Abstract
Background
Cardiotonic steroids, including marinobufagenin, are a group of new steroid hormones found in plasma and urine of patients with congestive heart failure, myocardial infarction, and chronic renal failure. In animal studies partial nephrectomy induces marinobufagenin elevation, cardiac hypertrophy and fibrosis. The objective of this study is to test the effect of renal ischemia on marinobufagenin levels in humans with renal artery stenosis.
Method and Results
Plasma marinobufagenin levels were measured in patients with renal artery stenosis of the RESIST trial, non-renal artery stenosis patient controls who were scheduled for coronary angiography, and normal healthy individuals. Marinobufagenin levels were significantly higher in patients with renal artery stenosis compared to the other two groups. Multivariate analysis shows that occurrence of renal artery stenosis is independently related with marinobufagenin levels. In addition, renal artery revascularization by stenting partially reversed marinobufagenin levels in the patients with renal artery stenosis (0.77±0.06 nM at baseline, 0.66±0.06 nM at 24 hour and 0.61±0.05 nM at 1 month).
Conclusion
Marinobufagenin levels are increased in patients with renal artery stenosis whereas reversal of renal ischemia by stenting treatment reduces marinobufagenin levels. These results suggest that renal artery stenosis-induced renal ischemia may be a major cause for marinobufagenin release.
Keywords: Renal Artery Stenosis, Hypertension, Cardiotonic Steroids, Marinobufagenin, Renal Artery Stenting
Introduction
Cardiotonic steroids (CTS) are a group of steroid hormones that have been recently found in mammals including humans 1. There is evidence demonstrating that these compounds can be synthesized endogenously and possess identical structures as their plant- and amphibian-originated counterparts 2, 3. In humans an endogenous CTS, marinobufagenin (MBG), was isolated and identified from the urine of myocardial infarction patients4, and from uremic plasma 2. Both in vitro and in vivo studies demonstrated that epinephrine, angiotensin II and ACTH could induce adrenal cortical cells to release endogenous CTS 5–7. Other factors which can stimulate CTS include physical exercise 8, hypoxia 9 and behavioral stress 10.
Renal artery stenosis (RAS) is a major cause for secondary hypertension in the United States 11. Importantly, RAS-induced hypertension has a 3 times higher incidence of adverse cardiovascular (CV) events than those with essential hypertension when matched with equivalent blood pressure 12–14. It has been reported that CTS levels increase in patients and animals with volume expanded hypertension or pre-eclampsia 15, 16 as well as in renal failure 2. We have recently demonstrated in animal models that partial nephrectomy (PNx) increases plasma MBG levels and induces hypertension and cardiac fibrosis 17. Neutralization of MBG by active immunization against an MBG-albumin conjugate attenuates the pathological cardiac fibrosis in rats 18, 19. The objective of the current study is to test whether renal ischemia induced by RAS alters MBG levels in humans.
Methods
Subjects
All subjects provided a written informed consent from a protocol approved by an Institutional Review Board. Subject groups: 1) RAS subjects were from RESIST trial (ClinicalTrials.gov identifier NCT00234585) which was conducted by the Clinical Coordinating Center at the University of Toledo 20. The inclusion criteria were patients with hypertension and one or more RAS of ≥50% and <100%, treatable with stenting. The primary exclusion criteria were a systolic blood pressure greater than 200 mmHg or diastolic greater than 120 mmHg on the day of randomization, age <18, pregnancy, dialysis, kidney transplant, kidney size < 8cm, restenosis, or stroke, major surgery, congestive heart failure (CHF), major trauma, and myocardial infarction within a short period of time of planned enrollment. All patients successfully received stenting treatment. 2) Patient control subjects were adult patients who have history of hypertension or angina scheduled for coronary angiography and no RAS. 3) Normal healthy control subjects were healthy individuals (age>18) who have no history of hypertension, angina or RAS.
Blood Sample Collection
All peripheral venous blood samples were collected in lithium heparin plasma separator tubes, spun at 1000 × g for 15 minutes, and the obtained plasma samples were stored at −80°C until analysis. RAS patients also had samples collected at 24-hour and 1-month post stenting during the RESIST trial.
Measurement of Plasma MBG
The plasma sample extraction was based on the method described before 21. The disposable Sep-Pak C-18 columns (Waters, Milford, MA) was activated by 10 ml 100% acetonitrile and washed once with 5 ml distilled water. Plasma sample (0.5ml) was then loaded to the column. After another washing step with 5 ml distilled water, the column was eluted by 7ml 20% acetonitrile followed by 7ml 80% acetonitrile. The eluates were combined, lyophilized and re-suspended in TBS buffer (50 mM Trizma, 150 mM NaCl, 7.7 mM NaN3, pH 7.4). The concentration of MBG was then determined using a competitive enzyme-linked immunosorbent assay (ELISA) based on a 4G4 anti-MBG murine monoclonal antibody 22. Briefly, 100 µl of MBG standards or sample eluates were mixed with 100 µl anti-MBG monoclonal antibody (1:1000 dilution in TBS buffer with 1% bovine serum albumin and 0.25% Tween-20). The mixture was then added to MBG-thyroglobulin-coated and 1% BSA-blocked ELISA plate. After 1 h incubation, plates were washed 3 times and secondary anti-mouse antibody conjugated with alkaline phosphatase (1:10,000 dilution in TBS buffer with 1% bovine serum albumin and 0.25% Tween-20) was added and incubated for another 1h. A fluorescent signal amplifier FDP, the substrate of alkaline phosphatase from ANASpec (San Jose, CA), was used to detect the signals after washing out the secondary antibody. The sample MBG concentrations were calculated based on the standard curve using purified MBG compound. MBG was purified from parotid secretion of Bufo marinus toad and MBG-thyroglobulin was synthesized as reported previously 23. The secondary anti-mouse antibody was purchased from Sigma (St. Louis, MO).
Other Laboratory Analyses
The baseline biomarkers were measured as described before 20. Glomerular filtration rate (GFR) was calculated from the modified MDRD equation 20, 24 and was used as the primary measure of renal function.
Statistical Analysis
Study data are presented as continuous (mean±SE) and categorical data. Because the MBG data are not normally distributed in RAS patients, the Kruskal-Wallis test was used for the analysis to detect the significance among the RAS patients and the control subjects. Repeated measure ANOVA was used to test the changes between the baseline, 24-hour and 1-month post stenting for the RAS patients. We also performed Fisher's least significant difference (LSD) procedure to detect the pairwise difference. Multivariate analysis was conducted using linear logistic regression. All analyses were performed with SAS 9.1 or JMP software (SAS Inc, Cary, NC).
Results
RAS-induced renal ischemia increases plasma MBG levels
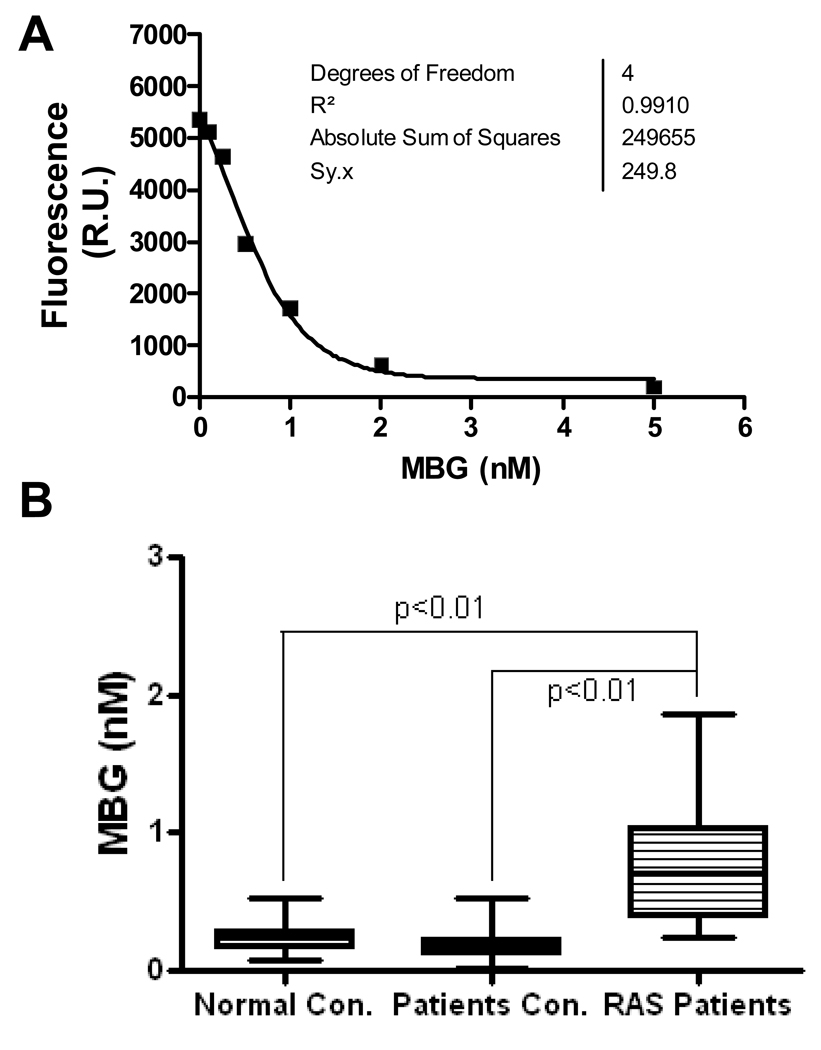
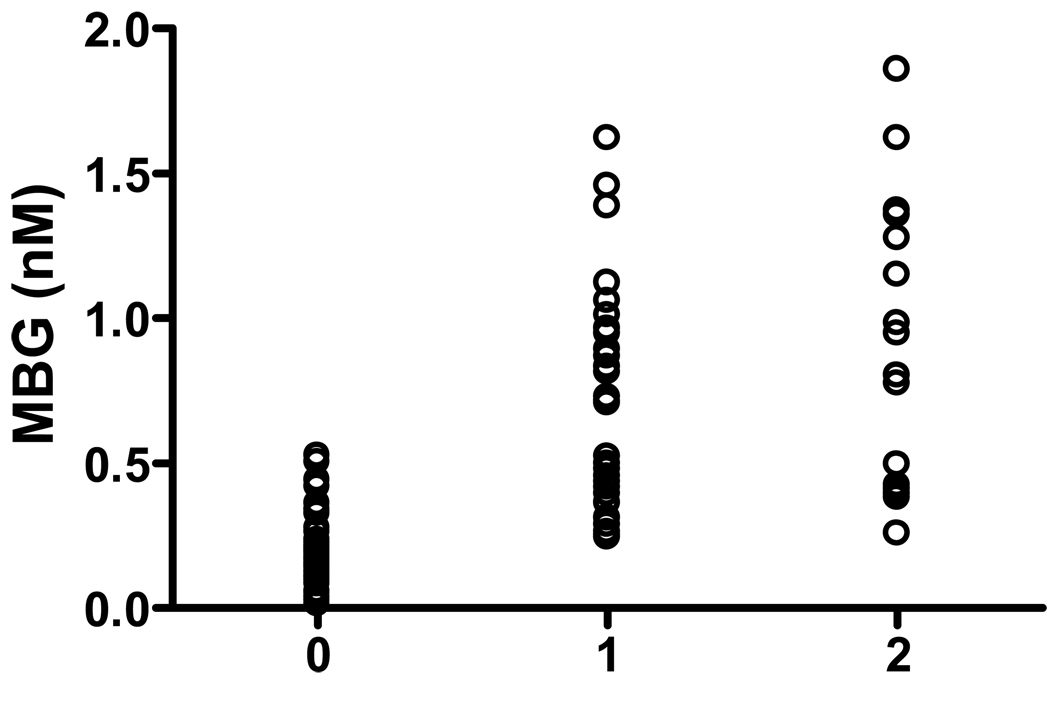
To test if RAS-induced renal ischemia increases plasma MBG levels, we first compared the plasma MBG concentration in RAS patients to that in normal healthy individuals (age: 30–55, no hypertension, angina, or RAS history). The result demonstrated that MBG levels are significantly higher in RAS patients (0.77 ± 0.06 nM, n=49 vs 0.25 ± 0.02 nM, n=26 in control subjects; p<0.01; Figure 1). Since MBG levels were also found elevated in patients with myocardial infarction and hypertension 4, 15, we then compared 60 non-RAS hypertensive patients who were scheduled for coronary angiography as an additional control group to test if renal ischemia specifically contributes to the increased MBG in RAS patients. To eliminate the confounding factors, we compared the basic characteristics of the RAS and non-RAS patients including their age, gender, medical history, blood pressure, kidney function, medications, and other risk factors. As shown in Table 1, the MBG concentration is significantly higher in RAS patients than in non-RAS patient controls (0.77±0.06 vs 0.20±0.06 nM, p<0.01). Other basic characteristics of the RAS and non-RAS patients were listed in Table 1. Among these variables, the age, systolic blood pressure, presence of hypertension, GFR, use of diuretics and use of ACEi/ARB were significantly different between the two groups, and serum creatinine was close to significance. To test if these factors are independently associated with the MBG elevation, we performed the multivariate analysis. All variables in Table 1 with p ≤ 0.1 were included in the multivariate analysis. As shown in Table 2, the occurrence of RAS and use of ACEi/ARB are independently associated with the increased plasma MBG levels in the multivariate model. In patients with RAS, the plasma MBG levels are significantly higher in patients receiving ACEi/ARB treatment than in patients without receiving ACEi/ARB treatment (0.93±0.08 nM, n=26 vs 0.63±0.08 nM, n=23, p<0.05). Figure 2 shows that MBG levels in patient plasma samples are correlated with the severity of the RAS. The average MBG concentrations are 0.20±0.06 nM in patients without RAS, 0.69±0.07 nM in patients with unilateral RAS (n=32), and 0.88±0.12 nM in patients with bilateral RAS (n=16) respectively.
Figure 1.
Plasma Marinobufagenin (MBG) levels in RAS patients and control subjects. The MBG concentration was measured in plasma samples from RAS patients or control subjects as described in the Methods section. Panel A shows a representative standard curve of MBG measurement using purified MBG compound; Panel B shows the distribution and mean MBG levels in normal healthy controls (Normal con.), hypertensive patient controls (Patients con.), and patients with renal artery stenosis (RAS patients).
Table 1.
Basic characteristics of RAS patients and non-RAS patient controls
| Non-RAS patients (n=60) |
RAS patients (n=49) |
P-Value Non-RAS vs RAS |
|
|---|---|---|---|
| MBG (nM) | 0.20 ± 0.06 | 0.77 ± 0.06 | <0.01 |
| Demographic characteristics | |||
| Age (y) | 61.4 ± 1.5 | 70.5 ± 1.3 | <0.01 |
| Female, n (%) | 28 (46) | 30 (61) | 0.18 |
| Caucasian, n (%) | 52 (87) | 45 (92) | 0.54 |
| BMI | 31.1 ± 1.1 | 28.9 ± 0.8 | 0.10 |
| Systolic BP (mm Hg) | 131 ± 2 | 159 ± 5 | <0.01 |
| Diastolic BP (mm Hg) | 77 ± 1 | 76 ± 2 | 0.57 |
| Heart Rate (bpm) | 68 ± 1 | 67 ± 2 | 0.63 |
| Indications for treatment | |||
| Hypertension | 43 (72) | 48 (98) | <0.01 |
| Angina | 24 (40) | 23 (47) | 0.56 |
| Laboratory Values | |||
| Serum Creatinine (mg/dL) | 0.98 ± 0.03 | 1.11 ± 0.06 | 0.07 |
| MDRD GFR | 78.3 ± 4.4 | 64.7 ± 3.7 | 0.02 |
| Risk Factors, n (%) | |||
| CAD | 32 (53) | 34 (69) | 0.08 |
| Diabetes Mellitus (DM) | 15 (25) | 11 (22) | 0.82 |
| History of Smoking | 31 (52) | 34 (69) | 0.08 |
| Medications, n (%) | |||
| ACE inhibitors/ARB | 18 (31) | 23 (47) | 0.08 |
| Diuretics | 14 (24) | 23 (47) | 0.02 |
BMI, body mass index; BP, blood pressure; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers, and CAD, coronary artery disease; RAS, renal artery stenosis. Values are mean ± SE or number and percentage of patients.
Table 2.
Multivariate analysis for predictors of plasma MBG levels.
| Model | Unstandardized Coefficients |
Standardized Coefficients |
95.0% Confidence Interval for B |
||||
|---|---|---|---|---|---|---|---|
| B | Std. Error |
Beta | t | Sig. | Lower Bound |
Upper Bound |
|
| (Constant) | .364 | .447 | .814 | .418 | −.524 | 1.252 | |
| AGE | 3.667E-5 | .003 | .001 | .012 | .990 | −.006 | .006 |
| BMI | −.005 | .005 | −.077 | −.950 | .345 | −.015 | .005 |
| Serum Creatinine | −.214 | .150 | −.188 | −1.424 | .158 | −.512 | .085 |
| MDRD_Est.GFR | .000 | .002 | −.024 | −.182 | .856 | −.004 | .003 |
| Systolic BP | .001 | .001 | .103 | 1.262 | .210 | −.001 | .004 |
| RAS_ Severity | .348 | .047 | .633 | 7.478 | .000 | .255 | .440 |
| ACEi/ARB | .152 | .065 | .178 | 2.351 | .021 | .024 | .281 |
| Diuretics | .009 | .066 | .010 | .136 | .892 | −.122 | .140 |
Dependent Variable: Baseline_MBG. BMI, body mass index; Systolic BP, systolic blood pressure; ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blockers; RAS, renal artery stenosis.
Figure 2.
Plasma MBG levels in patients categorized by the severity of RAS (0, stands for patients without RAS; 1 stands for patient with unilateral RAS; and 2 stands for patients with bilateral RAS or RAS patients with only a solitary kidney). The average MBG concentrations are 0.20±0.06 nM in patients without RAS, 0.69±0.07 nM in patients with unilateral RAS (n=32), and 0.88±0.12 nM in patients with bilateral RAS (n=16) respectively.
Reversal of renal ischemia by stenting reduces MBG levels
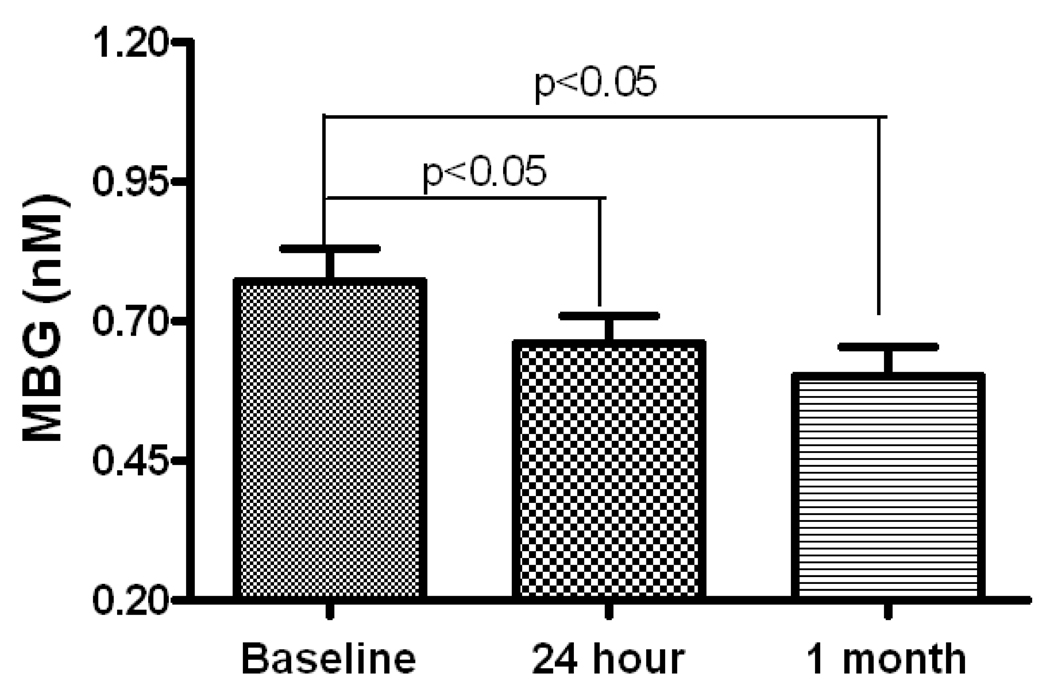
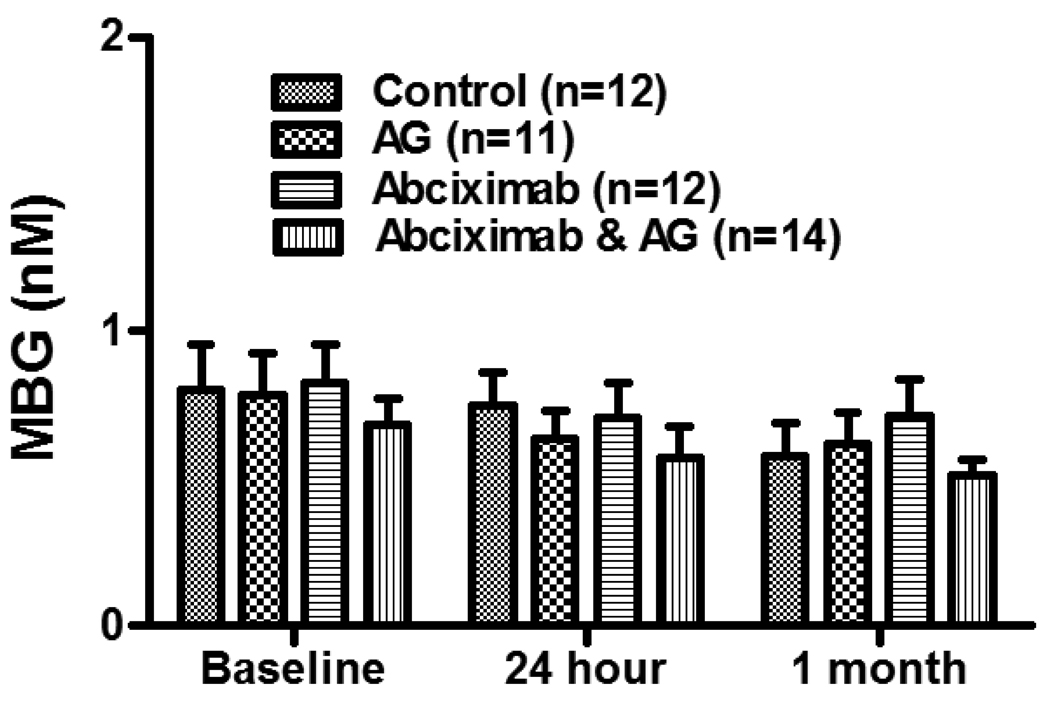
To further confirm that renal ischemia is a cause of MBG elevation in RAS patients, we measured the plasma MBG levels from RAS patients at 24-hour and 1-month after the stenting. A total of 49 available paired samples were tested. The result demonstrated that MBG levels decreased after stenting (0.77±0.06 nM baseline vs 0.66±0.05nM at 24-hour, and 0.60±0.05 nM at 1-month, p<0.05, Figure 3). MBG levels at 24 hour and 1 month were significantly lower than the baseline levels but no further reduction was seen from 24-hour to 1-month. Since the RESIST patients were randomized into 4 groups (control, Angioguard® only, abciximab only, and Angioguard® plus abciximab) before receiving the renal artery stenting treatment, we also compared the changes of MBG between these groups and found no significant differences between these groups (Figure 4).
Figure 3.
MBG levels at baseline, 24 hour and 1 month after renal artery stenting in RAS patients. The MBG levels were measured in the plasma samples collected from patients at three time points. The baseline sample was collected immediately before the patient receiving the renal artery stenting and the post stenting samples were collected at 24 hour and 1 month after receiving the renal artery stenting respectively.
Figure 4.
MBG levels in the randomized groups at baseline, 24 hour, and 1 month. Patients were randomly assigned into 4 groups before receiving the renal artery stenting as previously described 20. The control group receives neither Angioguard® nor abciximab); the Angioguard® only group (AG) receives Angioguard during the stenting procedure; the abciximab only group (Abciximab) received abciximab treatment before the stenting procedure; and the Angioguard® & abciximab group (Abciximab & AG) received both abciximab treatment before stenting and the Angioguard® during the stenting procedure. The plasma MBG concentrations were analyzed in the above 4 groups at their baseline and 24 hour and 1 month post stenting.
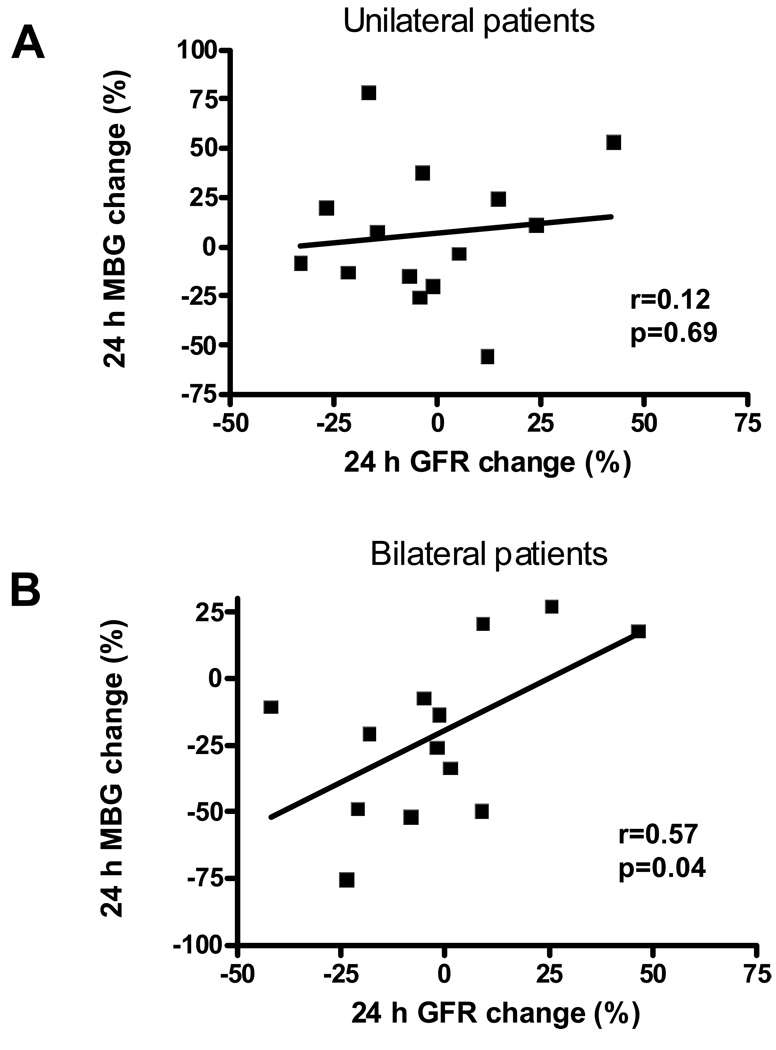
To test if MBG levels were related to renal function, we analyzed the creatinine concentrations in plasma samples and calculated the GFR from RAS patients at baseline, 24 hour and 1 month post stenting. As shown in Figure 5, MBG changes after stenting correlated with the GFR changes in patients with bilateral RAS (r=0.57, p<0.05) but not in patients with unilateral RAS (r=0.12, p>0.05).
Figure 5.
Correlation between MBG and glomerular filtration rate (GFR) changes in RAS patients after renal artery stenting. The percentage change of MBG and GFR were calculated using the following formula: 24 h change (%) = (24 h value - baseline value)/baseline value × 100%. Negative values indicate a decrease in MBG or GFR after stenting. Panel A shows the correlation of 24 h change between MBG and GFR in patients with unilateral RAS; Panel B shows the correlation of 24 h change between MBG and GFR in patients with bilateral RAS.
Discussion
In the current study we observed that patients with RAS appear to have elevated plasma levels of MBG when contrasted against either healthy adults or a comparator group of hypertensive patients (Figure 1 and Table 1). MBG is a bufadienolide type of CTS originally found from parotid secretion of Bufo marinus toad 23. It has been demonstrated that MBG can also be synthesized in animal adrenal glands 25 and in cultured adrenocortical cells 26. Like other CTS, MBG inhibits Na/K-ATPase activity and may potentially regulate the sodium re-absorption and kidney function in conditions of high-salt loading or plasma volume expansion 27, 28. Increased levels of CTS including MBG have been reported in patients with hypertension, myocardial infarction and heart failure 4, 29, 30 as well as in patients with end-stage renal disease and chronic renal diseases 2, 31. There are two lines of evidence from our study showing that renal ischemia induced by RAS may be a major stimulus to the release of plasma MBG in these patients. First, we noticed a good correlation between the severity of RAS and the plasma MBG levels in the RAS patients as shown in Figure 2. Second, reversal of renal ischemia by stenting reduces the plasma MBG levels in these patients. The MBG reduction after stenting was observed in all four groups regardless of the pretreatment of anti-coagulants as shown in Figure 4, which indicates that stenting may be the major factor for reduced MBG levels. These results are consistent with our animal experiments. For example, in partial nephrectomy-induced renal ischemia animals and in high salt-loaded animals, both the plasma MBG level and the urine MBG excretion increased 17, 19, 32. Since the MBG was found mainly synthesized in adrenal gland 25, the damage of kidney tissue may trigger the release of hormones from kidney and thus stimulate the release of MBG from adrenal gland. Actually, elevation of angiotensin II has been reported to regulate CTS release from adrenal cortex 6, 8, 27. Our current study also indicates that renin-angiotensin system may be involved in regulation of MBG release. RAS patients in need of ACEi/ARB treatment have higher plasma MBG levels compared to the patients without ACEi/ARB treatment as described in the result section. However, since the MBG levels before ACEi/ARB treatment in these patients are not available, it is not clear how these medications affect the MBG release. It is also not known whether reduced GFR in these patients have any effects on the MBG excretion, which may merit further studies to measure the 24 hour urine MBG excretion in the RAS patients.
On the other hand, MBG has been found to have natriuretic effect in animal models with high salt-induced volume expansion 32, 33. MBG as well as other CTS compounds can induce the protein endocytosis of the kidney proximal tubule Na/K-ATPase 34, 35. Reduced Na/K-ATPase protein and activity on the basolateral membrane of kidney proximal tubules blunt the sodium re-absorption and therefore increase the natriuresis. The current study has not shown an independent association between the renal function (plasma creatinine level or GFR) and the baseline MBG levels. However, as shown in Figure 5, the reduction of MBG at 24 hour after renal artery stenting seems correlated with a decreased GFR in patients with bilateral RAS but not in patients with unilateral RAS. We hypothesize that MBG may help maintain the GFR in global renal ischemia and acute reduction of MBG can cause the decrease of GFR. However, it will be prudent to obtain more data before making such a conclusion.
Renal dysfunction, mild or severe, is associated with increased rates of CV events 36–39 and increased CV mortality 40, 41. Wollenweber 42 described a 6-year CV-event-free survival of 53%, with risk related to the severity of the renal stenosis. Several others have suggested that the risk of adverse CV events is high and occurs in excess of the hypertension severity 43–45. More recently a significant difference in 4-year survival was seen between those with incidental RAS compared to those without, with a graded effect on mortality, according to the severity of RAS 46. In RAS patients specifically, renal dysfunction is associated with increased CV event rates and increased mortality 47, 48. Ventricular dysfunction and overt CHF are common in patients with RAS just as RAS is common in patients with CHF 49. The elevation of endogenous CTS has now been linked with a variety of CV and renal disease settings 50–55. Animal experiments using rats and mice have demonstrated that renal ischemia induced by partial nephrectomy increases MBG and causes diastolic dysfunction and cardiac fibrosis 17–19. Importantly, the cardiac fibrosis seen in such animals can be prevented by immunization against MBG, whereas infusion of MBG results in a similar pathologic lesion. Our result of MBG elevation may indicate that MBG is an important attributor to the increased CV events in RAS patients.
Perspectives
The current study shows that renal ischemia is associated with high levels of a circulating endogenous cardiotonic steroid, MBG. Recent work in this area demonstrates that CTS are likely important intermediaries in the linkage between chronic kidney disease and the development of cardiac hypertrophy and fibrosis. Thus the MBG elevation may in part attribute to the high CV events in patients with RAS and the measurement of plasma MBG levels may serve as a biomarker for the cardio-renal syndrome.
Acknowledgements
The RAS patient samples were from RESIST trial sponsored by The University of Toledo, Health Sciences Campus, Toledo, Ohio and funded by Centocor Inc and Cordis Corp, both Johnson & Johnson companies. A special acknowledgement is to Christina Eisenhauer, RN, Deborah Repass, MA and Kelly Walter, MA for their help in collecting blood samples.
Sources of Funding:
This work is supported by American Heart Association (0980027N). The work of Drs. Alexei Y. Bagrov and Olga V. Fedorova was supported by Intramural Research Program, National Institute on Aging, NIH.
Footnotes
Clinical trial registration information: NCT00234585
Disclosures
Dr. Christopher J. Cooper and Pamela Brewster have received research grants from the Centocor Inc and Cordis Corp. for the RESIST study and from the NIH for the CORAL study, 5U01HL071556-06. The other authors report no other conflicts.
References
- 1.Schoner W, Scheiner-Bobis G. Role of endogenous cardiotonic steroids in sodium homeostasis. Nephrol Dial Transplant. 2008;23:2723–2729. doi: 10.1093/ndt/gfn325. [DOI] [PubMed] [Google Scholar]
- 2.Komiyama Y, Dong XH, Nishimura N, Masaki H, Yoshika M, Masuda M, Takahashi H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin Biochem. 2005;38:36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Komiyama Y, Nishimura N, Munakata M, Mori T, Okuda K, Nishino N, Hirose S, Kosaka C, Masuda M, Takahashi H. Identification of endogenous ouabain in culture supernatant of PC12 cells. J Hypertens. 2001;19:229–236. doi: 10.1097/00004872-200102000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Bagrov AY, Fedorova OV, Dmitrieva RI, Howald WN, Hunter AP, Kuznetsova EA, Shpen VM. Characterization of a urinary bufodienolide Na+,K+-ATPase inhibitor in patients after acute myocardial infarction. Hypertension. 1998;31:1097–1103. doi: 10.1161/01.hyp.31.5.1097. [DOI] [PubMed] [Google Scholar]
- 5.Laredo J, Hamilton BP, Hamlyn JM. Ouabain is secreted by bovine adrenocortical cells. Endocrinology. 1994;135:794–797. doi: 10.1210/endo.135.2.8033829. [DOI] [PubMed] [Google Scholar]
- 6.Laredo J, Shah JR, Lu ZR, Hamilton BP, Hamlyn JM. Angiotensin II stimulates secretion of endogenous ouabain from bovine adrenocortical cells via angiotensin type 2 receptors. Hypertension. 1997;29:401–407. doi: 10.1161/01.hyp.29.1.401. [DOI] [PubMed] [Google Scholar]
- 7.Fedorova OV, Anderson DE, Bagrov AY. Plasma marinobufagenin-like and ouabain-like immunoreactivity in adrenocorticotropin-treated rats. Am J Hypertens. 1998;11:796–802. doi: 10.1016/s0895-7061(98)00042-9. [DOI] [PubMed] [Google Scholar]
- 8.Bauer N, Muller-Ehmsen J, Kramer U, Hambarchian N, Zobel C, Schwinger RH, Neu H, Kirch U, Grunbaum EG, Schoner W. Ouabain-like compound changes rapidly on physical exercise in humans and dogs: effects of beta-blockade and angiotensin-converting enzyme inhibition. Hypertension. 2005;45:1024–1028. doi: 10.1161/01.HYP.0000165024.47728.f7. [DOI] [PubMed] [Google Scholar]
- 9.De Angelis C, Haupert GT., Jr Hypoxia triggers release of an endogenous inhibitor of Na(+)-K(+)-ATPase from midbrain and adrenal. Am J Physiol. 1998;274:F182–F188. doi: 10.1152/ajprenal.1998.274.1.F182. [DOI] [PubMed] [Google Scholar]
- 10.Weidemann H, Salomon N, Avnit-Sagi T, Weidenfeld J, Rosen H, Lichtstein D. Diverse effects of stress and additional adrenocorticotropic hormone on digitalis-like compounds in normal and nude mice. J Neuroendocrinol. 2004;16:458–463. doi: 10.1111/j.1365-2826.2004.01181.x. [DOI] [PubMed] [Google Scholar]
- 11.Derkx FH, Schalekamp MA. Renal artery stenosis and hypertension. Lancet. 1994;344:237–239. doi: 10.1016/s0140-6736(94)93002-3. [DOI] [PubMed] [Google Scholar]
- 12.Davis BA, Crook JE, Vestal RE, Oates JA. Prevalence of renovascular hypertension in patients with grade III or IV hypertensive retinopathy. N Engl J Med. 1979;301:1273–1276. doi: 10.1056/NEJM197912063012307. [DOI] [PubMed] [Google Scholar]
- 13.Losito A, Fagugli RM, Zampi I, Parente B, de Rango P, Giordano G, Cao P. Comparison of target organ damage in renovascular and essential hypertension. Am J Hypertens. 1996;9:1062–1067. doi: 10.1016/0895-7061(96)00199-9. [DOI] [PubMed] [Google Scholar]
- 14.Simon N, Franklin SS, Bleifer KH, Maxwell MH. Clinical characteristics of renovascular hypertension. Jama. 1972;220:1209–1218. [PubMed] [Google Scholar]
- 15.Lopatin DA, Ailamazian EK, Dmitrieva RI, Shpen VM, Fedorova OV, Doris PA, Bagrov AY. Circulating bufodienolide and cardenolide sodium pump inhibitors in preeclampsia. J Hypertens. 1999;17:1179–1187. doi: 10.1097/00004872-199917080-00018. [DOI] [PubMed] [Google Scholar]
- 16.Vu HV, Ianosi-Irimie MR, Pridjian CA, Whitbred JM, Durst JM, Bagrov AY, Fedorova OV, Pridjian G, Puschett JB. Involvement of marinobufagenin in a rat model of human preeclampsia. Am J Nephrol. 2005;25:520–528. doi: 10.1159/000088461. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–495. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 18.Elkareh J, Kennedy DJ, Yashaswi B, Vetteth S, Shidyak A, Kim EG, Smaili S, Periyasamy SM, Hariri IM, Fedorova L, Liu J, Wu L, Kahaleh MB, Xie Z, Malhotra D, Fedorova OV, Kashkin VA, Bagrov AY, Shapiro JI. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. 2007;49:215–224. doi: 10.1161/01.HYP.0000252409.36927.05. [DOI] [PubMed] [Google Scholar]
- 19.Kennedy DJ, Elkareh J, Shidyak A, Shapiro AP, Smaili S, Mutgi K, Gupta S, Tian J, Morgan E, Khouri S, Cooper CJ, Periyasamy SM, Xie Z, Malhotra D, Fedorova OV, Bagrov AY, Shapiro JI. Partial Nephrectomy as a Model for Uremic Cardiomyopathy in the Mouse. Am J Physiol Renal Physiol. 2008;294:F450–F454. doi: 10.1152/ajprenal.00472.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper CJ, Haller ST, Colyer W, Steffes M, Burket MW, Thomas WJ, Safian R, Reddy B, Brewster P, Ankenbrandt MA, Virmani R, Dippel E, Rocha-Singh K, Murphy TP, Kennedy DJ, Shapiro JI, D'Agostino RD, Pencina MJ, Khuder S. Embolic protection and platelet inhibition during renal artery stenting. Circulation. 2008;117:2752–2760. doi: 10.1161/CIRCULATIONAHA.107.730259. [DOI] [PubMed] [Google Scholar]
- 21.Bagrov AY, Fedorova OV, Austin-Lane JL, Dmitrieva RI, Anderson DE. Endogenous marinobufagenin-like immunoreactive factor and Na+, K+ ATPase inhibition during voluntary hypoventilation. Hypertension. 1995;26:781–788. doi: 10.1161/01.hyp.26.5.781. [DOI] [PubMed] [Google Scholar]
- 22.Fedorova OV, Simbirtsev AS, Kolodkin NI, Kotov AY, Agalakova NI, Kashkin VA, Tapilskaya NI, Bzhelyansky A, Reznik VA, Frolova EV, Nikitina ER, Budny GV, Longo DL, Lakatta EG, Bagrov AY. Monoclonal antibody to an endogenous bufadienolide, marinobufagenin, reverses preeclampsia-induced Na/K-ATPase inhibition and lowers blood pressure in NaCl-sensitive hypertension. J Hypertens. 2008;26:2414–2425. doi: 10.1097/HJH.0b013e328312c86a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bagrov AY, Roukoyatkina NI, Pinaev AG, Dmitrieva RI, Fedorova OV. Effects of two endogenous Na+,K(+)-ATPase inhibitors, marinobufagenin and ouabain, on isolated rat aorta. Eur J Pharmacol. 1995;274:151–158. doi: 10.1016/0014-2999(94)00735-p. [DOI] [PubMed] [Google Scholar]
- 24.Stevens LA, Coresh J, Greene T, Levey AS. Assessing kidney function--measured and estimated glomerular filtration rate. N Engl J Med. 2006;354:2473–2483. doi: 10.1056/NEJMra054415. [DOI] [PubMed] [Google Scholar]
- 25.Fedorova OV, Talan MI, Agalakova NI, Lakatta EG, Bagrov AY. Endogenous ligand of alpha(1) sodium pump, marinobufagenin, is a novel mediator of sodium chloride--dependent hypertension. Circulation. 2002;105:1122–1127. doi: 10.1161/hc0902.104710. [DOI] [PubMed] [Google Scholar]
- 26.Dmitrieva RI, Bagrov AY, Lalli E, Sassone-Corsi P, Stocco DM, Doris PA. Mammalian bufadienolide is synthesized from cholesterol in the adrenal cortex by a pathway that Is independent of cholesterol side-chain cleavage. Hypertension. 2000;36:442–448. doi: 10.1161/01.hyp.36.3.442. [DOI] [PubMed] [Google Scholar]
- 27.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61:9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fedorova OV, Doris PA, Bagrov AY. Endogenous marinobufagenin-like factor in acute plasma volume expansion. Clin Exp Hypertens. 1998;20:581–591. doi: 10.3109/10641969809053236. [DOI] [PubMed] [Google Scholar]
- 29.Liu ZQ, Ma AQ, Zhang L, Yang DY. Intra-cellular electrolyte changes and levels of endogenous digoxin-like substance within the plasma in patients with congestive heart failure. Int J Cardiol. 1990;27:47–53. doi: 10.1016/0167-5273(90)90190-g. [DOI] [PubMed] [Google Scholar]
- 30.Manunta P, Maillard M, Tantardini C, Simonini M, Lanzani C, Citterio L, Stella P, Casamassima N, Burnier M, Hamlyn JM, Bianchi G. Relationships among endogenous ouabain, alpha-adducin polymorphisms and renal sodium handling in primary hypertension. J Hypertens. 2008;26:914–920. doi: 10.1097/HJH.0b013e3282f5315f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonick HC, Ding Y, Vaziri ND, Bagrov AY, Fedorova OV. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin Exp Hypertens. 1998;20:617–627. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- 32.Fedorova OV, Lakatta EG, Bagrov AY. Endogenous Na,K pump ligands are differentially regulated during acute NaCl loading of Dahl rats. Circulation. 2000;102:3009–3014. doi: 10.1161/01.cir.102.24.3009. [DOI] [PubMed] [Google Scholar]
- 33.Bagrov AY, Fedorova OV, Dmitrieva RI, French AW, Anderson DE. Plasma marinobufagenin-like and ouabain-like immunoreactivity during saline volume expansion in anesthetized dogs. Cardiovasc Res. 1996;31:296–305. [PubMed] [Google Scholar]
- 34.Liu J, Periyasamy SM, Gunning W, Fedorova OV, Bagrov AY, Malhotra D, Xie Z, Shapiro JI. Effects of cardiac glycosides on sodium pump expression and function in LLC-PK1 and MDCK cells. Kidney Int. 2002;62:2118–2125. doi: 10.1046/j.1523-1755.2002.00672.x. [DOI] [PubMed] [Google Scholar]
- 35.Periyasamy SM, Liu J, Tanta F, Kabak B, Wakefield B, Malhotra D, Kennedy DJ, Nadoor A, Fedorova OV, Gunning W, Xie Z, Bagrov AY, Shapiro JI. Salt loading induces redistribution of the plasmalemmal Na/K-ATPase in proximal tubule cells. Kidney Int. 2005;67:1868–1877. doi: 10.1111/j.1523-1755.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 36.Culleton BF, Larson MG, Wilson PW, Evans JC, Parfrey PS, Levy D. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 37.Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 38.Parfrey PS, Foley RN. The clinical epidemiology of cardiac disease in chronic renal failure. J Am Soc Nephrol. 1999;10:1606–1615. doi: 10.1681/ASN.V1071606. [DOI] [PubMed] [Google Scholar]
- 39.Shulman NB, Ford CE, Hall WD, Blaufox MD, Simon D, Langford HG, Schneider KA. Prognostic value of serum creatinine and effect of treatment of hypertension on renal function. Results from the hypertension detection and follow-up program. The Hypertension Detection and Follow-up Program Cooperative Group. Hypertension. 1989;13:I80–I93. doi: 10.1161/01.hyp.13.5_suppl.i80. [DOI] [PubMed] [Google Scholar]
- 40.Al Suwaidi J, Reddan DN, Williams K, Pieper KS, Harrington RA, Califf RM, Granger CB, Ohman EM, Holmes DR., Jr Prognostic implications of abnormalities in renal function in patients with acute coronary syndromes. Circulation. 2002;106:974–980. doi: 10.1161/01.cir.0000027560.41358.b3. [DOI] [PubMed] [Google Scholar]
- 41.McCullough PA, Soman SS, Shah SS, Smith ST, Marks KR, Yee J, Borzak S. Risks associated with renal dysfunction in patients in the coronary care unit. J Am Coll Cardiol. 2000;36:679–684. doi: 10.1016/s0735-1097(00)00774-9. [DOI] [PubMed] [Google Scholar]
- 42.Wollenweber J, Sheps SG, Davis GD. Clinical course of atherosclerotic renovascular disease. Am J Cardiol. 1968;21:60–71. doi: 10.1016/0002-9149(68)90014-3. [DOI] [PubMed] [Google Scholar]
- 43.Isles C, Main J, O'Connell J, Brown I, Findlay J, Stewart R, Wilkinson R. Survival associated with renovascular disease in Glasgow and Newcastle: a collaborative study. Scott Med J. 1990;35:70–73. doi: 10.1177/003693309003500303. [DOI] [PubMed] [Google Scholar]
- 44.Sheps SG, Osmundson PJ, Hunt JC, Schirger A, Fairbairn JF., 2nd Hypertension and renal artery stenosis: seral observations on 54 patients treated medically. Clin Pharmacol Ther. 1965;6:700–709. doi: 10.1002/cpt196566700. [DOI] [PubMed] [Google Scholar]
- 45.Valentine RJ, Clagett GP, Miller GL, Myers SI, Martin JD, Chervu A. The coronary risk of unsuspected renal artery stenosis. J Vasc Surg. 1993;18:433–439. discussion 439–440. [PubMed] [Google Scholar]
- 46.Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001;60:1490–1497. doi: 10.1046/j.1523-1755.2001.00953.x. [DOI] [PubMed] [Google Scholar]
- 47.Dorros G, Jaff M, Mathiak L, Dorros, Lowe A, Murphy K, He T. Four-year follow-up of Palmaz-Schatz stent revascularization as treatment for atherosclerotic renal artery stenosis. Circulation. 1998;98:642–647. doi: 10.1161/01.cir.98.7.642. [DOI] [PubMed] [Google Scholar]
- 48.Johansson M, Herlitz H, Jensen G, Rundqvist B, Friberg P. Increased cardiovascular mortality in hypertensive patients with renal artery stenosis. Relation to sympathetic activation, renal function and treatment regimens. J Hypertens. 1999;17:1743–1750. doi: 10.1097/00004872-199917120-00012. [DOI] [PubMed] [Google Scholar]
- 49.MacDowall P, Kalra PA, O'Donoghue DJ, Waldek S, Mamtora H, Brown K. Risk of morbidity from renovascular disease in elderly patients with congestive cardiac failure. Lancet. 1998;352:13–16. doi: 10.1016/s0140-6736(97)11060-1. [DOI] [PubMed] [Google Scholar]
- 50.Bagrov AY, Agalakova NI, Kashkin VA, Fedorova OV. Endogenous Cardiotonic Steroids and Differential Patterns of Sodium Pump Inhibition in NaCl-Loaded Salt-Sensitive and Normotensive Rats. Am J Hypertens. 2009 doi: 10.1038/ajh.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bagrov YY, Manusova NB, Frolova EV, Egorova IA, Kashkin VA, Tapilskaya NI, Fedorova OV, Bagrov AY. Endogenous sodium pump inhibitors, diabetes mellitus and preeclampsia Preliminary observations and a hypothesis. Pathophysiology. 2007;14:147–151. doi: 10.1016/j.pathophys.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dostanic-Larson I, Van Huysse JW, Lorenz JN, Lingrel JB. The highly conserved cardiac glycoside binding site of Na,K-ATPase plays a role in blood pressure regulation. Proc Natl Acad Sci U S A. 2005;102:15845–15850. doi: 10.1073/pnas.0507358102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fridman AI, Matveev SA, Agalakova NI, Fedorova OV, Lakatta EG, Bagrov AY. Marinobufagenin, an endogenous ligand of alpha-1 sodium pump, is a marker of congestive heart failure severity. J Hypertens. 2002;20:1189–1194. doi: 10.1097/00004872-200206000-00032. [DOI] [PubMed] [Google Scholar]
- 54.Hamlyn JM, Ringel R, Schaeffer J, Levinson PD, Hamilton BP, Kowarski AA, Blaustein MP. A circulating inhibitor of (Na+ + K+)ATPase associated with essential hypertension. Nature. 1982;300:650–652. doi: 10.1038/300650a0. [DOI] [PubMed] [Google Scholar]
- 55.Schoner W. Salt abuse: the path to hypertension. Nat Med. 2008;14:16–17. doi: 10.1038/nm0108-16. [DOI] [PubMed] [Google Scholar]