Abstract
Objective
We hypothesized that adiponectin and tumor necrosis factor-alpha (TNFα) reciprocally regulate their expression, thereby synergistically affecting both coronary and aortic endothelial dysfunction in type 2 diabetes.
Methods and Results
We examined endothelium-dependent and –independent vasodilation/vasorelaxation of coronary arterioles and aortas in control mice (m Leprdb), diabetic mice (Leprdb) and Leprdb treated with adiponectin or neutralizing antibody to TNFα (anti-TNFα). Endothelium-dependent vasodilation to acetylcholine (ACh) in both coronary arterioles and aortas was blunted in Leprdb compared with m Leprdb. Endothelium-independent vasodilation to sodium nitroprusside (SNP) was comparable. Adiponectin and anti-TNFα improved ACh-induced vasodilation of coronary arterioles and aortas in Leprdb without affecting dilator response to SNP. Adiponectin protein expression was significantly reduced and TNFα protein expression was significantly greater in coronary arterioles and aortas of Leprdb compared to m Leprdb. Immunofluorescence staining results indicate that adiponectin was colocalized with endothelial cells. Anti-TNFα treatment up-regulated adiponectin protein expression in Leprdb coronary arterioles and aortas. Adiponectin administration reduced TNFα protein expression in Leprdb. Although adiponectin receptor 1 (AdipoR1) protein expression in coronary arterioles and aortas was similar between control and diabetic mice, adiponectin receptor 2 (AdipoR2) expression was significantly reduced in Leprdb. Both adiponectin and anti-TNFα inhibited IκBα phosphorylation and nuclear factor-kappa B (NFκB) protein expression in Leprdb, suggesting that adiponectin and TNFα signaling may converge on NFκB to reciprocally regulate their expression.
Conclusions
These results indicate a reciprocal suppression occurs between adiponectin and TNFα that fundamentally affects the regulation of coronary and aortic endothelial function in type 2 diabetic mice.
Keywords: coronary circulation, cytokines, reactive oxygen species, vasodilation
Introduction
The growing epidemic of cardiovascular disease in developed countries is closely associated with an increased prevalence of obesity and type 2 diabetes.1-2 Much of the recent work on obesity has highlighted the key role of adipose tissue as an endocrine organ that secretes a number of factors, termed adipokines, which mediate many of the vascular and metabolic complications of obesity.3 An adverse adipokine expression profile characterized by diminished production of protective factors such as adiponectin and increased detrimental adipokines such as tumor necrosis factor-alpha (TNFα), has been suggested in obese and type 2 diabetic patients.4-7
Adiponectin, also known as ACRP30, or AdipoQ, is an adipokine that is secreted from adipocytes.8 Adiponectin plays an important role in the regulation of glucose and lipid metabolism.9 Paradoxically, serum concentration of adiponectin is decreased in obese and type 2 diabetic patients despite an increased adiposity.10 In contrast, plasma TNFα levels are elevated in such subjects, suggesting that there may be an imbalance between the production of adiponectin and TNFα in obesity.11 In 3T3-L1 adipocytes, decreased adiponectin mRNA levels by TNFα were partially recovered by treatment with a c-JUN N-terminal kinase (JNK) inhibitor, suggesting that the JNK signaling pathway, activated by TNFα, is involved in the regulation of adiponectin expression in adipocytes.7 Another study shows that adiponectin decreases leptin-induced TNFα expression by murine macrophages through suppressing phosphorylation of extracellular signal-regulated kinase (ERK1/2) and P38 mitogen-activated protein kinases (p38 MAPK) pathways.12 Thus, there may be a reciprocal association between adiponectin and TNFα.
Adipose-derived adipokines actively participate in the regulation of vascular function; i.e., TNFα contributes to the impairment of coronary and aortic vascular function in type 2 diabetic mice.13-14 However: 1) the role of adiponectin in regulating coronary and aortic vascular function in type 2 diabetic mice; or 2) if there is a reciprocal association between adiponectin and TNFα, has yet to be resolved. Thus, the goal of this study was to examine the nature and mechanisms of putative reciprocal suppressive effects between adiponectin and TNFα in coronary microvessels and aortas in type 2 diabetic mice and how this reciprocal regulation can contribute to the pathogenesis of diabetes-associated vascular dysfunction.
Methods
Animals
The procedures followed were in accordance with approved guidelines set by the Laboratory Animal Care Committee at the University of Missouri. Heterozygote control mice (m Leprdb) (Background Strain: C57BLKS/J), homozygote type 2 diabetic mice (Leprdb) (Background Strain: C57BLKS/J) and Leprdb null for TNFα (dbTNF-/dbTNF-) (Background Strain: C57BL/6J) were purchased from Jackson Laboratory and Adiponectin knockout mice (APN−/−) (Background Strain: C57BL/6J) were obtained from Dr. William P. Fay’ laboratory. All of these mice were maintained on a normal rodent chow diet. Male, 20-35g m Leprdb and APN−/−, 40-60 g Leprdb, and dbTNF-/dbTNF- mice of either sex were used in this study. The cross of Leprdb with TNFα knockout (TNF KO) is heterozygous for Leprdb and homozygous for TNF KO (TNF−/−). These dbTNF-/dbTNF- mice show the phenotypes of hyperglycemia and obesity, the diabetic phenotype that is consistent with the penetrance of the leptin receptor mutation. The obese mice from the second round of breeding of Leprdb and TNF−/− were used in experimentation.13
Treatment with Adiponectin, TNFα Neutralization, or Recombinant TNFα
At 12-16 weeks of age, Leprdb mice were treated with the recombinant murine globular adiponectin (30 μg/day, s.c. twice daily for 10 days, PeproTech). The neutralizing antibody to TNFα is 2E2 monoclonal antibody (2E2 MAb. 94021402, NCI Biological Resources Branch). Leprdb mice received the neutralizing anti-TNFα (0.625 mg/ml/kg/day, i.p. for 10 days).13 m Leprdb control mice received murine recombinant TNFα (10 μg/day, i.p. for 3 days, R&D).
Functional Assessment of Isolated Coronary Arterioles
The techniques for identification and isolation of coronary microvessels were described in detail previously.13 Coronary arterioles (40 to 100 μm in diameter) from mouse heart were carefully dissected for in vitro study. To determine whether adiponectin plays a role in vascular dysfunction in type 2 diabetes, vasodilation to endothelium-dependent vasodilator acetylcholine (ACh, 0.1 nmol/L to 10 μmol/L), endothelium-independent vasodilator sodium nitroprusside (SNP, 0.1 nmol/L to 10 μmol/L), or flow-induced dilation (NO-mediated, endothelium-dependent, but agonist-independent; 4 to 60 cm H2O) were assessed in isolated coronary arterioles in m Leprdb, Leprdb and Leprdb mice treated with adiponectin. At the end of each experiment, the vessel was relaxed with 100 μmol/L SNP to obtain its maximal diameter at 60 cm H2O intraluminal pressure. All diameter changes in response to agonists were normalized to the vasodilation in response to 100 μmol/L SNP and expressed as a percentage of maximal dilation.
Functional Assessment of Murine Aortas
2 mm of aortic rings were isometrically mounted in a myograph (model 610M, DMT, Denmark) and an optimal passive tension (15 mN) was applied. Aortic rings were precontracted with 1 μmol/L phenylephrine (PE). Dose-response curve was obtained by cumulative addition of ACh (1 nmol/L to 10 μmol/L), and SNP (1 nmol/L to 10 μmol/L). Relaxation at each concentration was measured and expressed as the percentage of force generated in response to PE.14 The contribution of NO in vasorelaxation was assessed by incubating the vessels with NOS (eNOS and neuronal NOS) inhibitor NG-nitro-L-arginine methyl ester (L-NAME; 100 μmol/L for 20min).15
Protein Expression by Western Blot Analyses
Coronary arterioles (4-6 vessels per sample) or aortas were homogenized in lysis buffer (Cellytic™ MT Mammalian Tissue Lysis/Extraction Reagent, Sigma). Protein concentrations were assessed with a BCA™ Protein Assay Kit (Pierce) and samples were separated by SDS-PAGE and transferred to PVDF membranes. TNFα, IκBα, phospho-IκBα (Santa Cruz), Adiponectin (R&D), AdipoR1 and AdipoR2 (Alpha Diagnostics), and NFκB (Abcam) were determined. Signals were visualized by enhanced chemiluminescence (ECL, Amersham), and quantified by Fuji film imaging software.13 Housekeeping gene β-actin was used as the loading control and we validated that no significant variation was detected between control and experimental groups as well as across samples.13-14 The relative amounts of protein expression in various groups were quantified and normalized to those of the corresponding m Leprdb control, which were set to a value of 1.0.13
Immunofluorescence Staining
Immunohistochemistry was used to identify and localize proteins in sections of vessels or myocardial tissue. Hearts or aortas were embedded in OCT and sectioned at 5 μm. Slides were incubated with blocking solution (10% donkey serum in PBS). Primary antibodies for adiponectin (goat polyclonal, R&D, AF1119), and endothelial cell marker, von Willebrand factor (rabbit polyclonal, Abcam), or smooth muscle α-actin (rabbit polyclonal, Abcam) or fibroblast (rat monoclonal, Novus Biologicals) were used for sequential double immunofluorescence staining. Secondary fluorescent antibodies were either FITC or Texas Red conjugated. For negative controls, primary antibodies were replaced with IgG-isotype controls at the same concentration. Sections were finally mounted in an anti-fading agent (Slowfade gold with DAPI, Invitrogen). Slides were observed and analyzed using a fluorescence microscope with a 40×objective (IX81, Olympus).16
Data Analysis
All data are presented as mean±SEM except as specifically stated. Statistical comparisons under various treatments were performed with one-way ANOVA, and intergroup differences were tested with LSD inequality. Significance was accepted at P < 0.05. Detailed methods can be found in the supplemental materials (please see http://atvb.ahajournals.org).
Results
Effects of adiponectin and anti-TNFα on Body Weight, Abdominal Girth, Blood Glucose and Insulin/Insulin Resistance
Body weight and abdominal girth were greater in Leprdb vs. m Leprdb. Non-fasting blood glucose level, plasma insulin level and HOMA-IR were elevated in Leprdb. Adiponectin and anti-TNFα treatment did not affect the above parameters in Leprdb. (Supplemental Table I)
Role of Adiponectin in Type 2 Diabetes-Induced Coronary Endothelial Dysfunction
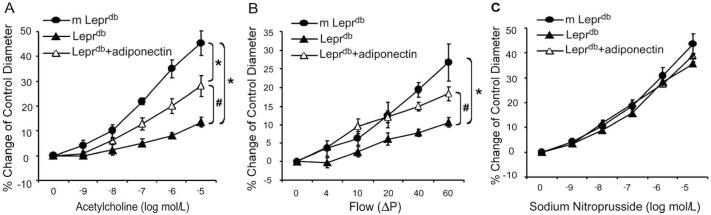
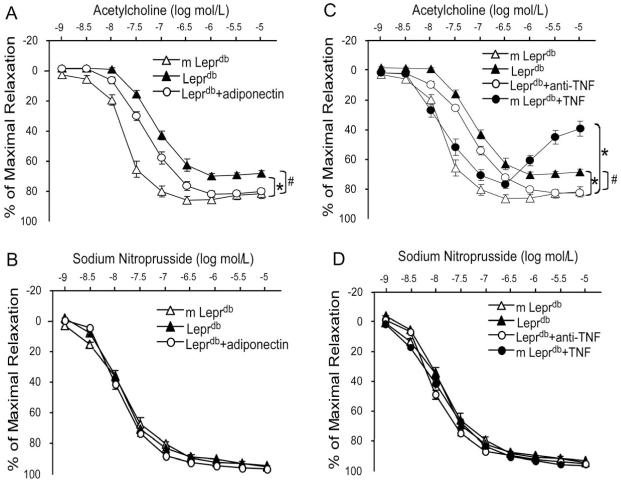
Vasodilation to endothelium-dependent vasodilator ACh was impaired in coronary arterioles of Leprdb (Figure 1A). Conversely, adiponectin partially restored ACh-induced vasodilation in Leprdb (Figure 1A). Moreover, flow-induced vasodilation (FID) was diminished in coronary arterioles of Leprdb, but was rescued by adiponectin (Figure 1B). In contrast, SNP-induced endothelium-independent vasodilation was similar among m Leprdb, Leprdb and Leprdb treated with adiponectin (Figure 1C).
Figure 1.
Role of adiponectin in improving coronary arteriolar endothelium-dependent and - independent vasodilation in type 2 diabetic mice. (A) and (B), ACh-induced vasodilation and flow-induced vasodilation (FID) were blunted in coronary arterioles of Leprdb, and adiponectin partially restored NO-mediated coronary arteriolar dilation to ACh and FID in Leprdb. (C) Endothelium-independent vasodilation of coronary arterioles to sodium nitroprusside (SNP) was not different among m Leprdb, Leprdb, and Leprdb+adiponectin. Data shown as mean±SEM. n=6 mice. *p<0.05 vs. m Leprdb; #p<0.05 vs. Leprdb.
Role of Adiponectin and TNFα in Type 2 Diabetes-Induced Aortic Endothelial Dysfunction
Endothelium-dependent vasorelaxation in response to ACh was significantly impaired in aortas of Leprdb. Adiponectin and anti-TNFα partially restored impaired vasorelaxation (Figure 2A and 2C, and Supplemental Table II). Endothelium-independent vasorelaxation to SNP was not statistically different among any of the groups (Figure 2B and 2D). Recombinant TNFα treatment impaired aortic function of m Leprdb control mice (Figure 2C and Supplemental Table II).
Figure 2.
Role of adiponectin and TNFα in aortic endothelium-dependent and - independent vasorelaxation in type 2 diabetic mice. (A) and (B), Endothelium-dependent vasorelaxation in response to ACh was significantly impaired in aortas of Leprdb. Adiponectin partially restored impaired vasorelaxation. Endothelium-independent vasorelaxation of aortic rings to SNP were similar among m Leprdb, Leprdb, and Leprdb+adiponectin. (C) and (D) Anti-TNFα (anti-TNF) improved endothelium-dependent vasorelaxation in Leprdb, but recombinant TNFα (TNF) treatment impaired ACh-induced vasorelaxation in m Leprdb. SNP-induced vasorelaxation of aortic rings were not different among m Leprdb, Leprdb, Leprdb+anti-TNF, and m Leprdb+TNF. Data represent mean±SEM. n=4-10 mice. *p<0.05 vs. m Leprdb; #p<0.05 vs. Leprdb.
The Reciprocal Association between Adiponectin and TNFα in Coronary Arterioles and Aortas of Diabetic Mice
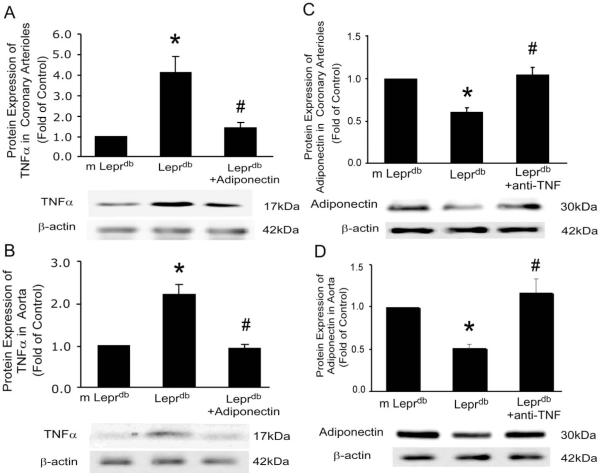
Protein expressions of adiponectin and TNFα from isolated coronary arterioles and aortas were analyzed in m Leprdb, Leprdb and Leprdb mice treated with anti-TNFα or adiponectin. Western blot analysis (Figure 3) revealed that anti-TNFα markedly increased adiponectin expression, while adiponectin reduced TNFα expression in both coronary arterioles and aortas of diabetic mice.
Figure 3.
Reciprocal regulation between adiponectin and TNFα. (A) and (B), Protein expression of TNFα in isolated coronary arterioles and aortas was higher in Leprdb vs. m Leprdb, but adiponectin attenuated TNFα expression in Leprdb. (C) and (D), Adiponectin expression in coronary arterioles and aortas was reduced in Leprdb vs. m Leprdb, but anti-TNFα increased adiponectin expression in Leprdb. Data represent mean±SEM. n=4 separate experiments. *p<0.05 vs. m Leprdb; # p<0.05 vs. Leprdb.
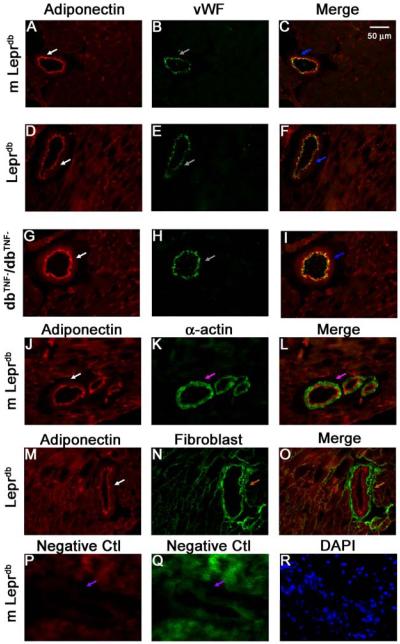
Cellular Source of Adiponectin Expression in Type 2 Diabetes
Immunostaining showed that adiponectin protein expression (red) was present in endothelial cells, but not in vascular smooth muscle cells in both coronary microvessels (Figure 4) and aortas (Supplemental Figure II). Adiponectin staining was absent in APN−/− mice, which validated the specificity of staining (Supplemental Figure III).
Figure 4.
Co-localization and regulation of adiponectin expression in coronary microvessels. Dual fluorescence combining adiponectin with markers for endothelial cells [von Willebrand factor (vWF)], vascular smooth muscle (α-actin) and fibroblast marker with the use of specific primary antibodies followed by fluorescent-labeled secondary antibodies. A, B and C, dual labeling of adiponectin (red) and vWF (green) in control mouse heart tissue. D, E and F, dual labeling of adiponectin (red) and vWF (green) in Leprdb mouse heart tissue. G, H and I, dual labeling of adiponectin (red) and vWF (green) in dbTNF-/dbTNF- heart tissue. Blue arrows in C, F and I show the colocalization of adiponectin and endothelial cells (yellow). J, K and L, dual labeling of adiponectin (red) and α-actin (green) in control mouse heart tissue. The pink arrow in L shows the specific α-actin staining with absence of adiponectin staining. M, N and O, dual labeling of adiponectin (red) and marker of fibroblast in Leprdb mice heart tissue. The brown arrow in O shows the specific fibroblast staining with absence of adiponectin staining. P and Q, negative control: the purple arrows show an absence of staining in vessels with isotype control IgG and without primary antibodies. R shows nuclear staining with DAPI (blue) in control mice heart tissue. Magnification×40. Data shown are representative of 4 separate experiments.
Adiponectin Receptor Expression in Coronary Arterioles and Aortas of Diabetic Mice
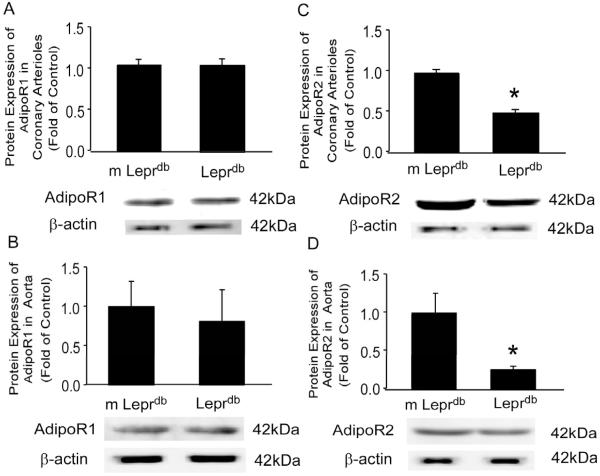
Adiponectin receptor 1 (AdipoR1) protein expression was not significantly different between m Leprdb and Leprdb in both coronary arterioles and aortas (Figure 5A and 5B), while adiponectin receptor 2 (AdipoR2) protein expression was greatly decreased in Leprdb coronary arterioles and aortas (Figure 5C and 5D).
Figure 5.
Protein expression of adiponectin receptors in coronary arterioles and aortas. (A) and (B), Adiponectin receptor 1 (AdipoR1) protein expression was not significantly different between m Leprdb and Leprdb in both coronary arterioles and aortas. (C) and (D), adiponectin receptor 2 (AdipoR2) protein expression was remarkably decreased in Leprdb coronary arterioles and aortas. Data represent mean±SEM. n=4 separate experiments. *p<0.05 vs. m Leprdb; #p<0.05 vs. Leprdb.
Adiponectin and TNFα Signaling Converged on NFκB to Reciprocally Regulate their Expression
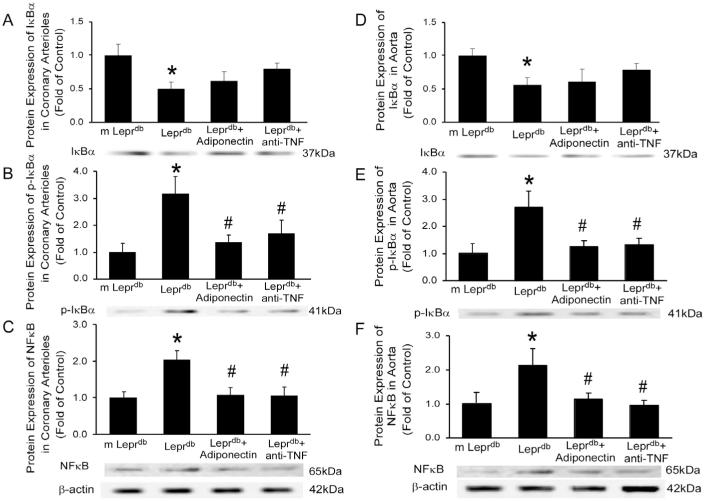
In both coronary arterioles and aortas, IκBα (Inhibitor of NFκB) expression was decreased in Leprdb (Figure 6A and 6D) while phospho-IκBα was greatly elevated (Figure 6B and 6E). Both adiponectin and anti-TNFα inhibited IκBα phosphorylation (Figure 6B and 6E) without affecting the total IκBα expression (Figure 6A and 6D). Nuclear factor-kappa B (NFκB) p65 protein expression was significantly higher in Leprdb. Adiponectin and anti-TNFα decreased NFκB protein expression (Figure 6C and 6F).
Figure 6.
Adiponectin and anti-TNFα inhibit IκBα phosphorylation and NFκB expression. In both coronary arterioles and aortas, total inhibitor of NFκB (IκBα) expression was decreased in Leprdb (A and D) while phospho-IκBα was greatly elevated (B and E). Both adiponectin and anti-TNFα inhibited IκBα phosphorylation (B and E) without affecting the total IκBα expression (A and D). (C) and (F), Nuclear factor-kappa B (NFκB) p65 protein expression was significantly increased in Leprdb. Adiponectin and anti-TNFα decreased NFκB protein expression. Data represent mean±SEM. n=4 separate experiments. *P<0.05 vs. m Leprdb; #P<0.05 vs. Leprdb.
Discussion
This is the first in vivo evidence for a reciprocal regulation occurring between adiponectin and TNFα in micro- and macrocirculation in type 2 diabetic mice. The circulatory protein adiponectin protects against diabetes-induced coronary arteriolar and aortic vascular dysfunction, and this protection involves, at least in part, the downregulation of TNFα. Furthermore, inhibition of TNFα is associated with upregulation of adiponectin in micro- and macrovessels. Taken together, these data suggest that the reciprocal suppression of adiponectin and TNFα functionally contributes to the regulation of diabetes-associated vascular dysfunction.
The Vascular Effects of TNFα and Adiponectin in Type 2 Diabetes
TNFα and adiponectin are important adipose-derived factors. TNFα is a key proinflammatory cytokine mainly secreted by non-fat cells in adipose tissue.17 We have previously demonstrated that TNFα contributed to endothelial dysfunction in type 2 diabetes by inducing activation of NAD(P)H oxidase and production of reactive oxygen species (ROS) in both aortas and coronary microcirculation.13-14, 18 Adiponectin is a relatively abundant plasma protein specifically secreted by adipocytes. Adiponectin exists in the circulation as a full-length protein (fAd) as well as a putative proteolytic cleavage fragment consisting of the globular C-terminal domain (gAd), which may have enhanced potency.19-20 The biological activities of gAd is controversial due to its proinflammatory effects in cardiac fibroblasts,21 but it appears that globular adiponectin is significantly more potent in reversing insulin resistance and exerts vascular protective effects by enhancing NO availability in endothelial cells.22-23 gAd incubation (2 mg/ml) for 2 hours improved endothelium-dependent relaxation and total production of nitric oxide as a result of enhanced eNOS activity.24 In Leprdb mice, serum adiponectin levels are significantly reduced compared with that in control mice (Supplemental Table I). By treating the mice with recombinant globular adiponectin, we found that chronic adiponectin administration rescues both coronary microvascular and aortic macrovascular dysfunction in type 2 diabetic mice (Figure 1 and Figure 2). This vasoprotection by adiponectin may be partly through the direct vascular effects on stimulating endothelial nitric oxide (NO) production and ameliorating oxidative stress based on previous studies using adiponectin knockout mice (APN−/−). In APN−/−, ACh-induced vasodilation in aortas was impaired, accompanied by increased superoxide and peroxynitrite production. eNOS expression was conserved in APN−/− mice, but NO production and endothelial NO synthase (eNOS) phosphorylation were significantly reduced.25 Adiponectin also causes endothelium-independent vasodilation by opening voltage-gated K channels (Kv).26 However, the endothelium-independent vasodilatory effects of adiponectin do not represent a common pathway for the regulation of vascular dysfunction in type 2 diabetic mice since sodium nitroprusside (SNP)-induced vasodilation is similar among control mice, diabetic mice and diabetic mice treated with adiponectin, or anti-TNFα (Figure 1C, Figure 2B and 2D).
The Reciprocal Regulation between Adiponectin and TNFα
The reciprocal regulation between adiponectin and TNFα has been studied in various tissues and cells. Adiponectin suppresses lipopolysaccharide (LPS)-stimulated TNFα production in cultured cardiac myocytes and macrophages,7, 27-28 whereas adiponectin deficiency leads to an increase in circulating TNFα in mouse models.29 TNFα also has a regulatory effect on adiponectin. By incubating human visceral adipose tissue with TNFα in vitro, the mRNA and protein expression of adiponectin were significantly reduced.30 However, there are no in vivo studies examining the reciprocal association between adiponectin and TNFα in vasculature, nor has the role of this reciprocal regulation in the pathogenesis of diabetes induced micro- and macrovascular dysfunction been investigated. Our results suggest that anti-TNFα treatment upregulates adiponectin expression, but adiponectin treatment inhibits TNFα expression in coronary arterioles and aortas of diabetic mice (Figure 3). Thus, adiponectin and TNFα not only exert vascular effects independently by affecting vascular NO bioavailability and oxidative stress,13-14, 25 but also act interactively to participate in the complex regulation of their combined vascular expression in type 2 diabetes.
Adiponectin and Adiponectin Receptor Expression in Coronary Microvessels and Aortas
Two receptor forms have been cloned for adiponectin and the receptors have unique distributions and affinities for the molecular forms of adiponectin. AdipoR1 is a high affinity receptor for gAd with very low affinity for fAd, and AdipoR2 has intermediate affinity for both forms of adiponectin.3 Interestingly, AdipoR1 is abundantly expressed in skeletal muscle and at moderate levels in other tissues, whereas AdipoR2 is predominantly expressed in the liver.20, 31-32 Aortic endothelial cells express both adiponectin isoforms, but appear to preferentially express mRNA of AdipoR1.33 In human umbilical vein endothelial cells (HUVECs), globular adiponectin-induced phosphorylation of eNOS at Ser1177 and NO production22 were abrogated when expression of AdipoR1 and AdipoR2 were simultaneously suppressed.34 Overexpression of AdipoR1 and AdipoR2 in HUVECs significantly enhanced the suppressive effect of an otherwise symptomless dose of globular adiponectin on TNFα-induced intercellular adhesion molecule 1 (ICAM-1) expression and NFκB activation, suggesting the involvement of adiponectin receptors in adiponetin-induced vasoprotection against the proinflammatory effects of TNFα.35 Our study provides the first in vivo documentation that AdipoR1 expression is similar between control and diabetic mice, but AdipoR2 expression is significantly reduced in both coronary arterioles and aortas in diabetic mice (Figure 5). Furthermore, AdipoR2 expression in aortas of diabetic mice is only 25% of that in aortas of control mice, but AdipoR2 expression in coronary arterioles of diabetic mice is about 47% of that in coronary vessels of control mice. The mechanisms accounting for the reduced AdipoR2, but not AdipoR1 expression in diabetes mice vasculature, and the possible differences in AdipoR2 expression between vascular beds remain unknown. However, our results suggest that, in addition to the reduced circulating level of adiponectin in diabetic mice (Supplemental Table I), suppressed AdipoR2 expression and the receptor-mediated response may also contribute to the impaired adiponectin-mediated vascular protective effects. Moreover, increased serum TNFα level (Supplemental Table I) and TNFα receptor 1 (TNFR1) expression in diabetic mice synergistically exacerbated the detrimental effects of TNFα on vascular function.36 Thus, in addition to modulating adiponectin and TNFα production and circulatory levels, treatments mediating AdipoR2 and TNFR1 expression may have potential therapeutic applications for vascular complications associated with the metabolic syndrome and diabetes.
Adiponectin and TNFα Converge on NFκB to Regulate Their Reciprocal Suppression
Adiponectin-mediated suppression of TNFα expression and inflammatory responses by the inhibition of NFκB signaling functionally contributes to the beneficial actions of adiponectin.37-38 fAd suppresses TNFα-induced inflammatory changes in human aortic endothelial cells (HAECs) by blocking IκBα phosphorylation and NFκB activation without affecting TNFα-mediated activation of c-JNK, p38, and protein kinase B (Akt).38 gAd has been shown to attenuate LPS-stimulated TNFα production in macrophages by suppression of NFκB activation.37 TNFα per se suppresses adiponectin secretion in 3T3-L1 adipocytes, and this suppression was reversed by IκB kinase-beta (IKKβ) inhibitor, IMD-0354.39 These data suggest that NFκB signaling may act as a pivot for a reciprocal association between adiponectin and TNFα.
Our studies demonstrate this pivotal role of NFκB signaling in the reciprocal association between adiponectin and TNFα in both coronary microcirculation and aortas in the type 2 diabetic murine model. The results reveal that adiponectin and anti-TNFα treatment remarkably inhibit IκBα phosphorylation and NFκB expression in coronary arterioles and aortas of Leprdb without affecting total IκBα expression (Figure 6). Therefore, adiponectin and TNFα may converge on NFκB signaling to reciprocally regulate their expression and function in coronary microvessels and aortas in type 2 diabetic mice. In conclusion, although TNFα is a key adipokine promoting endothelial dysfunction, adiponectin may prevent vascular injury. This paradigm for adipokine regulation of endothelial function may have important therapeutic implications in diabetes-associated vascular complications.
Condensed Abstract.
We tested if there is a reciprocal relationship between adiponectin and TNFα in the regulation of coronary and aortic endothelial function in type 2 diabetic mice. Our results demonstrated that adiponectin and TNFα may converge on NFκB signaling to reciprocally regulate their expression and function, which in turn affects coronary and aortic endothelial dysfunction in type 2 diabetes.
Supplementary Material
Acknowledgments
We deeply appreciate Dr. William P. Fay’s support on providing adiponectin knockout (APN−/−) mice for this study.
Sources of Funding
This study was supported by grants from Pfizer Atorvastatin Research Award (2004-37, to C.Z.), American Heart Association SDG (110350047A, to C.Z.) and NIH grants (RO1-HL077566 and RO1-HL085119, to C.Z.). H.Z. was supported by an NIH Clinical Biodetective Training Grant (R90DK70105).
Footnotes
Disclosures
None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Meetoo D, McGovern P, Safadi R. An epidemiological overview of diabetes across the world. Br J Nurs. 2007;16:1002–1007. doi: 10.12968/bjon.2007.16.16.27079. [DOI] [PubMed] [Google Scholar]
- 2.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–787. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 3.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 4.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, Iwahashi H, Kuriyama H, Ouchi N, Maeda K, Nishida M, Kihara S, Sakai N, Nakajima T, Hasegawa K, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Hanafusa T, Matsuzawa Y. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–1599. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 5.MacLaren R, Cui W, Simard S, Cianflone K. Influence of obesity and insulin sensitivity on insulin signaling genes in human omental and subcutaneous adipose tissue. J Lipid Res. 2008;49:308–323. doi: 10.1194/jlr.M700199-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Gomez-Ambrosi J, Catalan V, Diez-Caballero A, Martinez-Cruz LA, Gil MJ, Garcia-Foncillas J, Cienfuegos JA, Salvador J, Mato JM, Fruhbeck G. Gene expression profile of omental adipose tissue in human obesity. FASEB J. 2004;18:215–217. doi: 10.1096/fj.03-0591fje. [DOI] [PubMed] [Google Scholar]
- 7.Kim KY, Kim JK, Jeon JH, Yoon SR, Choi I, Yang Y. c-Jun N-terminal kinase is involved in the suppression of adiponectin expression by TNF-alpha in 3T3-L1 adipocytes. Biochem Biophys Res Commun. 2005;327:460–467. doi: 10.1016/j.bbrc.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 8.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 9.Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- 10.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 11.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao T, Hou M, Xia M, Wang Q, Zhu H, Xiao Y, Tang Z, Ma J, Ling W. Globular adiponectin decreases leptin-induced tumor necrosis factor-alpha expression by murine macrophages: involvement of cAMP-PKA and MAPK pathways. Cell Immunol. 2005;238:19–30. doi: 10.1016/j.cellimm.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 14.Zhang H, Zhang J, Ungvari Z, Zhang C. Resveratrol Improves Endothelial Function. Role of TNF{alpha} and Vascular Oxidative Stress. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.187146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X, Zhang H, McAfee SR, Zhang C. The Reciprocal Relationship between Adiponectin and LOX-1 in the Regulation of Endothelial Dysfunction in ApoE Knockout Mice. Am J Physiol Heart Circ Physiol. 2010 doi: 10.1152/ajpheart.01096.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao X, Zhang H, Belmadani S, Wu J, Xu X, Elford H, Potter BJ, Zhang C. Role of TNF-{alpha}-induced reactive oxygen species in endothelial dysfunction during reperfusion injury. Am J Physiol Heart Circ Physiol. 2008;295:H2242–2249. doi: 10.1152/ajpheart.00587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 18.Zhang H, Park Y, Wu J, Chen X, Lee S, Yang J, Dellsperger KC, Zhang C. Role of TNF-alpha in vascular dysfunction. Clin Sci (Lond) 2009;116:219–230. doi: 10.1042/CS20080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fruebis J, Tsao TS, Javorschi S, Ebbets-Reed D, Erickson MR, Yen FT, Bihain BE, Lodish HF. Proteolytic cleavage product of 30-kDa adipocyte complement-related protein increases fatty acid oxidation in muscle and causes weight loss in mice. Proc Natl Acad Sci U S A. 2001;98:2005–2010. doi: 10.1073/pnas.041591798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 21.Hattori Y, Hattori S, Akimoto K, Nishikimi T, Suzuki K, Matsuoka H, Kasai K. Globular adiponectin activates nuclear factor-kappaB and activating protein-1 and enhances angiotensin II-induced proliferation in cardiac fibroblasts. Diabetes. 2007;56:804–808. doi: 10.2337/db06-1405. [DOI] [PubMed] [Google Scholar]
- 22.Hattori Y, Suzuki M, Hattori S, Kasai K. Globular adiponectin upregulates nitric oxide production in vascular endothelial cells. Diabetologia. 2003;46:1543–1549. doi: 10.1007/s00125-003-1224-3. [DOI] [PubMed] [Google Scholar]
- 23.Yamauchi T, Kamon J, Waki H, Imai Y, Shimozawa N, Hioki K, Uchida S, Ito Y, Takakuwa K, Matsui J, Takata M, Eto K, Terauchi Y, Komeda K, Tsunoda M, Murakami K, Ohnishi Y, Naitoh T, Yamamura K, Ueyama Y, Froguel P, Kimura S, Nagai R, Kadowaki T. Globular adiponectin protected ob/ob mice from diabetes and ApoE-deficient mice from atherosclerosis. J Biol Chem. 2003;278:2461–2468. doi: 10.1074/jbc.M209033200. [DOI] [PubMed] [Google Scholar]
- 24.Deng G, Long Y, Yu YR, Li MR. Adiponectin directly improves endothelial dysfunction in obese rats through the AMPK-eNOS Pathway. Int J Obes (Lond) 2010;34:165–171. doi: 10.1038/ijo.2009.205. [DOI] [PubMed] [Google Scholar]
- 25.Cao Y, Tao L, Yuan Y, Jiao X, Lau WB, Wang Y, Christopher T, Lopez B, Chan L, Goldstein B, Ma XL. Endothelial dysfunction in adiponectin deficiency and its mechanisms involved. J Mol Cell Cardiol. 2009;46:413–419. doi: 10.1016/j.yjmcc.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–727. doi: 10.1016/j.cardiores.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, Walsh K. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1035–1042. doi: 10.1152/ajplung.00397.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, Komuro R, Ouchi N, Kihara S, Tochino Y, Okutomi K, Horie M, Takeda S, Aoyama T, Funahashi T, Matsuzawa Y. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- 30.Hector J, Schwarzloh B, Goehring J, Strate TG, Hess UF, Deuretzbacher G, Hansen-Algenstaedt N, Beil FU, Algenstaedt P. TNF-alpha alters visfatin and adiponectin levels in human fat. Horm Metab Res. 2007;39:250–255. doi: 10.1055/s-2007-973075. [DOI] [PubMed] [Google Scholar]
- 31.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 32.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang Cc C, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc Natl Acad Sci U S A. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Motoshima H, Wu X, Mahadev K, Goldstein BJ. Adiponectin suppresses proliferation and superoxide generation and enhances eNOS activity in endothelial cells treated with oxidized LDL. Biochem Biophys Res Commun. 2004;315:264–271. doi: 10.1016/j.bbrc.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 34.Cheng KK, Lam KS, Wang Y, Huang Y, Carling D, Wu D, Wong C, Xu A. Adiponectin-induced endothelial nitric oxide synthase activation and nitric oxide production are mediated by APPL1 in endothelial cells. Diabetes. 2007;56:1387–1394. doi: 10.2337/db06-1580. [DOI] [PubMed] [Google Scholar]
- 35.Zhang P, Wang Y, Fan Y, Tang Z, Wang N. Overexpression of adiponectin receptors potentiates the antiinflammatory action of subeffective dose of globular adiponectin in vascular endothelial cells. Arterioscler Thromb Vasc Biol. 2009;29:67–74. doi: 10.1161/ATVBAHA.108.178061. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Park Y, Picchi A, Potter BJ. Maturation-induces endothelial dysfunction via vascular inflammation in diabetic mice. Basic Res Cardiol. 2008;103:407–416. doi: 10.1007/s00395-008-0725-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park PH, Huang H, McMullen MR, Mandal P, Sun L, Nagy LE. Suppression of lipopolysaccharide-stimulated tumor necrosis factor-alpha production by adiponectin is mediated by transcriptional and post-transcriptional mechanisms. J Biol Chem. 2008;283:26850–26858. doi: 10.1074/jbc.M802787200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ouchi N, Kihara S, Arita Y, Okamoto Y, Maeda K, Kuriyama H, Hotta K, Nishida M, Takahashi M, Muraguchi M, Ohmoto Y, Nakamura T, Yamashita S, Funahashi T, Matsuzawa Y. Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102:1296–1301. doi: 10.1161/01.cir.102.11.1296. [DOI] [PubMed] [Google Scholar]
- 39.Kamon J, Yamauchi T, Muto S, Takekawa S, Ito Y, Hada Y, Ogawa W, Itai A, Kasuga M, Tobe K, Kadowaki T. A novel IKKbeta inhibitor stimulates adiponectin levels and ameliorates obesity-linked insulin resistance. Biochem Biophys Res Commun. 2004;323:242–248. doi: 10.1016/j.bbrc.2004.08.083. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.