 ng/ml was inhibited in the presence of a NOS-inhibitor (L-NNA), an NO scavenger (PTIO), a phosphoinositol 3-phosphate kinase inhibitor (WM, Wortmannin), and a guanylyl cyclase inhibitor (ODQ). Non-directional cell movement, without addition of chemokines, remained unaffected by these inhibitors, suggesting that the observed lack of chemotaxis is due to specific inhibition of the pathways in question, and cannot be explained by unspecific cell toxicity or globally disabled cell motility. Furthermore, our experiments show that the mechanisms involved in CAC chemotaxis are similar in HUVECs. Interestingly, L-arginine in the upper and lower chambers (no gradient) not only increased chemotaxis toward the VEGF gradient but enhanced chemokinesis.
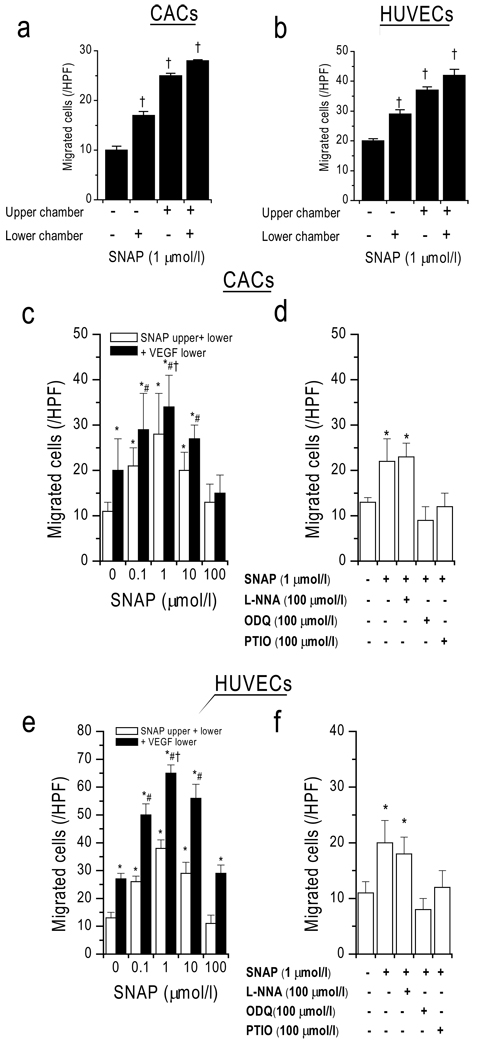
NO donor induces chemokinesis and enhances chemotaxis
In order to test how NO itself affects CAC motility and whether CACs follow a gradient of NO, we performed migration assays with the NO-donor SNAP (Figure 3). As opposed to VEGF, SNAP induced a strong increase in random cell movement (chemokinesis). However, the number of migrated cells when SNAP (1 µmol/l; measured NO concentration in medium 2.7 and 0.8 nmol/L at 0 h and 3 h, respectively) was present in both the upper and lower chambers was greater than when SNAP was present only in the lower chamber, suggesting that NO is a stronger inductor of chemokinesis than chemotaxis. Dose-dependent chemokinesis at 0.01–50 µmol/l showed a maximum at 1 µmol/l (28±3 cells/HPF). Directional cell movement toward a VEGF gradient was additionally present at these SNAP concentrations. The highest absolute number of migrated cells was observed with a VEGF gradient (50 ng/ml) in the presence of SNAP at 1 µmol/l (34±4 cells/HPF). These findings illustrate that SNAP-mediated chemokinesis acts synergistically with VEGF-induced chemotaxis to enhance the number of net migrated cells, suggesting that exogenous NO facilitated directional cell movement to a chemokine stimulus. Addition of ODQ and PTIO but not L-NNA inhibited the chemokinetic SNAP (1 µmol/l) response, suggesting guanylate cyclase dependence, NO specificity, and NOS-independence. This shows that both CACs and HUVECs similarly distribute faster in the presence of NO but are still responsive toward chemotactic stimuli.
Figure 3. NO donor SNAP causes dose-dependent chemokinesis and enhances VEGF-mediated chemotaxis.
(a,b) SNAP stimulated random cell movement (chemokinesis). (c,e) Dose-dependent stimulation of chemokinesis and chemotaxis toward VEGF. (d,f) Inhibition by ODQ (guanylyl-cyclase-inhibitor) and PTIO (NO-scavenger) but not by NOS inhibitor L-NNA. † p<0.05 vs. respective column to the left, *p<0.05 vs control, #p<0.05 vs respective white column, †p<0.05 vs VEGF alone.
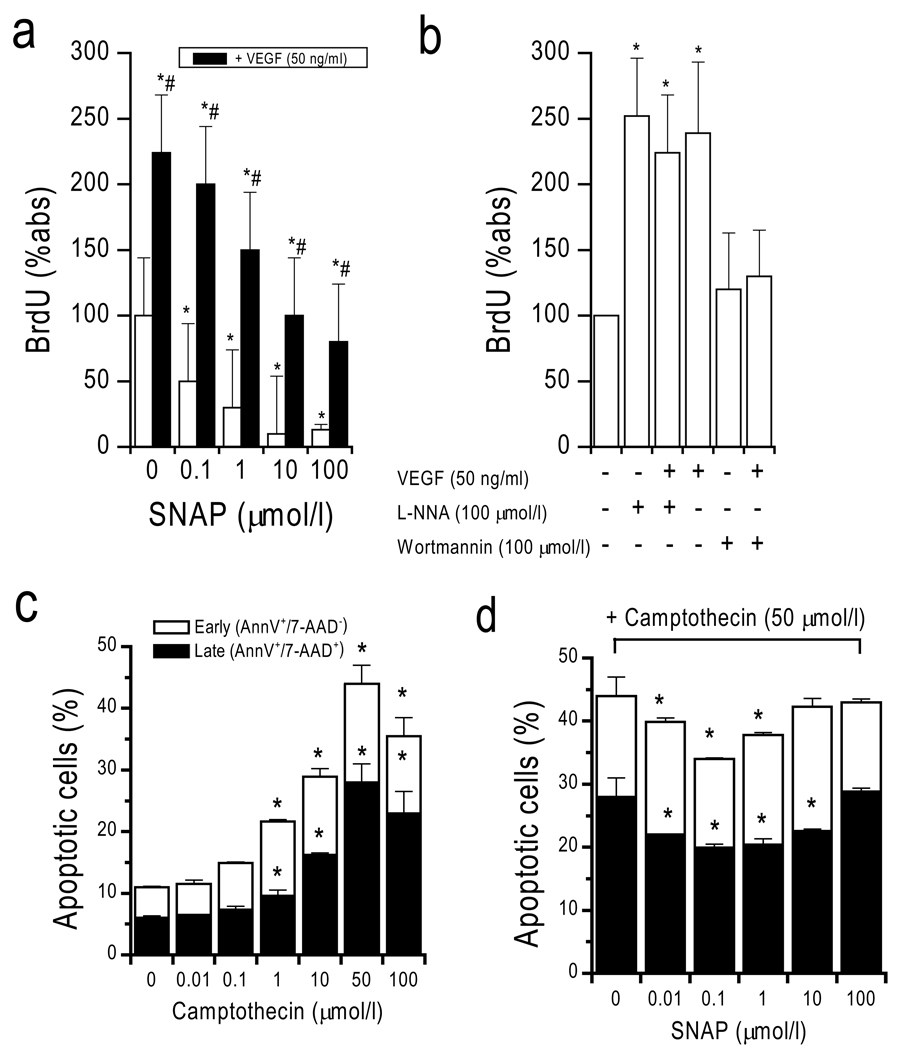
In addition to its effects on cell migration, SNAP dose-dependently inhibited both VEGF-induced and spontaneous CAC proliferation (Figure 4). Conversely, inhibition of NOS by L-NNA stimulated proliferation to a similar degree as VEGF, suggesting opposite effects of VEGF and NO with respect to proliferation as previously shown.23 VEGF-induced proliferation was inhibited by wortmannin, suggesting dependence of this effect on phosphoinositol 3-phosphate kinase but not NOS.
Figure 4. Effect of SNAP on CAC proliferation and apoptosis.
(a) Proliferation of CACs. (b) VEGF-induced proliferation was increased by addition of L-NNA (NOS-inhibitor), and inhibited by WM (PI3K-inhibitor). (c) Dose-finding for camptothecin-induced apoptosis (early apoptotic cells: AnnV+/7-AAD− [white columns]; late apoptotic cells: AnnV+/7-AAD+ [black columns]). (d) SNAP dose dependently inhibited apoptosis.
SNAP also dose-dependently inhibited camptothecin-induced apoptosis of CACs (Figure 4). Early apoptosis was identified by annexinV-binding (AnnV+) along with 7-AAD exclusion (7-AAD−) showing that the cell membrane was intact. AnnexinV binding along with 7-AAD uptake (i.e. disrupted membrane) identified late apoptosis. Vital cells were identified as being negative for annexin V-binding and 7-AAD. Camptothecin dose-dependently induced apoptosis in CACs. Maximal apoptosis of CACs was achieved with >10 µmol/l camptothecin at 3 h (40% early apoptosis AnnV+/7-AAD 20% late apoptosis AnnV+/7-AAD+). Coincubation of SNAP at 0.1–100 µmol/l led to dose-dependent inhibition of camptothecin-induced apoptosis at 50 µmol/l with significantly higher numbers of vital cells (AnnV−/7-AAD−). Maximal effects were observed at 1 µmol/l SNAP. The degree of apoptosis inhibition was similar to that induced by VEGF (50 ng/ml), which is known to inhibit apoptosis. Similar results were obtained when apoptosis was induced by staurosporin (data not shown).
Impaired CAC migration and NOS expression in CAD patients
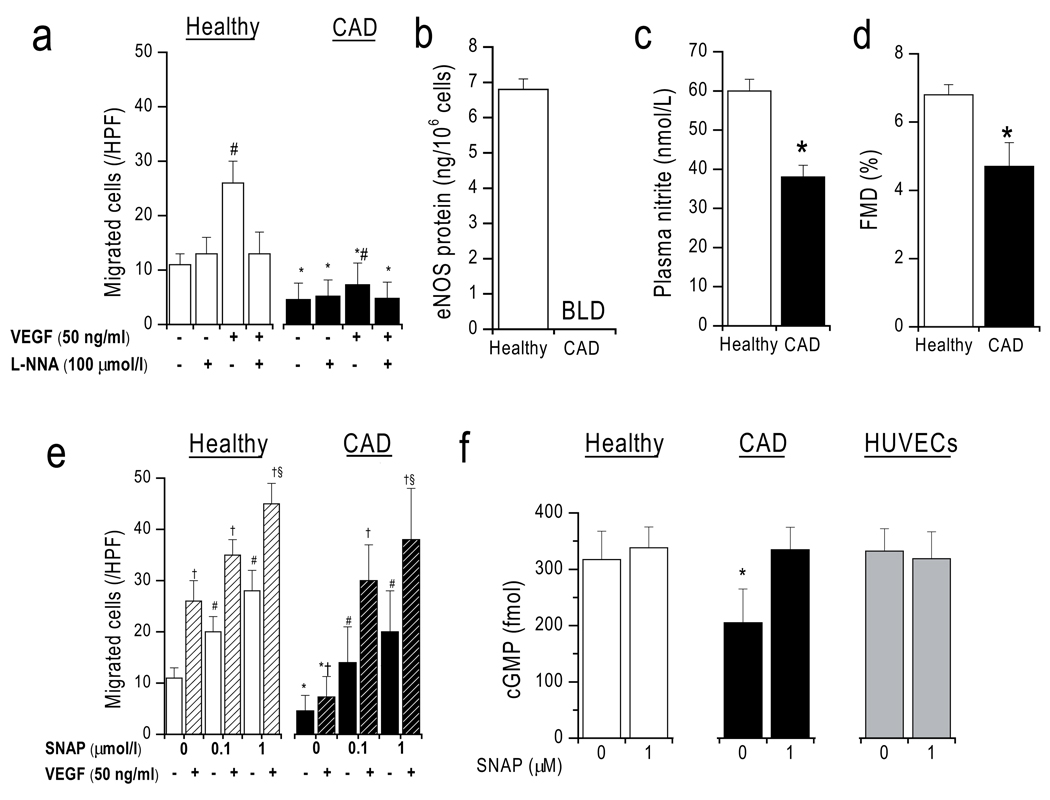
In order to show the clinical relevance of these findings to human cardiovascular disease, we measured CAC migration as a read-out of therapeutic potency and quantitated eNOS protein in CACs from older CAD patients as compared to those from younger healthy subjects (age: 56±3 vs 30±2 years, p<0.001). Table 1 (online supplement) summarizes the clinical baseline characteristics. Flow-mediated vasodilation (FMD; 4.7±0.7 vs. 6.8±0.3%, p<0.001, Figure 5) and plasma nitrite (38±3 vs. 60±3 nmol/l, p<0.001) were significantly lower in the patients, demonstrating endothelial dysfunction and impaired systemic NO bioavailability in these patients despite optimal medical therapy including statins.
Figure 5. Impaired chemotaxis and eNOS expression in CAD patients.
(a) L-NNA inhibitable CAC migration is significantly reduced in CAD patients as compared healthy controls. (b) eNOS protein expression in CAD CACs is below limit of detection (BLD). (c) Plasma nitrite and (d) flow-mediated vasodilation (FMD) were impaired in CAD, reflecting endothelial dysfunction. (e) CAC random cell movement and chemotaxis (toward 50 ng/ml VEGF; hatched columns) dose-dependently increase in CACs from both groups. (f) respective cGMP levels. *p<0.05 vs respective condition in healthy group, #p<0.05 vs random cell movement without additives same group. †p<0.05 vs respective no VEGF condition. §p <0.05 vs VEGF alone same group.
In vitro assays showed that in CAD both unstimulated random CAC movement and chemotaxis toward VEGF (5±3 and 7±4 cells/HPF, respectively) were significantly impaired compared to healthy subjects (11±2 and 26±4 cells/HPF, each p<0.001) (Figure 5). Importantly, eNOS levels were also significantly reduced (below the detection limit of the assay) in older CAD patients as compared to healthy volunteers, offering a feasible explanation for the decreased functional capacity of these patients’ CACs. SNAP added to both chambers at 0–1 µmol/l led to a similar dose-dependent chemokinetic migratory response in both groups and enhanced chemotaxis to VEGF (healthy n=10, CAD n=5 due to insufficient number of cells from 5 of the patients). In the presence of SNAP at >0.1 µmol/l, the migratory response was not significantly different between healthy and CAD patients. Whereas baseline intracellular cGMP levels were significantly lower in CAD, there was no significant difference between healthy and CAD after incubation with SNAP (1 µmol/l). No significant differences were seen in CD45 (98±2%, 97±1%) and CD31 (26±4%, 34±9%) expression by FACS, and iNOS mRNA was not detected in either kind of cell (data not shown). This suggests that response to exogenous NO was preserved in CAD patients despite a reduction in NOS-dependent response to endogenous NO.
Discussion
Our data show that both endogenous NOS activity and exogenous NO modulate CAC motility. NOS activity is required for chemotactic migration of CACs to angiogenic chemokines, whereas exogenous NO induces chemokinesis, enhancing directional chemotaxis toward VEGF, without directly acting as a chemoattractant itself. Notably, the effects of the NO donor SNAP and NOS on the CACs were qualitatively similar to their effects on HUVECs, despite the presumption that these early pro-angiogenic CACs do not function as direct endothelial precursors. We show clinical relevance in that CAC migration in CAD patients is limited by decreased endogenous NOS activity due to impaired expression rather than impaired response to exogenous NO.
NOS plays an important regulatory role in vascular biology and defective endothelial NO synthesis may limit angiogenesis in patients with endothelial dysfunction.27 An impairment of the endogenous NO signaling in endothelium is coupled to inability to produce an angiogenic response to VEGF.28,29 The effects of NO on CACs, which are important cells for endothelial repair, are not well understood. We describe here how the presence or absence of NO affects CAC motility. Corroborating previous studies,17,23,30 we demonstrate that CACs migrate to a number of chemokines NOS-dependently. This supports the notion that NOS represents an integral pathway for cell migration. We have recently shown that CACs migrate to a gradient of pleiotrophin (PTN) in a manner that is dependent on NOS, cGMP, NO, and PI3 Kinase.23 Similarly, it was previously shown by others that SDF-1α induces CAC migration in an eNOS, Akt, and PI3K dependent manner.30 We show here that the migration to VEGF involves the same pathways as migration to PTN and SDF-1α. This is important because a number of risk factors promoting arteriosclerosis and poor tissue regeneration, including smoking, aging, diabetes, hypertension, and hypercholesterolemia, have been shown to also inhibit NO production.2,17 These factors may mediate part of their vascular pathology by affecting vascular maintenance exerted by lowered NOS activity potentially via oxidative stress not only in endothelial cells, but also in CACs leading to dysfunction of these cells. This is supported by several papers in animal models and human clinical studies. In a recent clinical paper, we have shown that passive smoke may decrease CAC migration by blocking NO production.17 Animal hindlimb ischemia experiments have revealed that angiogenesis is impaired in eNOS −/− mice and that the eNOS substrate L-arginine can enhance angiogenesis in rabbits.27 Another study suggests that diabetes may impair re-endothelialization by impaired CAC function due to decreased eNOS expression.31 More recently, it was shown in diabetic rats and patients that diabetes may impair CAC functions by uncoupling eNOS.15 Taken together, our data suggest that dysfunctional CAC migration in CAD patients may be due to lower eNOS expression rather than impaired response to exogenous NO in CACs.
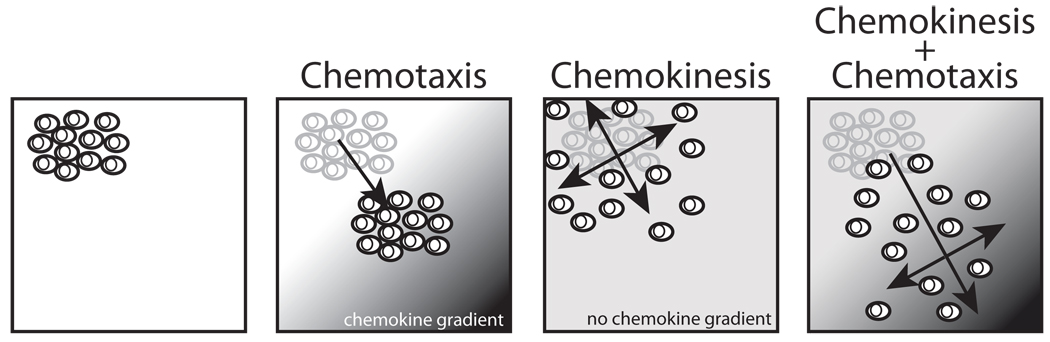
To our knowledge, this is the first paper to show the effect of exogenous NO on CAC migratory function. We show that an NO donor induces chemokinesis. It is important to note that this does not impair the CACs’ capacity to sense chemoattractant gradients and follow them, but actually significantly and additionally increases the net number of migrated cells at the site of higher chemokine concentration (Figure 6). This is in agreement with previously published results by others showing that HUVECs cGMP-dependently migrate towards a gradient of NO using different NO-donors, DEA/NO and DETA/NO, which have a longer half life as compared to SNAP.32 To methodically exclude the possibility that SNAP merely increases cell proliferation at the lower side of the membrane, we performed proliferation assays showing that NO in fact decreases proliferation.33 Corroborating results by others, we show that SNAP also decreased apoptosis.34 In the context of the present study, we cannot exclude the possibility that NO increases survival of CACs and may thereby explain part of the migration results, potentially contributing to more cells recovered at the lower side of the membrane. Mechanistically, both CACs and HUVECs release NO and chemotaxis of both cell types is enhanced by NO-related chemokinesis. This suggests that NO may serve as a signal coordinating and potentially stimulating endothelial and pro-angiogenic cell interactions. NO and chemokines released by pro-angiogenic CACs that have homed to sites of injury may further attract new cells in a positive feedback loop via facilitating chemotaxis and chemokinesis. Once cells reach each other, higher NO levels may enhance adhesion (unpublished results Heiss et al.), and inhibit proliferation and apoptosis while facilitating even dispersal via chemokinesis of CACs and endothelial cells. As the observed effects were dose-dependent, the effect may likely differ between sites with different NO levels, such as inflammation with expression of high output iNOS (micromolar range) or vascular endothelium (low nanomolar range). Furthermore, these results may have clinical importance in disease states with lowered NO bioavailability, e.g. decreased levels of plasma S-nitrosothiols or nitrite which represent physiologic ‘NO donors’ with cardiovascular risk factors.2,24,35,36
Figure 6.
Schematic to illustrate the proposed additive effects of directional (chemotaxis) and non-directional random cell movement (chemokinesis).
Our data further support the concept that the NOS/NO pathway is a strong modulator of CAC functions as it is in endothelial cells. CAC functions are likely to be affected both by factors that impair this pathway of endothelial cells in patients with cardiovascular disease in vivo and by reduced NO bioavailability.2
Supplementary Material
Acknowledgements
The authors thank Dr. Sourabh Kharait for helpful discussion and Dr. Matthias Totzeck for technical assistance.
Sources of Funding
This work was supported by awards from the American Heart Association (0525078Y) and Forschungskommission, University Duesseldorf to CH, the Wayne and Gladys Valley Foundation to WG, the National Institutes of Health (HL086917) to MLS and YY, the UCSF Academic Senate Committee on Research and the American Heart Association (0535244N) to MLS, and by the Deutsche Forschungsgemeinschaft (RA969/4-1) to TR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
MLS has received research funding from N30 Pharmaceuticals.
References
- 1.Cooke JP, Losordo DW. Nitric oxide and angiogenesis. Circulation. 2002;105:2133–2135. doi: 10.1161/01.cir.0000014928.45119.73. [DOI] [PubMed] [Google Scholar]
- 2.Heiss C, Lauer T, Dejam A, Kleinbongard P, Hamada S, Rassaf T, Matern S, Feelisch M, Kelm M. Plasma nitroso compounds are decreased in patients with endothelial dysfunction. J Am Coll Cardiol. 2006;47:573–579. doi: 10.1016/j.jacc.2005.06.089. [DOI] [PubMed] [Google Scholar]
- 3.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 4.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 5.Heiss C, Kleinbongard P, Dejam A, Perré S, Schroeter H, Sies H, Kelm M. Acute consumption of flavanol-rich cocoa and the reversal of endothelial dysfunction in smokers. J Am Coll Cardiol. 2005;46:1276–1283. doi: 10.1016/j.jacc.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 6.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24(No. 6):1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 8.Dimmeler S, Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82:671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 9.Hristov M, Weber C. The therapeutic potential of progenitor cells in ischemic heart disease--Past, present and future. Basic Res Cardiol. 2006;101:1–7. doi: 10.1007/s00395-005-0573-0. [DOI] [PubMed] [Google Scholar]
- 10.Murasawa S, Asahara T. Endothelial Progenitor Cells for Vasculogenesis. Physiology. 2005;20:36–42. doi: 10.1152/physiol.00033.2004. [DOI] [PubMed] [Google Scholar]
- 11.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rehman J, Li J, Orschell CM, March KL. Peripheral blood "endothelial progenitor cells" are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 13.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 14.Keymel S, Kalka C, Rassaf T, Yeghiazarians Y, Kelm M, Heiss C. Impaired endothelial progenitor cell function predicts age-dependent carotid intimal thickening. Basic Res Cardiol. 2008;103:582–586. doi: 10.1007/s00395-008-0742-z. [DOI] [PubMed] [Google Scholar]
- 15.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas d, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 16.Michaud SE, Dussault S, Haddad P, Groleau J, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423–432. doi: 10.1016/j.atherosclerosis.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Heiss C, Amabile N, Lee AC, Real WM, Schick SF, Lao D, Wong ML, Jahn S, Angeli FS, Minasi P, Springer ML, Hammond SK, Glantz SA, Grossman W, Balmes JR, Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J Am Coll Cardiol. 2008;51:1760–1771. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 18.Thum T, Tsikas d, Stein S, Schultheiss M, Eigenthaler M, Anker SD, Poole-Wilson PA, Ertl G, Bauersachs J. Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol. 2005;46:1693–1701. doi: 10.1016/j.jacc.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 19.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 20.Smith SC, Jr, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation. 2006;113:2363–2372. doi: 10.1161/CIRCULATIONAHA.106.174516. [DOI] [PubMed] [Google Scholar]
- 21.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 22.Falk W, Goodwin RH, Jr, Leonard EJ. A 48-well micro chemotaxis assembly for rapid and accurate measurement of leukocyte migration. J Immunol Methods. 1980;33:239–247. doi: 10.1016/0022-1759(80)90211-2. [DOI] [PubMed] [Google Scholar]
- 23.Heiss C, Wong ML, Block VI, Lao D, Real WM, Yeghiazarians Y, Lee RJ, Springer ML. Pleiotrophin induces nitric oxide dependent migration of endothelial progenitor cells. J Cell Physiol. 2007;215:366–373. doi: 10.1002/jcp.21313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rassaf T, Preik M, Kleinbongard P, Lauer T, Heiss C, Strauer BE, Feelisch M, Kelm M. Evidence for in vivo transport of bioactive nitric oxide in human plasma. J Clin Invest. 2002;109:1241–1248. doi: 10.1172/JCI14995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 26.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morbidelli L, Donnini S, Ziche M. Role of nitric oxide in the modulation of angiogenesis. Curr Pharm Des. 2003;9:521–530. doi: 10.2174/1381612033391405. [DOI] [PubMed] [Google Scholar]
- 29.Kimura H, Esumi H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim Pol. 2003;50:49–59. [PubMed] [Google Scholar]
- 30.Zheng H, Fu G, Dai T, Huang H. Migration of endothelial progenitor cells mediated by stromal cell-derived factor-1alpha/CXCR4 via PI3K/Akt/eNOS signal transduction pathway. J Cardiovasc Pharmacol. 2007;50:274–280. doi: 10.1097/FJC.0b013e318093ec8f. [DOI] [PubMed] [Google Scholar]
- 31.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 32.Isenberg JS, Ridnour LA, Thomas DD, Wink DA, Roberts DD, Espey MG. Guanylyl cyclase-dependent chemotaxis of endothelial cells in response to nitric oxide gradients. Free Radic Biol Med. 2006;40:1028–1033. doi: 10.1016/j.freeradbiomed.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 33.Bussolati B, Dunk C, Grohman M, Kontos CD, Mason J, Ahmed A. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol. 2001;159:993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng H, Dai T, Zhou B, Zhu J, Huang H, Wang M, Fu G. SDF-1alpha/CXCR4 decreases endothelial progenitor cells apoptosis under serum deprivation by PI3K/Akt/eNOS pathway. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 35.Cannon RO, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lundberg JO, Weitzberg E. NO generation from nitrite and its role in vascular control. Arterioscler Thromb Vasc Biol. 2005;25:915–922. doi: 10.1161/01.ATV.0000161048.72004.c2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.