Abstract
In a murine model of inflammatory bowel disease (IBD), treatment of colitis in IL-10 gene deficient mice with the parasitic helminth, Heligmosomoides polygyrus, ameliorates colonic inflammation. The cellular and molecular mechanisms driving this therapeutic host response are being studied vigorously. One proposed mechanism is that H. polygyrus infection favors the outgrowth or suppression of certain bacteria, which in turn help modulate host immunity. To begin to address this hypothesis, we quantified the effect of H. polygyrus treatment on the composition of the gastrointestinal (GI) tract microbiota in the absence of inflammation, using wild-type C57BL/6 mice. Here, we present evidence that a significant shift in the abundance and relative distribution of bacterial species in the ileum of mice is associated with H. polygyrus infection. Members of the bacterial family, Lactobacillaceae, significantly increased in abundance in the ileum of infected mice reproducibly in two independent experiments despite having different microbiotas present at the outset of each experiment. These data support the concept that helminth infection shifts the composition of intestinal bacteria. The clinical consequences of these shifts in intestinal flora are yet to be explored.
Keywords: Inflammatory bowel diseases, Microbial ecology, Heligmosomoides polygyrus, Microbiota, Microbiome
Introduction
The etiology of inflammatory bowel disease (IBD) is complex and influenced by genetic, microbiologic, and environmental factors. Lifestyle changes accompanying the modernization of developing countries have been epidemiologically linked to an increase in IBD incidence (1–6). These observations have led to the IBD hygiene hypothesis, which states that raising children in extremely hygienic environments negatively affects immune development and predisposes them to immunological diseases later in life (6).
Empirical support for the IBD hygiene hypothesis has been developed in mouse and rat models (7–9). These studies revealed that intestinal parasites and parasite products modulate host immunity and decrease IBD-like inflammation (6, 10). Likewise, Trichuris suis (pig whipworm) treatment was effective in decreasing symptoms of IBD in human clinical trials (11, 12).
It is not entirely understood how helminthic parasites modulate the host mucosal immune response and alter susceptibility to immunological diseases (10, 13). One unexplored hypothesis is that helminth infection changes the abundance and/or distribution of gastrointestinal tract bacteria (the microbiota), resulting in an overall decrease in the pro-inflammatory cytokine milieu associated with the chronically inflamed state (6). To begin to address this hypothesis, we studied the effect of helminthic infection on the composition of the GI tract microbiota of their host.
We found that the murine roundworm, Heligmosomoides polygyrus, changes the microbiota of the GI tract of otherwise healthy wild-type mice. We used clone library analysis of the bacterial 16S rRNA encoding gene (16S) to quantify changes that occur in the microbiota associated with the distal small intestine (ileum) and the tip of the cecum. In two independent experiments, we found that the ileal microbiota from H. polygyrus-infected mice was significantly different from uninfected controls. Also, the total bacterial load was greater in infected mice versus controls, and the dominant bacteria associated with the ileum of treated mice were also associated with adult worms that had been removed at the time of necropsy. These data support the hypothesis that parasites alter the abundance and relative distribution of GI tract bacteria and provide the basis for future experiments that investigate the overall importance that this alteration has on host immunity.
Materials and Methods
Mouse experiments and sample collection
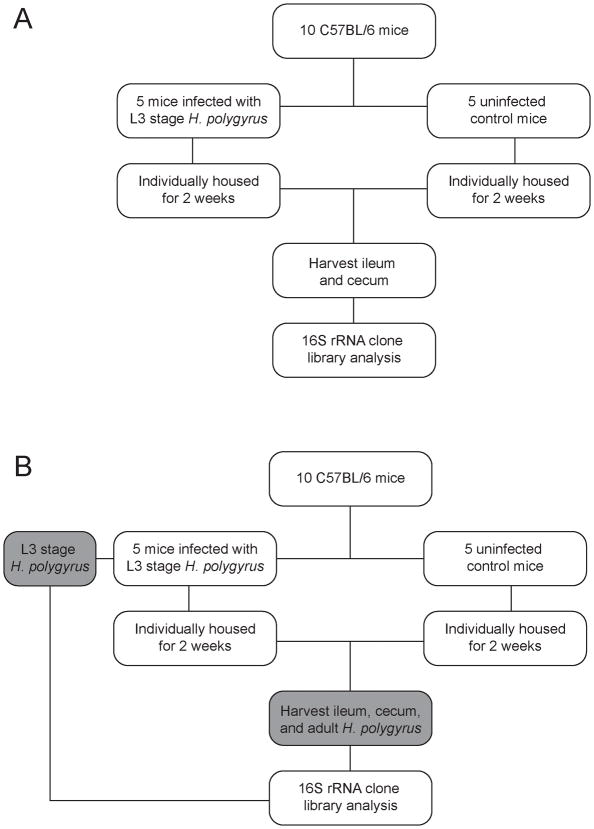
Two independent mouse experiments were completed as outlined in Figure 1. In each experiment, 5 of 10 C57BL/6 mice were infected with L3 stage H. polygyrus for 2 weeks. Upon necropsy, approximately 2 cm of the distal ileum and 1–1.5 cm of the cecal tip were collected from all animals, rinsed with phosphate buffered saline to remove luminal contents, added to Dry Bead Tubes (MoBio Laboratories Inc.), snap frozen in liquid nitrogen, and cryopreserved at −80°C for DNA extraction. During the second experiment, infective L3 stage H. polygyrus and adult worms from the duodenum of infected mice were also obtained for analysis.
Figure 1.
Overview of two independent murine infection experiments. The difference between the first experiment (A) and the second (B) was the inclusion of the L3 larval stage and adult H. polygyrus worms.
16S clone library construction and analyses
Total, bulk DNA was extracted from tissue samples obtained during each mouse experiment. In the first experiment, we used a developed protocol that combined bead-beating with the Qiagen DNeasy Blood & Tissue kit (14). In the second experiment, we again used bead-beating, but combined this with the MagNA Pure Compact System (Roche Diagnostics Corporation) to isolate the DNA. Briefly, a mixture of MagNA Pure Bacterial Lysis Buffer (BLB, Roche Diagnostics Corporation) and sterile phosphate-buffere saline (PBS) was added to frozen samples at a ratio of 9 parts BLB to 10 parts PBS. Samples were homogenized using a Mini-Beadbeater (BioSpec Products Inc.) for 1 minute. Samples were centrifuged at ~13,000 RPM in a microcentrifuge for 1 minute after which 40 μL of Proteinase K (>600 mAU/mL solution; Qiagen) was added. The solution was incubated at 65°C for 10 minutes and homogenized a second time on the Mini-Beadbeater for 1 minute. The remaining liquid was decanted after a 1 minute spin in a microcentrifuge (~13,000 RPM) and added to a fresh sample tube. DNA was extracted using the MagNA Pure Compact System per the manufacturer’s instructions (default Tissue program, 50 μL final elution volume).
Clone libraries of the 16S rRNA-encoding gene were generated as previously described (15). Briefly, broad range primers (8F and1492R) were used to PCR amplify approximately 1484 base pairs of the 16S rRNA-encoding gene. Amplicons were purified and ligated into plasmids, which were transformed into competent E. coli cells and grown overnight on LB agar (Topo® TA cloning, Invitrogen, Inc., Carlsbad, CA). Single E. coli colonies were randomly sampled and screened for inserts of the correct size using plasmid specific PCR primers. Approximately 96 inserts were selected, purified, and submitted for Sanger-style sequencing for each sample (cecum, ileum, and/or adult worm). Partial sequences (~800 bp) were obtained using the 8F primer for the 5-prime initiation of sequencing reads.
For OTU-based analyses, a matrix of all pairwise sequence identities (p-distance, or the proportion of nucleotide differences between sequences) was generated and downloaded using the Ribosomal Database Project (RDP) website (16, 17) and the RDP Download tool. This matrix was loaded into the DOTUR program (18) to identify and bin sequences according to an idealized bacterial group, called an operational taxonomic unit or OTU. The OTU definition used here considered all sequences ≥97% identical to be the same OTU. This definition is based on numerous microbial population and community analyses that suggest that this level of sequence similarity (≥97%) among 16S rRNA encoding genes defines a bacterial species (19, 20). An OTU-count matrix (a square matrix, where communities are rows, OTUs are columns, and each element of the matrix is a count of the number of times the OTU was observed in a particular community) was generated from the binned sequences and loaded into the EstimateS (21) program for the generation of a Bray-Curtis similarity matrix. The Bray-Curtis metric is a pairwise numerical representation of community similarity based on the abundance of relative distribution of OTUs in the communities being compared (22). To visualize Bray-Curtis similarities, a principal coordinates analysis was generated using the Numerical Taxonomy System, NTSYSpc version 2.2e (Exeter Software, Inc., Setauket, NY). For taxonomic classification, sequences were uploaded and aligned using the RDP Classifier tool (23). Taxonomic classifications in each sample were made at the 80% confidence level.
Quantitative PCR
Quantitative PCRs were used to measure the amount of 16S rRNA gene operons in each sample relative to the single-copy mouse housekeeping gene, TNF-α (tumor necrosis factor alpha) as previously described (14, 24, 25). Individual PCR assays were done for each target using the LightCycler 480 Probes Master reaction mixture (Roche Diagnostics Corporation) and the LightCyler 480 instrument (Roche Diagnostics Corporation). The comparative CT method was used to compare the bacterial load in each sample (26).
Statistical analyses
Differences in delta CT (relative difference in the copy numbers of bacterial 16S rRNA and murine TNFα operons) were tested using the Student’s T-Test and SAS statistical software (SAS Institute, Cary, NC). An analysis of variance (ANOVA) was conducted on the family-level taxonomic classification of samples in each experiment using the GLM procedure and SAS statistical software. The Bonferroni correction was used to adjust p-values for multiple comparisons.
Ethical Considerations
All animal experiments were completed in accordance with protocols approved by use and care of animals committees at both the University of Michigan and Tufts University.
Results
Two independent experiments were conducted. In the first experiment, no data were collected from the H. polygyrus L3 infective larvae or adult worms. In the second experiment, the microbiota associated with both L3 larvae and adult worms were analyzed. All mice treated with H. polygyrus L3 infective larvae became colonized, and adult worms were observed upon necropsy in both experiments. Adult worms were only present in the proximal small bowel.
H. polygyrus increases bacterial abundance in the ileum
Significantly more bacterial 16S rRNA operons were detected by quantitative PCR in cecal samples compared to ileal samples (experiment 1 = 9.5 fold greater; experiment 2 = 20.2 fold greater), indicating an overall difference in the total bacterial load (overall abundance of bacteria) of these two intestinal locations. H. polygyrus infection did not statistically effect bacterial load in the cecum of mice (t = −1.74, df = 17, p = 0.099). However, 1.8 fold more 16S rRNA operons were detected in the ileum of infected mice compared to uninfected mice (t = −2.33, df = 18, p = 0.031), indicating that parasitic infection increased the abundance of bacteria in this part of the GI tract.
Differences in mouse gut microbiota
A total of 1,647 and 1,706 16S sequences were generated from samples in each experiment and an average of 87 and 89 sequences were obtained per sample (GI location) per animal, respectively. A cecum sample from an uninfected mouse in experiment 1 was lost before clone library construction was completed and an ileum sample from an uninfected mouse in experiment 2 yielded insufficient bacterial DNA to allow amplification of 16S. For all other samples, two approaches were used to characterize differences within and between the microbiota. The first approach was OTU-based, meaning that the analysis was based on the abundance and distribution of groups of nucleotide sequences that were ≤3% identical (i.e. a theoretically-based sequence similarity cut-off for bacterial species). The second approach was also based on nucleotide sequence data, but instead of binning all similar sequences together, we determined the taxonomic classification for each sequence by making comparisons to an archived and curated 16S sequence database (see Materials and Methods section).
OTU-based characterization
To visualize the variation in bacterial communities, we generated Bray-Curtis similarity values from an OTU-count matrix. A principal coordinates analysis (PCoA) was used to visualize these data by positioning each sample in 3-D space (Figure 2). The more similar two communities are, the closer they appear in the figure. The microbiotas of uninfected mice grouped together in 3D coordinate space according to experiment, suggesting that the baseline composition at the outset of each experiment was different. Within each experiment, cecal samples were similar to each other, and there was only a modest difference between the communities from H. polygyrus (Hp) infected and uninfected mice. In contrast, ileal bacterial communities tended to be more variable, and there was a clearer distinction between the intestinal microbiotas of Hp infected and uninfected animals.
Figure 2.
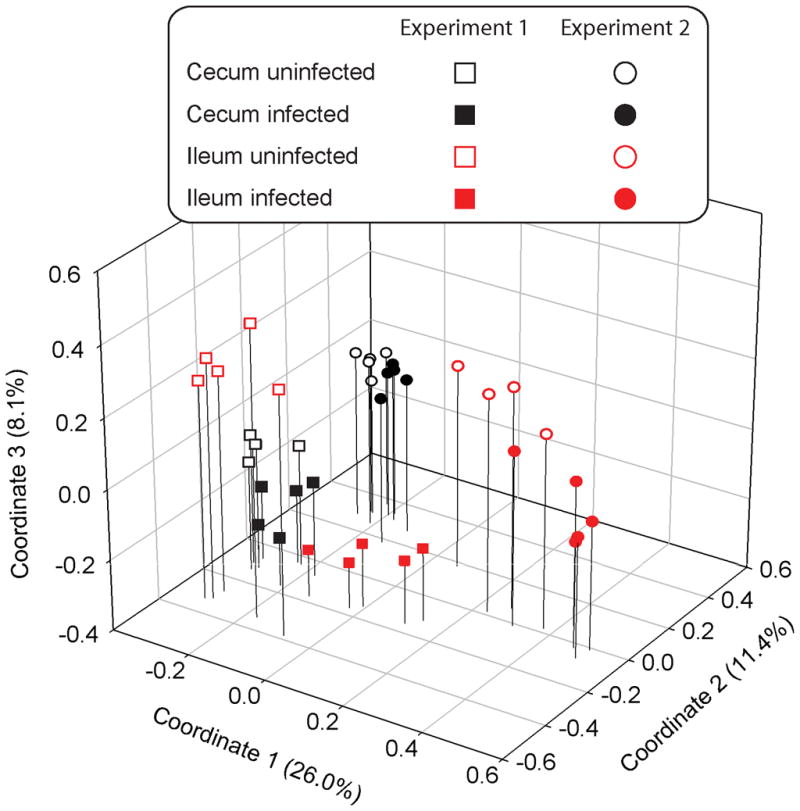
Principal coordinates analysis of the structure of the GI tract microbiotas of H. polygyrus infected and uninfected mice. Cecal and ileal microbiotas are represented by symbols of different color (cecum = black, ileum = red); experiment 1 and 2 are represented by symbols of different shape (square = experiment 1, circle = experiment 2); and microbiotas from infected and uninfected mice are represented by filled and unfilled symbols (uninfected = unfilled, infected = filled). Cecal microbiotas grouped according to the experiments. Ileal microbiotas from infected mice were different from uninfected mice in both experiments.
Taxonomic differences
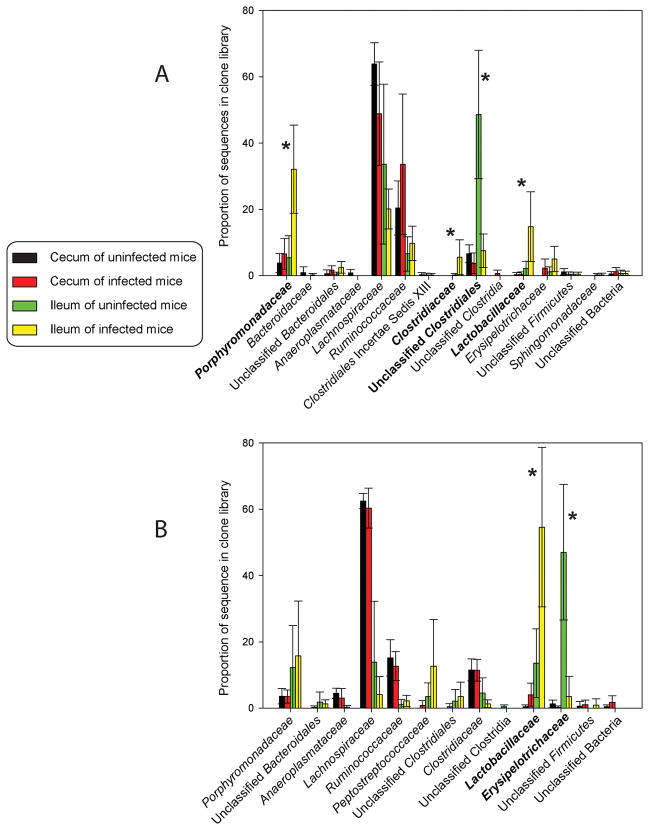
The PCoA in figure 2 is useful for visualizing how similar the samples were to each other based solely on sequence similarity. To understand how samples were different based on bacterial taxonomy, we analyzed the variation in the abundance of bacterial families in each sample (Table 1). Within each experiment, there were significant differences between cecal and ileal microbiotas, regardless of whether the mice were infected with Hp. For example, members of the Lachnospiraceae and Ruminococcaceae families were more abundant in the cecum of mice in both experiments (Figure 3). After correcting for multiple comparisons, no differences were found between the cecal microbiotas of Hp infected and uninfected mice, supporting the observation from the PCoA that these communities are highly similar (Figure 2).
Table 1.
Statistically significant differences in the abundance of bacterial families for each mouse experiment.
| Bacterial Family | F value | d.f. | Pr > F | Significant Difference |
|---|---|---|---|---|
| Experiment 1 | ||||
| Porphyromonadaceae | 13.1 | 3 | 0.0002 | abundant in ileum of infected mice |
| Lachnospiraceae | 6.71 | 3 | 0.0043 | abundant in cecum of mice |
| Ruminococcaceae | 5.31 | 3 | 0.0108 | abundant in cecum of mice |
| Clostridiaceae | 4.94 | 3 | 0.0139 | abundant in ileum of infected mice |
| Unclassified Clostridiales | 19.94 | 3 | <0.0001 | abundant in ileum of uninfected mice |
| Lactobacillaceae | 7.66 | 3 | 0.0025 | abundant in ileum of infected mice |
| Experiment 2 | ||||
| Anaeroplasmataceae | 7.37 | 3 | 0.0029 | abundant in cecum of mice |
| Lachnospiraceae | 51.99 | 3 | <0.0001 | abundant in cecum of mice |
| Ruminococcaceae | 16.25 | 3 | <0.0001 | abundant in cecum of mice |
| Unclassified Clostridiales | 11.74 | 3 | 0.0003 | abundant in ileum of mice |
| Lactobacillaceae | 17.26 | 3 | <0.0001 | abundant in ileum of infected mice |
| Erysipelotrichaceae | 23.67 | 3 | <0.0001 | abundant in ileum of uninfected mice |
Figure 3.
Abundance of bacterial families in microbiotas from the first (A) and second (B) experiments. Samples are represented by differently colored bars (see legend). Error bars represent the standard deviation in abundance among the mice. No differences were detected between infected and uninfected cecal samples. Significant differences between the infected and uninfected ileal samples are indicated with asterisks and bolded bacterial family name.
In contrast, the abundance of multiple bacterial families in the ileum of Hp infected and uninfected animals was significant different in each experiment (Figure 3). In the first experiment, Hp infected mice had a greater abundance of Porphyromonadaceae, Lactobacillaceae, and Clostridiaceae in their ileum compared to uninfected mice (p = 0.0007, p = 0.0167, and p = 0.0442 respectively). A similar difference in Lactobacilliaceae abundance was seen in the ileum of Hp infected mice in the second experiment (p < 0.0022). Compared to Hp infected animals, uninfected mice in the first experiment had more Clostridiales (p = 0.0001) in their ileum, while uninfected mice from the second experiment had more Erysipelotrichaceae (p < 0.0001).
H. polygyrus-associated microbiota
In the second experiment, L3 larvae and adult worms were sampled to determine the microbiota carried by (or associated with) H. polygyrus. The level of bacterial DNA extracted from L3 stage larvae was low, and only 28 16S sequences were obtained even after multiple PCR reactions were pooled. The amount of bacterial DNA in these samples was also extremely low by quantitative PCR (cycle threshold of 30.8 cycles). In contrast, bacterial DNA in the adult worms harvested from the proximal small bowel was abundant, and we had little problem cloning and sequencing 16S amplicons from these samples.
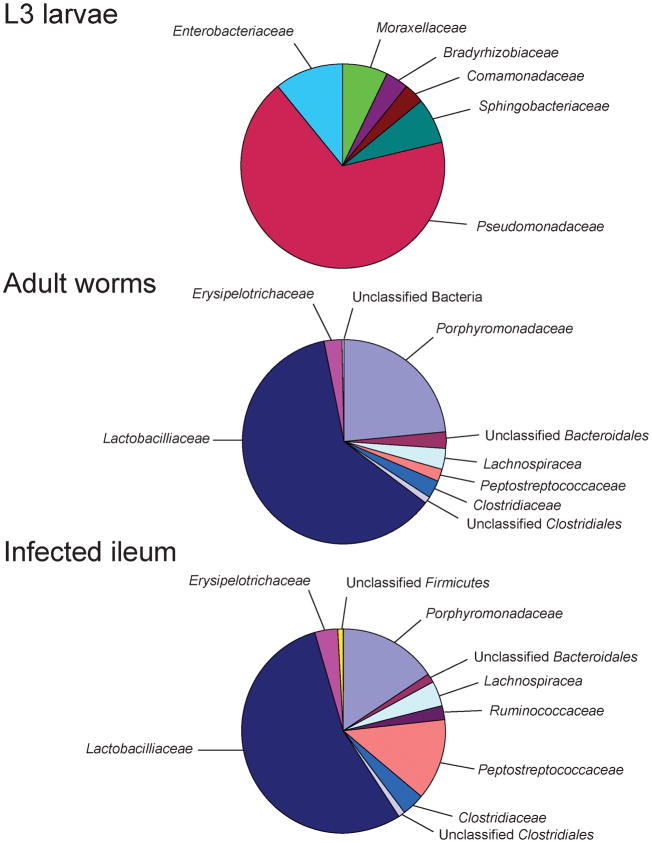
The microbiota associated with L3 stage larvae was completely unique (Figure 4). None of the sequences from L3 stage larvae were found in mice or adult worms, suggesting that Hp worm develop a unique microbiota as they mature into adults. Although only 28 sequences were obtained from the L3 stage larvae, 6 bacterial families were detected, suggesting that these worms had a complex microbiota at the time of infection. In contrast to the uniqueness of the L3 microbiota, the microbiota associated with adult worms looked remarkably similar to the microbiota of the ileum of their murine hosts suggesting that the microbiotas of Hp infected animals and adult Hp worms are highly related.
Figure 4.
Worm-associated microbiotas. Pie charts display the proportion of bacterial families in sequences from the L3 larvae (28 sequences), adult worms from infected mice (473 sequences), and samples from the ileum of mice (422 sequences). On average, the microbiota associated with adult worms is highly similar to the microbiota from ileal tissue samples.
Discussion
H. polygyrus L3 stage larvae invade the mucosa of the mouse jejunum, where they develop into adults and emerge into the intestinal lumen 8 days after ingestion. Infection induces a polarizing T helper cell type 2 (Th2) host immune response characterized by the production of IL-4 and IL-13, as well as activation of the STAT-6 pathway (27). These events activate mast cells, induce the alternatively activated phenotype in tissue-resident macrophages, increase mucus secretion by goblet cells, and enhance intestinal smooth muscle contraction (28, 29). During a primary H. polygyrus infection, the host Th2 response does not result in rapid clearance of the adult worms; rather it has a significant deleterious effect on H. polygyrus fecundity, as measured by the number of eggs shed per gram of feces (28, 30).
H. polygyrus infection has a profound influence on a number of immunoregulatory signals that decrease intestinal inflammation. H. polygyrus infection increases the production of the global transcription factor, FoxP3, in T cells of the lamina propria, and these cells decrease intestinal inflammation upon transfer to mice with established colitis (31). In the terminal ileum of mice, H. polygyrus induces mucosal T cells to express toll-like receptor 4 (TLR4) (32). TLR4 signaling by bacterial lipopolysaccharide (LPS) normally stimulates cells to produce pro-inflammatory cytokines, like TNFα and IL-12. In contrast to what happens normally, mucosal T cells from H. polygyrus infected mice do not produce TNFα or IL-12, but instead produce the regulatory cytokine TGFβ (32). In the absence of this TGFβ signaling, there is insufficient control of IFNγ production by IL-10 and colitis is maintained (33).
To our knowledge, it has never been examined if H. polygyrus infection alters the composition of the GI tract microbiota. A number of observations from experiments with germfree mice suggest that H. polygyrus needs GI tract bacteria to establish a robust infection (34). Compared to mice with a conventional microbiota, total worm burden was lower in germfree mice 13 days post-inoculation. Moreover, germfree mice rapidly cleared these worms between days13 through 30 post inoculation, while worm burden remained high in conventional mice. Also, a robust intestinal mucosal eosinophilia developed in germfree mice, which was absent in conventional mice suggesting that the host immune response to H. polygyrus differs in the absence of intestinal bacteria. Interestingly, mortality in mice given a “heavy” infection (1600 L3 infective larvae) was only observed in conventional mice. These observations suggest that the GI microbiota is necessary for the normal host response to H. polygyrus.
Infection of mice with H. polygyrus resulted in an increased abundance of various bacteria, suggesting that some species find conditions in the ileum of H. polygyrus-infected mice favorable. This observation is what one would expect from a microbial-helminth mutualism, where both bacteria and worm benefit from symbiosis. Interestingly, members of the Lactobacillaceae family increased in both experiments. The Lactobacillaceae family is composed of the genera Lactobacillus, Paralactobacillus, Pediococcus, and other bacteria that are not yet classified by microbial taxonomists. Most of the known Lactobacillaceae species belong to the genus Lactobacillus, which is a diverse group of Gram-positive, facultative anaerobic species commonly known as lactic acid bacteria because most convert sugars into lactic acid. Certain Lactobacillus species, like L. rhamnosus (35) and L. casei (36), decrease intestinal inflammation in murine models of IBD, and it is tempting to speculate that Lactobacillus species play a role in the anti-inflammatory/immune modulatory effects of H. polygyrus infection. More studies are needed to elucidate the role of this potentially important mutualism.
Identifying members of the GI tract microbiota that help to control gut inflammation is an active area of research (see (37) for a list of bacteria). Such organisms span much of the genetic breadth of the GI tract bacterial community, and there likely is no single species responsible for all of these microbially-mediated effects. Even within a genus, different species have different ways of modulating the host immune system. For example, L. rhamnosus inhibits NF-κB activation by producing reactive oxygen species (35), while cell wall components of L. casei inhibit IL-6 production (36). These observations suggest that the particular influence of the microbiota on host immunity depends on the structure of the microbiota (i.e. their abundance and relative distribution) present at the outset of helminth infection.
It is well established that the GI tract microbiotas of mammals are highly complex and variable among individuals (38). In fact, mice from different commercial vendors (e.g. Taconic Farms and Jackson Laboratory) have different GI tract microbiotas despite having the same genetic (wild-type C57BL/6) background (39). These observations support the notion that the GI tract microbiota within an individual or group of individuals is constantly evolving. Not surprisingly, the intestinal microbiotas of the uninfected mice in the experiments presented here were significantly different at the outset of each experiment. This result says little about the evolutionary dynamics of the microbiota of C57BL/6 mice, but since the two experiments were separated by ~2 years, it does suggest that change can evolve rapidly. Whether the initial microbiota structure influences the host immune response to H. polygyrus infection remains to be investigated. However, the observation that organisms of the Lactobacillaceae family increased in abundance in both experiments, regardless of different initial microbiotas, suggests that there may be compositional similarities among H. polygyrus infection that ultimately influence host immunomodulation.
Relevance of this study to helminth treatment of IBD
Normally, Th1/Th17 driven inflammation characterized by the production of proinflammatory cytokines (IL-12, IL-23, IL-17, and interferon γ) is held in check by the production of immunoregulatory cytokines and transcription factors (e.g. TGFβ, IL-10, and FoxP3). In patients with IBD, homeostatic regulatory signals are impaired, and a chronic inflammation develops that is perpetuated by antigens from the commensal GI tract microbiota (40). A number of therapies commonly used in IBD like azathiaprine, anti-TNF monoclonal antibodies and corticosteroids suppress this inflammatory cascade (41). Some studies suggest that helminths may prove useful for the treatment of both Crohn’s disease and ulcerative colitis (11, 12). The data presented here demonstrate that H. polygyrus infection is associated with a significant shift in the abundance and relative distribution of GI tract bacteria. The relatively large effect size of the shift among ileal-associated microbiotas compared to cecal-associated microbiotas suggests that either the helminth directly influenced the microbiota or that the host immune response to the infection mediated the observed alterations. The dynamic interactions among H. polygyrus, the host mucosal immune response, and the intestinal microbiota require more study.
Acknowledgments
This work was supported by the following grants and fellowships from the US National Institutes of Health: DK38327 (JVW), DK058755 (JVW), DK070875 (VBY), and a Ruth L. Kirschstein National Research Service Award T32 HL07749-15 (STW). Additional support for this project was supplied by the Broad Foundation (JVW), Schneider family (JVW), Friedman family (JVW), Gilman family (JVW), Michigan Institute for Clinical and Health Research Postdoctoral Translational Scholars Program (STW), a Crohn’s and Colitis Foundation of America Student Research Fellowship (SAE), and a Crohn’s and Colitis Foundation of America Senior Investigator Award (VBY).
References
- 1.Asakura H, Suzuki K, Kitahora T, et al. Is there a link between food and intestinal microbes and the occurrence of Crohn’s disease and ulcerative colitis? J Gastroenterol Hepatol. 2008;23:1794–1801. doi: 10.1111/j.1440-1746.2008.05681.x. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein CN, Shanahan F. Disorders of a modern lifestyle: reconciling the epidemiology of inflammatory bowel diseases. Gut. 2008;57:1185–1191. doi: 10.1136/gut.2007.122143. [DOI] [PubMed] [Google Scholar]
- 3.Frangos CC. Inflammatory bowel disease: reviewing an old study under a new perspective. Gut. 2007;56:1638–1639. [PMC free article] [PubMed] [Google Scholar]
- 4.Goh K, Xiao SD. Inflammatory bowel disease: a survey of the epidemiology in Asia. J Dig Dis. 2009;10:1–6. doi: 10.1111/j.1751-2980.2008.00355.x. [DOI] [PubMed] [Google Scholar]
- 5.Shanahan F, Bernstein CN. The evolving epidemiology of inflammatory bowel disease. Curr Opin Gastroenterol. 2009;25:301–305. doi: 10.1097/MOG.0b013e32832b12ef. [DOI] [PubMed] [Google Scholar]
- 6.Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15:128–133. doi: 10.1002/ibd.20633. [DOI] [PubMed] [Google Scholar]
- 7.Elliott DE, Li J, Blum A, et al. Exposure to schistosome eggs protects mice from TNBS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G385–391. doi: 10.1152/ajpgi.00049.2002. [DOI] [PubMed] [Google Scholar]
- 8.Moreels TG, Nieuwendijk RJ, De Man JG, et al. Concurrent infection with Schistosoma mansoni attenuates inflammation induced changes in colonic morphology, cytokine levels, and smooth muscle contractility of trinitrobenzene sulphonic acid induced colitis in rats. Gut. 2004;53:99–107. doi: 10.1136/gut.53.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ruyssers NE, De Winter BY, De Man JG, et al. Therapeutic potential of helminth soluble proteins in TNBS-induced colitis in mice. Inflamm Bowel Dis. 2009;15:491–500. doi: 10.1002/ibd.20787. [DOI] [PubMed] [Google Scholar]
- 10.Weinstock JV, Summers RW, Elliott DE. Role of helminths in regulating mucosal inflammation. Springer Semin Immunopathol. 2005;27:249–271. doi: 10.1007/s00281-005-0209-3. [DOI] [PubMed] [Google Scholar]
- 11.Summers RW, Elliott DE, Urban JF, Jr, et al. Trichuris suis therapy in Crohn’s disease. Gut. 2005;54:87–90. doi: 10.1136/gut.2004.041749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Summers RW, Elliott DE, Urban JF, Jr, et al. Trichuris suis therapy for active ulcerative colitis: a randomized controlled trial. Gastroenterology. 2005;128:825–832. doi: 10.1053/j.gastro.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Jackson JA, Friberg IM, Little S, et al. Review series on helminths, immune modulation and the hygiene hypothesis: immunity against helminths and immunological phenomena in modern human populations: coevolutionary legacies? Immunology. 2009;126:18–27. doi: 10.1111/j.1365-2567.2008.03010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonopoulos DA, Huse SM, Morrison HG, et al. Reproducible community dynamics of the gastrointestinal microbiota following antibiotic perturbation. Infect Immun. 2009;77:2367–2375. doi: 10.1128/IAI.01520-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young VB, Schmidt TM. Antibiotic-associated diarrhea accompanied by large-scale alterations in the composition of the fecal microbiota. J Clin Microbiol. 2004;42:1203–1206. doi: 10.1128/JCM.42.3.1203-1206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole JR, Chai B, Farris RJ, et al. The ribosomal database project (RDP-II): introducing my RDP space and quality controlled public data. Nucleic Acids Res. 2007;35:D169–172. doi: 10.1093/nar/gkl889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole JR, Wang Q, Cardenas E, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009;37:D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schloss PD, Handelsman J. Introducing DOTUR, a computer program for defining operational taxonomic units and estimating species richness. Appl Environ Microbiol. 2005;71:1501–1506. doi: 10.1128/AEM.71.3.1501-1506.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konstantinidis KT, Ramette A, Tiedje JM. The bacterial species definition in the genomic era. Philos Trans R Soc Lond B Biol Sci. 2006;361:1929–1940. doi: 10.1098/rstb.2006.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konstantinidis KT, Tiedje JM. Genomic insights that advance the species definition for prokaryotes. Proc Natl Acad Sci U S A. 2005;102:2567–2572. doi: 10.1073/pnas.0409727102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colwell RK. EstimateS: Statistical estimation of species richness and shared species from samples. 2006 Available at: Persistent URL < purl.oclc.org/estimates>.
- 22.Beals EW. Bray-Curtis Ordination: An effective strategy for analysis of multivariate ecological data. In: MacFadyen A, Ford ED, editors. Advances in Ecological Research. Orlando, FL: Academic Press, Inc; 1984. pp. 1–56. [Google Scholar]
- 23.Wang Q, Garrity GM, Tiedje JM, et al. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nadkarni MA, Martin FE, Jacques NA, et al. Determination of bacterial load by real-time PCR using a broad-range (universal) probe and primers set. Microbiology. 2002;148:257–266. doi: 10.1099/00221287-148-1-257. [DOI] [PubMed] [Google Scholar]
- 25.Nitsche A, Becker M, Junghahn I, et al. Quantification of human cells in NOD/SCID mice by duplex real-time polymerase-chain reaction. Haematologica. 2001;86:693–699. [PubMed] [Google Scholar]
- 26.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 27.Chen CC, Louie S, McCormick B, et al. Concurrent infection with an intestinal helminth parasite impairs host resistance to enteric Citrobacter rodentium and enhances Citrobacter-induced colitis in mice. Infect Immun. 2005;73:5468–5481. doi: 10.1128/IAI.73.9.5468-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hashimoto K, Uchikawa R, Tegoshi T, et al. Immunity-mediated regulation of fecundity in the nematode Heligmosomoides polygyrus - the potential role of mast cells. Parasitology. 2009:1–7. doi: 10.1017/S0031182009991673. [DOI] [PubMed] [Google Scholar]
- 29.Weng M, Huntley D, Huang IF, et al. Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J Immunol. 2007;179:4721–4731. doi: 10.4049/jimmunol.179.7.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urban JF, Jr, Katona IM, Finkelman FD. Heligmosomoides polygyrus: CD4+ but not CD8+ T cells regulate the IgE response and protective immunity in mice. Exp Parasitol. 1991;73:500–511. doi: 10.1016/0014-4894(91)90074-7. [DOI] [PubMed] [Google Scholar]
- 31.Elliott DE, Setiawan T, Metwali A, et al. Heligmosomoides polygyrus inhibits established colitis in IL-10-deficient mice. Eur J Immunol. 2004;34:2690–2698. doi: 10.1002/eji.200324833. [DOI] [PubMed] [Google Scholar]
- 32.Ince MN, Elliott DE, Setiawan T, et al. Heligmosomoides polygyrus induces TLR4 on murine mucosal T cells that produce TGFbeta after lipopolysaccharide stimulation. J Immunol. 2006;176:726–729. doi: 10.4049/jimmunol.176.2.726. [DOI] [PubMed] [Google Scholar]
- 33.Ince MN, Elliott DE, Setiawan T, et al. Role of T cell TGF-beta signaling in intestinal cytokine responses and helminthic immune modulation. Eur J Immunol. 2009;39:1870–1878. doi: 10.1002/eji.200838956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wescott RB. Experimental Nematospiroides dubius infection in germfree and conventional mice. Exp Parasitol. 1968;22:245–249. doi: 10.1016/0014-4894(68)90099-4. [DOI] [PubMed] [Google Scholar]
- 35.Lin PW, Myers LE, Ray L, et al. Lactobacillus rhamnosus blocks inflammatory signaling in vivo via reactive oxygen species generation. Free Radic Biol Med. 2009;47:1205–1211. doi: 10.1016/j.freeradbiomed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsumoto S, Hara T, Nagaoka M, et al. A component of polysaccharide peptidoglycan complex on Lactobacillus induced an improvement of murine model of inflammatory bowel disease and colitis-associated cancer. Immunology. 2009;128:e170–180. doi: 10.1111/j.1365-2567.2008.02942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Round JL, O’Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2009 doi: 10.1016/j.jaut.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ivanov II, Frutos Rde L, Manel N, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Balfour Sartor R. Bacteria in Crohn’s disease: mechanisms of inflammation and therapeutic implications. J Clin Gastroenterol. 2007;41 (Suppl 1):S37–43. doi: 10.1097/MCG.0b013e31802db364. [DOI] [PubMed] [Google Scholar]
- 41.Afif W, Loftus EV., Jr Safety profile of IBD therapeutics: infectious risks. Gastroenterol Clin North Am. 2009;38:691–709. doi: 10.1016/j.gtc.2009.07.005. [DOI] [PubMed] [Google Scholar]