Abstract
Background
New-onset diabetes after transplantation (NODAT) is a major post-transplant complication associated with lower allograft and recipient survival. Our objective was to determine if metabolic syndrome pre-transplant is independently associated with NODAT development.
Methods
We recruited 640 consecutive incident non-diabetic renal transplant recipients from 3 academic centers between 1999 and 2004. NODAT was defined as use of hypoglycemic medication, a random plasma glucose >200 mg/dL, or 2 fasting glucose levels ≥126 mg/dL beyond 30 days post-transplant.
Results
Metabolic syndrome was common pre-transplant (57.2 %). NODAT developed in 31.4% of recipients one year post-transplant. Participants with metabolic syndrome were more likely to develop NODAT compared to recipients without metabolic syndrome (34.4% v. 27.4%, p=0.057). Recipients with increasing number of positive metabolic syndrome components were more likely to develop NODAT (metabolic syndrome score-prevalence at 1 year: 0-0.0%, 1-24.2, 2-29.3%, 3-31.0%, 4-34.8%, and 5-73.7%, p=0.001). After adjustment for demographics, age by decade (HR-1.34 (1.20-1.50), p<0.0001), African American race (HR-1.35 (1.01-1.82), p=0.043), cumulative prednisone dosage (HR-1.18 (1.07-1.30), p=0.001), and metabolic syndrome (HR-1.34 (1.00-1.79), p=0.047) were independent predictors of development of NODAT at 1 year post-transplant. In a multivariable analysis incorporating the individual metabolic syndrome components themselves as covariates, the only pre-transplant metabolic syndrome component to remain an independent predictor of NODAT was low HDL (HR-1.37 (1.01-1.85), p=0.042).
Conclusions
Metabolic syndrome is an independent predictor for NODAT and is a possible target for intervention to prevent NODAT. Future studies to evaluate if modification of metabolic syndrome factors pre-transplant reduces NODAT development are needed.
Keywords: Renal Transplant, NODAT, Metabolic Syndrome
INTRODUCTION
New-onset diabetes mellitus after transplantation (NODAT) is a widely recognized and serious complication in renal transplant recipients.(1) It has been associated with reduced patient and graft survival, impaired graft function,(2-5) cardiovascular disease (CVD),(5-8) and atherosclerotic events.(9) Incidence rates vary from 2 to 50% during first post-transplantation year, depending on the inclusion and diagnostic criteria.(1) Established risk factors include African American or Hispanic ethnicity,(1, 4, 10) family history of diabetes,(10-11) obesity,(4,10) pre-transplant glucose intolerance, hepatitis C infection,(4, 10, 12) cytomegalovirus infection,(13-14) adult onset polycystic kidney disease,(15-17) immunosuppressive regimen,(10,18) recipients of deceased donor kidneys,(10-11) and older age.(4, 10)
In the general population, metabolic syndrome has been associated with the development of diabetes mellitus.(19-21) However, the impact of metabolic syndrome on NODAT is not well established.
The study objective was to evaluate the association of pre-transplant metabolic syndrome with incidence of NODAT. In addition, we were interested in determining which components of the metabolic syndrome were most relevant. Determining modifiable risk factors for NODAT would be crucial to improve screening, diagnosing, and management of this post-transplant complication.
MATERIALS AND METHODS
Recipients
Our cohort consisted of 640 non-diabetic renal transplant recipients from 3 academic adult transplant centers in the Philadelphia area recruited between 1999 and 2004. Recipients with other solid organ transplants were excluded. The study was approved by the Institutional Review Board of all 3 centers, and each patient provided written informed consent.
Immunosuppression
The majority (62.3%) of recipients were on a tacrolimus based regimen as their initial calcineurin inhibitor. Doses were titrated to levels of 8-10 ng/mL during the first 3-4 months and titrated to 5 ng/mL by the first year. A cyclosporine based initial regimen was used in 20.6% of recipients. Ideally, doses were titrated to levels of 150-200 ng/mL during the first 3-4 months and titrated to 50-100 ng/mL by the first year. Recipients were also prescribed mycophenolate mofetil (MMF) along with prednisone at time of transplant that was tapered off. Usually by 3 months post-transplant, recipients were downwardly titrated to 5 mg of prednisone. However, over time all centers transitioned to tacrolimus based regimens and by 60 months post-transplant, 92% of recipients were on a tacrolimus based regimen. Recipients undergo CMV prophylaxis based on donor and recipient CMV serostatus. Typically CMV +/- receive gancyclovir 1000mg three times a day for 6 mos. CMV-/+ and CMV +/+ receive the same medication for 3 months. CMV -/- receive acyclovir.
As for mTOR inhibitors, usage is relatively low with only 69 recipients being on either sirolimus during the first 6 months post-transplant.
Study variables
EDTA-anticoagulated plasma obtained on the day of transplant was used to assay for total cholesterol, triglycerides, HDL, calculated LDL, apoA-I and apoB immediately prior to transplantation. All plasma lipid assays were analyzed using commercially available reagents from Sigma Diagnostics (St. Louis, MO). In recipients where the sample could not be obtained on the day of transplant, we included a clinically obtained lipid profile drawn within one month prior to transplantation. All assays were run using commercially available reagents on a Cobas Fara II autoanalyzer (Roche Diagnostics, New Brunswick, NJ). The triglyceride assay was an enzymatic assay. The sensitivity of the triglyceride assay was 10 mg/dL with an interassay coefficient of variation of 6%. The high-density lipoprotein (HDL) cholesterol assay was a heparin manganese precipitation method. The sensitivity of the HDL assay was 2 mg/dL, and the interassay coefficient of variation was 5%. The low-density lipoprotein (LDL) was calculated using the Friedewald formula following standard protocols. Demographics, past medical history and immunosuppressant regimen were obtained from patient interviews and medical chart abstraction.
Adapting the National Cholesterol Education Program Adult Treatment Panel III guidelines,(22) metabolic syndrome was defined as the presence of three or more of the following five components: (1) obesity with body mass index (BMI) ≥30 kg/m2; (2) triglycerides ≥150 mg/dL or on treatment; (3) HDL <40 mg/dL in men and <50 mg/dL in women; (4) systolic blood pressure ≥130 mmHg, diastolic blood pressure ≥85 mmHg, or antihypertensive therapy; and (5) fasting glucose ≥100 mg/dL. As waist circumference was not available for all recipients, BMI was used as a surrogate, which has been shown to correlate in prior studies.(23)
NODAT was defined as two measurements of fasting plasma glucose ≥126 mg/dL, a single plasma glucose >200 mg/dL or the use of insulin or an oral hypoglycemic agent between 30 days post-transplant and 1 year post-transplant.(24) We classified recipients based on the first criteria for NODAT identified. Therefore, recipients may have met one or more of the criteria for definition of NODAT. Of the 201 recipients with NODAT at 1 year, 36% met the criteria by initiation of drug treatment and the remainder by abnormal glucose as defined by ADA criteria. Any patient meeting the glucose criteria for diabetes mellitus pre-transplant was deemed undiagnosed diabetic, and thus excluded from the main cohort. There were 16 recipients with undiagnosed pre-transplant diabetes and used in a sensitivity analysis, which determined they had significantly different outcomes and should not be included in the analysis.
Statistics
Student's t-tests and χ2 tests were used to compare continuous patient measures and characteristics for recipients with and without metabolic syndrome prior to transplant as well as with and without NODAT development post transplant. A survival analysis was performed using Cox proportional hazard models to determine univariate hazard ratios and significance of the individual predictors of NODAT. Both backward and forward selection methods were used to select the components for the presented multivariable Cox regression models. Previous known associations with NODAT and variables with an association of p<0.2 were entered into a multivariable Cox regression model to determine if the relationship between NODAT and metabolic syndrome persisted. Metabolic syndrome was initially included in the model as an independent term. A second set of multivariable Cox regression models was executed substituting the individual binary metabolic syndrome components (1 for present, 0 for not present). Both stepwise-additive and backward-elimination regression model building techniques were used to evaluate the sub-components. Note in the component analysis, if a single component was missing for an individual in the subcomponent analysis, an individual would not be included in the model. Those with insufficient data to determine a metabolic syndrome score were excluded from the cohort. However, criteria for the presence of metabolic syndrome can be determined for someone missing up to 2 components if all other components are positive (or negative). Therefore, some individuals included in the presence of metabolic syndrome score models were missing from the component models. To aid in interpretation of the hazard ratio, age by decade was used. All statistical analyses were executed using SAS version 9.1 (SAS Institute, Cary, NC, USA).
RESULTS
The average age of study participants was 46.4 (standard deviation, 12.8) years. African American recipients were younger compared to non-African Americans (44.1 (11.9) v. 47.3 (13.4), p=0.004). The majority of recipients were male (58.0%), white (63.1%) while approximately a third of recipients (31.6%) were African American. The prevalence of hepatitis C seropositivity was 10.9%. The majority of patients received deceased donor transplant (60.3%). The average plasma glucose level immediately prior to transplantation was 91.5 (26.2) mg/dL.
Metabolic syndrome was common (57.2%) at time of transplant. Unadjusted associations between metabolic syndrome and baseline continuous and categorical variables, including individual components of metabolic syndrome, are listed in Table 1. Recipients with metabolic syndrome, when compared to recipients without metabolic syndrome were less likely to be African American (27.0% v. 37.6%, p=0.005 and more likely to have polycystic kidney disease as etiology of kidney disease (15.8% v. 9.5%, p=0.009).
TABLE 1.
Demographic and laboratory parameters by presence of metabolic syndrome at time of transplant
| No Metabolic Syndrome (N=274) | Metabolic Syndrome (N=366) | p-value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age (years) | 45.5 | 13.0 | 47.1 | 13.1 | 0.11 |
| Male gender (%) | 56.2% | 59.3% | 0.43 | ||
| African American (%) | 37.6% | 27.0% | 0.005 | ||
| Hepatitis C (%) | 11.3% | 10.7% | 0.79 | ||
| CMV (%) | 67.2% | 70.4% | 0.42 | ||
| Etiology of ESRD | |||||
| HTN (%)a | 75.0% | 69.9% | 0.34 | ||
| PKD (%)a | 9.5% | 15.8% | 0.009 | ||
| Deceased donor (%) | 58.4% | 61.7% | 0.39 | ||
| Preemptive transplant (%) | 15.7% | 14.9% | 0.45 | ||
| BMI (kg/m2) | 26.7 | 7.7 | 31.5 | 10.0 | <0.0001 |
| Glucose (mg/dL) | 86.1 | 23.5 | 95.5 | 27.7 | <0.0001 |
| Triglycerides (mg/dL) | 118.8 | 62.7 | 246.4 | 127.4 | <0.0001 |
| SBP (mm Hg) | 159.2 | 22.9 | 158.2 | 20.9 | 0.59 |
| DBP (mm Hg) | 90.8 | 13.9 | 88.4 | 12.5 | 0.03 |
| HDL (mg/dL) | 49.3 | 14.8 | 36.3 | 12.6 | <0.0001 |
| cLDL (mg/dL) | 93.6 | 32.7 | 97.4 | 40.3 | 0.27 |
| Total cholesterol (mg/dL) | 163.7 | 40.3 | 176.1 | 52.5 | 0.004 |
| Apo A1 (mg/dL) | 125.5 | 30.0 | 113.3 | 30.2 | <0.0001 |
| Apo B (mg/dL) | 72.4 | 21.7 | 85.4 | 28.3 | <0.0001 |
| Ratio of Apo B to Apo A1 | 0.60 | 0.21 | 0.78 | 0.25 | <0.0001 |
| White Blood Cells (x109/L) | 7.6 | 3.3 | 8.1 | 4.9 | 0.15 |
| Calcium (mg/dL) | 9.07 | 1.37 | 9.01 | 3.74 | 0.80 |
| Phosphate (mg/dL) | 5.29 | 1.84 | 5.28 | 1.72 | 0.94 |
| Hematocrit (%) | 35.0% | 4.9% | 35.0% | 6.0% | 0.95 |
| Hemoglobin (g/dL) | 11.7 | 1.6 | 11.8 | 2.1 | 0.50 |
| Platelet Count (x109/L) | 198.4 | 68.1 | 198.6 | 79.1 | 0.98 |
| Blood urea nitrogen (mg/dL) | 48.9 | 22.3 | 47.7 | 20.5 | 0.48 |
| Creatinine (mg/dL) | 8.37 | 4.08 | 8.23 | 3.75 | 0.66 |
| Albumin (g/dL) | 4.14 | 0.47 | 4.16 | 0.43 | 0.88 |
| Weight Gain, 3 months (kg) | 4.97 | 5.90 | 4.16 | 9.01 | 0.48 |
| Weight Gain, 6 months (kg) | 5.82 | 8.36 | 6.60 | 9.32 | 0.60 |
BMI- body mass index, CMV- cytomegalovirus, ESRD- end-stage renal disease, HTN- hypertension, PKD- polycystic kidney disease
The majority (92.3%) recipients were on at least one antihypertensive or had systolic blood pressure ≥130 mmHg or diastolic blood pressure ≥85 mmHg on the day they received their transplant. Eighty-three percent of recipients were on antihypertensive medications. Interestingly, 60.0% still had systolic blood pressure above 140 mmHg and 32.5% had diastolic blood pressure above 90 mmHg on the day of transplant. Statin was prescribed for 17.0% of recipients pre-transplant, but by 6 months post-transplant 58% of recipients were on this class of drug.
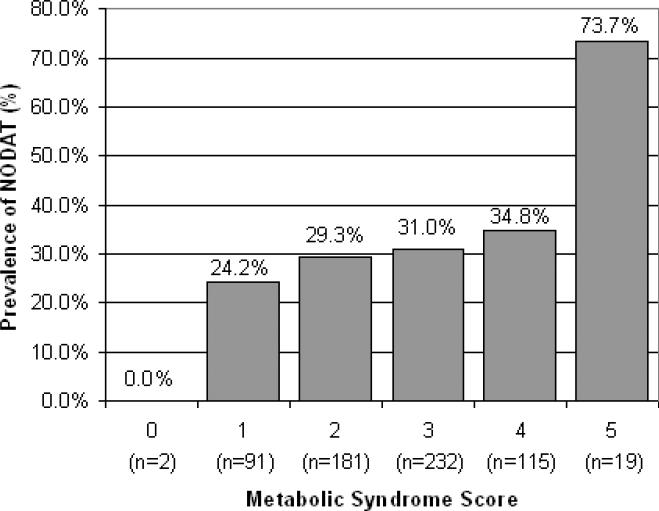
NODAT developed in 31.4% of recipients by the end of the first year post-transplant. Over half (58.7%) of the recipients who developed NODAT over the course of the 5 year follow-up period developed NODAT within the first year. Recipients with metabolic syndrome were more likely to develop NODAT than those without metabolic syndrome (34.4% v. 27.4%, p=0.057). The majority of recipients who developed NODAT within 1 year (62.7%) had metabolic syndrome at baseline. The likelihood of developing NODAT increased with the number of positive metabolic syndrome components (Figure 1). For example, 24.2% of recipients with 1 component present developed NODAT while 73.7% of recipients with all 5 components developed this complication.
FIGURE 1.
Prevalence of NODAT at 1 year by Metabolic Syndrome Score
Participants that developed NODAT were older at time of transplant (50.1 v. 44.8 years, p<0.0001) and more likely to have Hepatitis C (14.9% v. 9.1%, p=0.029). Gender did not significantly differ among the groups with and without NODAT (p=0.07). Weight gain by 3 months post-transplant was significantly different between the two groups (1.91 kg v. 5.78 kg, p=.001). However, by 6 months post-transplant, weight gain was not significantly different between the two groups (5.84 kg v. 6.42 kg, p=0.72). Additionally, African American recipients had a higher proportion of Hepatitis C seropositivity, (21.0% v. 6.4%, p<0.0001).
Table 2 provides the unadjusted hazard ratios for a priori selected variables. Female gender (HR 0.78 (0.59-1.04), p=0.09), cytomegalovirus seropositivity (HR 1.12 (0.81-1.54), p=0.50), polycystic kidney disease as etiology of ESRD (HR 1.01 (0.67-1.53), p=0.95), and use of tacrolimus (HR 1.29 (0.75-2.22), p=0.36) were not independent predictors of NODAT. In a multivariable analysis adjusted for demographics and utilizing presence of metabolic syndrome, age by decade (HR 1.34 (1.20-1.50), p<0.0001), African American race (HR 1.35 (1.01-1.82), p=0.043), cumulative prednisone dose (HR 1.18 (1.07-1.30), p=0.001), and metabolic syndrome (HR 1.34 (1.00-1.79), p=0.047) were independent predictors of development of NODAT. In a multivariable analysis incorporating the individual metabolic syndrome components themselves as covariates, only pre-transplant low HDL (HR 1.37 (1.01-1.85), p=0.042) remained an independent predictor of NODAT adjusted for age, prednisone usage, and African American race (Table 2). Using the metabolic syndrome components as continuous variables (HDL, Glucose, and triglycerides) only HDL (HR 0.985 (0.975-0.995), p=0.004) and glucose (HR 1.006 (1.002-1.011), p=0.005) were significantly associated with development of NODAT in the univariate analysis. This association persisted after adjustment for race and age [HDL (HR 0.982 (0.972-0.992), p<0.001) and glucose (HR 1.006 (1.001-1.011), p=0.02)].
TABLE 2.
Multivariable analysis of Metabolic Syndrome and its components and NODAT
| Unadj. HR (lcl – ucl) | p | Adj. HR Met. Syndrome | p | Adj. HR Components of Met. Syndrome | p | |
|---|---|---|---|---|---|---|
| Age by decade | 1.31 (1.18- 1.46) | <0.0001 | 1.34 (1.20 - 1.50) | <0.0001 | 1.35 (1.21- 1.51) | <0.0001 |
| African American | 1.19 (0.89- 1.59) | 0.23 | 1.35 (1.01 - 1.82) | 0.043 | 1.37 (1.01 - 1.85) | 0.043 |
| Female Gender | 0.78 (0.59- 1.04) | 0.09 | ||||
| Hepatitis C seropositive | 1.52 (1.03- 2.24) | 0.03 | ||||
| Cytomegalovirus seropositive | 1.12 (0.81- 1.54) | 0.50 | ||||
| Tacrolimus use | 1.29 (0.75- 2.22) | 0.36 | ||||
| Metabolic Syndrome | 1.36 (1.02- 1.82) | 0.03 | 1.34 (1.00 - 1.79) | 0.047 | ||
| BMI ≥ 30 | 1.32 (0.99- 1.75) | 0.06 | ||||
| Glucose ≥ 100 | 1.36 (1.00- 1.86) | 0.05 | ||||
| HDL<50 W or 40 M | 1.35 (1.00- 1.81) | 0.05 | 1.37 (1.01 - 1.85) | 0.042 | ||
| Triglycerides ≥ 150 | 1.27 (0.96- 1.69) | 0.09 | ||||
| Systolic BP | 1.01 (1.00- 1.02) | 0.01 | ||||
| Albumin | 0.73 (0.32- 1.68) | 0.46 | ||||
| Apo A1 | 1.00 (0.99- 1.00) | 0.22 | ||||
| Etiology of PKD | 1.01 (0.67- 1.53) | 0.95 | ||||
| Deceased Donor | 1.33 (0.99- 1.78) | 0.05 | ||||
| Prednisone usea | 1.16 (1.05- 1.28) | 0.005 | 1.18 (1.07 - 1.30) | 0.001 | 1.18 (1.07 - 1.30) | 0.001 |
Prednisone use is defined as cumulative prednisone dosage in grams
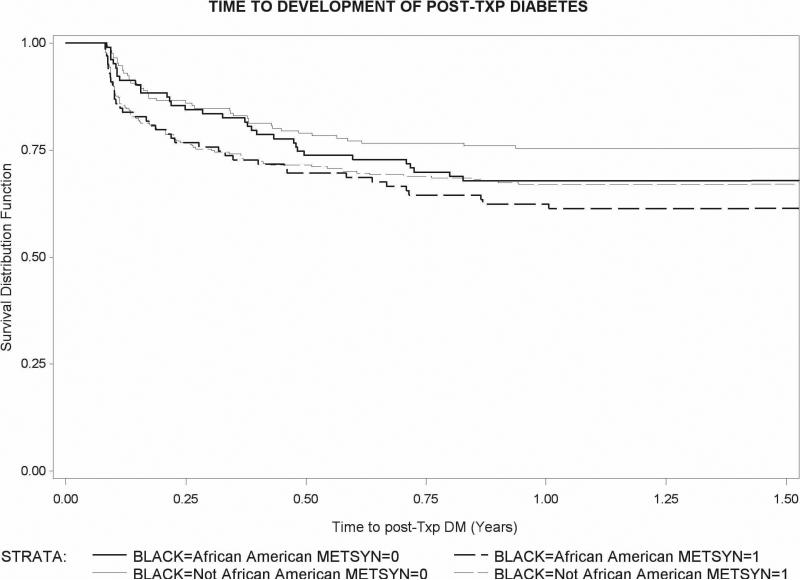
Figure 2 shows the Kaplan Meier survival curves of time to develop NODAT for recipients with and without metabolic syndrome at transplant stratified by race. African Americans with metabolic syndrome at transplant developed NODAT in higher proportion than other groups. Tests of association between these variables and time were not significant.
FIGURE 2.
Time to NODAT post-transplant; Survival Distribution Function
DISCUSSION
Our study examines the relationship between the pre-transplant metabolic syndrome and NODAT in a large cohort of incident renal recipients representative of the US ethnic background. The major findings are: (1) the presence of metabolic syndrome pre-transplantation is significantly associated with the development of NODAT; (2) age, African American race, prednisone, and presence of metabolic syndrome pre-transplant were independently associated with development of NODAT; (3) the risk for NODAT increased as the number of metabolic syndrome components that were abnormal increased; (4) of the specific pre-transplant metabolic syndrome components, only low HDL was independently associated with development of NODAT.
The prevalence of metabolic syndrome in our population at the time of transplant approximates that reported in other non-diabetic CKD(25-26) and renal transplant(1) cohorts in the US. Porrini et al. demonstrated that metabolic syndrome present at one year post-transplantation is a risk factor for subsequent NODAT beyond the second year post-transplant.(27) However, several studies have indicated that recipients are at greatest risk for NODAT within the first six months post-transplant,(28-29) with most cases occurring within one year.(1, 30) In our study, 26.4% of recipients developed NODAT within 6 months, 31.4% had developed it by one year, and 46.3% developed NODAT within 5 years post-transplant.
An interesting finding was the variation in the strength of the association between individual metabolic syndrome components and NODAT in this cohort. HDL was the only significant component of metabolic syndrome which predicted NODAT controlling for age, prednisone dosage, and African American race. In the general population, fasting glucose is the most robust component of the metabolic syndrome in predicting diabetes.(20-21, 31-33) Glucose was a significant risk factor for NODAT only when evaluated as a continuous variable. We used stringent criteria to exclude undiagnosed diabetes in our cohort. Undiagnosed pre-transplant diabetes would overestimate the association found between metabolic syndrome and NODAT and could potentially skew glucose as a more potent predictor of NODAT.
BMI or waist circumference, triglycerides, and glucose have been found to predict the development of diabetes in the general population.(19) Some studies do not support an association of triglycerides with development of diabetes,(20) and others find heightened risk with all of the metabolic syndrome components.(34) BMI(4, 35) and triglycerides have been associated with NODAT in the renal transplant population.(27)
NODAT was associated with hepatitis C seropositivity in the univariate analysis but we did not find that it was an independent predictor after adjusting for metabolic syndrome status, race, prednisone dosage, and age. Hepatitis C has effects on glucose dysregulation. While the mechanisms remain largely unknown, there is evidence that hepatitis C promotes insulin resistance.(36) This diabetogenicity is exacerbated through viral hindering of hepatic glucose uptake, glycogenesis, and insulin secretion.(37-39)
The type of immunosuppression explains 74% of the variability in incidence of NODAT.(1) Our study did not reveal a specific association between NODAT and tacrolimus use as in other studies.(15, 40-41) This is not a universal finding.(4, 35, 42-44) A possible reason to consider is the majority of recipients received tacrolimus-based immunosuppression making it more difficult to observe an independent relationship. Hypertension was quite prevalent as shown in other studies.(45)
Our study confirmed that African American race was associated with development of NODAT. African Americans with metabolic syndrome were at highest risk for NODAT. Gender was not a significant risk factor. This finding supports the work of Hamer and colleagues, who found no association between gender and NODAT.(15)
We found a higher proportion of recipients with PKD in the group with metabolic syndrome. Insulin resistance with compensatory hyperinsulinemia has been described in patients with PKD.(46) Increase membrane permeability and abnormalities in erythrocyte Na/Li transport both associated with insulin resistance have been postulated as likely culprits by the same research group.(47) In a recent report from a small cohort of Polish patients with PKD and normal kidney function, patients with PKD had higher blood pressure, abdominal obesity, and higher fasting glycemia compared to controls. However, they did not find a statistical difference in the prevalence of metabolic syndrome.(48)
There are important limitations to this study. The presence of metabolic syndrome was based on values of metabolic syndrome closest to transplant within one month. Therefore, there may have been some misclassification bias. The study population is representative of the renal recipients from our geographic area, but may not be representative of other areas with different ethnic composition. In addition, while the use of three academic centers increased our sample size, it is likely that there are health system differences in the care of renal recipients that we are not able to capture and adjust in our models. We were not able to test for other associations with metabolic syndrome that have been described such as magnesium.
In summary, our study has demonstrated that pre-transplant metabolic syndrome is an important potentially modifiable risk factor for NODAT, with a risk escalation that is directly related to the number of individual abnormal metabolic syndrome components. Future studies evaluating if modification of metabolic syndrome factors pre-transplant reduces the development of NODAT are imperative.
ACKNOWLEDGEMENTS
Presented in part at the American Heart Association; March 11-14, 2009; Palm Harbor, FL.
Support
This project was supported in part by grant K08 05656 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Rosas receives salary support from NIH grant R01 DK080033 and VA Merit Award IIR 05-247. The project also was supported by Grant Number UL1RR024134 from the National Center for Research Resources.
Abbreviations
- NODAT
New-onset diabetes after transplantation
Footnotes
Conflict of Interest
None of the authors have any conflict of interest or financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Montori VM, Basu A, Erwin PJ, Velosa JA, Gabriel SE, Kudva YC. Posttransplantation diabetes: a systematic review of the literature. Diabetes Care. 2002;25(3):583. doi: 10.2337/diacare.25.3.583. [DOI] [PubMed] [Google Scholar]
- 2.Miles AM, Sumrani N, Horowitz R, et al. Diabetes mellitus after renal transplantation: as deleterious as non-transplant-associated diabetes? Transplantation. 1998;65(3):380. doi: 10.1097/00007890-199802150-00014. [DOI] [PubMed] [Google Scholar]
- 3.Cosio FG, Pesavento TE, Kim S, Osei K, Henry M, Ferguson RM. Patient survival after renal transplantation: IV. Impact of post-transplant diabetes. Kidney Int. 2002;62(4):1440. doi: 10.1111/j.1523-1755.2002.kid582.x. [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Snyder JJ, Gilbertson D, Matas AJ. Diabetes mellitus after kidney transplantation in the United States. Am J Transplant. 2003;3(2):178. doi: 10.1034/j.1600-6143.2003.00010.x. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez-Fresnedo G, Escallada R, de Francisco AL, et al. Posttransplant diabetes is a cardiovascular risk factor in renal transplant patients. Transplant Proc. 2003;35(2):700. doi: 10.1016/s0041-1345(03)00052-6. [DOI] [PubMed] [Google Scholar]
- 6.Ojo AO. Cardiovascular complications after renal transplantation and their prevention. Transplantation. 2006;82(5):603. doi: 10.1097/01.tp.0000235527.81917.fe. [DOI] [PubMed] [Google Scholar]
- 7.Hjelmesaeth J, Hartmann A, Leivestad T, et al. The impact of early-diagnosed new-onset post-transplantation diabetes mellitus on survival and major cardiac events. Kidney Int. 2006;69(3):588. doi: 10.1038/sj.ki.5000116. [DOI] [PubMed] [Google Scholar]
- 8.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol. 2005;16(2):496. doi: 10.1681/ASN.2004070580. [DOI] [PubMed] [Google Scholar]
- 9.Ducloux D, Kazory A, Chalopin JM. Posttransplant diabetes mellitus and atherosclerotic events in renal transplant recipients: a prospective study. Transplantation. 2005;79(4):438. doi: 10.1097/01.tp.0000151799.98612.eb. [DOI] [PubMed] [Google Scholar]
- 10.Pham PT, Danovitch GM, Pham PC. The medical management of the renal transplant recipient. In: Johnson RJ, Feehally J, editors. Comprehensive clinical nephrology. Mosby; Philadelphia: 2007. p. 1085. [Google Scholar]
- 11.Sumrani NB, Delaney V, Ding ZK, et al. Diabetes mellitus after renal transplantation in the cyclosporine era--an analysis of risk factors. Transplantation. 1991;51(2):343. doi: 10.1097/00007890-199102000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Bloom RD, Rao V, Weng F, Grossman RA, Cohen D, Mange KC. Association of hepatitis C with posttransplant diabetes in renal transplant patients on tacrolimus. J Am Soc Nephrol. 2002;13(5):1374. doi: 10.1097/01.asn.0000012382.97168.e0. [DOI] [PubMed] [Google Scholar]
- 13.Hjelmesaeth J, Muller F, Jenssen T, Rollag H, Sagedal S, Hartmann A. Is there a link between cytomegalovirus infection and new-onset posttransplantation diabetes mellitus? Potential mechanisms of virus induced beta-cell damage. Nephrol Dial Transplant. 2005;20(11):2311. doi: 10.1093/ndt/gfi033. [DOI] [PubMed] [Google Scholar]
- 14.van der Werf N, Kroese FG, Rozing J, Hillebrands JL. Viral infections as potential triggers of type 1 diabetes. Diabetes Metab Res Rev. 2007;23(3):169. doi: 10.1002/dmrr.695. [DOI] [PubMed] [Google Scholar]
- 15.Hamer RA, Chow CL, Ong AC, McKane WS. Polycystic kidney disease is a risk factor for new-onset diabetes after transplantation. Transplantation. 2007;83(1):36. doi: 10.1097/01.tp.0000248759.37146.3d. [DOI] [PubMed] [Google Scholar]
- 16.Ducloux D, Motte G, Vautrin P, Bresson-Vautrin C, Rebibou JM, Chalopin JM. Polycystic kidney disease as a risk factor for post-transplant diabetes mellitus. Nephrol Dial Transplant. 1999;14(5):1244. doi: 10.1093/ndt/14.5.1244. [DOI] [PubMed] [Google Scholar]
- 17.de Mattos AM, Olyaei AJ, Prather JC, Golconda MS, Barry JM, Norman DJ. Autosomal-dominant polycystic kidney disease as a risk factor for diabetes mellitus following renal transplantation. Kidney Int. 2005;67(2):714. doi: 10.1111/j.1523-1755.2005.67132.x. [DOI] [PubMed] [Google Scholar]
- 18.Reisaeter AV, Hartmann A. Risk factors and incidence of posttransplant diabetes mellitus. Transplant Proc. 2001;33(5A Suppl):8S. doi: 10.1016/s0041-1345(01)02229-1. [DOI] [PubMed] [Google Scholar]
- 19.Sattar N, Gaw A, Scherbakova O, et al. Metabolic syndrome with and without C-reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation. 2003;108(4):414. doi: 10.1161/01.CIR.0000080897.52664.94. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, Bombelli M, Facchetti R, et al. Long-term risk of diabetes, hypertension and left ventricular hypertrophy associated with the metabolic syndrome in a general population. J Hypertens. 2008;26(8):1602. doi: 10.1097/HJH.0b013e328302f10d. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care. 2008;31(9):1898. doi: 10.2337/dc08-0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143. [PubMed] [Google Scholar]
- 23.Meigs JB, Wilson PW, Nathan DM, D'Agostino RB, Sr., Williams K, Haffner SM. Prevalence and characteristics of the metabolic syndrome in the San Antonio Heart and Framingham Offspring Studies. Diabetes. 2003;52(8):2160. doi: 10.2337/diabetes.52.8.2160. [DOI] [PubMed] [Google Scholar]
- 24.Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(Suppl 1):S42. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 25.Young DO, Lund RJ, Haynatzki G, Dunlay RW. Prevalence of the metabolic syndrome in an incident dialysis population. Hemodial Int. 2007;11(1):86. doi: 10.1111/j.1542-4758.2007.00158.x. [DOI] [PubMed] [Google Scholar]
- 26.Lea J, Cheek D, Thornley-Brown D, et al. Metabolic syndrome, proteinuria, and the risk of progressive CKD in hypertensive African Americans. Am J Kidney Dis. 2008;51(5):732. doi: 10.1053/j.ajkd.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 27.Porrini E, Delgado P, Bigo C, et al. Impact of metabolic syndrome on graft function and survival after cadaveric renal transplantation. Am J Kidney Dis. 2006;48(1):134. doi: 10.1053/j.ajkd.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 28.Cosio FG, Pesavento TE, Osei K, Henry ML, Ferguson RM. Post-transplant diabetes mellitus: increasing incidence in renal allograft recipients transplanted in recent years. Kidney Int. 2001;59(2):732. doi: 10.1046/j.1523-1755.2001.059002732.x. [DOI] [PubMed] [Google Scholar]
- 29.Davidson J, Wilkinson A, Dantal J, et al. New-onset diabetes after transplantation: 2003 International consensus guidelines.. Proceedings of an international expert panel meeting.; Barcelona, Spain. 19 February 2003; [DOI] [PubMed] [Google Scholar]; Transplantation. 2003;75(10 Suppl):SS3. doi: 10.1097/01.TP.0000069952.49242.3E. [DOI] [PubMed] [Google Scholar]
- 30.Abbott KC, Lentine KL, Bucci JR, et al. Impact of diabetes and hepatitis after kidney transplantation on patients who are affected by hepatitis C virus. J Am Soc Nephrol. 2004;15(12):3166. doi: 10.1097/01.ASN.0000145439.48387.BF. [DOI] [PubMed] [Google Scholar]
- 31.Sattar N, McConnachie A, Shaper AG, et al. Can metabolic syndrome usefully predict cardiovascular disease and diabetes? Outcome data from two prospective studies. Lancet. 2008;371(9628):1927. doi: 10.1016/S0140-6736(08)60602-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang JJ, Hu G, Miettinen ME, Tuomilehto J. The metabolic syndrome and incident diabetes: assessment of four suggested definitions of the metabolic syndrome in a Chinese population with high post-prandial glucose. Horm Metab Res. 2004;36(10):708. doi: 10.1055/s-2004-826020. [DOI] [PubMed] [Google Scholar]
- 33.Cameron AJ, Magliano DJ, Zimmet PZ, et al. The metabolic syndrome as a tool for predicting future diabetes: the AusDiab study. J Intern Med. 2008;264(2):177. doi: 10.1111/j.1365-2796.2008.01935.x. [DOI] [PubMed] [Google Scholar]
- 34.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 35.Fabrizi F, Lampertico P, Lunghi G, Mangano S, Aucella F, Martin P. Review article: hepatitis C virus infection and type-2 diabetes mellitus in renal diseases and transplantation. Aliment Pharmacol Ther. 2005;21(6):623. doi: 10.1111/j.1365-2036.2005.02389.x. [DOI] [PubMed] [Google Scholar]
- 36.Delgado-Borrego A, Casson D, Schoenfeld D, et al. Hepatitis C virus is independently associated with increased insulin resistance after liver transplantation. Transplantation. 2004;77(5):703. doi: 10.1097/01.tp.0000114283.04840.3a. [DOI] [PubMed] [Google Scholar]
- 37.Aytug S, Reich D, Sapiro LE, Bernstein D, Begum N. Impaired IRS-1/PI3-kinase signaling in patients with HCV: a mechanism for increased prevalence of type 2 diabetes. Hepatology. 2003;38(6):1384. doi: 10.1016/j.hep.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 38.Masini M, Campani D, Boggi U, et al. Hepatitis C virus infection and human pancreatic beta-cell dysfunction. Diabetes Care. 2005;28(4):940. doi: 10.2337/diacare.28.4.940. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi T, Yoshida T, Harada M, et al. Hepatitis C virus down-regulates insulin receptor substrates 1 and 2 through up-regulation of suppressor of cytokine signaling 3. Am J Pathol. 2004;165(5):1499. doi: 10.1016/S0002-9440(10)63408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.First MR, Gerber DA, Hariharan S, Kaufman DB, Shapiro R. Posttransplant diabetes mellitus in kidney allograft recipients: incidence, risk factors, and management. Transplantation. 2002;73(3):379. doi: 10.1097/00007890-200202150-00011. [DOI] [PubMed] [Google Scholar]
- 41.Rike AH, Mogilishetty G, Alloway RR, et al. Cardiovascular risk, cardiovascular events, and metabolic syndrome in renal transplantation: comparison of early steroid withdrawal and chronic steroids. Clin Transplant. 2008;22(2):229. doi: 10.1111/j.1399-0012.2007.00779.x. [DOI] [PubMed] [Google Scholar]
- 42.Crutchlow MF, Bloom RD. Transplant-associated hyperglycemia: a new look at an old problem. Clin J Am Soc Nephrol. 2007;2(2):343. doi: 10.2215/CJN.03671106. [DOI] [PubMed] [Google Scholar]
- 43.Burroughs TE, Swindle J, Takemoto S, et al. Diabetic complications associated with new-onset diabetes mellitus in renal transplant recipients. Transplantation. 2007;83(8):1027. doi: 10.1097/01.tp.0000259617.21741.95. [DOI] [PubMed] [Google Scholar]
- 44.Heisel O, Heisel R, Balshaw R, Keown P. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant. 2004;4(4):583. doi: 10.1046/j.1600-6143.2003.00372.x. [DOI] [PubMed] [Google Scholar]
- 45.Armstrong KA, Campbell SB, Hawley CM, Nicol DL, Johnson DW, Isbel NM. Obesity is associated with worsening cardiovascular risk factor profiles and proteinuria progression in renal transplant recipients. Am J Transplant. 2005;5(11):2710. doi: 10.1111/j.1600-6143.2005.01073.x. [DOI] [PubMed] [Google Scholar]
- 46.Vareesangthip K, Tong P, Wilkinson R, Thomas TH. Insulin resistance in adult polycystic kidney disease. Kidney Int. 1997;52(2):503. doi: 10.1038/ki.1997.360. [DOI] [PubMed] [Google Scholar]
- 47.Vareesangthip K, Thomas TH, Wilkinson R. Abnormal effect of thiol groups on erythrocyte Na/Li countertransport kinetics in adult polycystic kidney disease. Nephrol Dial Transplant. 1995;10(12):2219. doi: 10.1093/ndt/10.12.2219. [DOI] [PubMed] [Google Scholar]
- 48.Pietrzak-Nowacka M, Safranow K, Byra E, Binczak-Kuleta A, Ciechanowicz A, Ciechanowski K. Metabolic syndrome components in patients with autosomal-dominant polycystic kidney disease. Kidney Blood Press Res. 2009;32(6):405. doi: 10.1159/000260042. [DOI] [PubMed] [Google Scholar]